Abstract
Studies suggest that microRNA (miR)-34c may serve a role in cognitive function in rodent and primate groups. A previous study demonstrated an increase in miR-34c expression in chronic epileptic rats with memory disorders, induced by pentylenetetrazol (PTZ). However, the mechanism underlying the effects of miR-34c on cognitive function in epileptic rats remains unclear. Therefore, the present study investigated alterations in cognitive function in temporal lobe epileptic rats, induced by repeated injections of PTZ, following treatment with an miR-34c agomir compared with a scramble group. Increased expression of miR-34c was observed in the agomir group, in addition to an increased deficit in learning and memory function in the Morris water maze test. Glutamate receptor ionotropic N-methyl-D-aspartate (NMDA) 2B (NR2B), phosphorylated (p)-reduced nicotinamide-adenine dinucleotide phosphate-dependent diflavin oxidoreductase 1 (NR1) and p-glutamate receptor 1 (GluR1) protein expression was detected in the hippocampus using western blotting. Additionally, the downregulation of NR2B, p-NR1 and p-GluR1 in the miR-34c agomir group demonstrated that miR-34c may serve a negative role in cognitive function in epileptic seizures, by dysregulating NMDA and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors, which are associated with long-term potentiation.
Keywords: epilepsy, microRNA-34c, cognitive function, long-term potentiation, glutamate receptor ionotropic N-methyl-D-aspartate 2B
Introduction
Epilepsy may cause cognitive dysfunction and is increasingly becoming a focus of research. Previous studies have demonstrated that the abnormal expression and function of microRNAs (miRs/miRNAs) is associated with the learning and memory impairments observed in disorders of the nervous system, including epilepsy and Alzheimer's disease (1,2). However, the mechanism through which this impairment occurs remains unclear. miRs are a type of endogenous single-stranded small molecule RNA that exist in animal and plant cells, and cause target mRNA degradation or translational inhibition by acting on specific mRNA 3′ untranslated regions (3). miR-34c is a member of the miR-34 family. It has been hypothesized that miR-34c may be a regulatory factor of cognitive function (4). Previous studies have demonstrated that, in neurodegenerative disease, the expression of miR-34c decreases following treatment with an inhibitor and that this may improve learning ability (5,6). miR-34c expression was observed to be downregulated by silencing miR-34c, and this improved the cognitive impairment caused by ketamine (7).
Long-term potentiation (LTP) is a form of synaptic plasticity and is hypothesized to be one of the major cellular mechanisms underlying learning and memory (8,9). Previous studies have demonstrated that miRNAs serve a role in LTP by regulating the synthesis of proteins associated with LTP maintenance, including N-methyl-D-aspartate receptors (NMDARs) and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors (AMPARs). Previous studies have observed that miR-34a and miR-132 regulate LTP maintenance by acting on LTP-associated specific mRNAs (10,11). NMDARs serve an important role in switching synaptic activity-specific patterns into long-term changes in synaptic function and structure associated with learning and memory (12).
miR-34c serves an important role in numerous cognitive disorders, but few studies have focused on the role of miR-34c in cognitive impairment caused by epilepsy. In a previous study (13), the expression of miR-34c in pentylenetetrazol (PTZ)-induced epileptic rats with memory impairment was demonstrated to be increased; however, the potential regulatory effects of miR-34c on cognitive function in epilepsy, via the regulation of LTP maintenance, remain unknown. Therefore, the present study aimed to use a PTZ-induced epileptic rat model to investigate the association between miR-34c and memory impairment in epileptic rats, and the underlying mechanism. Repetitive PTZ kindling was performed to induce the temporal lobe epilepsy (TLE) model and an miR-34c agomir was used as an exacerbating treatment, in place of an miR-34c inhibitor, in order to explore the mechanisms, as a previous study reported an incidence of 19.23% (10/52) TLE rats with cognitive deficits (13).
Materials and methods
Animals and grouping
A total of 36 healthy male Sprague Dawley rats (9–10 weeks-old, 220–240 g) were provided by the Animal Experimental Center of Guangxi Medical University (Nanning, China). Animals were housed in groups of five under the following conditions: 22–26°C room temperature, 50–60% humidity, under a 12 h light/dark cycle, with lights on at 8:00 a.m. The rats were given free access to food and water. Animals were handled according to the guidelines on animal experimentation of the Council for International Organizations of Medical Sciences (World Health Organization, Geneva, Switzerland), and the Guangxi Medical University Animal Care and Use Committee approved the animal protocols.
Epileptic rats were randomly divided into 2 groups of 12 rats, including the epilepsy group and the miR-34c agomir group. Additionally, 12 rats were used as the sham group, receiving treatment with an equal amount of saline solution administered into the abdominal cavity.
Model establishment and drug delivery
The rats were treated with 60 mg/kg PTZ (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) by intraperitoneal (i.p.) injection. All of the rats were observed for 20 min following PTZ injection and behavioral alterations were recorded and graded according to the Racine grading system as follows: 0, No reaction; grade I, face and ears twitching; grade II, nodding, neck and back spasms; grade III, unilateral forelimb clonus; grade IV, bilateral forelimb clonus and standing; and grade V, systemic seizure and loss of balance. Rats with grade IV or V seizures were selected as successful epilepsy models. These rats were secured in stereotaxic apparatus (Shanghai Alcott Biotech Co., Ltd., Shanghai, China) following anesthetization with 10% chloral hydrate (3.5 ml/kg; i.p.), on the 2nd day following treatment with PTZ. The skin was cut along the middle of the head and the anterior fontanel was exposed. The right ventricle was positioned (AP=−0.9 mm, R=−1.3 mm, V=−3.5 mm) in the stereotaxic atlas according to Paxinos et al (14). A 10 µl micro syringe was fixed on the micro injection pump pointing to the hole of the right ventricle drilled on the skull with a syringe needle. The speed of injection was set at 3 min/µl. Rats in the miR-34c agomir group were injected with Rattus norvegicus-miR-34c agomir (cat. no. miR40004723-1-10, Guangzhou RiboBio Co., Ltd., Guangzhou, China), while rats in the epilepsy group were injected with an equivalent amount of scramble miR (cat. no. miR04101-1-10, Guangzhou RiboBio Co., Ltd.) into the right ventricle. The needle remained in place for 10 min subsequent to the injection. The rats received a repeated i.p. injection of 35 mg/kg PTZ every other 48 h from the 3rd day following surgery, with a total of 14 injections, to rekindle the seizures.
Morris water maze test
The Morris water maze test was used to evaluate the cognitive function of the rats. The Morris water maze included a round pool (diameter, 120 cm; height, 50 cm; depth, 25 cm). Additionally, there was a platform with a diameter of 10 cm, located 2 cm below the water level. The water in the maze was kept at a temperature of 22±1°C. A digital camera was connected to the computer monitor screen above the pool, and the data were acquired and processed using the SLY-WMS Morris water maze system (version 2.1, Panlab, S.L.U. Barcelona, Spain).
The place navigation test lasted for 5 days, and training occurred at 9:00 a.m. every day. The rats were placed into the water in each the four quadrants, sequentially in a clockwise manner. The time that the rats took to find the platform was observed and recorded (escape latency). If a rat failed to find the platform within 90 sec, they were guided to and kept on the platform for 10 sec and the escape latency was recorded as 90 sec.
The spatial probe test was used to evaluate the ability of the rats to remember spatial locations, subsequent to the rats having learned how to find the platform. On the 6th day, the platform was removed and the rats were observed for 90 sec in the water. The frequency with which the rat travelled to the original position of the platform (crossing time), and the time and distance percentages that they remained in the target quadrant, were recorded.
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR) analysis of miR-34c
After 1 h finishing the final water maze test, the rats were anesthetized by injecting 10% chloral hydrate (300 mg/kg) intraperitoneally, and were perfused by normal saline intracardially. The hippocampal tissue was isolated on ice for PCR and western blotting. The expression of miR-34c was detected by RT-qPCR to verify the effect of the agomir. The miR-34c primers were designed and synthesized by Takara Biotechnology Co., Ltd. (cat. no. MQA174, Takara, Dalian, China). Total RNA was extracted using TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA). The concentration and purity of the RNA were measured using the Scientific NanoDrop Thermo 2000 spectrophotometer (Thermo Fisher Scientific, Inc., Wilmington, DE, USA). The amplification of miR-34c cDNA was performed using an Mir-X™ miRNA First-Strand Synthesis kit (Takara Biotechnology Co., Ltd.). The reaction system was at a volume of 10 µl, and the cDNA was stored at −20°C. Using an Mir-X miRNA RT-qPCR SYBR kit (Takara, Biotechnology Co., Ltd.), the PCR was performed using the Light Cycler 480 Real-time PCR system (denaturation at 95°C for 10 sec; 40 cycles of amplification at 95°C for 5 sec and 60°C for 20 sec). U6 (forward, 5′-GCTTCGGCAGCACATATACTAAAAT-3′ and reverse 5′-CGCTTCACGAATTTGCGTGTCAT-3′) was used as an internal control. The relative amount of miR-34c in the hippocampal tissue was analyzed using the relative quantification 2−ΔΔCq method (15).
miR-34c target gene prediction and functional analysis
Potential target genes of miR-34c were predicted using MicroCosm (https://www.microcosm.com/), miRanda (http://34.236.212.39/microrna/home.do), and miRDB (http://www.mirdb.org/). The Gene Ontology (GO; www.geneontology.org/) and Kyoto Encyclopedia of Genes and Genomes (KEGG; www.genome.jp/keg/) databases were used to obtain information regarding the functions of the predicted target genes. GO and KEGG analysis was performed on the predicted target genes of miR-34c to investigate the regulatory mechanism of the overexpressed miR-34c in response to cognitive impairment in epilepsy.
Immunofluorescence staining
After perfusion with normal saline intracardially, the rats were sacrificed and brain tissue was removed, postfixed overnight in the same solution at 4°C, embedded in paraffin wax, and sectioned coronally at 3 µm for immunofluorescence staining. In brief, the slices were immune-labeled using primary antibody specific to glutamate receptor ionotropic, NMDA 2B (NR2B; 1:1,000; cat. no. 14544, Cell Signaling Technology, Inc., Danvers, MA, USA) diluted in PBS overnight at 4°C. Following washing with PBS three times, the slices were incubated with the secondary antibody (goat anti-rabbit immunoglobulin G; 1:1,000; cat. no. A8275, Sigma-Aldrich; Merck KGaA) at room temperature for 1 h. Fluorescence images were acquired using an Olympus fluorescence microscope (Olympus Corporation, Tokyo, Japan) and an image capture system.
Western blotting
Expression of NR2B protein in whole cell lysate, and phosphorylated (p)-NADPH-dependent diflavin oxidoreductase 1 (NR1) and p-glutamate receptor 1 (GluR1) protein at the postsynaptic density (PSD), were detected using western blotting. The total protein was extracted using radioimmunoprecipitation assay buffer (Beyotime Institute of Biotechnology, Haimen, China), and was detected using a bicinchoninic acid protein assay kit. Isolated protein harvested from 150 g fresh brain tissue was heat-denatured at 100°C for 5 min, subjected to electrophoresis on a 10% SDS-PAGE gel for 2.5 h and transferred to a 0.45 mm polyvinylidene fluoride membrane (EMD Millipore, Billerica, MA, USA) using the semi-dry transfer apparatus. The membranes were blocked in 5% skimmed milk for 1 h at 24°C and incubated on ice overnight with the following primary antibodies: Anti-NR2B (1:1,000), with anti-GAPDH (1:5,000; cat. no. 2118, Cell Signaling Technology, Inc.) as an internal control; anti-NR1 (1:1,000; cat. no. 5704, Cell Signaling Technology, Inc.), with anti-β-actin (1:5,000; cat. no. 8457, Cell Signaling Technology, Inc.) as an internal control; and anti-GluR1 (1:1,000; cat. No. 8850, Cell Signaling Technology, Inc.), with anti-β-actin (1:5,000) as an internal control. The membranes were incubated with an Alexa Fluor® secondary anti-rabbit antibody (1:5,000; cat. no. 8889, Cell Signaling Technology, Inc.) for 1 h following washing at room temperature. The bands were scanned using the LI-COR Odyssey imaging system (LI-COR Biosciences, Lincoln, NE, USA), and analyzed using LI-COR Odyssey software (version 3.0; LI-COR Biosciences).
Statistical analysis
Data are presented as the mean ± standard deviation. SPSS statistical software (version 16.0; SPSS, Inc., Chicago, IL, USA) was used to analyze the data and single factor analysis of variance followed by the Least-Significant Difference post-hoc test, which was performed to compare the groups. P<0.05 was considered to indicate a statistically significant difference.
Results
miR-34c serves a role in impairing cognitive function
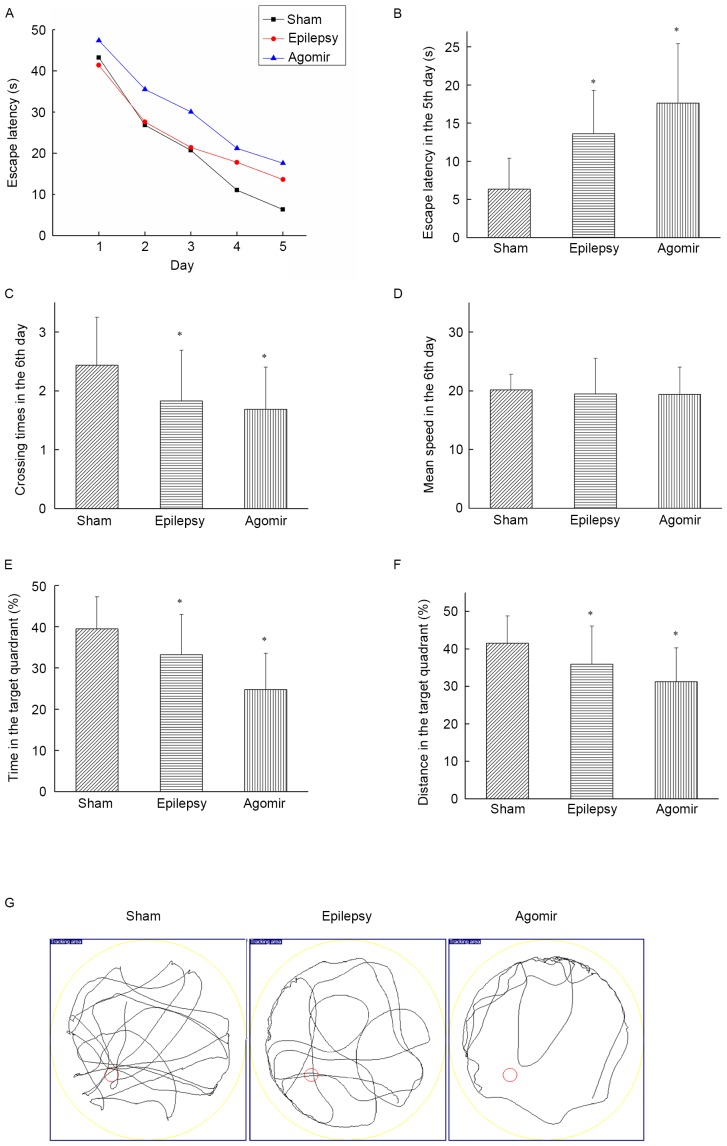
The place navigation test may be used to analyze learning ability in animals. The results of the present study demonstrated that the escape latency of each group decreased as the number of training days increased. The miR-34c agomir group exhibited increased escape latencies at each time point compared with the sham and epilepsy groups (Fig. 1A). As presented in Fig. 1B, compared with the sham group, the escape latency on the 5th day was significantly increased in the epilepsy and miR-34c agomir groups (both P<0.05), indicating a decreased learning ability following epileptic seizures. Additionally, rats in the miR-34c agomir group exhibited an impairment in studying ability.
Figure 1.
Results of the Morris water maze test. (A) The trend of escape latency during 5 days. (B) The escape latency of each group on the 5th day. (C) Crossing times of each group on the 6th day. (D) The mean speed of each group on the 6th day. (E) The percentage of time that rats stayed in target quadrant. (F) The percentage of distance that rats stayed in target quadrant. (G) Track plot from the 6th day. Data are presented as the mean ± standard deviation. *P<0.05 vs. sham group.
On the 6th day, the platform was removed to perform the spatial probe test. As presented in Fig. 1C, the epilepsy and miR-34c agomir groups exhibited significantly decreased crossing times compared with the sham group (both P<0.05); however, no significant differences were observed between the epilepsy and agomir groups. As presented in Fig. 1D, no differences were observed in mean speed among the three groups on the 6th day. The time and distance for which the rats remained in the target quadrant in the epilepsy and miR-34c agomir groups were decreased compared with the sham group (all P<0.05; Fig. 1E and F). In the miR-34c agomir group, the time and distance for which the rats remained in the target quadrant were significantly decreased compared with the epilepsy group (both P<0.05). The results of the present study demonstrated that treatment with the miR-34c agomir led to memory impairment. The track plot from the 6th day is presented in Fig. 1G.
miR-34c is upregulated following epilepsy induced by PTZ
As presented in Fig. 2, compared with the sham group, the miR-34c expression in the epilepsy group was significantly upregulated (P<0.05), demonstrating that miR-34c was increased following epilepsy induced by PTZ. In addition, the expression of miR-34c in the miR-34c agomir group was also significantly increased compared with the sham group (P<0.05).
Figure 2.
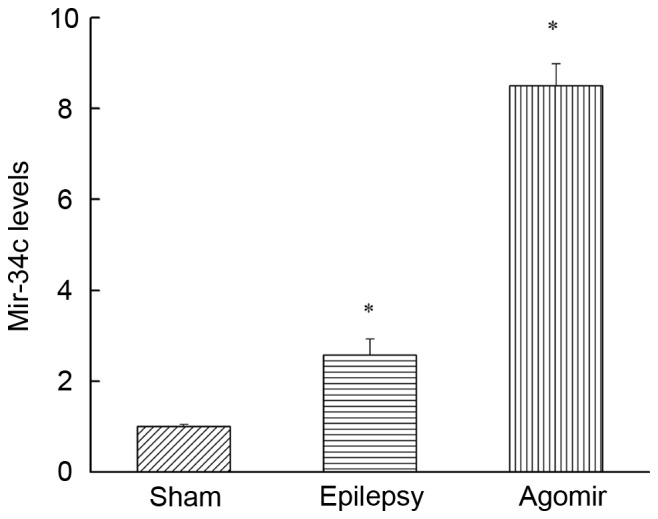
Expression of miR-34c. Data are presented as the mean ± standard deviation. *P<0.05 vs. sham group. miR, microRNA.
Functional analysis of miR-34c
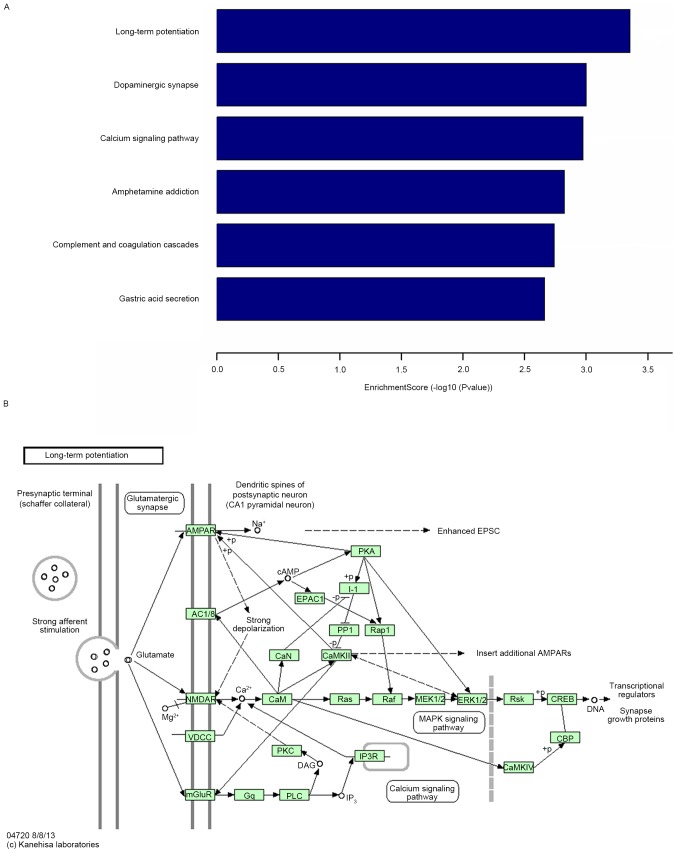
Following KEGG pathway enrichment analysis, the predicted target genes of miR-34c were primarily enriched in six KEGG pathways, including LTP, dopaminergic synapse, calcium signaling pathway and amphetamine addiction. It is known that LTP serves an important role in cognitive function (Fig. 3).
Figure 3.
Functional analysis of miR-34c. (A) The enrichment score of the main pathways of predicted target genes of miR-34c. (B) Long-term potentiation pathway. Data are presented as the mean ± standard deviation. An enrichment score >2 was taken as the threshold. miR, microRNA.
Expression of NR2B in the cortex
As demonstrated by counting positive cells in the cortex, rats in the epilepsy and miR-34c agomir groups exhibited decreased NR2B expression compared with the sham group, and the number of NR2B-positive cells in miR-34c agomir group was decreased compared with the sham group (Fig. 4).
Figure 4.
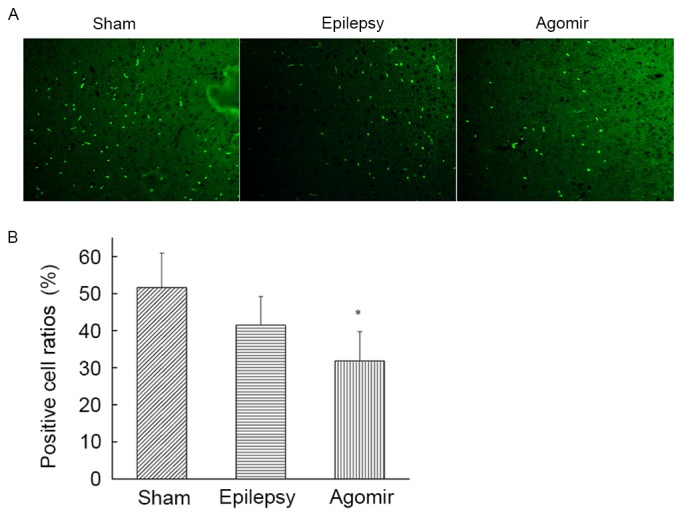
Detection of NR2B protein in the cortex by immunofluorescence. (A) Representative samples (magnification, ×200) from each group (n=6). Green staining indicates NR2B expression in the cells. (B) Ratio of NR2B-positive cells in each group. Data are presented as the mean ± standard deviation. *P<0.05 vs. sham group. NR2B, Glutamate receptor ionotropic, N-methyl-D-aspartate 2B.
Expression of NR2B, p-GluR1 and p-NR1 in the hippocampus
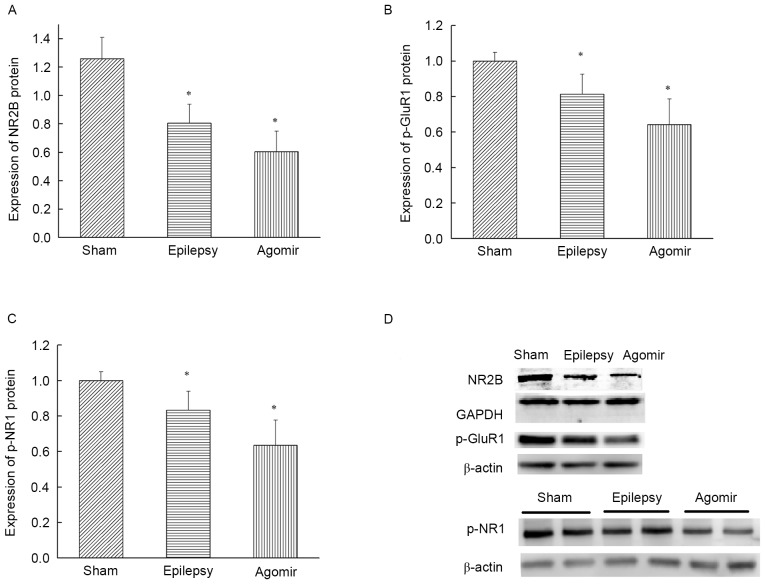
As presented in Fig. 5A, the expression of NR2B in the epilepsy group was significantly downregulated compared with the sham group (P<0.05), indicating that NR2B expression in the hippocampus was decreased following epilepsy. The expression of NR2B protein in the miR-34c agomir group was also decreased compared with the sham group (P<0.05), which demonstrated that the levels of NR2B in the hippocampus were consistent with those in the cortex.
Figure 5.
Expression of NR2B, p-GluR1 and p-NR1 in the hippocampus. (A) Total NR2B protein levels in the hippocampus. (B) Expression of p-GluR1 at the PSD of the hippocampus. (C) Expression of p-NR1 at the PSD of the hippocampus. (D) Representative samples from western blotting. Data are presented as the mean ± standard deviation. *P<0.05 vs. sham group. NR2B, Glutamate receptor ionotropic, N-methyl-D-aspartate 2B; p-GluR1, phosphorylated glutamate receptor 1; NR1, NADPH-dependent diflavin oxidoreductase 1; PSD, postsynaptic density.
Compared with the sham group, the levels of p-GluR1 and p-NR1 in the PSD of the hippocampus in the epilepsy group were significantly decreased (P<0.05). In addition, the expression of p-GluR1 and p-NR1 in the PSD of the hippocampus in the miR-34c agomir group were also decreased compared with the sham group (P<0.05) (Fig. 5B and C). The results of the western blot analysis are presented in Fig. 5D.
Discussion
In the clinical manifestation of epilepsy, particularly TLE, 30–40% of patients develop memory impairment, attention dispersion and other cognitive disorders (16). PTZ-induced epileptic rats are similar to humans with temporal lobe epilepsy. PTZ-induced rats are frequently used to study the mechanisms of cognitive impairment in epilepsy. Cognitive impairment due to TLE has been demonstrated to be associated with lesions of the limbic system, including the hippocampus. Studies have demonstrated that the synaptic plasticity of the hippocampus, represented by LTP (17) and long term depression (18,19), is associated with cognitive function.
The chronic epilepsy modal generated in the present study began with an injection of PTZ (60 mg/kg) as a preconditioning treatment, followed by a total of 14 injections of PTZ (35 mg/kg), instead of the standard 15 injections, to mimic a prominent feature of clinical patients with epilepsy. miRNA agomir is a chemically-modified antisense strand containing 2 phosphorothioates at the 5′ end, 4 phosphorothioates and 4 cholesterol groups at the 3′ end, and a full-length nucleotide 2′-methoxy modification. Agomirs are able to mimic mature endogenous miRNAs and stimulate miRNA activity following transfer into cells in vivo. Agomirs and antagomirs have been widely-used in vivo to investigate the role of miRNAs (20).
The results of the present study demonstrated that LTP is one of the principal pathways of miR-34c, via KEGG pathway enrichment analysis. LTP in the hippocampus is a cellular mechanism hypothesized to underlie memory formation, and previous studies have demonstrated that stress may acutely and chronically impair memory acquisition and LTP induction (21,22). When glutamate activates NMDARs on the postsynaptic membrane, the influx of Ca2+ is increased, causing a series of cascade reactions and leading to LTP. NMDARs are voltage-dependent ligand-gated ionotropic glutamate receptors and exhibit an increased permeability to calcium ions. NMDARs, including NR1, NR2A-D and NMDA receptor subunit 3, are expressed in the cerebral cortex and hippocampus. NR2A-D act as regulatory subunits. NR2B is the primary regulatory subunit of NMDAR, causing the change in Ca2+ permeability and serving an important role in learning and memory. It is frequently termed a ‘smart gene’ (23). Functional NMDARs consist of 1–2 constitutive glycine-binding NR1 subunits and 1–2 NR2 glutamate-binding subunits (24,25). A previous study demonstrated that the specific deletion of the NR1 subunit of NMDAR may lead to hippocampus-specific manipulation of LTP and an increase in a behavioral phenotype which impairs spatial working memory (26). Previous studies have observed that when rats were injected with an NMDA receptor antagonist, spatial working memory was impaired (27,28), while learning ability was improved by increasing the level of NR2B (29). Genetic deletion of the NR2B subunit in the hippocampus or forebrain may lead to memory deficits and impaired LTP (30). Similar results demonstrated that acute administration of ghrelin increased NR2B protein levels in the hippocampus and may result in increased LTP generation and long-term memory (31). The results of the present study indicated that, when treated with miR-34c agomir, cognitive function in epileptic rats was impaired and the expression of NR2B in the cortex, and p-NR1 and NR2B in the hippocampus, were decreased. It was hypothesized that miR-34c may exert an effect on cognitive dysfunction in epileptic rats induced by PTZ, which may account for the impaired LTP.
GluR1 (also termed GluR-A or GluA1) acts as an AMPAR subunit. GluR1-containing AMPARs serve a role in hippocampus-dependent forms of learning and memory (32). Previous studies have demonstrated that GluR1 deletion may impair spatial working memory. The influx of Ca2+, activated by NMDARs, stimulates calcium/calmodulin-dependent protein kinase type II subunit γ (CaMKII), which phosphorylates the GluR1 subunit of the AMPARs (24). The upregulation of NR2B subunits may increase the influx of Ca2+ and activation of CaMKII (31). NMDAR activation and downstream signaling events induced by Ca2+ influx result in the phosphorylation of GluR1-containing AMPARs (33). It was hypothesized that downregulation of NR2B subunits may cause a decrease in Ca2+ influx and activation of CaMKII, leading to p-GluR1 expression at the PSD of the hippocampus and resulting in LTP impairment.
In conclusion, the present study used a PTZ-induced epileptic rat model and caused miR-34c overexpression using treatment with miR-34c agomir. The results of the present study demonstrated that, following epileptic seizures, the levels of miR-34c were upregulated and cognitive function was impaired, possibly due to the increased expression of miR-34c. It was hypothesized that miR-34c may serve a negative role in cognitive function in a PTZ-induced epileptic model, by reducing the expression of NR2B, p-NR1 and p-GluR1 proteins associated with LTP, in pathways including the NMDARs and AMPARs, which may elucidate a potential anabolic strategy for treating cognitive dysfunction in epilepsy.
Acknowledgements
The present study was supported by the National Natural Science Foundation of China (grant nos. 81360201 and 81160167).
References
- 1.Fineberg SK, Kosik KS, Davidson BL. MicroRNAs potentiate neural development. Neuron. 2009;64:303–309. doi: 10.1016/j.neuron.2009.10.020. [DOI] [PubMed] [Google Scholar]
- 2.Smalheiser NR, Lugli G. microRNA regulation of synaptic plasticity. Neuromolecular Med. 2009;11:133–140. doi: 10.1007/s12017-009-8065-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ouellet DL, Perron MP, Gobeil LA, Plante P, Provost P. MicroRNAs in gene regulation: When the smallest governs it all. J Biomed Biotechnol. 2006;2006:69616. doi: 10.1155/JBB/2006/69616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parsons MJ, Grimm CH, Paya-Cano JL, Sugden K, Nietfeld W, Lehrach H, Schalkwyk LC. Using hippocampal microRNA expression differences between mouse inbred strains to characterise miRNA function. Mamm Genome. 2008;19:552–560. doi: 10.1007/s00335-008-9116-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haramati S, Navon I, Issler O, Ezra-Nevo G, Gil S, Zwang R, Hornstein E, Chen A. MicroRNA as repressors of stress-induced anxiety: The case of amygdalar miR-34. J Neurosci. 2011;31:14191–14203. doi: 10.1523/JNEUROSCI.1673-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zovoilis A, Agbemenyah HY, Agis-Balboa RC, Stilling RM, Edbauer D, Rao P, Farinelli L, Delalle I, Schmitt A, Falkai P, et al. microRNA-34c is a novel target to treat dementias. EMBO J. 2011;30:4299–4308. doi: 10.1038/emboj.2011.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cao SE, Tian J, Chen S, Zhang X, Zhang Y. Role of miR-34c in ketamine-induced neurotoxicity in neonatal mice hippocampus. Cell Biol Int. 2015;39:164–168. doi: 10.1002/cbin.10349. [DOI] [PubMed] [Google Scholar]
- 8.Ryan B, Joilin G, Williams JM. Plasticity-related microRNA and their potential contribution to the maintenance of long-term potentiation. Front Mol Neurosci. 2015;8:4. doi: 10.3389/fnmol.2015.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stepan J, Dine J, Eder M. Functional optical probing of the hippocampal trisynaptic circuit in vitro: Network dynamics, filter properties, and polysynaptic induction of CA1 LTP. Front Neurosci. 2015;9:160. doi: 10.3389/fnins.2015.00160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bowden JB, Abraham WC, Harris KM. Differential effects of strain, circadian cycle, and stimulation pattern on LTP and concurrent LTD in the dentate gyrus of freely moving rats. Hippocampus. 2012;22:1363–1370. doi: 10.1002/hipo.20972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Joilin G, Guévremont D, Ryan B, Claudianos C, Cristino AS, Abraham WC, Williams JM. Rapid regulation of microRNA following induction of long-term potentiation in vivo. Front Mol Neurosci. 2014;7:98. doi: 10.3389/fnmol.2014.00098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cercato MC, Colettis N, Snitcofsky M, Aguirre AI, Kornisiuk EE, Baez MV, Jerusalinsky DA. Hippocampal NMDA receptors and the previous experience effect on memory. J Physiol Paris. 2014;108:263–269. doi: 10.1016/j.jphysparis.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 13.Liu X, Wu Y, Huang Q, Zou D, Qin W, Chen Z. Grouping pentylenetetrazol-induced epileptic rats according to memory impairment and MicroRNA expression profiles in the hippocampus. PLoS One. 2015;10:e0126123. doi: 10.1371/journal.pone.0126123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paxinos G, Watson CR, Emson PC. AChE-stained horizontal sections of the rat brain in stereotaxic coordinates. J Neurosci Methods. 1980;3:129–149. doi: 10.1016/0165-0270(80)90021-7. [DOI] [PubMed] [Google Scholar]
- 15.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 16.Sayin U, Sutula TP, Stafstrom CE. Seizures in the developing brain cause adverse long-term effects on spatial learning and anxiety. Epilepsia. 2004;45:1539–1548. doi: 10.1111/j.0013-9580.2004.54903.x. [DOI] [PubMed] [Google Scholar]
- 17.Kerchner GA, Nicoll RA. Silent synapses and the emergence of a postsynaptic mechanism for LTP. Nat Rev Neurosci. 2008;9:813–825. doi: 10.1038/nrn2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pierrefiche O. Long term depression in rat hippocampus and the effect of ethanol during fetal life. Brain Sci. 2017;7:pii: E157. doi: 10.3390/brainsci7120157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ramachandran B, Ahmed S, Dean C. Long-term depression is differentially expressed in distinct lamina of hippocampal CA1 dendrites. Front Cell Neurosci. 2015;9:23. doi: 10.3389/fncel.2015.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Izumi Y, O'Dell KA, Zorumski CF. Corticosterone enhances the potency of ethanol against hippocampal long-term potentiation via local neurosteroid synthesis. Front Cell Neurosci. 2015;9:254. doi: 10.3389/fncel.2015.00254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liao Y, Huang Y, Liu X, Luo C, Zou D, Wei X, Huang Q, Wu Y. MicroRNA-328a regulates water maze performance in PTZ-kindled rats. Brain Res Bull. 2016;125:205–210. doi: 10.1016/j.brainresbull.2016.07.008. [DOI] [PubMed] [Google Scholar]
- 22.Tabassum H, Frey JU. The effect of acute swim stress and training in the water maze on hippocampal synaptic activity as well as plasticity in the dentate gyrus of freely moving rats: Revisiting swim-induced LTP reinforcement. Hippocampus. 2013;23:1291–1298. doi: 10.1002/hipo.22166. [DOI] [PubMed] [Google Scholar]
- 23.Cui Y, Jin J, Zhang X, Xu H, Yang L, Du D, Zeng Q, Tsien JZ, Yu H, Cao X. Forebrain NR2B overexpression facilitating the prefrontal cortex long-term potentiation and enhancing working memory function in mice. PLoS One. 2011;6:e20312. doi: 10.1371/journal.pone.0020312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Szczurowska E, Mareš P. NMDA and AMPA receptors: Development and status epilepticus. Physiol Res. 2013;62(Suppl 1):S21–S38. doi: 10.33549/physiolres.932662. [DOI] [PubMed] [Google Scholar]
- 25.Traynelis SF, Wollmuth LP, McBain CJ, Menniti FS, Vance KM, Ogden KK, Hansen KB, Yuan H, Myers SJ, Dingledine R. Glutamate receptor ion channels: Structure, regulation, and function. Pharmacol Rev. 2010;62:405–496. doi: 10.1124/pr.109.002451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Niewoehner B, Single FN, Hvalby Ø, Jensen V, Meyer zum Alten Borgloh S, Seeburg PH, Rawlins JN, Sprengel R, Bannerman DM. Impaired spatial working memory but spared spatial reference memory following functional loss of NMDA receptors in the dentate gyrus. Eur J Neurosci. 2007;25:837–846. doi: 10.1111/j.1460-9568.2007.05312.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Watson DJ, Herbert MR, Stanton ME. NMDA receptor involvement in spatial delayed alternation in developing rats. Behav Neurosci. 2009;123:44–53. doi: 10.1037/a0013633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Watson DJ, Stanton ME. Intrahippocampal administration of an NMDA-receptor antagonist impairs spatial discrimination reversal learning in weanling rats. Neurobiol Learn Mem. 2009;92:89–98. doi: 10.1016/j.nlm.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hawasli AH, Benavides DR, Nguyen C, Kansy JW, Hayashi K, Chambon P, Greengard P, Powell CM, Cooper DC, Bibb JA. Cyclin-dependent kinase 5 governs learning and synaptic plasticity via control of NMDAR degradation. Nat Neurosci. 2007;10:880–886. doi: 10.1038/nn1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.von Engelhardt J, Doganci B, Jensen V, Hvalby Ø, Göngrich C, Taylor A, Barkus C, Sanderson DJ, Rawlins JN, Seeburg PH, et al. Contribution of hippocampal and extra-hippocampal NR2B-containing NMDA receptors to performance on spatial learning tasks. Neuron. 2008;60:846–860. doi: 10.1016/j.neuron.2008.09.039. [DOI] [PubMed] [Google Scholar]
- 31.Ghersi MS, Gabach LA, Buteler F, Vilcaes AA, Schiöth HB, Perez MF, de Barioglio SR. Ghrelin increases memory consolidation through hippocampal mechanisms dependent on glutamate release and NR2B-subunits of the NMDA receptor. Psychopharmacology (Berl) 2015;232:1843–1857. doi: 10.1007/s00213-014-3817-6. [DOI] [PubMed] [Google Scholar]
- 32.Sanderson DJ, Good MA, Seeburg PH, Sprengel R, Rawlins JN, Bannerman DM. The role of the GluR-A (GluR1) AMPA receptor subunit in learning and memory. Prog Brain Res. 2008;169:159–178. doi: 10.1016/S0079-6123(07)00009-X. [DOI] [PubMed] [Google Scholar]
- 33.Balu DT, Coyle JT. Glutamate receptor composition of the post-synaptic density is altered in genetic mouse models of NMDA receptor hypo- and hyperfunction. Brain Res. 2011;1392:1–7. doi: 10.1016/j.brainres.2011.03.051. [DOI] [PMC free article] [PubMed] [Google Scholar]