Abstract
Osteoarthritis (OA) affects a large number of patients; however, human umbilical cord stem cells exhibit therapeutic potential for treating OA. The aim of the present study was to explore the interaction between human umbilical cord stem cells and degenerated chondrocytes, and the therapeutic potential of human umbilical cord stem cells on degenerated chondrocytes. Human umbilical cord-derived mesenchymal stem cells (hUC-MSCs) were harvested from human umbilical cords, and flow cytometry was used to analyze the surface antigen markers, in addition, chondrogenic, osteogenic and adipogenic differentiation on the cells was investigated. OA cells at P3 were cocultured with hUC-MSCs in a separated co-culture system, and reverse transcription-polymerase chain reaction and western blot were used to evaluate the mRNA, and protein expression of collagen type II (Col2), SRY-box 9 (sox-9) and aggrecan. The level of inflammatory cytokines, tumor necrosis factor-α, interleukin (IL)-1β, IL-6, IL-10, were analyzed by ELISA in the supernatant. hUC-MSCs grow in a fibroblastic shape with stable proliferation. hUC-MSCs expressed cluster of differentiation 44 (CD44), CD73, CD90, CD105; while did not express CD34, CD45, CD106, CD133. After multi-induction, hUC-MSCs were able to differatiate into adipogenic, osteogenic and chondrogenic lineage. hUC-MSCs inhibited the expression of matrix metalloproteinase-13, collagen type X α1 chain and cyclooxygenase-2 in OA chondrocytes, and enhanced the proliferation of OA chondrocytes, while OA chondrocytes stimulated the production of Col2, sox-9 and aggrecan and promoted hUC-MSCs differentiate into chondrocytes. Flow cytometry analysis demonstrated hUC-MSCs have a predominant expression of stem cell markers, while the hematopoietic and endothelial markers were absent. Osteogenic, chondrogenic and adipogenic differentiation was observed in certain induction conditions. hUC-MSCs improved the proliferation of OA chondrocytes and downregulated the expression of inflammatory cytokines, while OA chondrocytes promoted MSCs to differentiate into chondrocytes. Taken together, the co-culture of hUC-MSCs and OA chondrocytes may provide a therapeutic potential in OA treatment.
Keywords: human umbilical cord-derived mesenchymal stem cells, osteoarthritis, degenerated chondrocytes, differentiation, co-culture
Introduction
Osteoarthritis (OA) is the most common articular disease characterized by a progressive degradation of joint cartilage, resulting in loss of joint mobility and function at last, which was estimated to be the 4th leading cause of disability in Asia (1). OA affects the hands, feet, spine and large weight-bearing joints commonly, such as the hips and knees, while OA patients manifest joint pain, stiffness, tenderness, limited joint movement, and joint cracking. OA is a major disturbing in aging people as its incidence increases highly associated with age (2).
It is reported that OA occurred in 19.2% of adults participants age ≥45 in the Framingham Project and 27.8% in the Johnston County Osteoarthritis Survey, ~37% of participants age >60 years or older had radiographic knee OA in the third National Health and Nutrition Examination Study (3,4). According to a systematic review of Global Burden of Disease 2010 study, the global age-standardized prevalence of knee OA was 3.8%, and hip OA was 0.85% (5). Articular cartilage (AC) stand the mechanical distribution of loads across the joints, while cartilage degenerated and osteophyte formed in OA (6). Articular chondrocytes maintain proliferation and terminal differentiation in healthy articular cartilage. While hypertrophy, vascularization and calcification were observed in OA cartilage (7).
Despite the global increase in the incidence of OA, there are no effective pharmacotherapies capable of restoring the original structure and function of damaged articular cartilage (8). Pharmaceutical or surgical therapies (osteotomies, microfracture) have limited efficacy in reversing or halting OA progression, while stem cell-based cartilage tissue engineering and cartilage regeneration that may be an effective strategy in OA treatments (9–13). Numerous efforts have been made to develop tissue-engineered grafts or patches to repair focal chondral and osteochondral defects, and to date several researchers aim to implement clinical application of cell-based therapies for cartilage repair (14,15).
Mesenchymal stem cells (MSCs) are reported to show promising clinical applications in articular cartilage regeneration, and mesenchymal stem cells have a potential in treatment of OA (16,17).
Adipose mesenchymal stem cells have been reported to differentiate into chondrocytes in 3-dimensional culture express lubricin, and adipose tissue derived-mesenchymal stem cells cultured on collagen cell carrier scaffolds were to regenerated engineered cartilage (18,19).
Human umbilical stem cell populations was reported to be found in the umbilical cord, the cord lining, and perivascular tissue, as well as Wharton's jelly, so they are attractive autologous or allogenic cells to treat malignant and non-malignant solid and soft cancers, and they also can be the feeder layer for embryonic stem cells or other pluripotent stem cells (20,21).
Human umbilical cord-derived MSCs (hUC-MSCs) constitute an attractive alternative to bone marrow-derived MSCs for potential clinical applications because of easy preparation and lower risk of viral contamination, they can differentiate into the three germ layers that promote tissue and organ repair and modulate immune responses and anticancer properties (20).
However, the role of hUC-MSCs and degenerated chondrocytes in OA progression is unclear. Therefore, we explore the interaction between human umbilical cord stem cells and OA degenerated chondrocytes, and the therapeutic potential of human umbilical cord stem cells on degenerated chondrocytes.
Materials and methods
Patients
The study was approved by the Ethical Review Committee of 455th hospital of PLA (Shanghai, China). After obtaining informed consents from the mothers and family, the umbilical cords were harvested from the full-term natural delivery infants. WJ-MSC were isolated and cultured by Shanghai Omnicells Biotechnology Co., Ltd. (Shanghai, China) as described (22): Umbilical cords were washed with sterile phosphate-buffered saline (PBS) three times and diced into pieces, and the blood vessels in umbilical cords were dissected. Wharton's jelly was cut into small pieces and digested in DMEM/Hams's F-12 (1:1 vol/vol) culture medium containing 10% FBS, collagenase type II (1 µg/ml), penicillin (100 U/ml), streptomycin (100 µg/ml), and amphotericin-B (2.5 µg/ml) (all from Thermo Fisher Scientific, Inc., Waltham, MA, USA) at 37°C in a shaker for 4–6 h, and then centrifuged at 500 × g for 15 min. The cell pellet was resuspended in the above culture medium supplemented with 10 ng/ml basic fibroblast growth factor (bFGF; R&D Systems, Inc., Minneapolis, MN, USA), and cells were subcultured using 0.05% trypsin/0.02% EDTA (Thermo Fisher Scientific, Inc.) on reaching 80–90% confluence.
The cartilage samples were obtained from the knee joints of OA patients (n=12, KL grade >2) undergoing joint replacement surgery, and chondrocytes were harvested by enzymatic digestion, chondrocytes were cultured in low glucose DMEM at 37°C with 5% CO2 as previously described (23).
In the present study, the cells were divided into 2 groups: Experimental group (co-culture group) and control group. In proliferation assay, the medium in experimental group was changed with the supernatant from hUC-MSCs, while the medium in control group was changed with normal medium. In other experiments, OA chondrocytes and hUC-MSC were incubated in a noncontact co-culture system: Chondrocytes were cultured in the bottom well, and hUC-MSCs were cultured in a Transwell insert (Transwell; Corning Costar, Corning, NY, USA) in co-culture group; while chondrocytes were cultured alone in the bottom well in control group.
Cytoflow analysis
The expression of hUC-MSC surface markers was tested by using flow cytometry as described previously (24). hUC-MSCs were isolated and harvested by using 0.1% trypsin-EDTA treatment, and washed with PBS. Then the cells were incubated with the following antibodies: Rabbit polyclonal to CD44 (ab157107; 1/500 dilution), rabbit polyclonal to CD73 (ab175396; 1/500 dilution), rabbit monoclonal to CD90 (ab92574; 1/1,000 dilution), mouse monoclonal to CD105 (ab11414; 1/1,000 dilution), rabbit monoclonal to CD34 (ab81289; 1/1,000 dilution), rabbit polyclonal to CD45 (ab10559; 1/1,000 dilution), rabbit polyclonal to CD106 (ab134047; 1/1,000 dilution), rabbit polyclonal to CD133 (ab16518; 1/1,000 dilution) (all from Abcam, Cambridge, MA, USA), in the dark for 30 min at room temperature, then conjugated with either fluorescein PE or FITC [Goat Anti-Rabbit IgG H&L (FITC) (ab6717) or Goat Anti-Mouse IgG Secondary Antibody (PE) LS-C60691; 1/1,000 dilution; LifeSpan BioSciences, Seattle, WA, USA]. The labeled cells were washed and tested by flow cytometry (Becton-Dickinson, Franklin Lakes, NJ, USA).
Cell differentiation
A 6 well-plate was cultured with 5×104 cells/well. At 48 h, the medium was replaced by adipogenic medium (high glucose DMEM containing 10% FBS, 500 µM isobutylmethylxanthine, 5 µg/ml insulin, 200 µg/ml ascorpate-2-phosphate, 100 U/ml penicillin, 100 U/ml streptomycin, 1 µM dexamethasone), osteogenic medium (glucose DMEM containing 10% FBS, 100 U/ml penicillin, 100 U/ml streptomycin, 100 nM dexamethasone, 10 mM β-glycerophosphate and 50 µg/ml ascorbate-2-phosphate), and chondogenic medium (high glucose DMEM containing 10% FBS, 10 ng/ml TGF-β1, 100 nM dexamethasone, 50 ng/ml ascorbate-2-phosphate, 1 mM sodium pyruvate). All the reagents were purchased from Sigma-Aldrich; Merck KGaA (Darmstadt, Germany).
The induction medium was replaced every 2 days for 3 weeks, control group were cultured without induction. After 3 weeks, RNA extractions and RT-PCR were performed to evaluate the cell differentiation.
The special staining was further performed to show morphological changes of differentiated cells. After 3 weeks of adipogenic induction, cells were fixed in 4% paraformaldehyde at room temperature for 30 min, and washed with deionized water and then with 60% isopropanol, followed by staining with 0.5% Oil red O (Sigma-Aldrich; Merck KGaA) in isopropanol (wt/vol) for 30 min, and excess stain was removed and the cells were washed 3 times with deionized water.
For osteogenesis, cells were fixed in 4% paraformaldehyde at room temperature for 30 min, and washed with deionized water and stained with Alizarin Red Solution (no. 0223, Alizarin Red S staining kit; ScienCell Research Laboratories (San Diego, CA, USA) at room temperature for 15 min following the manufacturer protocol, and excess stain was removed and the cells were washed 3 times with deionized water.
For chondrogenisis, cells were fixed with 4% paraformaldehyde for 30 min at room temperature and then were stained with 1% Toluidine Blue Solution (sc-206058; Santa Cruz Biotechnology, Heidelberg, Germany), for 30 min, and excess stain was removed and the cells were washed 3 times with deionized water.
Reverse transcription-quantitative polymerase chain reaction
The total RNA was isolated from samples by using TRI Reagent® RNA Isolation Reagent (Sigma-Aldrich; Merck KGaA) according to the manufacturer's instructions and complementary DNA (cDNA) was prepared by using a First-Strand cDNA Synthesis kit (Pharmacia LKB, Uppsala, Sweden). Real-time quantitative PCR was performed using the TaqMan system (Applied Biosystems; Thermo Fisher Scientific, Inc.), and Real-time PCR amplification and product detection were performed using an ABI PRISM 7300 Sequence Detection System (Applied Biosystems; Thermo Fisher Scientific, Inc.) (25).
Each reaction is consisted of 10 µl SYBR Green (Applied Biosystems; Thermo Fisher Scientific, Inc.), 6 µl molecular grade water, 1 µl each forward primer and 1 µl reverse primer and 2 µl cDNA (Table I). The amplification was performed under the certain conditions: 10 min at 95°C, followed by 50 cycles, 10 sec at 95°C and 60 sec at 60°C. q-PCR was performed under standard conditions and all experiments were performed in triplicate. The quantitative gene expression was normalized to GAPDH, and the relative expression was determined by using the ΔΔCt method.
Table I.
Primer sequences.
| Gene | Sequence (5′->3′) | Product length, bp |
|---|---|---|
| OCN | 227 | |
| F | ACTCCATCTTCGTGCTCCTCA | |
| R | TCCGGACAGGAGAAAGGGTC | |
| ALP | 281 | |
| F | CTGTGCCTCAGCCAGCTC | |
| R | GGAGGATTCCAGAGGGGAGT | |
| RUNX2 | 225 | |
| F | CGCCTCACAAACAACCACAG | |
| R | TCACTGTGCTGAAGAGGCTG | |
| Aggrecan | 109 | |
| F | GTTTCCACAAGGGAGAGAGGG | |
| R | GTAGGTGGTGGCTAGGACGA | |
| Sox-9 | 110 | |
| F | AGGAGAACCCCAAGATGCAC | |
| R | GAGGCGTTTTGCTTCGTCAA | |
| COL2A1 | 241 | |
| F | GGTCCTGCAGGTGAACCC | |
| R | GAGGACCTCTAGGGCCAGAA | |
| adipoQ | 294 | |
| F | ATTCGGCACGAGGGATGCTA | |
| R | GCCCTTCAGCTCCTGTCATT | |
| aP2 | 181 | |
| F | TGAAAGAAGTGGGAGTGGGC | |
| R | CCTTTCCTGTCATCTGCGGT | |
| PPARγ | 123 | |
| F | GTGGAAGGCGAGCAGATGAT | |
| R | GGCAGATCTGGACTGGTAGC | |
| MMP13 | 230 | |
| F | CATGAGTTCGGCCACTCCTT | |
| R | CCTCGGAGACTGGTAATGGC | |
| COL10A1 | 143 | |
| F | CCAGCACGCAGAATCCATCTGA | |
| R | CTTGGTGTTGGGTAGTGGGC | |
| COX-2 | 297 | |
| F | TGTGAAAGGGTGTCCCTTCG | |
| R | AGTACAACACAGGAATCTTCACA |
F, forward; R, reverse.
Cell proliferation assay
The Cell Counting Kit-8 (CCK-8) assay (Dojindo Molecular Technologies, Inc., Rockville, MD, USA) was used to evaluate the cell proliferation. Chondrocytes from OA patients at passage 2 were isolated and harvested by using 0.1% trypsin-EDTA and plated into 96-well plates at a density of 103 cells/well. The plates were divided into 2 groups: Experimental (co-culture group) and control groups. The medium in experimental group was changed with the supernatant from hUC-MSCs, while the medium in control group was changed with normal medium. At day 1, 3, 5, 7, the medium was change with 100 µl of fresh medium containing 10 µl CCK-8 solution for 4 h in a 37°C incubator. The OD at 570 nm was measured, and the assay was repeated in a triplicate.
Western blotting
The protein expression was accessed by using western blotting. Cells were prepared in RIPA buffer containing a protease inhibitor cocktail, and then centrifuged at 12,000 × g for 10 min at 4°C, and the protein concentration was tested using the BCA protein assay kit (cat. no. 23225; Applied Biosystems; Thermo Fisher Scientific, Inc.). The certain protein was electrophoresis on a 10% SDS-PAGE gels and electrotransferred onto a nitrocellulose membrane (Bio-Rad Laboratories, Inc., Hercules, CA, USA). And then block with 5% non-fat milk (Sangon Biotech Co., Ltd., Shanghai, China) in TBS for 1 h at room temperature room, the membrane was incubated with first antibodies (mouse monoclonal to Cox-2, cat. no. sc-19999, dilution 1:1,000; Santa Cruz Biotechnology, Inc., Dallas, TX, USA) (mouse monoclonal to collagen X, cat. no. ab49945, dilution 1:1,000; Abcam) (mouse monoclonal to MMP13, cat. no. sc-101564, dilution 1:1,000; Santa Cruz Biotechnology, Inc.) (anti-β-actin antibody, cat. no. ab6276, dilution 1:1,000; Abcam) for 1 h. Then wash with TBST 3 times, the membrane was incubated with horseradish peroxidase-conjugated goat anti-mouse IgG (anti-mouse IgG, HRP-linked antibody, cat. no. 7076, dilution 1:1,000; Cell Signaling Technology, Inc., Danvers, MA, USA) for 1 h at RT. Then wash with TBST 3 times, a the ECL Plus detection kit (cat. no. 32132; Thermo Fisher Scientific, Inc.) was used to visualize the immunoreactive according to the manufacturer's instructions, and the relative protein expression was calculated by using ImageJ software (version 1.46; Wayne Rasband National Institutes of Health, Bethesda, MD, USA).
Detection of total factors in superatant
Enzyme-linked immunosorbent assay (ELISA) was performed to measure TNF-α, IL-1β, IL-6, IL-10 in supernatant. Briefly, antibodies specific for TNF-α (cat. no. SMTA00B), IL-1β (cat. no. SLB50), IL-6 (cat. no. S6050), and IL-10 (cat. no. S1000B) (all from R&D Systems, Inc.) was immobilized onto-96-well microtiter plates, and remove unbound antibody and add a blocking reagent. Then the sample were incubated with the solid phase antibodies, and washing unbound molecules away, followed by adding a detection antibody specific for TNF-α, IL-1β, IL-6, IL-10. The incubation and washing was followed by adding HRP-conjugated anti-mouse immunoglobulin. The plate was washed and a TMB substrate solution (Zymed Laboratories, San Francisco, CA, USA) was added for 30 min, and measured using a Beckman Coulter DU 800 spectrophotometer (Beckman Coulter, Fullerton, CA, USA) at 450 nm.
Statistical analysis
All the data are expressed as mean ± standard deviation. a one-way ANOVA with Tukey's HSD post hoc test was performed to comparise differences between groups. All the statistical evaluations were performed by using the SPSS 16.0 (SPSS, Inc., Chicago, IL, USA). P-values <0.05 were considered to indiacate statistically significant difference.
Results
Expression of the MSCs-surface markers
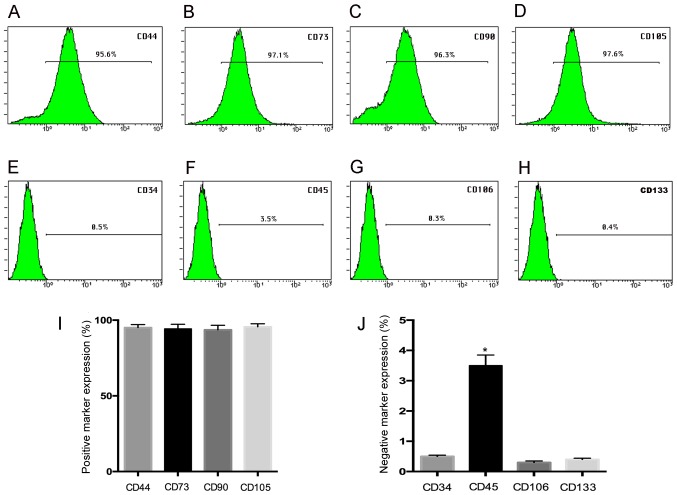
Flow cytometry assay demonstrated that hUC-MSC at passage 3 show a high expression (>95%) for the mesenchymal stem positive markers CD44 (95.6%), CD73 (97.1%), CD90 (96.3%), CD105 (97.6), while the negative expression of CD34 (0.5%), CD45 (3.5%), CD106 (0.3%), and CD133 (0.4%) was observed (Fig. 1A-H), and the histogram gives a clear picture of the expression ratio (Fig. 1I and J) suggesting our results was in agreement with previous studies (21).
Figure 1.
Surface marker expression by FACS analysis. The hUC-MSC at passage 3 demonstrated a high expression of (A) CD44 (95.6%), (B) CD73 (97.1%), (C) CD90 (96.3%), (D) CD105 (97.6%) by FACS analysis, but they express (E) CD34 (0.5%) and (F) CD45 (3.5%), (G) CD106 (0.3%), and (H) CD133 (0.4%) negatively; (I and J) the histogram gives a clear picture of the expression ratio. *P<0.05 when compared to control group.
Multi-differentiation assay
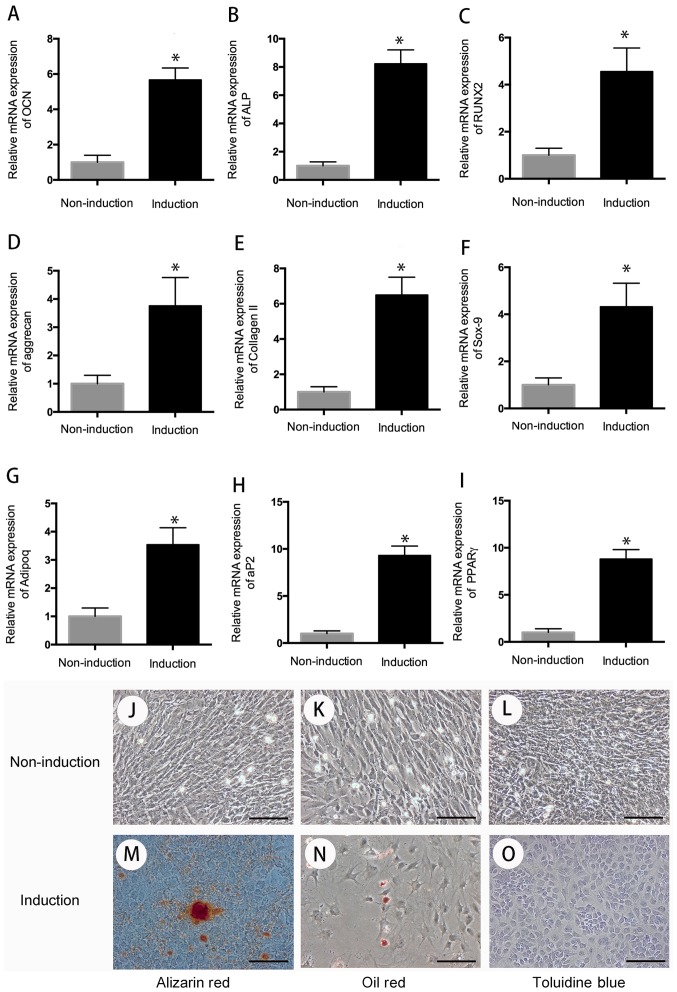
The multi-differentiation (chondrogenic, osteogenic, adipogenic differentiation) assay was used to evaluate the differentiation potential of hUC-MSC. After 3 weeks of induction toward the multi-lineage differentiation, the RNA was extracted, and quantitative RT-PCR was performed to evaluate the cell differentiation. The result indicated that compared with the cells in the control group, cells in the induction group have high expression of chondrogenic genes (aggrecan, collagen II, sox-9), osteogenic genes (OCN, ALP, RUNX2), and adipogenic genes (adipoq, aP2, PPARr) following 3 weeks of chondrogenic, osteogenic, and adipogenic differentiation (P<0.05) (Fig. 2).
Figure 2.
Cell differentiation. After 3 weeks of multi-differentiation induction, cells in the experiment group have higher expression of osteogenic genes, (A) OCN, (B) ALP, (C) RUNX2, chondrogenic genes, (D) aggrecan, (E) collagen II, (F) sox-9, and adipogenic genes, (G) adipoq, (H) aP2, (I) PPARγ than that in control group (P<0.05). There is no positive staining in control group (J-L), wheread, positive Alizarin Red staining was observed in osteogenic differentiation group for 21 days (M); Oil Red O-positive staining of lipid droplets were detected in induction group after 3 weeks of chondrogenic differentiation (N); Cells in chondrogenic induction group showed remarkable changes in cellular morphology and were stain positive for Toluidine Blue (O). *P<0.05 when compared to control group. Scale bar, 100 µm.
For special staining, Oil Red O-positive staining of lipid droplets were observed in adipogenic differentiation after 3 weeks of adipogenic induction. On day 21 of osteogenic differentiation, cells were detected with positive Alizarin Red staining. After 3 weeks of chondrogenic differentiation, cells showed remarkable changes in cellular morphology and were stain positive for toluidine blue (Fig. 2).
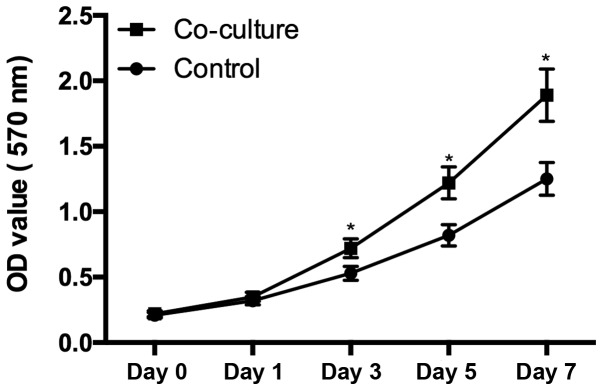
Cell proliferation
Chondrocytes harvested from OA patients seeded at a density of 1,000 cells/wells were cultured in the supernatant from hUC-MSC. From day 3, the cells cultured in the supernatant from hUC-MSC demonstrated significant higher proliferation rates than control group (P<0.05), suggested that the secretion of hUC-MSC enhanced the chondrocytes proliferation (Fig. 3).
Figure 3.
Cell proliferation. There is a significant difference of the chondrocytes proliferation between the cells cultured in the supernatant from hUC-MSC and cells culture alone (P<0.05), cells in coculture group demonstrated a high proliferation rate from day 3. *P<0.05 when compared to control group.
The quantitative RT-PCR assay
The quantitative RT-PCR assay was performed to evaluate the mRNA of hUC-MSC and chondrocytes in coculture system. As shown in Fig. 4, hUC-MSC demonstrated a significant increased mRNA expression of aggrecan, sox-9, collagen II, compared to the control group (P<0.05), indicating chondrocytes promoted chondrogenic differentiation of hUC-MSC (Fig. 4).
Figure 4.
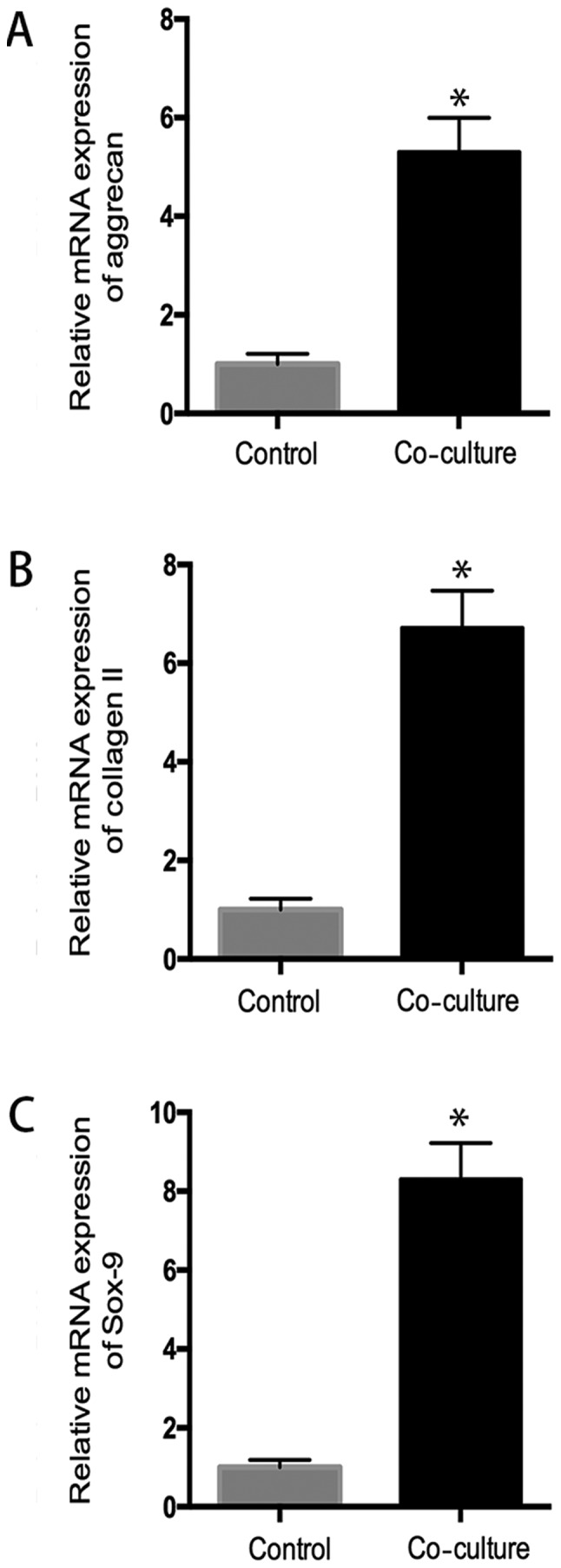
Chondrocytes promotes the chondrogenesis of hUC-MSC. RT-PCR analysis revealed increased compared with control group, aggrecan mRNA expression was increased 5.3-fold (A) and collagen II mRNA expression was increased 6.7-fold (B), as well as sox-9 mRNA expression was increased 8.2-fold (C) in co-culture system, indicating chondrocytes promoted the chondrogenic differentiation of hUC-MSC. *P<0.05 when compared to control group.
After coculture with hUC-MSC, chondrocytes from OA patients showed a significant decreased mRNA expression of cox2, collagen X, MMP13, compared to the control group (P<0.05), which suggested hUC-MSC inhibited inflammatory activity in OA chondrocytes (Fig. 5).
Figure 5.
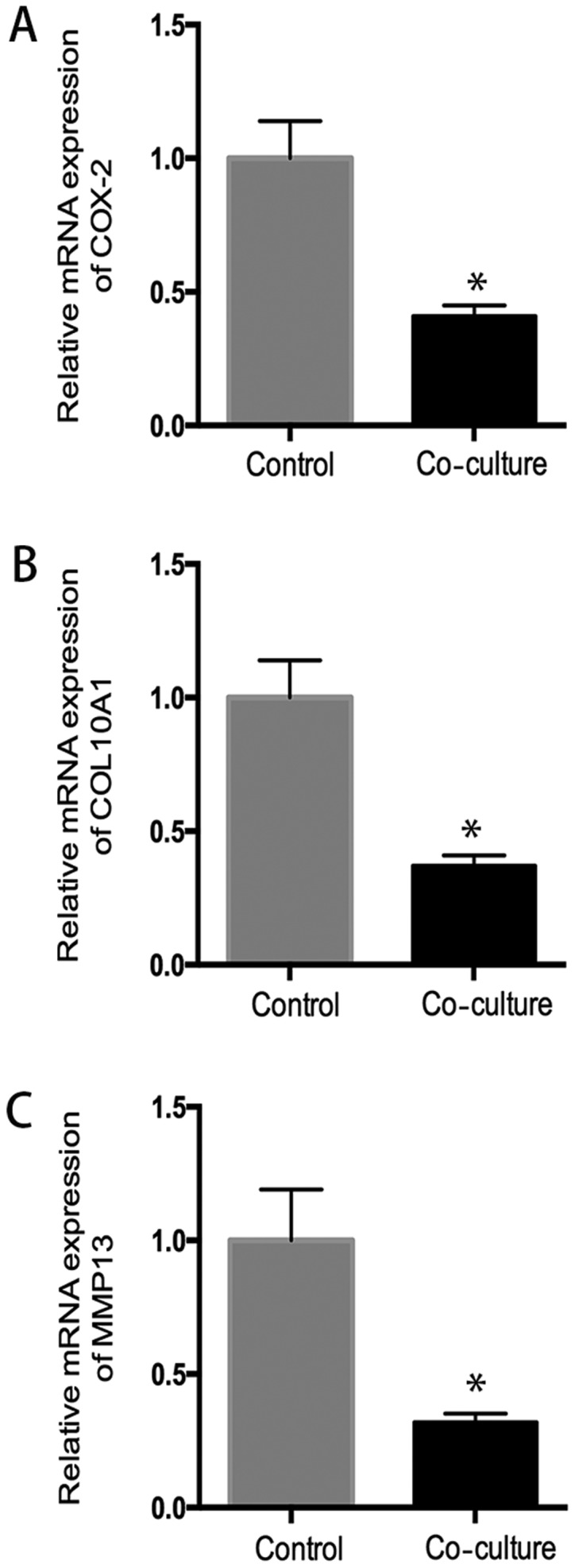
Effects of hUC-MSC on expression of genes related to inflammation and matrix degradation. In co-culture system, hUC-MSC downregulated (A) COX-2, (B) collagen 10A1, and (C) MMP13 gene expressions, compared to control group (P<0.05), indicating that hUC-MSC can have an inhibitory effect in genes expression related to inflammation and matrix degradation in human osteoarthritic chondrocytes. *P<0.05 when compared to control group.
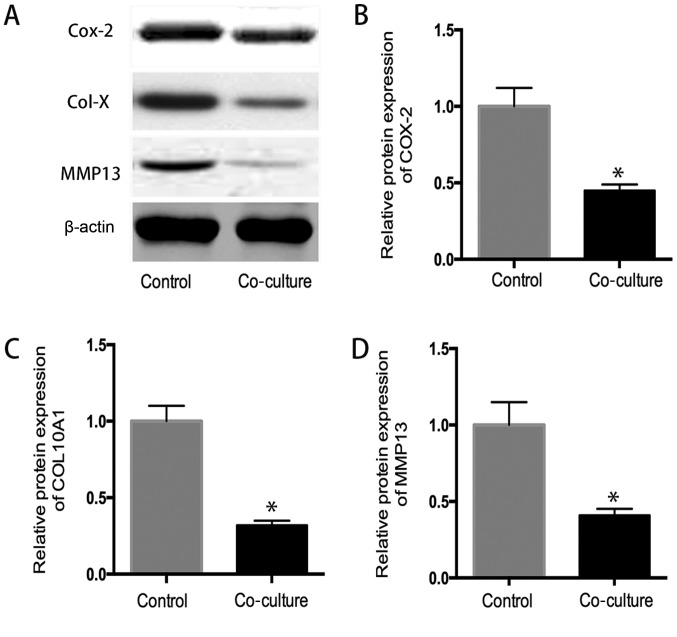
Western blot analysis
As shown in Fig. 6, the western blot analysis indicated that the protein expression of COX-2, collagen 10A1 and MMP13 in coculture system significantly decreased in comparison to that in control group determined by western blot analysis (P<0.05), suggested that hUC-MSC inhibit the expression inflammatory related protein in OA chondrocytes (Fig. 6).
Figure 6.
(A) The western blot analysis. The protein expression of (B) COX-2, (C) collagen 10A1 and (D) MMP13 in co-culture system was lower than that in control group (P<0.05), which confirmed the previously mRNA expression results. *P<0.05 when compared to control group.
Levels of inflammentary factors
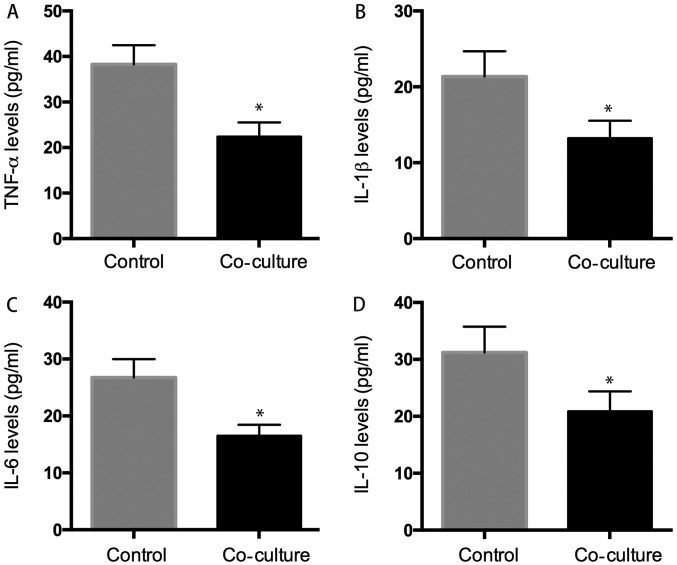
The supernatant was also collected for ELISA assay, and the result showed that the levels of TNF-α, IL-1β, IL-6, IL-10 were: 22.38±3.12, 13.23±2.32, 16.53±1.92, and 20.89±3.54 pg/ml in the supernatant of co-culture system, respectively which were significantly lower than those in the control group: 32.28±4.21, 21.37±3.31, 26.75±3.24, and 31.23±4.53 pg/ml (P<0.05) (Fig. 7).
Figure 7.
ELISA analysis. We evaluated whether hUC-MSC affected inflammatory cytokines secretion of osteoarthritic chondrocytes, the results indicated that the level of inflammatory cytokines (TNF-α, IL-1β, IL-6, IL-10) was 22.38±3.2 pg/ml (A), 13.23±2.3 pg/ml (B), 16.53±1.9 pg/ml (C), 20.89±3.5 pg/ml (D) in co-culture group decreased significant than that was 38.28±4.3 pg/ml (A), 21.37±3.3 pg/ml (B), 26.75±3.6 pg/ml (C), 31.23±4.5 pg/ml (D) in control group. *P<0.05 when compared to control group.
Discussion
OA is caused by degeneration of the joint cartilage and growth of new bone, cartilage and connective tissue, leading to pain disability and impaired quality of life (26). Knee OA is the most common type of OA, which results in major disability in senior people and more health care problems (27).
There is currently no best treatment for OA symptoms, while the application of stem cell as a novel therapy for discogenic pain, neuropathic pain, and OA becomes more common practice (28,29). It is reported that the application of MSCs, such as bone marrow mesenchymal stem cells, adipose-derived mesenchymal stem cells, synovial-derived mesenchymal stem cells and peripheral blood-derived mesenchymal stem cells, ameliorated the overall outcomes of patients with knee osteoathritis, including pain relief and functional improvement from basal evaluations, particularly at 12 and 24 months after follow-up (30).
It is indicated that mesenchymal stem cells isolated from the synovial membrane are reported that a candidate cell source in cartilage and menisci regenerative medicine and OA (31). Feng et al reported that intra-articular injection of allogeneic adipose-derived mesenchymal stem cells combined with hyaluronic acid could efficiently block OA progression and promote cartilage regeneration and allogeneic adipose-derived mesenchymal stem cells combined with HA might survive at least 14 weeks after intra-articular injection (32). Zhang et al the coculture of bone marrow stem cells with chondrocytes from patients with OA increases cell proliferation of chondrocytes and inhibits inflammatory activity in OA (33).
Due to a painless collection procedure and self-renewal properties, the human umbilical cord provides a promising source of mesenchymal stem cells, although the use of umbilical cord-derived stem cells in cell therapy was reported in other diseases (34–36), the effect of human umbilical cord stem cell for OA treatment has not been reported in the literature. In the present study, we explored the effect of human umbilical cord mesenchymal stem cells on chondrocytes from patients with OA was observed in a co-culture system, we found human umbilical cord mesenchymal stem cells and chondrocytes have mutual effect on each other, and human umbilical cord stem cell significantly attenuated OA in a co-culture system.
Human umbilical cord mesenchymal stem cells are reported to be positive for CD13, CD29, CD73, CD90, CD105 and HLA-ABC and are negative for CD34, CD45, CD133 and HLA-DR (37,38). As analyzed using flow cytometry in our study, we also found the umbilical cord mesenchymal stem cells have a high expression of CD29, CD73, CD90, CD105, and less expression of CD34, CD45 and CD133. In addition, human umbilical cord mesenchymal stem cells are pluripotent stem cells, they can differentiate into chondrogenic, osteogenic and adipogenic lineage. In our study, the cells have high expression of chondrogenic genes (aggrecan, collagen II and sox-9), osteogenic genes (OCN, ALP and RUNX2) and adipogenic genes (adipoq, aP2 and PPARr) after multi-lineage induction.
Some studies showed that chondrocytes promoted that chondrogenic differentiation of human umbilical cord blood-derived MSCs (39–42). Similar to previous study, our studies indicated that chondrocytes from patients with OA could promote the chondrogenesis of human umbilical cord stem cell. The mRNA analysis demonstrated that expression of collagen II, SOX-9, aggrecan, the specific marker of cartilage in human umbilical cord blood-derived MSCs, was increased in the co-culture with chondrocytes.
Some studies have shown that chondrocytes secrete the same cytokines and induce human stem cells to differentiate into chondrocytes (43,44). The present data was consistent with the previous study by Zheng et al that found chondrogenic differentiation of human umbilical cord blood-derived MSCs by co-culture with rabbit chondrocytes (41).
It is reported that intra-articular injection of mesenchymal stem cells significantly attenuated OA, as mesenchymal stem cells could downregulate some intrachondrogenic osteogenic genes and proteins (45). The present study indicated that human umbilical cord stem cell decreased the osteogenic genes (COX2, COL10A1 and MMP13) and production of some inflammatory factors (TNF-α, IL-1β, IL-6, IL-10), indicating human umbilical cord stem cell attenuated inflammatory response of OA. Zhu et al reported that human umbilical cord blood MSC transplantation suppresses inflammatory responses during early stage of focal cerebral ischemia in rabbits, ischemia-induced increases of IL-1β, IL-6 and TNF-α levels in the serum and peri-ischemic brain tissues within 6 h MCAO-reperfusion were markedly suppressed human umbilical cord stem cell transplantation (46). In another study, human umbilical cord mesenchymal stem cells were reported to decrease expression of MDA, GSSG, TNF-α, IFN-γ, TGF-β, IL-1, IL-2, IL-6, collagen type 1 mRNA and MMPs (47).
In addition, further experiments demonstrated that human umbilical cord stem cell significantly promoted chondrocyte proliferation, that may be explained by that human umbilical cord stem cell secreted many soluble factors, such as G-CSF, PDGF-BB and bFGF (48,49). In addition, human umbilical cord stem cell may inhibit apoptosis, and then promoted proliferaration, Zhang et al found human umbilical cord stem cell promoted proliferation and inhibited apoptosis of skin cells after heat-stress in vitro by secreting exosomes (50).
In conclusion, human umbilical cord stem cell could promote the proliferation of chondrocytes from patients with OA and downregulate inflammatory activity in OA, they would be promising autologous or allogenic cells in the treatment of OA, and this is a preliminary study and further studies are need to strenghten the presented results, such as the cell cycle changes in cell proliferation assay, and western blots in the differentiation assay, as well as COX-2 expression in inflammatory cells should be performed to strengthen the experiments. In addition, the isolation, purification, and expansion of human umbilical cord stem cell in vitro should be optimized before clinical application.
Acknowledgements
This study was supported by Medical and Health Research Foundation of PLA (grant no. 14ZD09), and Medical Science Foundation of Nanjing Military Area (grant nos. 14MS024 and 12MA019).
References
- 1.Fransen M, Bridgett L, March L, Hoy D, Penserga E, Brooks P. The epidemiology of osteoarthritis in Asia. Int J Rheum Dis. 2011;14:113–121. doi: 10.1111/j.1756-185X.2011.01608.x. [DOI] [PubMed] [Google Scholar]
- 2.van der Kraan PM, van den Berg WB. Chondrocyte hypertrophy and osteoarthritis: Role in initiation and progression of cartilage degeneration? Osteoarthritis Cartilage. 2012;20:223–232. doi: 10.1016/j.joca.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 3.Zhang Y, Jordan JM. Epidemiology of osteoarthritis. Clin Geriatr Med. 2010;26:355–369. doi: 10.1016/j.cger.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Neogi T, Zhang Y. Epidemiology of osteoarthritis. Rheum Dis Clin North Am. 2013;39:1–19. doi: 10.1016/j.rdc.2012.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cross M, Smith E, Hoy D, Nolte S, Ackerman I, Fransen M, Bridgett L, Williams S, Guillemin F, Hill CL, et al. The global burden of hip and knee osteoarthritis: Estimates from the global burden of disease 2010 study. Ann Rheum Dis. 2014;73:1323–1330. doi: 10.1136/annrheumdis-2013-204763. [DOI] [PubMed] [Google Scholar]
- 6.Akkiraju H, Nohe A. Role of chondrocytes in cartilage formation, progression of osteoarthritis and cartilage regeneration. J Dev Biol. 2015;3:177–192. doi: 10.3390/jdb3040177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dreier R. Hypertrophic differentiation of chondrocytes in osteoarthritis: The developmental aspect of degenerative joint disorders. Arthritis Res Ther. 2010;12:216. doi: 10.1186/ar3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mobasheri A, Kalamegam G, Musumeci G, Batt ME. Chondrocyte and mesenchymal stem cell-based therapies for cartilage repair in osteoarthritis and related orthopaedic conditions. Maturitas. 2014;78:188–198. doi: 10.1016/j.maturitas.2014.04.017. [DOI] [PubMed] [Google Scholar]
- 9.Bennell KL, Hunter DJ, Hinman RS. Management of osteoarthritis of the knee. BMJ. 2012;345:e4934. doi: 10.1136/bmj.e4934. [DOI] [PubMed] [Google Scholar]
- 10.Jakob R. The management of early osteoarthritis. Knee. 2014;21:799–800. doi: 10.1016/j.knee.2014.05.008. [DOI] [PubMed] [Google Scholar]
- 11.Kon E, Filardo G, Drobnic M, Madry H, Jelic M, van Dijk N, Della Villa S. Non-surgical management of early knee osteoarthritis. Knee Surg Sports Traumatol Arthrosc. 2012;20:436–449. doi: 10.1007/s00167-011-1713-8. [DOI] [PubMed] [Google Scholar]
- 12.Musumeci G, Loreto C, Castorina S, Imbesi R, Leonardi R, Castrogiovanni P. Current concepts in the treatment of cartilage damage. A review. Ital J Anat Embryol. 2013;118:189–203. [PubMed] [Google Scholar]
- 13.Musumeci G, Mobasheri A, Trovato FM, Szychlinska MA, Graziano AC, Lo Furno D, Avola R, Mangano S, Giuffrida R, Cardile V. Biosynthesis of collagen I, II, RUNX2 and lubricin at different time points of chondrogenic differentiation in a 3D in vitro model of human mesenchymal stem cells derived from adipose tissue. Acta Histochem. 2014;116:1407–1417. doi: 10.1016/j.acthis.2014.09.008. [DOI] [PubMed] [Google Scholar]
- 14.Musumeci G, Castrogiovanni P, Leonardi R, Trovato FM, Szychlinska MA, Di Giunta A, Loreto C, Castorina S. New perspectives for articular cartilage repair treatment through tissue engineering: A contemporary review. World J Orthop. 2014;5:80–88. doi: 10.5312/wjo.v5.i2.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Szychlinska MA, Stoddart MJ, D'Amora U, Ambrosio L, Alini M, PhD, Musumeci G. Mesenchymal stem cell-based cartilage regeneration approach and cell senescence: Can we manipulate cell aging and function? Tissue Eng Part B Rev. 2017 May 17; doi: 10.1089/ten.teb.2017.0083. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 16.Savkovic V, Li H, Seon JK, Hacker M, Franz S, Simon JC. Mesenchymal stem cells in cartilage regeneration. Curr Stem Cell Res Ther. 2014;9:469–488. doi: 10.2174/1574888X09666140709111444. [DOI] [PubMed] [Google Scholar]
- 17.Pers YM, Ruiz M, Noël D, Jorgensen C. Mesenchymal stem cells for the management of inflammation in osteoarthritis: State of the art and perspectives. Osteoarthritis Cartilage. 2015;23:2027–2035. doi: 10.1016/j.joca.2015.07.004. [DOI] [PubMed] [Google Scholar]
- 18.Musumeci G, Lo Furno D, Loreto C, Giuffrida R, Caggia S, Leonardi R, Cardile V. Mesenchymal stem cells from adipose tissue which have been differentiated into chondrocytes in three-dimensional culture express lubricin. Exp Biol Med (Maywood) 2011;236:1333–1341. doi: 10.1258/ebm.2011.011183. [DOI] [PubMed] [Google Scholar]
- 19.Szychlinska MA, Castrogiovanni P, Nsir H, Di Rosa M, Guglielmino C, Parenti R, Calabrese G, Pricoco E, Salvatorelli L, Magro G, et al. Engineered cartilage regeneration from adipose tissue derived-mesenchymal stem cells: A morphomolecular study on osteoblast, chondrocyte and apoptosis evaluation. Exp Cell Res. 2017;357:222–235. doi: 10.1016/j.yexcr.2017.05.018. [DOI] [PubMed] [Google Scholar]
- 20.Ding DC, Chang YH, Shyu WC, Lin SZ. Human umbilical cord mesenchymal stem cells: A new era for stem cell therapy. Cell Transplant. 2015;24:339–347. doi: 10.3727/096368915X686841. [DOI] [PubMed] [Google Scholar]
- 21.Gauthaman K, Yee FC, Cheyyatraivendran S, Biswas A, Choolani M, Bongso A. Human umbilical cord Wharton's jelly stem cell (hWJSC) extracts inhibit cancer cell growth in vitro. J Cell Biochem. 2012;113:2027–2039. doi: 10.1002/jcb.24073. [DOI] [PubMed] [Google Scholar]
- 22.Ali H, Al-Yatama MK, Abu-Farha M, Behbehani K, Al Madhoun A. Multi-lineage differentiation of human umbilical cord Wharton's Jelly Mesenchymal Stromal Cells mediates changes in the expression profile of stemness markers. PLoS One. 2015;10:e0122465. doi: 10.1371/journal.pone.0122465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fernandes AM, Herlofsen SR, Karlsen TA, Küchler AM, Fløisand Y, Brinchmann JE. Similar properties of chondrocytes from osteoarthritis joints and mesenchymal stem cells from healthy donors for tissue engineering of articular cartilage. PLoS One. 2013;8:e62994. doi: 10.1371/journal.pone.0062994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang ZH, Li XL, He XJ, Wu BJ, Xu M, Chang HM, Zhang XH, Xing Z, Jing XH, Kong DM, et al. Delivery of the Sox9 gene promotes chondrogenic differentiation of human umbilical cord blood-derived mesenchymal stem cells in an in vitro model. Braz J Med Biol Res. 2014;47:279–286. doi: 10.1590/1414-431X20133539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hata K, Watanabe Y, Nakai H, Hata T, Hoshiai H. Expression of the vascular endothelial growth factor (VEGF) gene in epithelial ovarian cancer: An approach to anti-VEGF therapy. Anticancer Res. 2011;31:731–737. [PubMed] [Google Scholar]
- 26.Puljak L, Marin A, Vrdoljak D, Markotic F, Utrobicic A, Tugwell P. Celecoxib for osteoarthritis. Cochrane Database Syst Rev. 2017;5:CD009865. doi: 10.1002/14651858.CD009865.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu Q, Chen B, Wang Y, Wang X, Han D, Ding D, Zheng Y, Cao Y, Zhan H, Zhou Y. The effectiveness of manual therapy for relieving pain, stiffness, and dysfunction in knee osteoarthritis: A systematic review and meta-analysis. Pain Physician. 2017;20:229–243. [PubMed] [Google Scholar]
- 28.Fellows CR, Matta C, Zakany R, Khan IM, Mobasheri A. Adipose, bone marrow and synovial joint-derived mesenchymal stem cells for cartilage repair. Front Genet. 2016;7:213. doi: 10.3389/fgene.2016.00213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chakravarthy K, Chen Y, He C, Christo PJ. Stem cell therapy for chronic pain management: Review of uses, advances, and adverse effects. Pain Physician. 2017;20:293–305. [PubMed] [Google Scholar]
- 30.Cui GH, Wang YY, Li CJ, Shi CH, Wang WS. Efficacy of mesenchymal stem cells in treating patients with osteoarthritis of the knee: A meta-analysis. Exp Ther Med. 2016;12:3390–3400. doi: 10.3892/etm.2016.3791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kohno Y, Mizuno M, Ozeki N, Katano H, Komori K, Fujii S, Otabe K, Horie M, Koga H, Tsuji K, et al. Yields and chondrogenic potential of primary synovial mesenchymal stem cells are comparable between rheumatoid arthritis and osteoarthritis patients. Stem Cell Res Ther. 2017;8:115. doi: 10.1186/s13287-017-0572-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Feng C, Luo X, He N, Xia H, Lv X, Zhang X, Li D, Wang F, He J, Zhang L, et al. Efficacy and persistence of allogeneic adipose-derived mesenchymal stem cells combined with hyaluronic acid in osteoarthritis after intra-articular injection in a sheep model. Tissue Eng Part A. 2017 Sep 27; doi: 10.1089/ten.TEA.2017.0039. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 33.Zhang Q, Chen Y, Wang Q, Fang C, Sun Y, Yuan T, Wang Y, Bao R, Zhao N. Effect of bone marrow-derived stem cells on chondrocytes from patients with osteoarthritis. Mol Med Rep. 2016;13:1795–1800. doi: 10.3892/mmr.2015.4720. [DOI] [PubMed] [Google Scholar]
- 34.Kim SW, Han H, Chae GT, Lee SH, Bo S, Yoon JH, Lee YS, Lee KS, Park HK, Kang KS. Successful stem cell therapy using umbilical cord blood-derived multipotent stem cells for Buerger's disease and ischemic limb disease animal model. Stem Cells. 2006;24:1620–1626. doi: 10.1634/stemcells.2005-0365. [DOI] [PubMed] [Google Scholar]
- 35.Baker CD, Abman SH. Umbilical cord stem cell therapy for bronchopulmonary dysplasia: Ready for prime time? Thorax. 2013;68:402–404. doi: 10.1136/thoraxjnl-2012-202661. [DOI] [PubMed] [Google Scholar]
- 36.Gu Z, Akiyama K, Ma X, Zhang H, Feng X, Yao G, Hou Y, Lu L, Gilkeson GS, Silver RM, et al. Transplantation of umbilical cord mesenchymal stem cells alleviates lupus nephritis in MRL/lpr mice. Lupus. 2010;19:1502–1514. doi: 10.1177/0961203310373782. [DOI] [PubMed] [Google Scholar]
- 37.Nagamura-Inoue T, He H. Umbilical cord-derived mesenchymal stem cells: Their advantages and potential clinical utility. World J Stem Cells. 2014;6:195–202. doi: 10.4252/wjsc.v6.i2.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mennan C, Brown S, McCarthy H, Mavrogonatou E, Kletsas D, Garcia J, Balain B, Richardson J, Roberts S. Mesenchymal stromal cells derived from whole human umbilical cord exhibit similar properties to those derived from Wharton's jelly and bone marrow. FEBS Open Bio. 2016;6:1054–1066. doi: 10.1002/2211-5463.12104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li X, Duan L, Liang Y, Zhu W, Xiong J, Wang D. Human umbilical cord blood-derived mesenchymal stem cells contribute to chondrogenesis in coculture with chondrocytes. Biomed Res Int. 2016;2016:3827057. doi: 10.1155/2016/3827057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang J, Li J, Deng N, Zhao X, Liu Y, Wang X, Zhang H. Transfection of hBMP-2 into mesenchymal stem cells derived from human umbilical cord blood and bone marrow induces cell differentiation into chondrocytes. Minerva Med. 2014;105:283–288. [PubMed] [Google Scholar]
- 41.Zheng P, Ju L, Jiang B, Chen L, Dong Z, Jiang L, Wang R, Lou Y. Chondrogenic differentiation of human umbilical cord blood-derived mesenchymal stem cells by co-culture with rabbit chondrocytes. Mol Med Rep. 2013;8:1169–1182. doi: 10.3892/mmr.2013.1637. [DOI] [PubMed] [Google Scholar]
- 42.Mennan C, Wright K, Bhattacharjee A, Balain B, Richardson J, Roberts S. Isolation and characterisation of mesenchymal stem cells from different regions of the human umbilical cord. Biomed Res Int. 2013;2013:916136. doi: 10.1155/2013/916136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu L, Prins HJ, Helder MN, van Blitterswijk CA, Karperien M. Trophic effects of mesenchymal stem cells in chondrocyte co-cultures are independent of culture conditions and cell sources. Tissue Eng Part A. 2012;18:1542–1551. doi: 10.1089/ten.tea.2011.0715. [DOI] [PubMed] [Google Scholar]
- 44.Meretoja VV, Dahlin RL, Kasper FK, Mikos AG. Enhanced chondrogenesis in co-cultures with articular chondrocytes and mesenchymal stem cells. Biomaterials. 2012;33:6362–6369. doi: 10.1016/j.biomaterials.2012.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jo CH, Lee YG, Shin WH, Kim H, Chai JW, Jeong EC, Kim JE, Shim H, Shin JS, Shin IS, et al. Intra-articular injection of mesenchymal stem cells for the treatment of osteoarthritis of the knee: A proof-of-concept clinical trial. Stem Cells. 2014;32:1254–1266. doi: 10.1002/stem.1634. [DOI] [PubMed] [Google Scholar]
- 46.Zhu Y, Guan YM, Huang HL, Wang QS. Human umbilical cord blood mesenchymal stem cell transplantation suppresses inflammatory responses and neuronal apoptosis during early stage of focal cerebral ischemia in rabbits. Acta Pharmacol Sin. 2014;35:585–591. doi: 10.1038/aps.2014.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Min F, Gao F, Li Q, Liu Z. Therapeutic effect of human umbilical cord mesenchymal stem cells modified by angiotensin-converting enzyme 2 gene on bleomycin-induced lung fibrosis injury. Mol Med Rep. 2015;11:2387–2396. doi: 10.3892/mmr.2014.3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Amable PR, Teixeira MV, Carias RB, Granjeiro JM, Borojevic R. Protein synthesis and secretion in human mesenchymal cells derived from bone marrow, adipose tissue and Wharton's jelly. Stem Cell Res Ther. 2014;5:53. doi: 10.1186/scrt442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen J, Liu Z, Hong MM, Zhang H, Chen C, Xiao M, Wang J, Yao F, Ba M, Liu J, et al. Proangiogenic compositions of microvesicles derived from human umbilical cord mesenchymal stem cells. PLoS One. 2014;9:e115316. doi: 10.1371/journal.pone.0115316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang B, Wang M, Gong A, Zhang X, Wu X, Zhu Y, Shi H, Wu L, Zhu W, Qian H, Xu W. HucMSC-exosome mediated-Wnt4 signaling is required for cutaneous wound healing. Stem Cells. 2015;33:2158–2168. doi: 10.1002/stem.1771. [DOI] [PubMed] [Google Scholar]