Abstract
MicroRNAs (miRs) have been proposed as minimally invasive prognostic markers for various types of cancer, including liver cancer, which is one of the most common cancers worldwide. In the present study, the expression of miR-34a in human liver cancer tissues and cell lines was evaluated and the effects of miR-34a on cell proliferation, invasion and glycolysis in hepatocellular carcinoma (HCC) cells were determined. The results indicated that miR-34a was downregulated in human liver cancer tissues. Overexpression of miR-34a significantly inhibited liver cancer cell proliferation and clone formation. In terms of the underlying mechanism, miR-34a was indicated to negatively regulate the expression of lactate dehydrogenase A (LDHA), which consequently inhibited LDHA-dependent glucose uptake in the cancer cells, as well as cell proliferation and invasion. Collectively, these data suggest that miR-34a functions as a negative regulator of glucose metabolism and may serve as a novel marker for liver cancer prognosis.
Keywords: micrRNA-34a, liver cancer, glucose metabolism
Introduction
Liver cancer is one of the most common malignancies worldwide, with an increasing incidence, especially in China (1–3). Hepatocellular carcinoma (HCC) is the most common type of primary liver cancer; it is estimated to be the second leading cause of cancer-related death in developing countries and the sixth leading cause in developed countries (4–6). Despite recent advances in HCC management, including surgical resection, radiofrequency ablation, liver transplantation, and transcatheter arterial chemoembolization (TACE), the prognosis of most HCC patients remains poor. In addition, the molecular pathogenesis of HCC is not well understood. Therefore, it is crucial to clarify the effector molecules and signaling pathways underlying HCC tumor progression and metastasis to develop novel therapeutic strategies and more effective treatments.
MicroRNAs (miRNAs/miRs) are endogenous small (19–25 nucleotide) non-coding RNAs. By binding to the 3′-untranslated regions (3′-UTRs) of targeted messenger RNAs (mRNAs) (7–9), miRNAs play important roles in post-transcriptional regulation and numerous biological processes such as proliferation, survival, and apoptosis (10,11). Recently, several studies have found that miRNAs are also critical for cancer invasion and metastasis. Dysregulated expression of miRNAs is correlated with many human cancers, including HCC. In addition, miRNAs are emerging as promising diagnostic and prognostic markers for human cancers (12,13).
miR-34a reportedly acts as a tumor suppressor in many cancers, including pancreatic cancer, prostate cancer, glioblastoma, colon cancer, and breast cancer (14–17). In pancreatic cancer, miR-34a inhibits stem cell self-renewal by downregulating Bcl-2 and Notch (18). In glioblastoma, miR-34a inhibits cell proliferation due to its regulation of the TGF-β signaling network (19). Several recent studies have shown that the expression of miR-34a is dramatically decreased in clinical HCC specimens, suggesting that miR-34a represents a potential target for HCC treatment (20–22). However, the biological effect and underlying mechanism of miR-34a in HCC tumorigenesis and metastasis remains to be elucidated. Thus, further exploration of miR-34a is of utmost significance.
Lactate dehydrogenase A (LDHA) plays an important role in tumor cell metabolism (23–25). It has recently been reported that LDHA expression is correlated with progression and survival outcomes in multiple cancers, including renal cancer, gastric cancer, esophageal squamous cell carcinoma, and pancreatic cancer. Moreover, several oncogenes and deacetylases, including HIF-1α, SIRT2, and MYC, contribute to the regulation of LDHA expression and post-transcriptional modification (26,27). Together, these studies indicate that LDHA could be a novel therapeutic target for multiple human cancers, including HCC.
In this study, we evaluated the expression of miR-34a in HCC tissues and cell lines. Furthermore, functional studies of the effects of miR-34a and LDHA on cell proliferation, invasion, and glycolysis in HCC cells were performed to explore the underlying connection between miR-34a and HCC.
Materials and methods
Cell lines and culture
The HCC cell lines Huh7, HCCLM3, Hep3B, Mahlavu, SNU475, and human hepatocyte line L02 were obtained from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China) and cultured in Dulbecco's modified Eagle's medium (DMEM) (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin (Gibco; Thermo Fisher Scientific, Inc.) at 37°C in a humidified atmosphere with 5% CO2.
Clinical samples
The study protocols were approved by the Ethics Committee of Qingdao No. 6 People's Hospital following the ethical standards outlined in the Declaration of Helsinki. Written informed consent was obtained from all patients. Tissue samples from 22 pairs of HCC tissues (HC) and their corresponding adjacent tissues (normal, located ~2 cm apart) were collected in the Department of Hepatology, Qingdao No. 6 People's Hospital. The clinical profiles of the 22 participants in the study, such as gender, age, BMI, tumor size, and Child-Pugh class were described in Table I. Tissue samples were obtained and immediately stored in liquid nitrogen for further reverse transcription-quantitative polymerase chain reaction (RT-qPCR analysis.
Table I.
The clinical profiles of the 22 patients with hepatocellular carcinoma.
| Clinical variable | No. of patients (n=22) |
|---|---|
| Gender | |
| Female | 8 |
| Male | 14 |
| Age | |
| Median | 58.7 |
| Range | 40–79 |
| HBV | |
| Positive | 22 |
| Negative | 0 |
| Tumor size | |
| ≤5 cm | 13 |
| >5 cm | 9 |
| Child-Pugh class | |
| A | 22 |
| B | 0 |
| BMI | |
| Median | 21.2 |
| Range | 16–26.8 |
HBV, hepatitis B virus; BMI, body mass index.
RNA extraction and RT-qPCR
Total RNA was extracted from the tissues and cell lines using TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.). RT was performed using a Prime-Script RT Reagent kit (Takara Biotechnology Co., Ltd., Dalian, China) and RT-qPCR was performed using SYBR Premix Ex Taq (Takara Biotechnology Co., Ltd.). MicroRNA extraction was conducted using a MicroRNA Extraction kit (Tiangen Biotech Co., Ltd., Beijing, China) and RT-qPCR was performed with SYBR Premix Ex Taq (Takara Biotechnology Co., Ltd.) according to the manufacturer's protocol. GAPDH and RNU6B were used as normalizing controls for mRNA and miRNA quantification, respectively. The primers were as follows: miR-34a forward, 5′-UGGCAGUGUCUUAGCUGGUUGU-3′ and reverse, 5′-AACCAGCUAAGACACUGCCAUU-3′; LDHA, forward, 5′-TTGGTCCAGCGTAACGTGAAC-3′ and reverse, 5′-CCAGGATGTGTAGCCTTTGAG-3′. The 2−ΔΔCq method was used to determine relative expression levels.
Cell proliferation assay
HuH7 and HCCLM3 cells were seeded in 96-well plates and incubated for 24 h before being transfected with miR-34a or scrambled mimics. After the cells were incubated for another 48 h, an MTT assay was performed according to the manufacturer's instructions (Molecular Probes; Thermo Fisher Scientific, Inc.). The absorbance at 570 nm was determined using a Spectra Max 250 spectrophotometer (Molecular Devices, Sunnyvale, CA, USA).
Foci formation assay
HuH7 and HCCLM3 cells were seeded in 6-well plates at a density of 2,000 cells per well and transfected with miR-34a or scrambled mimics. When the number of clones exceeded 50, the cells were stained with 0.06% crystal violet and the foci numbers were counted.
Cell invasion assay
A Transwell invasion assay was used to determine the invasion capacity of the tumor cells. Briefly, HuH7 and HCCLM3 cells were transfected with miR-34a or scrambled mimics, cultivated for 24 h, and seeded onto the Matrigel-coated chambers (24-well; BD Biosciences, San Jose, CA, USA) in serum-free DMEM. DMEM containing 10% FBS was added to the lower chamber. The matrix and non-invaded cells were removed after 48 h of incubation, while the invaded cells were fixed, stained, and counted.
Wound-healing assay
After transfection, HuH7 cells were seeded in 6-well plates and grown to 90% confluence. After 24 h, linear scratch wounds were created using pipette tips and the cells were washed three times with PBS. The cells were then incubated in DMEM containing 5% FBS. Cell movement at the wound site was monitored and photographed at 0 and 24 h. The percentage of wound closure was analyzed and compared as described previously (24).
Measurement of glucose uptake and lactate production
To assess glucose uptake and lactate production, HuH7 and HCCLM3 cells were transfected with miR-34a or scrambled mimics, and the cell culture medium was collected 48 h after transfection. An Amplex® Red Glucose/Glucose Oxidase Assay kit (Invitrogen; Thermo Fisher Scientific, Inc.) was then used to measure glucose uptake, and a lactate assay kit (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) was used to determine lactate production.
Western blotting
HuH7 and HCCLM3 cells were transfected with either miR-34a or scrambled mimics. After 48 h, protein was extracted using RIPA lysis buffer. Protein concentrations were quantified using a Protein BCA Assay kit (Pierce; Thermo Fisher Scientific, Inc.). The protein samples were separated by 10% SDS-PAGE then transferred to polyvinylidene difluoride membranes (EMD Millipore, Billerica, MA, USA). After blocking in 5% skim milk for 1 h at room temperature, the membranes were incubated with antibodies against LDHA and β-actin (Affinity Biosciences, Columbus, OH, USA) at 4°C overnight. A peroxidase-conjugated secondary antibody (dilution 1:1,500) was applied for 1 h at room temperature to visualize the target proteins. The target proteins were visualized using Western blotting detection reagents (Thermo Fisher Scientific, Inc.) and then exposed to X-ray film (Kodak, Inc., Rochester, NY, USA). The optical density (OD) (target proteins)/OD (β-actin) was used to quantify protein expression.
LDHA-expressing vector
Full-length LDHA cDNA was purchased from GeneCopeia (Rockville, MD, USA) and sub-cloned into the expression vector pcDNA3.1(+) (GeneCopeia). The vector pcDNA3.1(+) was used as a negative control.
Statistical analysis
All in vitro experiments were performed in triplicate. The results are presented as means ± standard deviation. Statistical comparisons between two groups were analyzed using t-tests and χ2 tests. Statistical comparisons between multiple groups were analyzed using one-way ANOVA followed by Newman-Keuls post-hoc comparison test. P<0.05 was considered to indicate a statistically significant difference (SPSS 16.0; SPPS, Inc., Chicago, IL, USA).
Results
miR-34a was significantly downregulated in HCC cell lines and clinical specimens
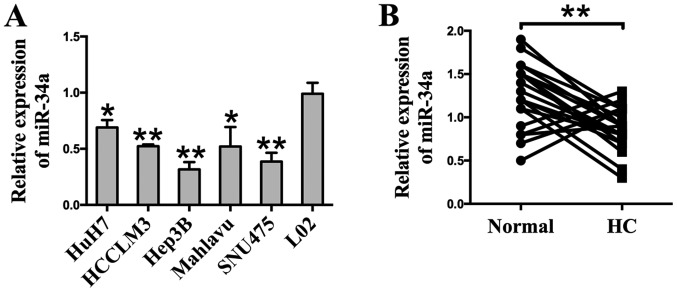
A RT-qPCR analysis was employed to detect the expression of miR-34a. The results show that the expression of miR-34a was markedly downregulated in six different HCC cell lines (Huh7, HCCLM3, Hep3B, Mahlavu, and SNU475) compared to the human hepatocyte cell line L02 (Fig. 1A). To determine the expression of miR-34a in clinical specimens, HCC tissues (HC) and their matched adjacent normal tissues (Normal) were examined through RT-qPCR analysis. Compared with adjacent normal tissues, we found that 77.3% (17 of 22 patients, P<0.01) of tumor tissues showed decreased miR-34a levels (Fig. 1B). Taken together, these results indicate that miR-34a is downregulated at a high frequency in HCC, and may be related to HCC carcinogenesis.
Figure 1.
miR-34a is downregulated in liver cancer cell lines and clinical HCC specimens. (A) RT-qPCR analysis revealed the expression level of miR-34a in six HCC cell lines (Huh7, HCCLM3, HepG2, Hep3B, Mahlavu, and SNU475) and human hepatocyte line L02. (B) RT-qPCR was performed to determine the expression of miR-34a in 22 HCC tissues (HC) and their matched adjacent normal tissues (Normal). These results indicated that the expression of miR-34a was downregulated in HCC cell lines and clinical specimens. *P<0.05 and **P<0.01 vs. L02.
miR-34a inhibits cell proliferation and invasion
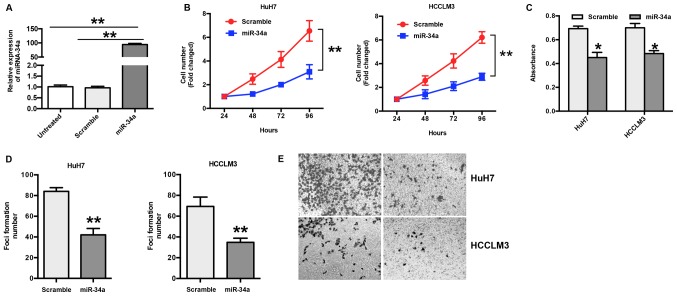
The expression of miR-34a was examined in HuH7 and HCCLM3 cells following transfection with miR-34a or scramble mimics. The RT-qPCR results show a significant increase in miR-34a (~94 fold) in transfected cells compared to scramble or untreated cells (P<0.001) (Fig. 2A). To explore the biological effects of miR-34a in HCC, HuH7 and HCCLM3 cells were transfected with miR-34a or scramble mimics, and the number of cells was counted. The results show that ectopic expression of miR-34a significantly suppressed the proliferation of HuH7 and HCCLM3 cells in a time-dependent manner (P<0.05) (Fig. 2B); this was further confirmed by an MTT assay (Fig. 2C). In addition, the results of the foci formation assay show that the overexpression of miR-34a led to decreased foci formation of HuH7 and HCCLM3 cells (P<0.01) (Fig. 2D). To further explore the function of miR-34a in HCC, a Transwell invasion assay was performed. The results show that overexpression of miR-34a significantly inhibited invasion in HuH7 and HCCLM3 cells compared with the scramble group (Fig. 2E).
Figure 2.
miR-34a inhibits cell proliferation and invasion. (A) The expression level of miR-34a was greatly increased by miR-34a mimics. **P<0.01. (B) The ectopic expression of miR-34a significantly suppressed the cell proliferation of HuH7 and HCCLM3 cells in a time dependent manner. **P<0.01 vs. Scramble. (C) The results of MTT assay showed miR-34a significantly suppressed cell proliferation in 48 h after transfection. *P<0.05 vs. Scramble. (D) The results of foci formation assay showed that overexpression of miR-34a significantly decreased foci formation of HuH7 and HCCLM3 cells. **P<0.01 vs. Scramble. (E) Representative images of three independent experiments are presented (magnification, ×100). The results of transwell invasion assay showed that overexpression of miR-34a significantly inhibited cell invasion of HuH7 and HCCLM3 cells compared with the scramble group.
miR-34a inhibits glycolysis in HCC
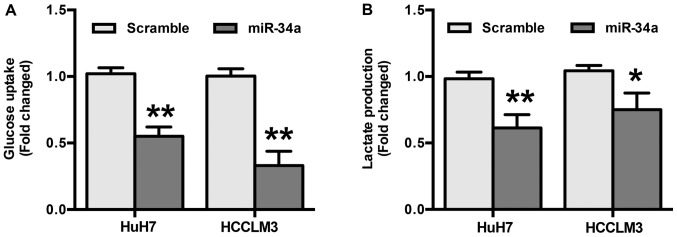
To explore the role miR-34a in glycolysis in HCC, differences in metabolic parameters were detected after HuH7 and HCCLM3 cells were transfected with miR-34a or scramble mimics. The results show that overexpression of miR-34a significantly decreased glucose uptake (P<0.01) (Fig. 3A). In addition, the results indicate that miR-34a mimics induced a decrease in the production of extracellular lactate (P<0.05) (Fig. 3B). Taken together, these results suggest that the inhibition of glycolysis by miR-34a may be responsible for the suppression of migration and invasion in HCC cells.
Figure 3.
miR-34a inhibits glycolysis in hepatocellular carcinoma. (A) HuH7 and HCCLM3 cells were transfected with miR-34a or scramble mimics. The glucose uptake levels were measured after transfection. (B) HuH7 and HCCLM3 cells were transfected as described before and the lactate production levels were measured. The results showed that overexpression of miR-34a largely decreased glucose uptake and extracellular lactate production. *P<0.05 and **P<0.01 vs. Scramble.
miR-34a inhibits LDHA expression in HCC
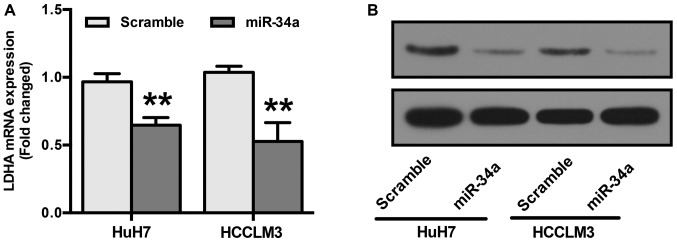
LDHA is reportedly a direct target of miR-34a (28). To confirm this, RT-qPCR and Western blot analyses were performed. HuH7 and HCCLM3 cells were transfected with miR-34a or scramble mimics before examining LDHA expression. Our results show that cells transfected with miR-34a mimics showed a significant reduction in both the mRNA and protein levels of LDHA (Fig. 4A and B).
Figure 4.
LDHA is a target of miR-34a. (A) HuH7 and HCCLM3 cells were transfected with miR-34a or scramble mimics. Overexpression of miR-34a significantly downregulated the levels of LDHA mRNA. (B) Overexpression of miR-34a significantly inhibited the LDHA protein expression. **P<0.01 vs. Scramble.
miR-34a inhibits LDHA-induced glycolysis, cell proliferation, and invasion
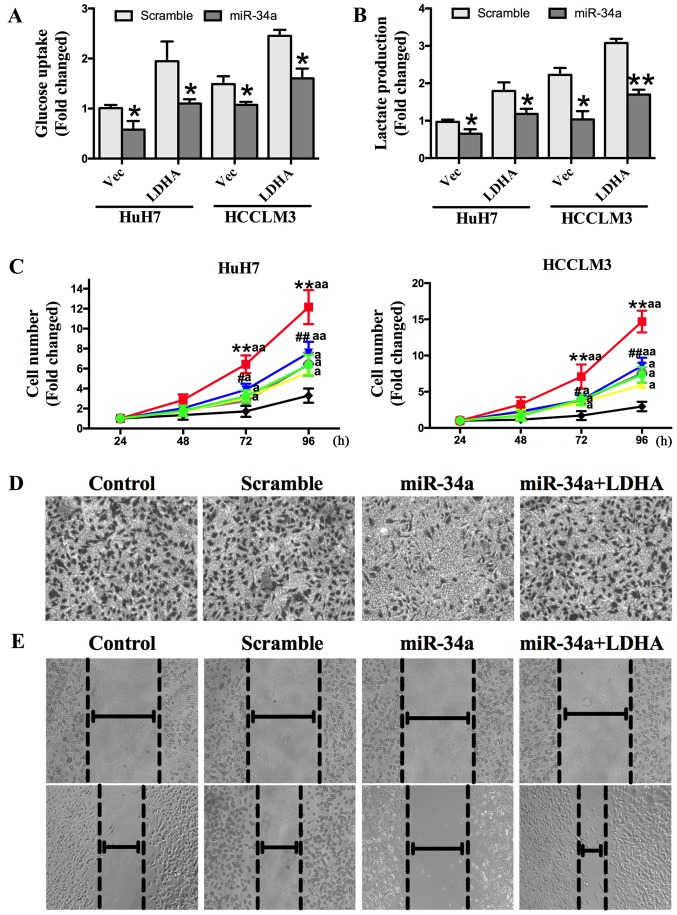
To confirm that miR-34a inhibited glycolysis, cell proliferation, and invasion of HCC cells by targeting LDHA, we transfected HuH7 and HCCLM3 cells with an LDHA-expressing vector, control vector, control vector + miR-34a mimics, control vector + a scrambled oligonucleotide, LDHA expressing vector + miR-34a mimics, or LDHA expressing vector + a scrambled oligonucleotide. The results show that overexpression of LDHA could increase HCC cell glucose uptake and lactate production, and that this effect was abolished by transfection with miR-34a mimics (Fig. 5A and B). We also found that the overexpression of LDHA could promote cell proliferation, while miR-34a inhibited cell proliferation. The increased cell proliferation induced by LDHA was significantly repressed by miR-34a (Fig. 5C). Transwell invasion and wound-healing assays showed that miR-34a mimics decreased invasion capacity, which was increased by the overexpression of LDHA (Fig. 5D and E). Together, these findings demonstrate that miR-34a inhibits HCC glycolysis, and cell proliferation and invasion in vitro by targeting LDHA.
Figure 5.
LDHA-induced glycolysis and cell proliferation can be inhibited by miR-34a. (A) Overexpression of LDHA could increase HCC cell glucose uptake, which was abrogated by miR-34a after cotransfection with miR-34a mimics. *P<0.05 vs. Scramble. (B) Increased lactate production by LDHA was abrogated by miR-34a. *P<0.05 and **P<0.01 vs. Scramble. (C) HuH7 and HCCLM3 cells were transfected as described before. After transfection, the increased cell proliferation of HuH7 and HCCLM3 cells by LDHA was suppressed by miR-34a in a time-dependent manner. **P<0.01 vs. Vec; #P<0.05 and ##P<0.01 vs. LDHA; aP<0.05 and aaP<0.01 vs. miR-34a. (D) miR-34a mimics reversed the invasion capacity which was increased by overexpressed LDHA (magnification, ×200). (E) miR-34a mimics reversed the migration capacity which was increased by overexpressed LDHA (magnification, ×100).
Discussion
Although outstanding advances in surgical techniques and radio-chemotherapy regimens have been achieved in recent decades, the prognosis for most patients with HCC remains poor. Therefore, novel therapeutic strategies are needed to treat HCC more effectively (3,4,6).
Increasing evidence has demonstrated that miRNAs play a vital role in the pathogenesis, clinical metastasis, and progression of multiple cancers, including HCC (29–31). Fang et al (32) reported that miR-383 was downregulated in HCC tissues compared with their adjacent normal tissues. In addition, overexpression of miR-383 significantly suppressed cell proliferation and invasion, indicating that miR-383 may act as a tumor suppressor in HCC. Kota et al (33) demonstrated that systemic administration of miR-26a resulted in significant inhibition of HCC cell proliferation, and induced protection against disease progression without toxicity. Thus, understanding the crucial role of miRNAs in HCC may provide novel diagnostic, prognostic, and therapeutic potential.
miR-34a is a member of the highly conserved miR-34 family. It is located at the chromosome lp36 locus, which is particularly susceptible to molecular events that may disturb the balance of proliferation and apoptosis (34,35). miR-34a is also one of multiple recently discovered miRNAs that are regulated by p53 (17,36). Studies have shown that miR-34a is an important molecule that inhibits the growth of many cancers. Decreased expression of miR-34a has been found in pancreatic cancer, cervical cancer, prostate cancer, glioblastoma, colon cancer, esophageal squamous cell carcinoma, breast cancer, and lung cancer (37–39). In cancers like pancreatic cancer and glioblastoma, miR-34a was reported to inhibit cell proliferation and induce apoptosis by downregulating Bcl-2 and Notch or the TGF-β signaling network (18,19,40). However, the role of miR-34a in the pathogenesis of HCC is unclear.
Several studies have shown that miR-34a may act as an oncogene (41). Pineau et al (42) found that miR-34a was highly expressed in liver cancer, and was positively related to HCC progression. In a tamoxifen-induced mouse liver cancer model, Pogribny et al (43) found that miR-34 expression was increased. However, more and more studies have recently shown that the expression of miR-34a is dramatically decreased in clinical HCC specimens, suggesting that miR-34a is a potential target for HCC treatment (20–22). Dang et al (44) detected miR-34a expression in 60 HCC tissues and adjacent normal tissues and found that miRNA-34a expression in HCC tissues was significantly lower than in normal tissues. Moreover, a miR-34a mimic was reported to inhibit HCC cell growth and induce apoptosis. However, the mechanism remains unknown. In this study, we found that miR-34a was downregulated in human liver cancer tissues, and that the upregulation of miR-34a inhibits liver cancer cell proliferation, migration, and invasion, consistent with previous studies (20–22,44). Moreover, we found that miR-34a negatively regulates the expression of LDHA in HCC cell lines, which consequently inhibits LDHA-dependent glucose uptake in cancer cells, leading to reduced cell proliferation and invasion.
Aberrant metabolism has been shown to play an important role in the progression and metastasis of multiple cancers (45–47). Among a series of enzymes involved in cancer metabolism, LDHA is reported to be of vital importance and is involved in proliferation and glycolysis in gastric cancer, breast cancer, and pancreatic cancer (23,25,27,48). Indeed, some references have already proved that miR-34a negatively regulates the expression level of LDHA in various cancers (23,25,28,49). By performing luciferase reporter assays, or cloning of 3′-UTRs, miR-34a was reported to have direct role on LDHA mRNA stability in various cell lines (23,28), including HCC cells (50). In a previous study based on HCC cells, Wang et al reported that miR-34a specifically bind to the 3′-UTR region of LDHA, acting as a negative regulator (50). In this study, we confirmed that LDHA is also involved in HCC metabolism, and an LDHA-expressing vector significantly improved HCC cell glycolysis, proliferation, migration, and invasion. Furthermore, we also confirmed that LDHA may be a target gene for miR-34a. The function of miR-34a in HCC may be partly due to its regulation of LDHA and the subsequent reprogramming of glucose metabolism. The increased cell glycolysis, proliferation, and invasion triggered by LDHA was effectively inhibited by miR-34a in HCC cell lines, which indicates that the miR-34a-LDHA axis could be a promising therapeutic target for more effective HCC treatment.
To the best of our knowledge, this is the first study of the effect of miR-34a on HCC glucose metabolism. However, some limitations of our study should be noted. First, this was an in vitro study that used clinical samples, but it lacked an in vivo evaluation of the potential impact of miR-34a. Second, the carcinogenesis of HCC is complicated, which means miR-34a may also target other genes. In addition, although the results of this study showed that LDHA was downregulated in miR-34a transfected HuH7 and HCCLM3 cells, further analysis about the direct role of miR-34a on LDHA mRNA stability was not conducted, which may decrease the robustness of the conclusion of our study. Nevertheless, our study provides useful insight into the effect of miR-34a on cell proliferation, invasion, and glycolysis in HCC cell lines. Future studies are needed.
In summary, the results of this study suggest that miR-34a inhibits HCC glycolysis, cell proliferation, and invasion in vitro by targeting LDHA. miR-34a functions as a negative regulator of glucose metabolism, which may serve as a novel marker for liver cancer prognosis.
The results of this study suggest that miR-34a inhibits HCC glycolysis, cell proliferation, and invasion in vitro by targeting LDHA.
References
- 1.Zhong GC, Liu Y, Chen N, Hao FB, Wang K, Cheng JH, Gong JP, Ding X. Reproductive factors, menopausal hormone therapies and primary liver cancer risk: A systematic review and dose-response meta-analysis of observational studies. Hum Reprod Update. 2016;23:126–138. doi: 10.1093/humupd/dmw037. [DOI] [PubMed] [Google Scholar]
- 2.Nio K, Yamashita T, Kaneko S. The evolving concept of liver cancer stem cells. Mol Cancer. 2017;16:4. doi: 10.1186/s12943-016-0572-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rao CV, Asch AS, Yamada HY. Frequently mutated genes/pathways and genomic instability as prevention targets in liver cancer. Carcinogenesis. 2017;38:2–11. doi: 10.1093/carcin/bgw118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rabinel P, Dousse D, Muscari F, Suc B. Management of liver cancer. The Surgeon's point of view. Rep Pract Oncol Radiother. 2017;22:176–180. doi: 10.1016/j.rpor.2017.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sia D, Villanueva A, Friedman SL, Llovet JM. Liver cancer cell of origin, molecular class, and effects on patient prognosis. Gastroenterology. 2017;152:745–761. doi: 10.1053/j.gastro.2016.11.048. [DOI] [PubMed] [Google Scholar]
- 6.Stroehl YW, Letzen BS, van Breugel JM, Geschwind JF, Chapiro J. Intra-arterial therapies for liver cancer: Assessing tumor response. Expert Rev Anticancer Ther. 2017;17:119–127. doi: 10.1080/14737140.2017.1273775. [DOI] [PubMed] [Google Scholar]
- 7.Ma J, Lin J, Qian J, Qian W, Yin J, Yang B, Tang Q, Chen X, Wen X, Guo H, Deng Z. miR-378 promotes the migration of liver cancer cells by down-regulating Fus expression. Cell Physiol Biochem. 2014;34:2266–2274. doi: 10.1159/000369669. [DOI] [PubMed] [Google Scholar]
- 8.Pang F, Zha R, Zhao Y, Wang Q, Chen D, Zhang Z, Chen T, Yao M, Gu J, He X. miR-525-3p enhances the migration and invasion of liver cancer cells by downregulating ZNF395. PLoS One. 2014;9:e90867. doi: 10.1371/journal.pone.0090867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yin J, Bai Z, Song J, Yang Y, Wang J, Han W, Zhang J, Meng H, Ma X, Yang Y, et al. Differential expression of serum miR-126, miR-141 and miR-21 as novel biomarkers for early detection of liver metastasis in colorectal cancer. Chin J Cancer Res. 2014;26:95–103. doi: 10.3978/j.issn.1000-9604.2014.02.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guo W, Qiu Z, Wang Z, Wang Q, Tan N, Chen T, Chen Z, Huang S, Gu J, Li J, et al. miR-199a-5p is negatively associated with malignancies and regulates glycolysis and lactate production by targeting hexokinase 2 in liver cancer. Hepatology. 2015;62:1132–1144. doi: 10.1002/hep.27929. [DOI] [PubMed] [Google Scholar]
- 11.Liu HB, Hua Y, Jin ZX. Effects of MicroRNA-132 transfection on the proliferation and apoptosis of human liver cancer cells in vitro and in vivo. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2015;37:30–36. doi: 10.3881/j.issn.1000-503X.2015.01.006. [DOI] [PubMed] [Google Scholar]
- 12.Lu Z, Zhang W, Gao S, Jiang Q, Xiao Z, Ye L, Zhang X. miR-506 suppresses liver cancer angiogenesis through targeting sphingosine kinase 1 (SPHK1) mRNA. Biochem Biophys Res Commun. 2015;468:8–13. doi: 10.1016/j.bbrc.2015.11.008. [DOI] [PubMed] [Google Scholar]
- 13.Sun B, Li J, Shao D, Pan Y, Chen Y, Li S, Yao X, Li H, Liu W, Zhang M, et al. Adipose tissue-secreted miR-27a promotes liver cancer by targeting FOXO1 in obese individuals. Onco Targets Ther. 2015;8:735–744. doi: 10.2147/OTT.S80945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dong P, Xiong Y, Watari H, Hanley SJ, Konno Y, Ihira K, Yamada T, Kudo M, Yue J, Sakuragi N. miR-137 and miR-34a directly target Snail and inhibit EMT, invasion and sphere-forming ability of ovarian cancer cells. J Exp Clin Cancer Res. 2016;35:132. doi: 10.1186/s13046-016-0415-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen W, Liu Y, Liang X, Huang Y, Li Q. Chondroitin sulfate-functionalized polyamidoamine as a tumor-targeted carrier for miR-34a delivery. Acta Biomater. 2017;57:238–250. doi: 10.1016/j.actbio.2017.05.030. [DOI] [PubMed] [Google Scholar]
- 16.Chen Y, Sun P, Guo X, Gao A. miR-34a, a promising novel biomarker for benzene toxicity, is involved in cell apoptosis triggered by 1,4-benzoquinone through targeting Bcl-2. Environ Pollut. 2017;221:256–265. doi: 10.1016/j.envpol.2016.11.072. [DOI] [PubMed] [Google Scholar]
- 17.Lin Y, Shen J, Li D, Ming J, Liu X, Zhang N, Lai J, Shi M, Ji Q, Xing Y. miR-34a contributes to diabetes-related cochlear hair cell apoptosis via SIRT1/HIF-1α signaling. Gen Comp Endocrinol. 2017;246:63–70. doi: 10.1016/j.ygcen.2017.02.017. [DOI] [PubMed] [Google Scholar]
- 18.Ji Q, Hao X, Zhang M, Tang W, Yang M, Li L, Xiang D, Desano JT, Bommer GT, Fan D, et al. MicroRNA miR-34 inhibits human pancreatic cancer tumor-initiating cells. PLoS One. 2009;4:e6816. doi: 10.1371/journal.pone.0006816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Genovese G, Ergun A, Shukla SA, Campos B, Hanna J, Ghosh P, Quayle SN, Rai K, Colla S, Ying H, et al. microRNA regulatory network inference identifies miR-34a as a novel regulator of TGF-β signaling in glioblastoma. Cancer Discov. 2012;2:736–749. doi: 10.1158/2159-8290.CD-12-0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tryndyak VP, Ross SA, Beland FA, Pogribny IP. Down-regulation of the microRNAs miR-34a, miR-127, and miR-200b in rat liver during hepatocarcinogenesis induced by a methyl-deficient diet. Mol Carcinog. 2009;48:479–487. doi: 10.1002/mc.20484. [DOI] [PubMed] [Google Scholar]
- 21.Chen Q, Li L, Tu Y, Zheng LL, Liu W, Zuo XY, He YM, Zhang SY, Zhu W, Cao JP, et al. miR-34a regulates apoptosis in liver cells by targeting the KLF4 gene. Cell Mol Biol Lett. 2014;19:52–64. doi: 10.2478/s11658-013-0115-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang XP, Zhou J, Han M, Chen CB, Zheng YT, He XS, Yuan XP. MicroRNA-34a regulates liver regeneration and the development of liver cancer in rats by targeting Notch signaling pathway. Oncotarget. 2017;8:13264–13276. doi: 10.18632/oncotarget.14807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaller M, Liffers ST, Oeljeklaus S, Kuhlmann K, Röh S, Hoffmann R, Warscheid B, Hermeking H. Genome-wide characterization of miR-34a induced changes in protein and mRNA expression by a combined pulsed SILAC and microarray analysis. Mol Cell Proteomics. 2011;10:M111.010462. doi: 10.1074/mcp.M111.010462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Du JY, Wang LF, Wang Q, Yu LD. miR-26b inhibits proliferation, migration, invasion and apoptosis induction via the downregulation of 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase-3 driven glycolysis in osteosarcoma cells. Oncol Rep. 2015;33:1890–1898. doi: 10.3892/or.2015.3797. [DOI] [PubMed] [Google Scholar]
- 25.Wang J, Wang H, Liu A, Fang C, Hao J, Wang Z. Lactate dehydrogenase A negatively regulated by miRNAs promotes aerobic glycolysis and is increased in colorectal cancer. Oncotarget. 2015;6:19456–19468. doi: 10.18632/oncotarget.3318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Song K, Kwon H, Han C, Zhang J, Dash S, Lim K, Wu T. Active glycolytic metabolism in CD133(+) hepatocellular cancer stem cells: Regulation by MIR-122. Oncotarget. 2015;6:40822–40835. doi: 10.18632/oncotarget.5812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ullmann P, Qureshi-Baig K, Rodriguez F, Ginolhac A, Nonnenmacher Y, Ternes D, Weiler J, Gabler K, Bahlawane C, Hiller K, et al. Hypoxia-responsive miR-210 promotes self-renewal capacity of colon tumor-initiating cells by repressing ISCU and by inducing lactate production. Oncotarget. 2016;7:65454–65470. doi: 10.18632/oncotarget.11772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xiao X, Huang X, Ye F, Chen B, Song C, Wen J, Zhang Z, Zheng G, Tang H, Xie X. The miR-34a-LDHA axis regulates glucose metabolism and tumor growth in breast cancer. Sci Rep. 2016;6:21735. doi: 10.1038/srep21735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang Q, Jiang W, Zhuang C, Geng Z, Hou C, Huang D, Hu L, Wang X. microRNA-22 downregulation of galectin-9 influences lymphocyte apoptosis and tumor cell proliferation in liver cancer. Oncol Rep. 2015;34:1771–1778. doi: 10.3892/or.2015.4167. [DOI] [PubMed] [Google Scholar]
- 30.Retraction notice to microarray analysis of microRNA expression in liver cancer tissues and normal control [GENE 523/2 (2014) 158–60] Gene. 2016;578:137. doi: 10.1016/j.gene.2015.10.057. [DOI] [PubMed] [Google Scholar]
- 31.Liu C, Wang C, Wang J, Huang H. miR-1297 promotes cell proliferation by inhibiting RB1 in liver cancer. Oncol Lett. 2016;12:5177–5182. doi: 10.3892/ol.2016.5326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fang Z, He L, Jia H, Huang Q, Chen D, Zhang Z. The miR-383-LDHA axis regulates cell proliferation, invasion and glycolysis in hepatocellular cancer. Iran J Basic Med Sci. 2017;20:187–192. doi: 10.22038/ijbms.2017.8246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kota J, Chivukula RR, O'Donnell KA, Wentzel EA, Montgomery CL, Hwang HW, Chang TC, Vivekanandan P, Torbenson M, Clark KR, et al. Therapeutic microRNA delivery suppresses tumorigenesis in a murine liver cancer model. Cell. 2009;137:1005–1017. doi: 10.1016/j.cell.2009.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cui H, Ge J, Xie N, Banerjee S, Zhou Y, Antony VB, Thannickal VJ, Liu G. miR-34a inhibits lung fibrosis by inducing lung fibroblast senescence. Am J Respir Cell Mol Biol. 2017;56:168–178. doi: 10.1165/rcmb.2016-0163OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fu BC, Lang JL, Zhang DY, Sun L, Chen W, Liu W, Liu KY, Ma CY, Jiang SL, Li RK, Tian H. Suppression of miR-34a expression in the myocardium protects against ischemia-reperfusion injury Through SIRT1 protective pathway. Stem Cells Dev. 2017;26:1270–1282. doi: 10.1089/scd.2017.0062. [DOI] [PubMed] [Google Scholar]
- 36.Jiang L, Hermeking H. miR-34a and miR-34b/c suppress intestinal tumorigenesis. Cancer Res. 2017;77:2746–2758. doi: 10.1158/0008-5472.CAN-16-2183. [DOI] [PubMed] [Google Scholar]
- 37.Liu YP, Hu H, Xu F, Wen JJ. Relation of miR-34a expression in diffuse large B cell lymphoma with clinical prognosis. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2017;25:455–459. doi: 10.7534/j.issn.1009-2137.2017.02.026. (In Chinese) [DOI] [PubMed] [Google Scholar]
- 38.Maroni P, Puglisi R, Mattia G, Care A, Matteucci E, Bendinelli P, Desiderio MA. In bone metastasis miR-34a-5p absence inversely correlates with Met expression, while Met oncogene is unaffected by miR-34a-5p in non-metastatic and metastatic breast carcinomas. Carcinogenesis. 2017;38:492–503. doi: 10.1093/carcin/bgx027. [DOI] [PubMed] [Google Scholar]
- 39.Song C, Lu P, Sun G, Yang L, Wang Z, Wang Z. miR-34a sensitizes lung cancer cells to cisplatin via p53/miR-34a/MYCN axis. Biochem Biophys Res Commun. 2017;482:22–27. doi: 10.1016/j.bbrc.2016.11.037. [DOI] [PubMed] [Google Scholar]
- 40.Chen AH, Qin YE, Tang WF, Tao J, Song HM, Zuo M. miR-34a and miR-206 act as novel prognostic and therapy biomarkers in cervical cancer. Cancer Cell Int. 2017;17:63. doi: 10.1186/s12935-017-0431-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sukata T, Sumida K, Kushida M, Ogata K, Miyata K, Yabushita S, Uwagawa S. Circulating microRNAs, possible indicators of progress of rat hepatocarcinogenesis from early stages. Toxicol Lett. 2011;200:46–52. doi: 10.1016/j.toxlet.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 42.Pineau P, Volinia S, McJunkin K, Marchio A, Battiston C, Terris B, Mazzaferro V, Lowe SW, Croce CM, Dejean A. miR-221 overexpression contributes to liver tumorigenesis; Proc Natl Acad Sci USA; 2010; pp. 264–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pogribny IP, Tryndyak VP, Boyko A, Rodriguez-Juarez R, Beland FA, Kovalchuk O. Induction of microRNAome deregulation in rat liver by long-term tamoxifen exposure. Mutat Res. 2007;619:30–37. doi: 10.1016/j.mrfmmm.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 44.Dang Y, Luo D, Rong M, Chen G. Underexpression of miR-34a in hepatocellular carcinoma and its contribution towards enhancement of proliferating inhibitory effects of agents targeting c-MET. PLoS One. 2013;8:e61054. doi: 10.1371/journal.pone.0061054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen B, Li H, Zeng X, Yang P, Liu X, Zhao X, Liang S. Roles of microRNA on cancer cell metabolism. J Transl Med. 2012;10:228. doi: 10.1186/1479-5876-10-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhao E, Maj T, Kryczek I, Li W, Wu K, Zhao L, Wei S, Crespo J, Wan S, Vatan L, et al. Cancer mediates effector T cell dysfunction by targeting microRNAs and EZH2 via glycolysis restriction. Nat Immunol. 2016;17:95–103. doi: 10.1038/ni.3313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xu W, Zhang Z, Zou K, Cheng Y, Yang M, Chen H, Wang H, Zhao J, Chen P, He L, et al. miR-1 suppresses tumor cell proliferation in colorectal cancer by inhibition of Smad3-mediated tumor glycolysis. Cell Death Dis. 2017;8:e2761. doi: 10.1038/cddis.2017.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Han RL, Wang FP, Zhang PA, Zhou XY, Li Y. miR-383 inhibits ovarian cancer cell proliferation, invasion and aerobic glycolysis by targeting LDHA. Neoplasma. 2017;64:244–252. doi: 10.4149/neo_2017_211. [DOI] [PubMed] [Google Scholar]
- 49.Zhang R, Su J, Xue SL, Yang H, Ju LL, Ji Y, Wu KH, Zhang YW, Zhang YX, Hu JF, Yu MM. HPV E6/p53 mediated down-regulation of miR-34a inhibits Warburg effect through targeting LDHA in cervical cancer. Am J Cancer Res. 2016;6:312–320. [PMC free article] [PubMed] [Google Scholar]
- 50.Wang J, Yan S, Zhang W, Zhang H, Dai J. Integrated proteomic and miRNA transcriptional analysis reveals the hepatotoxicity mechanism of PFNA exposure in mice. J Proteome Res. 2015;14:330–341. doi: 10.1021/pr500641b. [DOI] [PubMed] [Google Scholar]