Abstract
In the present study, the effects of hyaluronic acid (HA) combined with chitosan conduit on peripheral nerve scarring and regeneration were investigated in a rat model of peripheral nerve crush injury. A total of 60 Sprague-Dawley rats were randomly distributed into four groups (15 rats in each group), in which the nerve was either not treated (control group) or treated with chitosan conduit, hyaluronic acid, or chitosan conduit coupled with hyaluronic acid following clamp injury to the sciatic nerve. The surgical sites were evaluated by assessing the sciatic functional index, the degree of scar adhesions, the numbers of myelinated nerve fibers, the average diameter of myelinated nerve fibers and the myelin sheath thickness. Larger epineurial scar thickness was observed in the control groups compared with the treatment groups at 4, 8 and 12 weeks following surgery. There was no significant difference in scar adhesion among the four groups at 4 weeks following surgery. However, animals receiving chitosan coupled with HA demonstrated better neural recovery, as measured by reduced nerve adherence to surrounding tissues, less scar adhesion, increased number of axons, nerve fiber diameter and myelin thickness. In conclusion, the application of chitosan conduit combined with HA, to a certain extent, inhibited sciatic nerve extraneural scaring and adhesion, and promoted neural regeneration and recovery.
Keywords: chitosan, hyaluronic acid, sciatic nerve, regeneration, scar
Introduction
Peripheral nerve injury is a common clinical problem, and severe nerve injury has a devastating impact on the quality of life of patients. Extraneural scarring and adhesion are detrimental side effects of nerve injury, which can prevent axonal regeneration and recovery of nerve function and can even cause additional nerve damage (1). Therefore, preventing extraneural scarring and adhesion is a key research focus in the field of peripheral nerve injury recovery. Previous studies on the structure and function of the nerve regeneration chamber have suggested that therapeutic interventions on the microenvironment of nerve regeneration may effectively prevent scar and adhesion formation (1,2).
Hyaluronic acid (HA) gel is a type of natural and transparent polysaccharide composed of straight-chain polymers, which exists widely in the body as an important component of extracellular matrix. Considering that HA does not exhibit species or tissue specificity, and exogenous HA is biodegradable in vivo, HA is often applied to the study of peripheral nerve injury recovery as an ideal biomaterial. HA has been reported to effectively reduce scar formation following neurolysis (2). Smit et al (3) revealed that HA gel significantly reduces nerve adhesions following different types of peripheral nerve damage. However, no further studies have investigated the effect of HA on nerve regeneration.
Chitosan, a macromolecular polysaccharide and a type of artificial extracellular matrix material, has attracted increasing attention because of its availability and non-toxic biodegradability in vivo. Rosales-Cortes et al (4) reported that no obvious immune rejection response was observed for chitosan in female dogs. Chitosan has good biocompatibility, and it has been demonstrated to support the growth of nerve cells and Schwann cells, to promote axonal extension of nerve cells and to inhibit the growth of fibroblasts (5,6). Patel et al (7), using a novel evaluation method for function, reported that chitosan conduit not only benefits the morphology recovery of regenerated nerves, but also promotes the recovery of nerve function. Several studies have reported that chitosan, as a vector combined with supplements, such as brain-derived neurotropic factor and nerve growth factor, is important in bridging peripheral nerve defects and in enhancing the regeneration and functional recovery of peripheral nerves (8–11).
As previously reported, HA has inhibitory effects on scarring and adhesion and promotive effects on regeneration of injured nerves, while chitosan conduit enhances regeneration and restoration of injured peripheral nerves. Study from our group has demonstrated that chitosan conduit can prevent scarring, and promote the functional recovery of facial nerve in early stages (unpublished data). The purpose of the present study was to evaluate the effect of topical administration of HA around chitosan conduit on the formation of extraneural scarring, adhesion and regeneration and functional recovery of injured peripheral nerves using a rat sciatic nerve clamp injury model.
Materials and methods
Animals and surgical procedure
A total of 60 healthy adult Sprague-Dawley (SD) rats weighing 180–220 g and maintained under controlled lighting and temperature conditions were supplied by the Laboratory Animal Centre of Chinese PLA General Hospital (Beijing, China) in this experiment. All studies on rats were performed with approval from the Institutional Animal Care and Use Committee (IACUC) of the Laboratory Animal Centre of Chinese PLA General Hospital. Animals were housed in steel cages at room temperature 22–26°C under a 12/12 h light/dark cycle and were fed with pelleted standard feed and water. The 8-week-old rats (male or female) were randomly allocated to four groups, with 15 animals in each group. They were all anesthetized with 10% chloral hydrate (0.3 ml/100 g). Then, the fur of the right hind limbs was shaved off. The rats were kept in prone position. Following local povidone-iodine disinfection and covering with a paper towel, an oblique incision (3 cm) was performed on the right hind leg. The sciatic nerve was exposed through the spatium intermusculare prior to clamping the nerve (1 cm) with needle holders (J32220; Shanghai Medical Instruments Ltd., Shanghai, China) for 30 sec. In the present study, an identical needle clamp was used in all the animals, which, when fully closed, it generates the same length nerve clamp (1 cm) and during the same working time (30 sec) results in identical force injury in each group. The chitosan conduits were longitudinally split prior to immersing in physiological saline. The chitosan conduit was 1 mm long, 1 mm wide and 0.05 mm thick.
The four experimental groups were as follows: Chitosan group, the injury sites of sciatic nerves were covered with chitosan conduits and 6-0 sutures were used to anchor the conduits to the fascia around the nerves; chitosan/HA group, the animals were treated with the chitosan conduits which were fixed as described above, plus HA gel (0.2 ml; MW ~200 million Dalton; Shanghai Medical Instruments Ltd. (Shanghai, China) which was injected evenly onto the surface of the injury nerve and chitosan conduits; control group, no additional treatment was performed following clamping the nerve; and HA group, HA gel (0.2 ml) was injected at the site of injury. Following the appropriate treatment establishing the animal models, the incision was sutured with 6-0 sutures one layer simultaneously and appositionally. Finally, the skin was closed with subcuticular suture.
Sciatic functional index (SFI)
At 4, 8 and 12 weeks following surgery, evaluation of SFI was performed based on a method described previously (12,13). Prior to the test, the rats were trained to walk through the channel (length × width × height: 100×10×15 cm), from one end to the other. Following laying some white papers on the bottom of the walking channel and painting of hind paws of rats with ink, the rats walked through the channel once again, and at least 5 paw-prints of every rat were recorded. The paw-prints of every rat on the experimental operated side (E) and the contralateral normal side (N) were measured. PL is the length from the third toe to the heel, TS is the length from the first to the fifth toe, and IT is the length from the second to the fourth toe (Fig. 1). The SFI of each rat was then calculated by the following equation: SFI = −38.3 [(EPL-NPL)/NPL] + 109.5 [(ETS-NTS)/NTS) + 13.3 [(EIT-NIT)/NIT] - 8.8.
Figure 1.
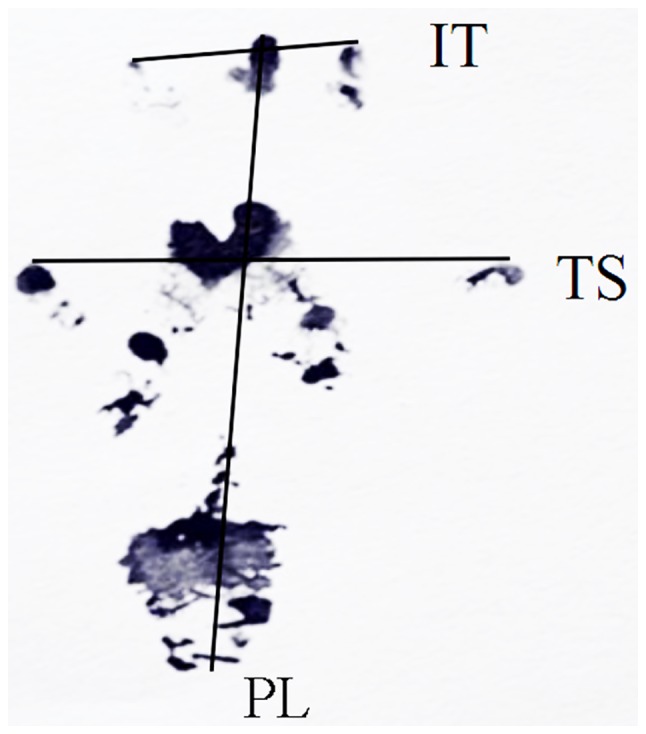
Rat footprint for SFI measurement. SFI, sciatic functional index; PL, length from the third toe to the heel; TS, length from the first to the fifth toe; IT, length from the second to the fourth toe.
In general, SFI equal to 0 represents normal nerve function, whereas an SFI oscillating around-100 indicates total dysfunction.
Scar adhesion analysis
After anesthetizing the rats with 10% chloral hydrate at 4, 8, 12 weeks following surgery, the nerves sections were exposed by incising the scar along the original incisions. According to the classification standard by Petersen et al (14), the force size of separating the skin and muscles, fascia and nerves was graded: 1, no need of or just in need of blunt dissection slightly; 2, need of blunt separation using a lot of force; and 3, need of point-sharp dissection.
Electrophysiological evaluation
At 12 weeks following surgery, all rats were subjected to electrophysiological measurement. The sciatic nerve sections clamped were re-exposed by surgical incision at the previous surgical site under general anesthesia. The stimulating electrodes were placed at the proximal and distal segments of the sciatic nerve section of clamping, while the recording electrodes were inserted into the gastrocnemius muscle. Then, electrophysiological instrument was used to test and record nerve-muscle action potential. The amplitude and latency period of sciatic nerves was recorded for the rats in each group, the distance between two electrodes was measured and the conduction velocity of the nerves separately was calculated.
Histological staining
At 4, 8, 12 weeks following surgery, the conduits and nerves of the anesthetized rats were exposed by incision of the skin and excision of both. Each group had 15 rats, with 5 rats for each time-point (4, 8 and 12 weeks), then tissues samples from each rat were divided into four blocks on average: Two middle parts were used in epoxy resin embedding [for toluidine blue staining and transmission electron microscopy (TEM)] and paraffin embedding [for hematoxylin and eosin (H&E) staining and Masson staining]. The epoxy resin-embedded samples were first fixed in glutaraldehyde (2.5%; 24 h at 4°C) and osmium (2%; 2 h at 4°C) and dehydrated through a gradient of propyl alcohol. Toluidine blue staining and TEM investigations for each specimen were performed as described below. The paraffin-embedded samples were first fixed in 4% formaldehyde solution for 8 h at room temperature prior to 4 µm sectioning for H&E staining and Masson staining.
Masson's trichrome stain was performed as previously described (15), and the resulting images were used for semi-quantitative analysis. Extraneural scars are composed primarily of collagen fibers. In the present research, the average thickness of collagen in the epineurium was determined in four random optical fields from 1 randomly selected section of each rat, using Image-Pro 6.0 Plus software (Media Cybernetics, Inc., Rockville, MD, USA).
Toluidine blue staining
The experimental samples were embedded in Epon resin prior to 1.5 µm sectioning. Then, the sections were treated with toluidine blue stain to label the myelin sheath. The mean number of myelinated axons was calculated in six randomly selected fields from 1 randomly selected section of each rat using Image-Pro 6.0 Plus software following observation using a light microscope.
TEM
The experimental samples were embedded in Epon resin prior to 70 nm ultrathin sections, saturated in uranyl acetate for 30 min at room temperature and stained with citric acid for 15 min at room temperature. The myelin sheath morphology, the cellular construction and the regeneration situation of cell organelles was evaluated in six randomly selected fields from 1 randomly selected section of each rat, observed using a transmission electron microscope (JEM-2000ex; JEOL, Ltd., Tokyo, Japan). The average diameter of myelinated fibers and myelin sheath thickness was calculated using Image-Pro 6.0 Plus.
Statistical analysis
Statistical analysis was performed using SPSS 19.0 (IBM Corp., Armonk, NY, USA). The data were presented as the mean ± standard deviation. One-way analysis of variance was used to compare differences among groups. P<0.05 was considered to indicate a statistically significant difference.
Results
SFI outcome
At 4 weeks following surgery, the SFI of each group exhibited no statistically significant difference (P>0.05; Fig. 2). At 8 weeks following surgery, the SFI of the chitosan group and the chitosan/HA group was significantly higher compared with the control group (P<0.05; Fig. 2). However, no significant difference was observed between the SFI of the chitosan and the chitosan/HA groups, suggesting that the chitosan alone treatment was equally beneficial to nerve recovery as the combined treatment. In addition, HA treatment alone exhibited no significant difference in SFI outcome compared with control at 8 weeks (Fig. 2). At 12 weeks following surgery, the results demonstrated that the recovery of the clamped nerves function in the control group was the worst in all four groups (P<0.05; Fig. 2).
Figure 2.
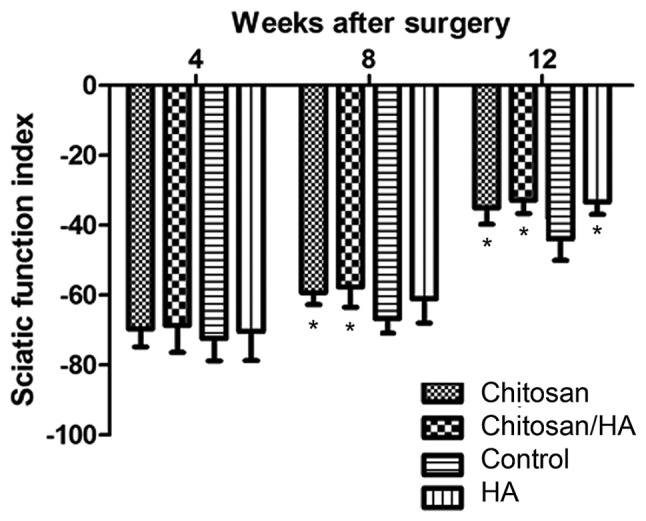
Effect of treatments on the SFI index. *P<0.05 vs. control. SFI, sciatic functional index; HA, hyaluronic acid.
Scar adhesions
Following anesthesia, the skin was incised along the original incision, and then the muscle, fascia and nerves were separated layer by layer (Fig. 3). At 4, 8 and 12 weeks following surgery, test results revealed that the skin, muscle and fascia of all the rats healed well without wound disruption and infection (Table I). At 4 weeks following surgery, the nerves in all four groups had only a mild adhesion with the surrounding muscle and fascia tissue, and were easy to separate from them. Statistically, there was no significant difference among the groups (P>0.05; Table I), as measured by the Petersen classification (14). At 8 and 12 weeks following surgery, the control group had a significantly heavier adhesion compared with the chitosan/HA group (P<0.05; Table I). No statistically significant differences were observed by either the chitosan alone group or the HA alone group when compared with the control group (P>0.05; Table I).
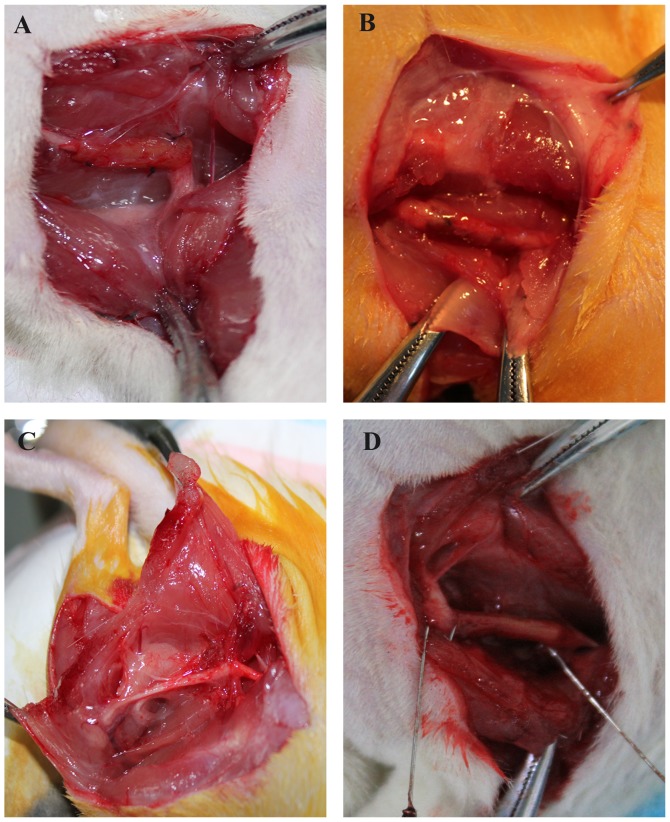
Figure 3.
Gross observation of scar adhesion at 12 weeks following surgery. (A) Chitosan group. (B) Chitosan/HA group. (C) Control group. (D) HA group. HA, hyaluronic acid.
Table I.
Evaluation of scar adhesion according to the Petersen classification.
| 4 weeks | 8 weeks | 12 weeks | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Skin | Muscle and fascia | Neurons | Skin | Muscle and fascia | Neurons | Skin | Muscle and fascia | Neurons | |
| Chitosan | 1 | 1 | 1.4±0.55 | 1 | 1.4±0.55 | 1.6±0.55 | 1 | 1.4±0.55 | 1.8±0.45 |
| Chitosan/HA | 1 | 1 | 1.2±0.45 | 1 | 1.2±0.45a | 1.2±0.45a | 1 | 1.2±0.45a | 1.6±0.55a |
| Control | 1 | 1 | 1.4±0.55 | 1 | 1.4±0.55 | 2.0±0.00 | 1 | 1.6±0.55 | 2.0±0.45 |
| HA | 1 | 1 | 1.2±0.45 | 1 | 1.2±0.45 | 1.6±0.55 | 1 | 1.4±0.55 | 2.0±0.00 |
HA, hyaluronic acid
P<0.05 compared with control group.
Histological findings
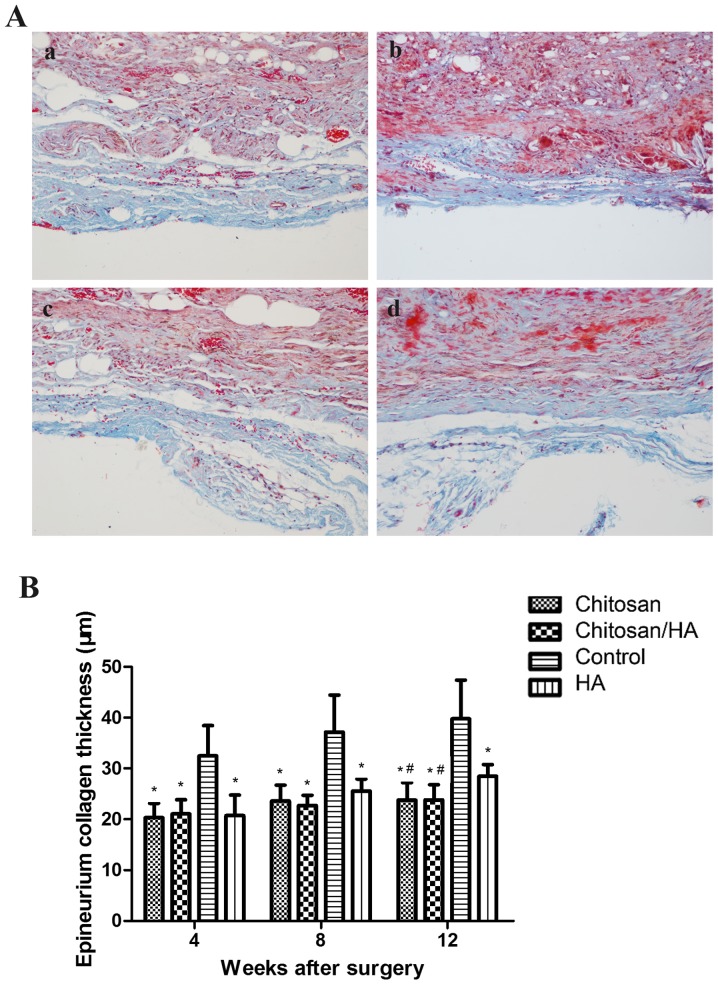
The status of extraneural scar hyperplasia was observed by Masson staining (Fig. 4A). At 4, 8, 12 weeks following surgery, the collagen of epineurium in the control group was noticeably thicker compared with the treatment groups. Quantification of the epineurium collagen thickness demonstrated that this was significantly higher in the control group compared with the treatment groups (P<0.05; Fig. 4B). At 12 weeks following surgery, the collagen of the epineurium in the HA group was significantly thicker compared with the chitosan group and the chitosan/HA group (P<0.05; Fig. 4B).
Figure 4.
Effect of treatments on epineurium collagen thickness. (A) Representative images from Masson staining of tissue sections from the four experimental groups (magnification, ×200); (a) chitosan group; (b) chitosan/HA group; (c) control group; and (d) HA group. (B) Quantification of epineurium collagen thickness at 4, 8 and 12 weeks following surgery. *P<0.05 vs. control; #P<0.05 vs. HA group. HA, hyaluronic acid.
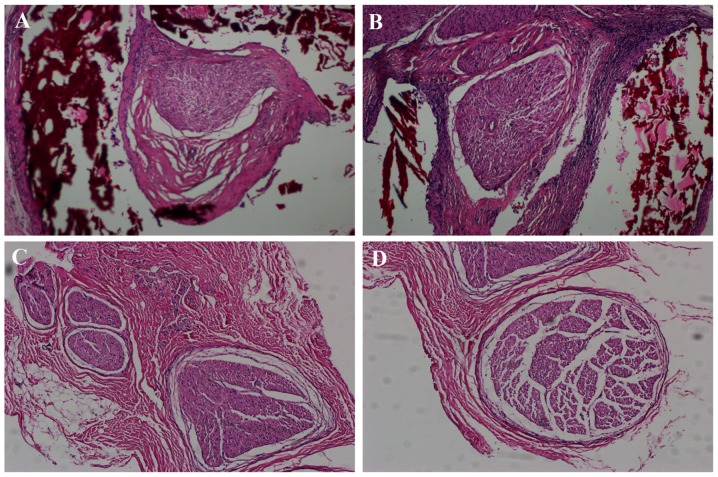
At 12 weeks following surgery, there were no significant inflammatory changes observed among the experimental groups (Fig. 5). H&E staining demonstrated that there were a large number of nerve fibers in each group, which grew in closely packed bundles, with the nerve fibers in the chitosan/HA group being the most compact (Fig. 5).
Figure 5.
Hematoxylin and eosin staining of tissue samples from the four experimental groups at 12 weeks following surgery. Representative images of (A) chitosan group, (B) chitosan/HA group, (C) control group and (D) HA group (magnification, ×100). The chitosan conduit (red color) covering the surface of the nerves in panels A and B had partial degradation. At 12 weeks following surgery, a large number of nerve fibers existed in the trauma area in all groups and no significant inflammatory manifestations were observed. HA, hyaluronic acid.
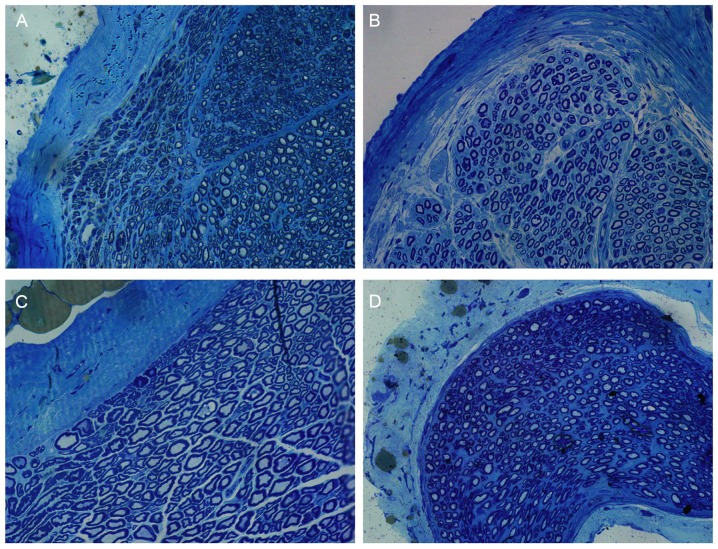
Toluidine blue staining results revealed that a number of myelinated nerve fibers in each group existed in the trauma area (Fig. 6). The myelinated nerve fibers in the chitosan/HA group displayed the most orderly arrangement (Fig. 6). The myelinated nerve fibers of the control group were arranged in an organized way but relatively sparse, and the morphology of the axons was the most irregular compared with the treatment groups (Fig. 6).
Figure 6.
Toluidine blue staining of tissue samples from the four experimental groups at 12 weeks following surgery. Representative images of (A) chitosan group, (B) chitosan/HA group, (C) control group and (D) HA group (magnification, ×400). The chitosan/HA group had more arranged and distinct axons compared with the other groups, as well as more bundled myelinated nerve fibers. HA, hyaluronic acid
TEM findings
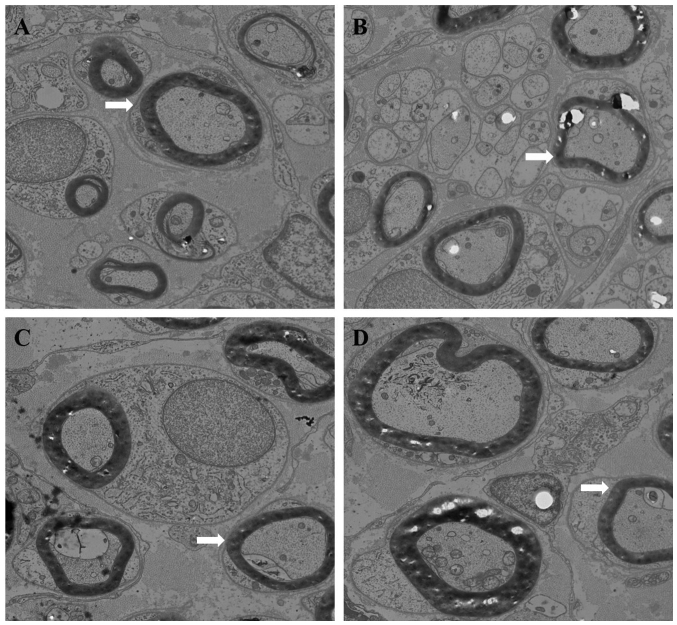
Using TEM, plenty of nerve fibers, myelinated nerve fibers and Schwann cells were observed in each group (Fig. 7). Microtubules, microfilaments and mitochondria were also abundant in the nerve axons. However, more nerve fibers were observed in the chitosan and the chitosan/HA groups compared with the other groups. Compared with the other three groups, the chitosan/HA group had the least connective tissue, the most myelinated nerve fibers and the most distinct morphology (Fig. 7). The control and HA groups exhibited less dense nerve fibers, irregular axon morphology, and a more dissolved myelin. The number of nerve fibers was calculated by counting the number of axons of slices stained with toluidine blue. The nerve fiber diameter and myelin sheath thickness were quantified using the images from the TEM analysis.
Figure 7.
Transmission electron microscopy analysis of tissue samples from the four experimental groups. Representative images of (A) chitosan group, (B) chitosan/HA group, (C) control group and (D) HA group (magnification, ×4,000). Arrow heads indicate the representative axons in each group. The diameter of axons and the thickness of myelin sheath in the control group appeared decreased compared with the treatment groups. Plenty of myelinated nerve fibers and Schwann cells were observed in each group. Axons contained a large number of neuronal microtubules, microfilaments, mitochondria. Compared with the other groups, the chitosan/HA group exhibited less connective tissue, the myelinated nerve fibers were arranged more tightly, the myelinated nerve fibers were more regular and uniform in diameter, and the shapes of axons were more equal and regular. HA, hyaluronic acid.
Nerve fiber number
At 4 weeks following surgery, the number of nerve fibers per section in either group had no obvious difference (P>0.05; Fig. 8). At 8 and 12 weeks following surgery, the nerve fiber number in the chitosan/HA group was significantly larger compared with the control group (P<0.05; Fig. 8).
Figure 8.
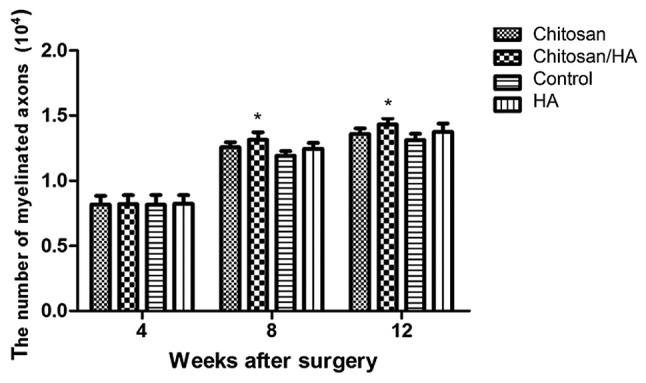
Chitosin/HA treatment increases the number of myelinated nerve fibers. Quantification of the number of myelinated nerve fibers per section at 4, 8 and 12 weeks following surgery following Toluidine blue staining. *P<0.05 vs. control. HA, hyaluronic acid.
Myelinated nerve fiber diameter
At 4 weeks following surgery, the average diameter of myelinated nerve fibers in each group had no significant difference (P>0.05; Fig. 9). However, at 8 and 12 weeks following surgery, the average diameter of myelinated nerve fibers in the control group was significantly smaller compared with the three treatment groups (Fig. 9). No significant difference was observed among the treatment groups (P>0.05; Fig. 9).
Figure 9.
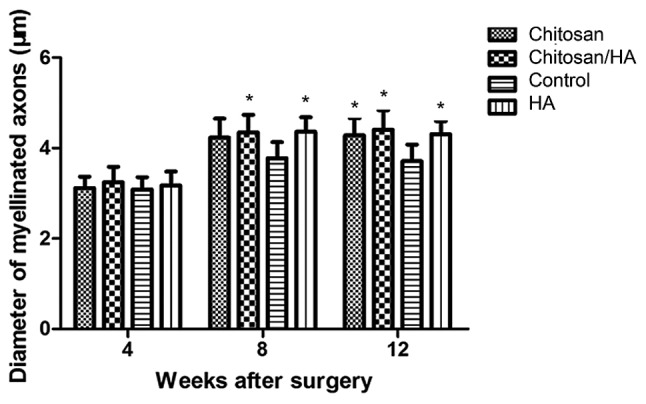
Effect of treatments on nerve fiber myelination. Quantification of the diameter of myelinated axons at 4, 8 and 12 weeks following surgery following transmission electron microscopy analysis. *P<0.05 vs. control. HA, hyaluronic acid.
Myelin sheath thickness
At 4 weeks following surgery, the average myelin sheath thickness was similar in the four experimental groups (Fig. 10). At 8 weeks following surgery, the myelin sheath thickness in the chitosan/HA group was slightly but significantly larger compared with the control group (Fig. 10). Finally, at 12 weeks following surgery, the thickness of the myelin sheath was significantly larger in all three treatment groups compared with the control (Fig. 10).
Figure 10.
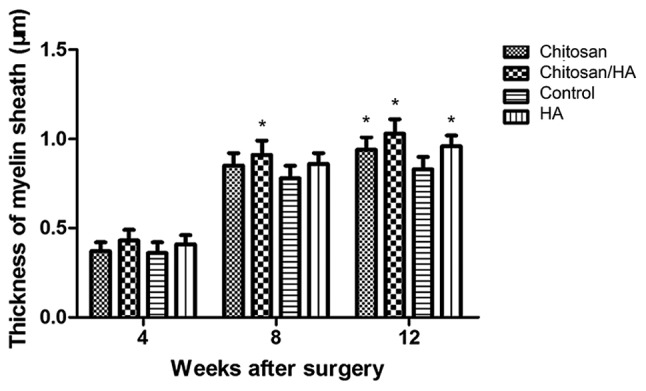
Chitosan/HA treatment increases the thickness of myelin sheath. Quantification of the thickness of myelin sheath at 4, 8 and 12 weeks following surgery following transmission electron microscopy analysis. *P<0.05 vs. control. HA, hyaluronic acid.
Electrophysiological measurement
At 12 weeks following surgery, nerve conduction velocity (NCV) and amplitude was measured in the experimental rats by electrophysiological assessment. As presented in Fig. 11, the results revealed that NCV in the control group was significantly slower compared with the treatment groups (P<0.05; Fig. 11A), and amplitude was significantly smaller compared with the treatment groups (P<0.05; Fig. 11B).
Figure 11.
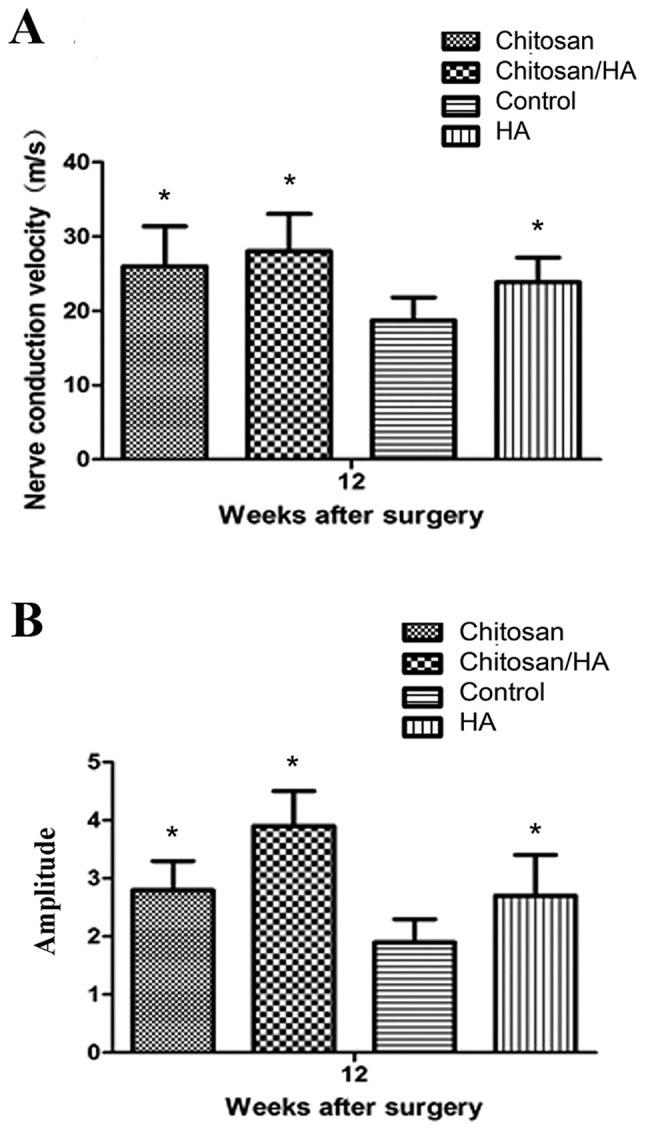
Effect of treatments on nerve function. Electrophysiological evaluation of nerve function was performed at 12 weeks following surgery in the four experimental groups. (A) Nerve conduction velocity. (B) Amplitude. *P<0.05 vs. control. HA, hyaluronic acid.
Discussion
Extraneural scarring results in tissue contracture around nerves and pressure on the nerve trunk, which may reduce the diameter of nerves by up to 80–90% in the three months following nerve injury (13). In addition, extraneural scarring may mechanically compress and then interrupt blood flow in the injury area, leading to hypoxic nerves and inhibiting nerve functional recovery (16,17).
Nerves adhered to peripheral tissue cannot slide normally, and this effect may disturb the recovery of nerves or lead to stretch injury at the proximal ends of nerves. If nerves are adhered to hard bone tissue, it can even cause new stretch injury to the nerve segments already repaired (1). Therefore, extraneural scarring and adhesion directly influences the functional recovery of peripheral nerves. To date, many methods have been explored in order to prevent extraneural scarring and adhesion, mainly involving drug intervention and physical barriers using autologous tissue flap or synthetic material to coat the injured nerves.
While many pharmaceutical agents have been used to prevent nerve scarring and adhesion in experimental studies, results have been poor. Doxorubicin (DXR), an anthracycline anticancer agent, is mainly used in treating lymphoma and soft-tissue sarcoma in clinical practice (18,19). Albayrak et al (20) reported that when DXR was placed around nerves that had complete epineurium for 5 min, the formation of extraneural scar and adhesion was reduced after 12 weeks. However, other experimental studies have reported that DXR may lead to neurotoxic effects due to anterograde axonal transport, even if it is administered by intraneural microinjection into the nerves (21,22). Methyl prednisolone acetate, an anti-inflammatory drug, has also been used in the surgical site following neurolysis, in order to reduce adhesion, however, Ikeda et al (2) reported that less intraneural and extraneural scar tissue existed in the HA treatment group compared with the methyl prednisolone acetate group, and the tensile strength needed to strip the sciatic nerve from surrounding tissue in the HA group was less compared with the other groups, suggesting that methyl prednisolone acetate did not significantly improve nerve scarring and adhesion.
HA-carboxymethylcellulose (HA-CMC), also known as Seprafilm, is a type of degradable biomembrane, which is a mixture of HA with carboxymethylcellulose (23,24). Several clinical studies have claimed that HA-CMC can effectively reduce adhesion following abdominal and pelvic surgeries. According to Magill et al (25), when Seprafilm was placed in proximity to nerves or used following nerve injury, no obvious deleterious effect was observed, while it markedly reduced extraneural scarring and increased the diameter of axonal fibers. However, results from walking track analysis revealed no improvement in nerve regeneration by seprafilm (25). Park et al (26) have also reported that HA-CMC can effectively reduce extraneural scarring and adhesion. Another study, however, reported that, under bacterial peritonitis conditions, Seprafilm may increase the inflammatory reaction and fibrosis and lead to increased adhesion (27). Hence, further study and validation is needed in order to fully understand whether HA-CMC is beneficial for nerve regeneration and its potential disadvantages.
Ozgenel et al (28) applied human amniotic membrane wrapping to the injury section of sciatic nerve and injected HA into nerves, and they reported that this treatment method was safe and effective in preventing extraneural scarring and adhesion (29,30). However, limited sources of amniotic membrane exist. Therefore, in the present study, chitosan, which is widely available, was used in combination with HA. Previous studies regarding application of chitosan in peripheral nerve injury repair, chitosan was used only as a physical channel for nerve regeneration, and other substances, such as growth factors, cells and hydrogels, were used in order to promote the regeneration of peripheral nerve defects (8,9). In the present study, however, we focused on the role of chitosan and HA gel in promoting neural regeneration during nerve repair. Chitosan was used to cover the sciatic nerve following the sciatic nerve clamp, and the HA gel was applied to cover the surface of the scaffold. Then the potential preventive effects of chitosan or HA or their combination were evaluated on extraneural scarring and adhesion, and on the regeneration and functional recovery of nerves following nerve injury using a rat sciatic nerve clamp injury model.
The present results demonstrated that the extraneural scarring was reduced by treatment with chitosan or HA or their combination. At 12 weeks following surgery, the chitosan group and the chitosan/HA group displayed better results compared with the HA group and the control group. Scar adhesion analysis revealed that chitosan combined with HA effectively reduced the sciatic nerve adhesion with surrounding tissue. Another study has also reported that HA can inhibit the formation of scar and reduce adhesion by histological examination and measuring the tensile strength that is needed to peel the sciatic nerve away from surrounding tissue (2). Assessment of the SFI revealed that, at 8 weeks following surgery, functional recovery of sciatic nerves in the chitosan group and the chitosan/HA group was obviously better compared with the control group. At 12 weeks following surgery, SFI analysis and electrophysiological measurement revealed that all three treatment groups recovered sciatic nerve function and increased their nerve conduction velocity compared with control. Due to the scar formation, the progress of nerve regeneration was most likely inhibited in the control group at this later stage. The present data demonstrated that both chitosan and HA had a role in nerve regeneration. Ishikawa et al (31) applied chitosan conduit to bridge a sciatic nerve gap in rats, and observed an increased number of regenerated axons using electron microscopy. The present experimental results of the combination treatment indicated that chitosan and HA might have a synergistic effect, including a stronger inhibition of neural scar and adhesion formation, and promotion of nerve regeneration. It should be noted that the effects from the combination group were not significantly better than the chitosan or HA alone groups in several of the functions tested, and this may be due to the small sample sizes in the present study. However, the chitosan/HA combination treatment exhibited markedly decreased scar adhesion compared with the chitosan or HA alone groups, which thus may provide a more favorable microenvironment for nerve regeneration. Since HA and chitosan both have roles in promoting nerve regeneration (29,30), using a combination treatment for nerve clamp injury may synergistically promote never regeneration.
In conclusion, both chitosan and HA inhibited extraneural scarring, promoted nerve regeneration, increased the nerve conduction velocity and improved the recovery of nerve function. Chitosan exhibited a more significant effect on inhibiting extraneural scarring compared with HA in the later phase of neural repair. In addition, chitosan conduit combined with HA may be more effective than either chitosan or HA alone to reduce extraneural scarring and adhesion, and to promote neural regeneration and repair. Further studies will be required in the future in order to fully investigate the mechanisms and effects of a chitosan conduit and HA combination in preventing nerve scarring and adhesion and promoting nerve regeneration.
Acknowledgements
This study was supported by grants from the National Natural Science Foundation of China (grant no. 81371116) and the Natural Science Foundation of Beijing (grant no. 13G30867).
References
- 1.Ip WY, Shibata T, Tang FH, Mak AF, Chow SP. Adhesion formation after nerve repair: An experimental study of early protected mobilization in the rabbit. J Hand Surg Br. 2000;25:582–584. doi: 10.1054/jhsb.2000.0480. [DOI] [PubMed] [Google Scholar]
- 2.Ikeda K, Yamauchi D, Osamura N, Hagiwara N, Tomita K. Hyaluronic acid prevents peripheral nerve adhesion. Br J Plast Surg. 2003;56:342–347. doi: 10.1016/S0007-1226(03)00197-8. [DOI] [PubMed] [Google Scholar]
- 3.Smit X, van Neck JW, Afoke A, Hovius SE. Reduction of neural adhesions by biodegradable autocrosslinked hyaluronic acid gel after injury of peripheral nerves: An experimental study. J Neurosurg. 2004;101:648–652. doi: 10.3171/jns.2004.101.4.0648. [DOI] [PubMed] [Google Scholar]
- 4.Rosales-Cortes M, Rosales-Cortés M, Peregrina-Sandoval J, Bañuelos-Pineda J, Sarabia-Estrada R, Gómez-Rodiles CC, Albarrán-Rodríguez E, Zaitseva GP, Pita-López ML. Immunological study of a chitosan prosthesis in the sciatic nerve regeneration of the axotomized dog. J Biomater Appl. 2003;18:15–23. doi: 10.1177/0885328203018001002. [DOI] [PubMed] [Google Scholar]
- 5.Yuan Y, Zhang P, Yang Y, Wang X, Gu X. The interaction of Schwann cells with chitosan membranes and fibers in vitro. Biomaterials. 2004;25:4273–4278. doi: 10.1016/j.biomaterials.2003.11.029. [DOI] [PubMed] [Google Scholar]
- 6.Freier T, Koh HS, Kazazian K, Shoichet MS. Controlling cell adhesion and degradation of chitosan films by N-acetylation. Biomaterials. 2005;26:5872–5878. doi: 10.1016/j.biomaterials.2005.02.033. [DOI] [PubMed] [Google Scholar]
- 7.Patel M, Vandevord PJ, Matthew H, Wu B, DeSilva S, Wooley PH. Video-gait analysis of functional recovery of nerve repaired with chitosan nerve guides. Tissue Eng. 2006;12:3189–3199. doi: 10.1089/ten.2006.12.3189. [DOI] [PubMed] [Google Scholar]
- 8.Meyer C, Wrobel S, Raimondo S, Rochkind S, Heimann C, Shahar A, Ziv-Polat O, Geuna S, Grothe C, Haastert-Talini K. Peripheral nerve regeneration through hydrogel-enriched chitosan conduits containing engineered schwann cells for drug delivery. Cell Transplant. 2016;25:159–182. doi: 10.3727/096368915X688010. [DOI] [PubMed] [Google Scholar]
- 9.Nawrotek K, Tylman M, Rudnicka K, Gatkowska J, Wieczorek M. Epineurium-mimicking chitosan conduits for peripheral nervous tissue engineering. Carbohydr. Polym. 2016;152:119–128. doi: 10.1016/j.carbpol.2016.07.002. [DOI] [PubMed] [Google Scholar]
- 10.Hsueh YY, Chang YJ, Huang TC, Fan SC, Wang DH, Chen JJ, Wu CC, Lin SC. Functional recoveries of sciatic nerve regeneration by combining chitosan-coated conduit and neurosphere cells induced from adipose-derived stem cells. Biomaterials. 2014;35:2234–2244. doi: 10.1016/j.biomaterials.2013.11.081. [DOI] [PubMed] [Google Scholar]
- 11.Ao Q, Fung CK, Tsui AY, Cai S, Zuo HC, Chan YS, Shum DK. The regeneration of transected sciatic nerves of adult rats using chitosan nerve conduits seeded with bone marrow stromal cell-derived Schwann cells. Biomaterials. 2011;32:787–796. doi: 10.1016/j.biomaterials.2010.09.046. [DOI] [PubMed] [Google Scholar]
- 12.Kabiri M, Oraee-Yazdani S, Shafiee A, Hanaee-Ahvaz H, Dodel M, Vaseei M, Soleimani M. Neuroregenerative effects of olfactory ensheathing cells transplanted in a multi-layered conductive nanofibrous conduit in peripheral nerve repair in rats. J Biomed Sci. 2015;22:35. doi: 10.1186/s12929-015-0144-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Farjah GH, Fazli F. The effect of chick embryo amniotic fluid on sciatic nerve regeneration of rats. Iran J Vet Res. 2015;16:167–171. [PMC free article] [PubMed] [Google Scholar]
- 14.Petersen J, Russell L, Andrus K, MacKinnon M, Silver J, Kliot M. Reduction of extraneural scarring by ADCON-T/N after surgical intervention. Neurosurgery. 1996;38:976–984. doi: 10.1097/00006123-199605000-00025. [DOI] [PubMed] [Google Scholar]
- 15.Sunderland S, Bradley KC. Endoneurial tube shrinkage in the distal segment of a severed nerve. J Comp Neurol. 1950;93:411–420. doi: 10.1002/cne.900930305. [DOI] [PubMed] [Google Scholar]
- 16.Rydevik B, Lundborg G, Nordborg C. Intraneural tissue reactions induced by internal neurolysis. An experimental study on the blood-nerve barrier, connective tissues and nerve fibres of rabbit tibial nerve. Scand J Plast Reconstr Surg. 1976;10:3–8. doi: 10.1080/02844317609169741. [DOI] [PubMed] [Google Scholar]
- 17.Wilgis EF, Murphy R. The significance of longitudinal excursion in peripheral nerves. Hand Clin. 1986;2:761–766. [PubMed] [Google Scholar]
- 18.Younes A. New treatment strategies for aggressive lymphoma. Semin Oncol. 2004;31(6 Suppl 15):S10–S13. doi: 10.1053/j.seminoncol.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 19.Pervaiz N, Colterjohn N, Farrokhyar F, Tozer R, Figueredo A, Ghert M. A systematic meta-analysis of randomized controlled trials of adjuvant chemotherapy for localized resectable soft-tissue sarcoma. Cancer. 2008;113:573–581. doi: 10.1002/cncr.23592. [DOI] [PubMed] [Google Scholar]
- 20.Albayrak BS, Ismailoglu O, Ilbay K, Yaka U, Tanriover G, Gorgulu A, Demir N. Doxorubicin for prevention of epineurial fibrosis in a rat sciatic nerve model: Outcome based on gross postsurgical, histopathological and ultrastructural findings. J Neurosurg Spine. 2010;12:327–333. doi: 10.3171/2009.9.SPINE09407. [DOI] [PubMed] [Google Scholar]
- 21.England JD, Rhee EK, Said G, Sumner AJ. Schwann cell degeneration induced by doxorubicin (adriamycin) Brain. 1988;111:901–913. doi: 10.1093/brain/111.4.901. [DOI] [PubMed] [Google Scholar]
- 22.England JD, Asbury AK, Rhee EK, Sumner AJ. Lethal retrograde axoplasmic transport of doxorubicin (adriamycin) to motor neurons. A toxic motor neuronopathy. Brain. 1988;111:915–926. doi: 10.1093/brain/111.4.915. [DOI] [PubMed] [Google Scholar]
- 23.Becker JM, Dayton MT, Fazio VW, Beck DE, Stryker SJ, Wexner SD, Wolff BG, Roberts PL, Smith LE, Sweeney SA, Moore M. Prevention of postoperative abdominal adhesions by a sodium hyaluronate-based bioresorbable membrane: A prospective, randomized, double-blind multicenter study. J Am Coll Surg. 1996;183:297–306. [PubMed] [Google Scholar]
- 24.Fazio VW, Cohen Z, Fleshman JW, van Goor H, Bauer JJ, Wolff BG, Corman M, Beart RW, Jr, Wexner SD, Becker JM, et al. Reduction in adhesive small-bowel obstruction by Seprafilm adhesion barrier after intestinal resection. Dis Colon Rectum. 2006;49:1–11. doi: 10.1007/s10350-005-0268-5. [DOI] [PubMed] [Google Scholar]
- 25.Magill CK, Tuffaha SH, Yee A, Luciano JP, Hunter DA, Mackinnon SE, Borschel GH. The short- and long-term effects of Seprafilm on peripheral nerves: A histological and functional study. J Reconstr Microsurg. 2009;25:345–354. doi: 10.1055/s-0029-1215526. [DOI] [PubMed] [Google Scholar]
- 26.Park JS, Lee JH, Han CS, Chung DW, Kim GY. Effect of hyaluronic acid-carboxymethylcellulose solution on perineural scar formation after sciatic nerve repair in rats. Clin Orthop Surg. 2011;3:315–324. doi: 10.4055/cios.2011.3.4.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kayaoglu HA, Ozkan N, Hazinedaroglu SM, Ersoy OF, Koseoglu RD. An assessment of the effects of two types of bioresorbable barriers to prevent postoperative intra-abdominal adhesions in rats. Surg Today. 2005;35:946–950. doi: 10.1007/s00595-004-3050-8. [DOI] [PubMed] [Google Scholar]
- 28.Ozgenel GY, Filiz G. Combined application of human amniotic membrane wrapping and hyaluronic acid injection in epineurectomized rat sciatic nerve. J Reconstr Microsurg. 2004;20:153–157. doi: 10.1055/s-2004-820772. [DOI] [PubMed] [Google Scholar]
- 29.Zor f, Deveci M, Kilic A, Ozdag MF, Kurt B, Sengezer M, Sönmez TT. Effect of VEGF gene therapy and hyaluronic acid film sheath on peripheral nerve regeneration. Microsurgery. 2014;34:209–216. doi: 10.1002/micr.22196. [DOI] [PubMed] [Google Scholar]
- 30.Chen Q, Lu H, Yang H. Chitosan prevents adhesion during rabbit flexor tendon repair via the sirtuin 1 signaling pathway. Mol Med Rep. 2015;12:4598–4603. doi: 10.3892/mmr.2015.4007. [DOI] [PubMed] [Google Scholar]
- 31.Ishikawa N, Suzuki Y, Ohta M, Cho H, Suzuki S, Dezawa M, Ide C. Peripheral nerve regeneration through the space formed by a chitosan gel sponge. J Biomed Mater Res A. 2007;83:33–40. doi: 10.1002/jbm.a.31126. [DOI] [PubMed] [Google Scholar]