Abstract
Liraglutide, as a glucagon-like peptide-1 analogue, is used to treat type 2 diabetes mellitus and obesity. Previous findings have demonstrated the effects of liraglutide on adipogenesis; however, the underlying mechanism involved in this process remains to be elucidated. In the present study, to certify the effect of liraglutide on adipogenesis and explore the possible underlying mechanism involved in this process, preadipocyte 3T3-L1 cells were cultured in adipocyte-inducing medium and treated with liraglutide. Subsequently, the expression levels of the master transcription factors and adipocyte-specific genes were measured by reverse transcription-quantitative polymerase chain reaction and immunoblotting analysis. Lipid droplet production was detected by Oil red O staining. Cell proliferation was determined by a Cell Counting Kit-8 assay and cell immunofluorescence for Ki67, and apoptosis was assessed by flow cytometry. Next, the expression levels of the core components in the Hippo-yes-associated protein (YAP) signaling pathway as well as YAP-specific target genes were measured. Finally, short interfering RNAs of mammalian ste20 kinase 1/2 (MST1/2), a key protein kinase in the Hippo-YAP pathway, were used to determine whether liraglutide regulated adipogenic differentiation via the Hippo-YAP pathway. It was demonstrated that liraglutide promoted adipogenic differentiation, suppressed proliferation, did not affect apoptosis of 3T3-L1 cells and activated the Hippo-YAP signaling pathway at the initial stage of adipogenesis. Silencing of MST1 counteracted the effect of increasing adipogenesis by liraglutide. These results suggested that liraglutide may activate the Hippo-YAP signaling pathway leading to the inhibition of proliferation of preadipocyte 3T3-L1 cells, and result in cells achieving transformation into mature adipocytes sooner. Taken together, the results of the present study may expand knowledge of the underlying mechanism of liraglutide facilitating adipogenesis, and may contribute to the development of GLP-1 receptor agonists for weight loss and increased insulin sensitivity.
Keywords: liraglutide, adipocyte, adipogenesis, Hippo-yes-associated protein pathway, 3T3-L1 cells
Introduction
According to the Report on the Status of Nutrition and Chronic Diseases of Chinese residents (2015) (1), the prevalence of diabetes mellitus (DM) reached up to 9.7% in 2012, which resulted in a heavy social and economic burden on individuals, families and the country (2). Therefore, research regarding the pathogenesis, prevention and therapy of DM is of primary concern. Substantial evidence implicates that obesity is a key risk factor for type 2 DM (T2DM) (3). The root etiology of obesity is chronic energy imbalance, which produces adipocyte hypertrophy and hyperplasia, and these processes lead to adipocyte dysfunction, induce insulin resistance and result in T2DM (4,5). Accordingly, exploring the characteristics of adipocytes may help the treatment of obesity and T2DM.
The Hippo-yes associated protein (YAP) signaling pathway has been identified to serve crucial roles in the regulation of cell proliferation, cell apoptosis and cell differentiation, and may modulate development, organ size, tissue homeostasis and tumorigenesis (6,7). The core of the mammalian Hippo-YAP pathway has been well established. In general, upon activation, the extracellular signals are transduced to the mammalian ste20 kinase 1/2 (MST1/2), then, the complex is assembled by MST1/2 and phosphorylates salvador homolog 1 (Sav1), which activates S/T protein kinase large tumor suppressor 1/2 (LATS1/2). Activated LATS1/2 in complex with Mob phosphorylates and inhibits two homologous transcription coactivators, namely, YAP and its paralog transcriptional co-activator with PDZ-binding motif (TAZ). Finally, YAP/TAZ regulates the expression of a large number of genes important for cell proliferation (8). Ankyrin repeat domain 1 (ANKRD1), connective tissue growth factor (CTGF) and cysteine rich angiogenic inducer 61 (Cyr61) are well-characterized YAP target genes that regulate cell proliferation (9). Several studies have indicated that the Hippo-YAP signaling pathway is involved in adipogenesis (10–12). At the early phase of adipogenic differentiation, adipogenesis is governed by the activity of a series of key transcription factors, especially peroxisome proliferator-activated receptor γ (PPARγ) and CCAAT/enhancer binding protein α (C/EBPα), which coordinates the expression of adipogenic genes characteristic of terminally differentiated adipocytes, including adipocyte protein 2 (aP2) (13).
Liraglutide is a glucagon-like peptide-1 (GLP-1) analogue widely used for the therapy of T2DM, and it is also recommended for the treatment of obesity (14). As an insulinotropic hormone, GLP-1 is secreted by intestinal L cells in response to nutrient ingestion, and acts on the GLP-1 receptor to facilitate glucose-dependent insulin secretion, decreases glucagon levels and improves β-cell neogenesis (14). GLP-1 and liraglutide have previously been reported to regulate adipocyte formation via extracellular signal-regulated kinase, protein kinase C, protein kinase B and Wnt signaling pathways (15–17). However, it is not clear whether other signaling pathways are involved in the regulation of adipogenic differentiation by liraglutide.
The present study investigated the effects and possible underlying mechanisms of liraglutide on preadipocyte 3T3-L1 cell adipogenesis. These findings may contribute to the development of novel drugs to aid weight loss and increase insulin sensitivity.
Materials and methods
Reagents
Liraglutide was purchased from Novo Nordisk (West Sussex, UK). Anti-β-actin (cat. no. A1978-100UL) for the western blotting was purchased from Sigma-Aldrich; Merck KGaA (Darmstadt, Germany). Anti-PPARγ (cat. no. 2435), anti-C/EBPα (cat. no. 2295), anti-aP2 (cat. no. 3544), anti-YAP (cat. no. 14074), anti-phosphorylated (p)-YAP (cat. no. 46931), anti-LATS1 (cat. no. 9153) and anti-MST1 (cat. no. 3682) for western blotting were all purchased from Cell Signaling Technology, Inc. (Danvers, MA, USA). Anti-Ki67 (cat. no. 27309-1-AP) for the immunofluorescence assay was purchased from ProteinTech Group, Inc. (Chicago, IL, USA).
Cell lines and cell culture
Preadipocyte 3T3-L1 cells were cultured in Dulbecco's modified Eagle's medium (HyClone; GE Healthcare Life Sciences, Logan, UT, USA) supplemented with 10% fetal bovine serum (FBS), 200 U/ml penicillin and 200 U/ml streptomycin, and were maintained in a humidified incubator containing 5% CO2 at 37°C.
Cell differentiation and treatment
For adipocyte differentiation, 3T3-L1 cells that reached ~90% confluence were cultured in an adipocyte-inducing medium (AIM; α-MEM containing 10% FBS, 0.5 µM dexamethasone, 0.25 mM methylisobutylxanthine, 5 µg/ml of insulin and 50 µM indomethacin) in a humidified atmosphere at 37°C with 5% CO2 for 3 days. For liraglutide treatment, cells were cultured in AIM with 0 nM (vehicle), 10, 100, or 1,000 nM liraglutide.
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from cells using TRIzol® reagent (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA). A total of 0.5 µg RNA of each sample was reverse-transcribed into cDNA with a high-capacity cDNA reverse transcription kit (Thermo Fisher Scientific, Inc.) with the following thermocycling parameters: 25°C for 10 min, 37°C for 2 h and 85°C for 5 min. qPCR was performed using SGExcel Fast SYBR Mixture kits (Sangon Biotech Co., Ltd., Shanghai, China) on the Light Cycler® 96 Real-Time PCR system (Roche Diagnostics GmbH, Mannheim, Germany), and the thermocycling parameters were followed as previously described (18), which consisted of 40 cycles (95°C for 10 sec, 60°C for 10 sec and 72°C for 10 sec) following an initial denaturation step (95°C for 2 min). The mRNA expression level of non-POU-domain-containing, octamer binding protein (NONO) was used as an internal control as previously reported (19). Relative expression was calculated using the comparative threshold cycle method (2−ΔΔCq) (20). The primer sequences used in the study were as follows: C/EBPα forward, 5′-CTGATTCTTGCCAAACTGAG-3′ and reverse, 5′-GAGGAAGCTAAGACCCACTAC-3′; PPARγ forward, 5′-CTTGACAGGAAAGACAACGG-3′ and reverse, 5′-GCTTCTACGGATCGAAACTG-3′; aP2 forward, 5′-AAATCACCGCAGACGACAGG-3′ and reverse, 5′-GGCTCATGCCCTTTCATAAAC-3′; ANKRD1 forward, 5′-CTTGATGACCTTCGGTGCG-3′ and reverse, 5′-GCTCTTCTGTTGGGAAATGCT-3′; CTGF forward, 5′-TCTCCACCCGAGTTACCAATG-3′ and reverse, 5′-TGCAGCCAGAAAGCTCAAAC-3′; Cry61 forward, 5′-GGAAAAGGCAGCTCACTGAAG-3′ and reverse, 5′-GCACTCTGGGTTGTCATTGGTA-3′; MST1 forward, 5′-TCCCTCAGGATGGAGACTATGA-3′ and reverse, 5′-AAGGCTGGGCTGGTGTTG-3′; NONO forward, 5′-TGCTCCTGTGCCACCTGGT-3′ and reverse, 5′-CCGGAGCTGGACGGTTGAAT-3′.
Western blotting
The cells were incubated in AIM or treated with different concentrations of liraglutide (0, 10, 100 or 1,000 nM) for 3 days. Following this, treated cells were lysed with radioimmunoprecipitation assay lysis buffer (Beyotime Institute of Biotechnology, Haimen, China) and protein concentration was determined with bicinchoninic acid protein assay. Immunoblotting was performed as previously described (21). Briefly, protein (20 µg/lane) was separated on a 12% SDS denatured polyacrylamide gel and then transferred onto a polyvinylidene difluoride membrane. The membranes were blocked with 5% skim milk at room temperature for 1 h, and were subsequently incubated with anti-β-actin, anti-PPARγ, anti-C/EBPα, anti-aP2, anti-YAP, anti-p-YAP, anti-LATS1 and anti-MST at 4°C overnight (all at 1:1,000). Membranes were washed in PBS with 0.1% Tween-20 and incubated with horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG and goat anti-rabbit IgG secondary antibodies (1:3,000, cat. no. ZDR-5307 and ZDR-5306; OriGene Technologies, Inc., Beijing, China) according to the manufacturer's instructions. Finally, the protein of interest was visualized using Immobilon Western Chemiluminescent HRP substrate (EMD Millipore, Billerica, MA, USA). Intensity analysis of images was performed using ImageJ software (version 1.48v; National Institutes of Health, Bethesda, MD, USA).
Oil red O staining
3T3-L1 cells that reached ~90% confluence were cultured in AIM or treated with different concentrations of liraglutide (0, 10, 100 or 1,000 nM) for 3 days. Following this, the treated adipocyte-induced 3T3-L1 cells were gently washed twice with cold PBS, and then fixed in 4% paraformaldehyde for 10 min at room temperature. Following this, the cells were washed twice with deionized water, and stained with 60% saturated Oil Red O for 5 min at room temperature. For Oil Red O quantification, 4% IGEPAL CA 630 (Sigma-Aldrich; Merck KGaA) in isopropanol was added to each well, and the plate was then rocked on a shaker for 15 min. Light absorbance by the extracted dye was measured at a wavelength of 520 nm.
Cell immunofluorescence
Adipocyte-induced 3T3-L1 cells were treated with different concentrations of liraglutide (0, 10, 100 or 1,000 nM) in a humidified atmosphere at 37°C with 5% CO2 for 2 days using the aforementioned procedure. Cells were fixed in 4% paraformaldehyde for 15 min and permeabilized with 1% Triton X-100 at room temperature for 15 min. Following blocking with 1% bovine serum albumin at room temperature for 2 h, cells were incubated with rabbit anti-Ki67 (1:200) at 4°C for 12 h and incubated with goat anti-rabbit Alexa Fluor 488 at room temperature for 1 h (1:3,000, cat. no. A-31565; Invitrogen; Thermo Fisher Scientific, Inc.). The images were acquired using the AV300-ASW confocal microscope (Olympus Corporation, Tokyo, Japan). Image magnification, ×100.
Cell proliferation assay
A Cell Counting Kit (CCK)-8 kit (Beyotime Institute of Biotechnology) was used for the evaluation of cell proliferation. A total of 3×103 cells/well were seeded into 96-well plates, which were cultured in AIM medium and treated with different concentrations of liraglutide (0, 0.1, 1, 10, 100 nM or 1, 10, 100 µM) in a humidified atmosphere at 37°C with 5% CO2 for 48 h. CCK-8 reagent was then added to each well and incubated for 2 h at 37°C. The absorbance was measured using a pan-wavelength microplate reader at a wavelength of 450 nm (SynergyMx; BioTek Instruments, Inc., Winooski, VT, USA).
Cell apoptosis assay by flow cytometry (FCM)
Adipocyte-inducing 3T3-L1 cells at ~90% confluence were treated with different concentrations of liraglutide (0, 10, 100 or 1,000 nM) in a humidified atmosphere at 37°C with 5% CO2 for 2 days. Subsequently, the cells were used for apoptosis analysis. For FCM analysis, an Annexin V-APC Apoptosis Detection kit from Tianjin Biotech Co., Ltd. (Beijing, China) was used. According to the manufacturer's instructions, the cells were washed twice with cold PBS and resuspended in 1X binding buffer at a concentration of 1×106 cells/ml. This solution (100 µl) was transferred to a 5 ml culture tube and 5 µl FITC-Annexin V and 5 µl PI was added. Cells were gently vortexed and incubated for 15 min at room temperature in the dark. Subsequently, 400 µl of 1X binding buffer was added to each tube and was analyzed by FCM (FACSAria I; BD Biosciences, Franklin Lakes, NJ, USA).
Cell transfection
According to the manufacturer's instructions, transfection was performed using Lipofectamine RNAiMAX in Opti-MEM® I Medium (both from Invitrogen; Thermo Fisher Scientific, Inc.) at 37°C for 12 h. Briefly, cells were counted and seeded in 12 well plates at 1.5×105 cells/well the day prior to transfection to ensure ~50% confluency on the day of transfection. For 3T3-L1 cell transfection, 50 nM MST1 siRNA (siMST1) or its negative control (siNC; Shanghai GenePharma Co., Ltd., Shanghai, China) were used. The siRNA sequences were as follows: siMST1-1, 5′-GGGACUAGAAUACCUUCAU-3′; siMST1-2, 5′-GGGAAUAACUGCCAUAGAA-3′; siMST1-3, 5′-GGAGAACUCAGAGGAGGAU-3′; siNC, 5′-UUCUCCGAACGUGUCACGU-3′.
Statistical analysis
The results are expressed as the mean ± standard deviation. A one-way analysis of variance was used followed by Tukey's or Dunnett's post hoc test to analyze the differences between multiple groups. Statistical analysis was performed using SPSS software, version 17 (SPSS, Inc., Chicago, IL, USA). P<0.05 was considered to indicate a statistically significant difference.
Results
Liraglutide promotes adipogenic differentiation of 3T3-L1 cells
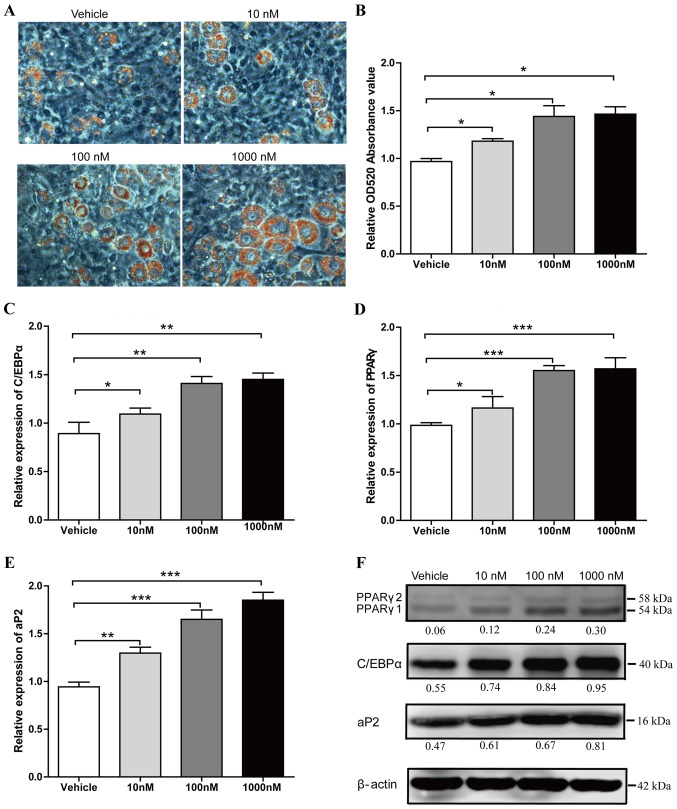
The authors of the present study previously demonstrated that adipogenic induced 3T3-L1 cells may produce a significant increase in lipid droplet numbers when treated with liraglutide (18). In the current study, the adipocyte formation effect of liraglutide was investigated at the early phase of adipogenic differentiation. Preadipocyte 3T3-L1 cells were cultured in AIM, and were treated with different concentrations of liraglutide (0, 10, 100 or 1,000 nM). Following 5 days treatment, Oil Red O staining was performed to examine the lipid droplet production. In a dose-dependent manner, 1,000 nM Liraglutide treatment led to a maximal increase of lipid droplet numbers (34% increase in Oil Red O staining compared with the vehicle group; P<0.05; Fig. 1A and B). Liraglutide also significantly increased the mRNA expression levels of adipocyte marker genes, including C/EBPα, PPARγ and aP2 compared with the levels in the vehicle group (Fig. 1C-E). When cells were treated with 10, 100 and 1,000 nM liraglutide, the mRNA expression levels of C/EBPα increased by 1.23-fold (P<0.05), 1.58-fold (P<0.01) and 1.63-fold (P<0.01), respectively (Fig. 1C), the mRNA expression levels of PPARγ increased by 1.18-fold (P<0.05), 1.54-fold (P<0.001) and 1.59-fold (P<0.001), respectively (Fig. 1D), and the mRNA expression levels of aP2 increased by 1.38-fold (P<0.01), 1.75-fold (P<0.001) and 1.96-fold (P<0.001), respectively (Fig. 1E). Enhanced expression of C/EBPα, PPARγ and aP2 was further verified by western blot analysis (Fig. 1F). These data suggested that liraglutide accelerates adipogenesis at an early stage of differentiation.
Figure 1.
Liraglutide promotes adipogenic differentiation of 3T3-L1 cells. Preadipocyte 3T3-L1 cells were cultured in adipocyte-inducing medium and treated with different concentrations of liraglutide. (A) Representative images of differentiated and treated cells were labeled with Oil Red O at day 5. (B) Oil Red O extracted with isopropanol was measured at OD520, the values represent the mean ± standard deviation. The mRNA expression levels of key transcription factors, (C) C/EBPα and (D) PPARγ, as well as (E) the adipocyte-specific gene aP2, were analyzed by reverse transcription-quantitative polymerase chain reaction at day 2. Results represent the mean ± standard deviation. (F) The protein expression levels of C/EBPα, PPARγ and aP2 were assessed by western blot analysis at day 3 of differentiation. The value under each lane indicates the relative expression level of the genes, which is represented by the intensity ratio between PPARγ, C/EBPα or aP2 and β-actin bands in each lane. β-actin was used as an internal control. Image magnification, ×200, and all data represent three separate experiments. One-way analysis of variance was used followed by Tukey's post hoc test to analyze the differences between the other groups with the vehicle group in B-E. *P<0.05, **P<0.01 and ***P<0.001 vs. vehicle group. OD, optical density; C/EBPα, CCAAT/enhancer binding protein α; PPARγ, peroxisome proliferator-activated receptor γ; ap2, adipocyte protein 2.
Liraglutide suppresses the proliferation of 3T3-L1 cells and does not affect apoptosis
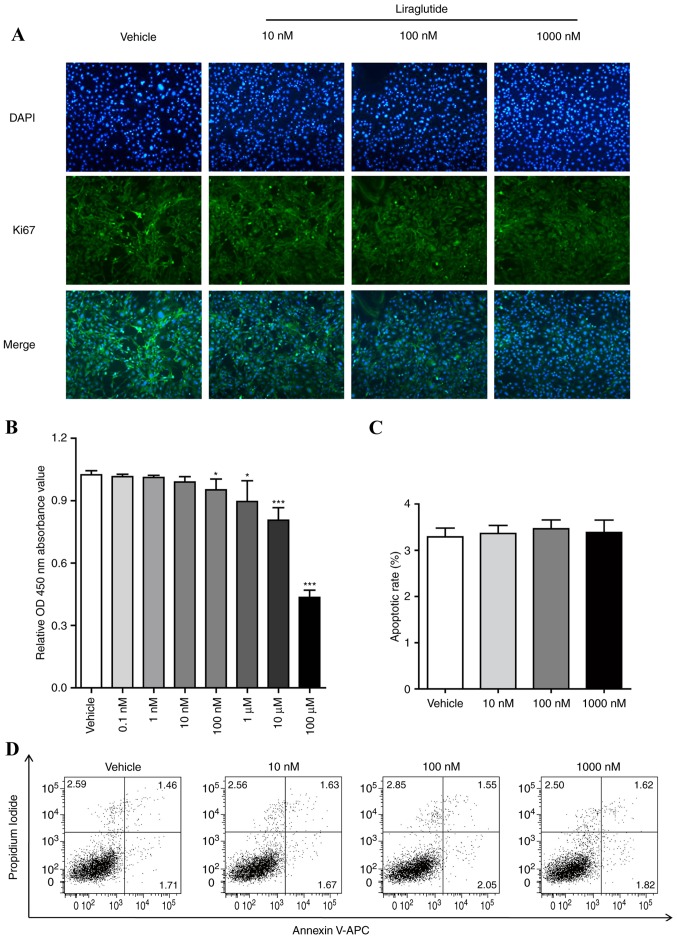
Considering that there is a stage of mitotic clonal expansion prior to the terminal differentiation stage of adipogenesis, the effect of liraglutide on cell proliferation of 3T3-L1 cells was examined. 3T3-L1 cells were treated with different concentrations of liraglutide (0, 10, 100 or 1,000 nM) for 48 h, the expression of Ki67, which is a marker for determining the growth fraction of a given cell population, was determined by immunostaining. As presented in Fig. 2A, the green fluorescence in the cell nucleus treated with different concentrations of liraglutide (0, 10, 100 or 1,000 nM) was decreased in a dose-dependent manner. 3T3-L1 cells were treated using the aforementioned procedure and CCK-8 analysis was performed to measure the cell proliferation. The results demonstrated that liraglutide reduced the cell growth in a dose-dependent manner (Fig. 2B). The effect of liraglutide on the cell apoptosis of preadipocyte 3T3-L1 was also examined. 3T3-L1 cells were treated with different concentrations of GLP-1 (0, 10, 100 or 1,000 nM) for 48 h, and cell apoptosis was assessed by FCM assay. As presented in Fig. 2C and D, liraglutide did not affect apoptosis of 3T3-L1 cells compared with the vehicle group. It was also observed that apoptosis of 3T3-L1 cells were not influenced when the cells were induced with AIM and liraglutide following 5 days (data not shown).
Figure 2.
Liraglutide suppresses the proliferation of 3T3-L1 cells and does not affect apoptosis. 3T3-L1 cells were treated with different concentrations of liraglutide for 2 days. (A) Immunostaining for determining cell growth marker gene Ki67 abundance in cell nuclei. Ki67 was labeled as green and nuclei of cells were stained with DAPI as blue. Representative images from three independent experiments are presented, and the image magnification is ×100. (B) Cell proliferation was assessed by Cell Counting Kit-8 assay. The values represent the mean ± standard deviation. *P<0.05 and ***P<0.001 vs. vehicle group. Three separate experiments were performed. (C) The effect of liraglutide on apoptosis was examined by flow cytometric analysis. Cells were stained with Annexin V-APC and propidium iodide. The statistical analysis for the percentage sum of PI- and PI+ cells in Annexin V+ cells is presented. Data represent the mean ± standard deviation from three independent experiments. (D) One representative flow cytometric experiment is presented. One-way analysis of variance was used followed by Tukey's post hoc test to analyze the differences between the other groups with the vehicle group in B and C. OD, optical density.
Liraglutide activates the Hippo-YAP signaling pathway during adipogenesis
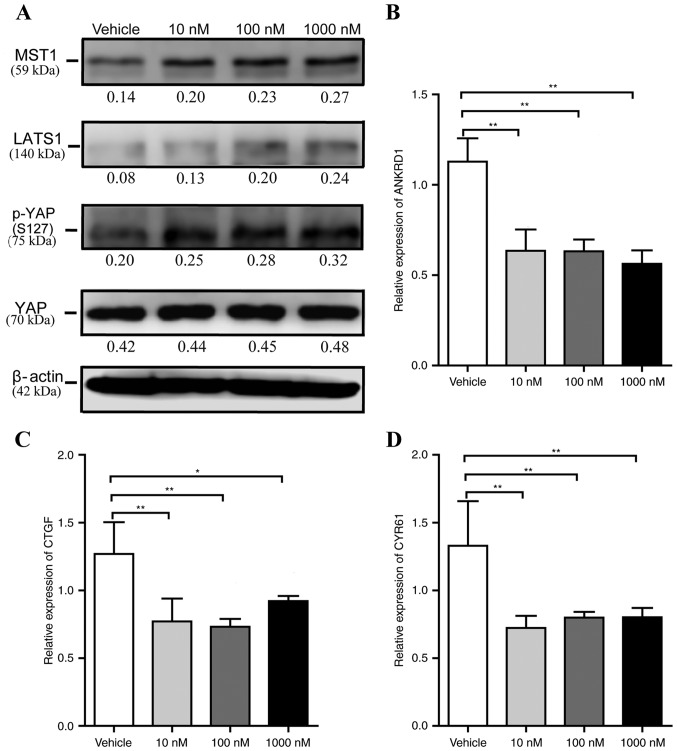
Previous investigations have demonstrated that the Hippo-YAP signaling pathway is involved in cell proliferation and adipogenesis. To clarify whether liraglutide affects this signaling pathway during adipogenesis, the expression of MST1, LATS1, YAP and Ser127 phosphorylated-YAP [p-YAP (S127)] were measured at the early phase of differentiation. 3T3-L1 cells were incubated in AIM containing 0, 10, 100 and 1,000 nM liraglutide for 48 h, then the cells were harvested and the expression levels of these proteins was measured by western blotting. As presented in Fig. 3A, no significant alteration in the total protein expression level of YAP was observed, and the levels of MST1, LATS1 and p-YAP (S127) in liraglutide-treated cells were markedly increased compared with vehicle control cells. ANKRD1, CTGF and Cyr61 are well-characterized YAP target genes, and therefore, it was next examined whether liraglutide altered the levels of their expression. Compared with the mRNA expression levels in the vehicle group, when cells were treated with 10, 100 and 1,000 nM liraglutide, the levels of ANKRD1 decreased by 0.56-fold (P<0.01), 0.56-fold (P<0.01) and 0.50-fold (P<0.01), respectively (Fig. 3B), the levels of CTGF decreased by 0.61-fold (P<0.01), 0.58-fold (P<0.01) and 0.73-fold (P<0.05), respectively (Fig. 3C), and the mRNA levels of Cyr61 decreased by 0.54-fold (P<0.01), 0.60-fold (P<0.01) and 0.60-fold (P<0.01), respectively (Fig. 3D). These data also suggested that liraglutide may reduce 3T3-L1 cell proliferation.
Figure 3.
Liraglutide activates the Hippo-YAP signaling pathway during adipogenesis. 3T3-L1 cells were cultured in adipocyte-inducing medium and treated with different concentrations of liraglutide, and then the cells were harvested at day 2. (A) Western blot analysis was used to assess the protein expression levels of the core components of the Hippo-YAP signaling pathway, including MST1, LATS1, p-YAP (S127) and total YAP. The value under each lane indicates the relative expression level of the gene, which is represented by the intensity ratio between MST1, LATS1, p-YAP (S127) or total YAP and β-actin bands in each lane. β-actin was used as an internal control. Reverse transcription-quantitative polymerase chain reaction was used to determine the mRNA expression levels of YAP specific target genes, including (B) ANKRD1, (C) CTGF and (D) Cyr61. The values represented the mean ± standard deviation. All data are representative of at least three independent experiments. One-way analysis of variance was applied followed by Dunnett's test to analyze the differences between the vehicle, 10, 100 and 1,000 nM groups in B-D. *P<0.05 and **P<0.01 vs. vehicle group. YAP, Yes-associated protein; p-YAP, phosphorylated yes-associated protein; MST1, mammalian ste20 kinase 1/2; LATS1/2, large tumor suppressor 1/2; ANKRD1, ankyrin repeat domain 1; CTGF, connective tissue growth factor; Cyr61, cysteine rich angiogenic inducer 61.
Silencing of MST1 attenuates liraglutide stimulation of adipogenic differentiation
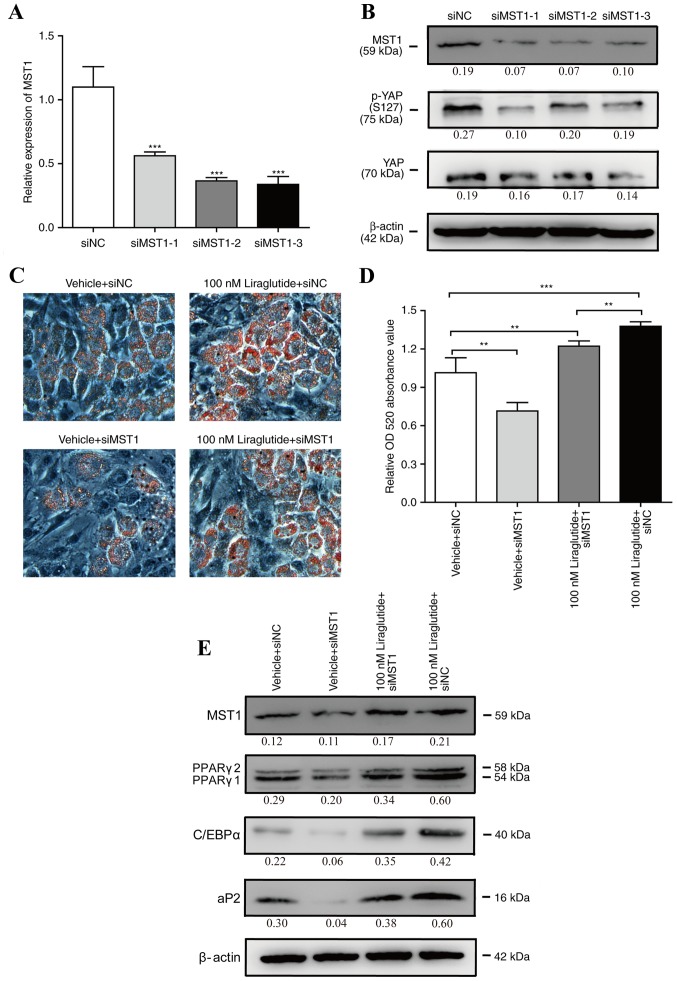
To further clarify whether the Hippo-YAP signaling pathway mediates liraglutide promotion of adipogenesis, liraglutide gain-of-function studies were performed on the background of MST1 silencing. 3T3-L1 cells were transfected with siMST1s or siNC for 48 h, and as presented in Fig. 4A and B, the mRNA and protein expression levels of MST1 were significantly reduced by all three siMST1s, and the expression of p-YAP (S127) was also decreased by MST1 siRNAs. Subsequently, one of three siMST1s was selected to perform further experiments. 3T3-L1 cells were transfected with siMST1 or siNC for 24 h, and then, the cells were incubated in AIM containing 0 or 100 nM liraglutide. As shown in Fig. 4C and D, knockdown of MST1 partially abrogated liraglutide-enhanced adipogenesis. Consistently, the protein expression levels of adipogenic differentiation marker genes including C/EBPα, PPARγ and aP2 were decreased in the cells transfected with siMST1 and incubated with 100 nM liraglutide, as compared with the cells transfected with siNC and incubated with 100 nM liraglutide (Fig. 4E). These results suggested that the Hippo-YAP signaling pathway may be involved in the process of liraglutide enforced adipogenesis during the early phase of differentiation.
Figure 4.
Silencing of MST1 attenuates liraglutide stimulation of adipogenic differentiation. 3T3-L1 cells were transfected with siMST1s or siNC. (A) Following 2 days, reverse transcription-quantitative polymerase chain reaction was used to monitor the mRNA expression levels of MST1, and NONO served as the internal control. The values represent the mean ± standard deviation. ***P<0.001 vs. siNC group (B); following 3 days, western blotting was used to measure the protein expression levels of MST1, p-YAP (S127) and total YAP. The value under each lane indicates the relative expression level of the gene, which is represented by the intensity ratio between MST1, p-YAP (S127) or total YAP and β-actin bands in each lane. β-actin was used as the internal control. 3T3-L1 cells were transfected with siMST1 or siNC for 24 h, and then, the cells were incubated in adipocyte-inducing medium containing 0 or 100 nM liraglutide. (C) Representative images of differentiated and treated cells were labeled with Oil Red O at day 5. (D) Oil Red O extracted with isopropanol was measured at OD520, and the values represented the mean ± standard deviation. **P<0.01 and ***P<0.001 vs. vehicle + siNC group. (E) The protein expression levels of MST1, C/EBPα, PPARγ and aP2 were assessed by western blot analysis at day 3 of differentiation. The value under each lane indicates the relative expression level of the gene, which is represented by the intensity ratio between MST1, PPARγ, C/EBPα or aP2 and β-actin bands in each lane. β-actin was used as an internal control. Image magnification, ×200, and all data represented three separate experiments. One-way analysis of variance followed by Tukey's post hoc test was used to analyze the differences between groups with the siNC group in A or the vehicle + siNC group in D. MST1, mammalian ste20 kinase 1; siMST1s, mammalian ste20 kinase 1 short interfering RNA; siNC, negative control short interfering RNA; p-YAP, phosphorylated-yes associated protein; YAP, yes associated protein; OD, optical density; C/EBPα, CCAAT/enhancer binding protein α; PPARγ, peroxisome proliferator-activated receptor γ; ap2, adipocyte protein 2.
Discussion
Obesity results in insulin resistance that results in individuals having an increased risk of developing T2DM, and insulin resistance may be partly induced by adipocyte hypertrophy, hyperplasia and dysfunction (5). It has been suggested by clinical studies that treatment with liraglutide controls body weight gain, improves insulin resistance and reduces adipocyte hyperplasia (22,23). A previous study revealed that liraglutide treatment may lead to adipogenic differentiated 3T3-L1 cells to produce a significant increase in lipid droplet numbers at the stage of terminal differentiation (18). The present study focused on the function and underlying mechanism of liraglutide in the regulation of adipogenesis at the early phase.
Adipocytes originate from multipotent mesenchymal stem cells (MSC). During adipogenesis, fibroblast-like pluripotent MSCs differentiate into mature lipid-laden and insulin-responsive adipocytes and the process of adipogenesis involves several stages, which includes mesenchymal precursor and committed preadipocyte proliferation, as well as their differentiation into terminally mature adipocytes (24). This differentiation is tightly regulated by an intricate network of transcriptional factors, which is governed to a large extent by an adipocyte-enriched nuclear receptor, PPARγ. PPARγ is both necessary and sufficient for adipogenesis, and PPARγ cooperates with C/EBPs to induce the expression of numerous downstream target genes important for terminal differentiation including aP2 (13). In the current study, it was observed that liraglutide increased the lipid droplet production of 3T3-L1 cells. It was also demonstrated that liraglutide promoted the expression of the master transcriptional factors C/EBPα and PPARγ, and enhanced the expression of the downstream adipocyte-specific gene aP2 in a dose-dependent manner. These results suggested that liraglutide may accelerate adipogenesis through a process that upregulates the expression of C/EBPα and PPARγ at the early phase of adipogenic differentiation, then promoted the expression of lipogenesis associated genes including aP2, and enhanced the accumulated of lipids.
Next, the present study demonstrated that liraglutide reduced 3T3-L1 cell growth in a dose-dependent manner and did not affect apoptosis at the early phase of adipogenic differentiation. Considering that there is a stage of committed preadipocyte proliferation prior to the terminal differentiation phase of adipogenesis, it was hypothesized that liraglutide may force preadipocyte 3T3-L1 cells to achieve transformation into mature adipocytes earlier, and enhance adipogenesis. Previously, the Hippo-YAP signaling pathway has been demonstrated to have an important role in the regulation of cell proliferation and differentiation. This pathway was initially defined through genetic studies in Drosophila for tumor suppressor genes (6). In mammalian systems, the core components of the Hippo-YAP signaling pathway initiate a kinase cascade, which acts on a transcriptional complex to regulate the expression of target downstream genes that control cell proliferation (25). Briefly, as STE20 family protein kinases, MST1/2 is associated with Sav1/WW45 to phosphorylate Mob1 and LATS1/2. Phosphorylated Mob1 binds to the autoinhibitory motif in LATS1/2, which activates their phosphorylation loop and kinase activity. Next, the active complex (combined LATS1/2 with Mob1) phosphorylates downstream effectors YAP/TAZ, and this leads to their cytoplasmic retention and inhibition. Dephosphorylated YAP/TAZ accumulates in the nucleus and binds to DNA-binding transcription factors to initiate the expression of growth-promoting and apoptosis-inhibiting genes (7,25). Therefore, the active Hippo-YAP signaling pathway induces the cytoplasmic accumulation of phosphorylated YAP and the inhibition of growth promoting genes. In the present study, it was demonstrated that liraglutide increased the levels of the core components of the Hippo-YAP signaling pathway, including MST1, LATS1 and p-YAP (S127) at the early phase of adipogenesis. Consistently, YAP specific target genes were downregulated in liraglutide-treated 3T3-L1 cells, including ANKRD1, CTGF and Cyr61. Silencing of MST1 reduced adipogenic differentiation of 3T3-L1 cells, and silencing of MST1 counteracted the effect of increasing adipogenesis by liraglutide. Previous studies have reported that MST2 interacts with Sav1 to activate PPARγ and augments PPARγ-induced adipocyte differentiation (26). LATS2 phosphorylated YAP and TAZ and retained them in the cytoplasm, leading to the reduction of cell proliferation and the promotion of cell adipogenic differentiation (10). The results of the present study and previous studies suggest that the activation of the Hippo-YAP signaling pathway may be involved in the process of liraglutide enhanced adipogenic differentiation.
In conclusion, the present study demonstrated that liraglutide promoted adipogenic differentiation of preadipocyte 3T3-L1 cells. In addition, liraglutide may activate the Hippo-YAP signaling pathway leading to proliferation inhibition of committed preadipocyte, and accordingly, 3T3-L1 cells achieve transformation into mature adipocytes sooner. The results may help to expand the knowledge regarding the underlying mechanism of liraglutide facilitating adipogenesis, and may provide a theoretical support for liraglutide in T2DM and obesity treatment.
Acknowledgements
The present study was supported by the National Natural Science Foundation of China (grant nos. 81501846 and 81270927), the Scientific Foundation of Tianjin Medical University (grant no. 2014KYM16), the Scientific Foundation of Tianjin Metabolic Diseases Hospital and Tianjin Institute of Endocrinology, Tianjin Medical University (grant no. 2014RC01) and the Tianjin Municipal Natural Science Foundation of China (grant no. 16JCYBJC26800).
References
- 1.Yu D, He Y, Guo Q, Fang H, Xu X, Fang Y, Li J, Zhao L. Trends of energy and nutrients intake among Chinese population in 2002–2012. J Hygiene Res. 2016;45:527–533. [PubMed] [Google Scholar]
- 2.Zhang N, Du SM, Ma GS. Current lifestyle factors that increase risk of T2DM in China. Eur J Clin Nutr. 2017;71:832–838. doi: 10.1038/ejcn.2017.41. [DOI] [PubMed] [Google Scholar]
- 3.Flier JS. Obesity wars: Molecular progress confronts an expanding epidemic. Cell. 2004;116:337–350. doi: 10.1016/S0092-8674(03)01081-X. [DOI] [PubMed] [Google Scholar]
- 4.Guilherme A, Virbasius JV, Puri V, Czech MP. Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nat Rev Mol Cell Biol. 2008;9:367–377. doi: 10.1038/nrm2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Ferranti S, Mozaffarian D. The perfect storm: Obesity, adipocyte dysfunction, and metabolic consequences. Clin Chem. 2008;54:945–955. doi: 10.1373/clinchem.2007.100156. [DOI] [PubMed] [Google Scholar]
- 6.Zhao B, Li L, Lei Q, Guan KL. The Hippo-YAP pathway in organ size control and tumorigenesis: An updated version. Genes Dev. 2010;24:862–874. doi: 10.1101/gad.1909210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yu FX, Guan KL. The Hippo pathway: Regulators and regulations. Genes Dev. 2013;27:355–371. doi: 10.1101/gad.210773.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang Y, Park HW, Jewell JL, Chen Q, Deng Y, Pan D, Taylor SS, Lai ZC, Guan KL. Protein kinase A activates the Hippo pathway to modulate cell proliferation and differentiation. Genes Dev. 2013;27:1223–1232. doi: 10.1101/gad.219402.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhao B, Panupinthu N, Jewell JL, Lian I, Wang LH, Zhao J, Yuan H, Tumaneng K, Li H, Fu XD, et al. Regulation of the Hippo-YAP pathway by G-protein-coupled receptor signaling. Cell. 2012;150:780–791. doi: 10.1016/j.cell.2012.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.An Y, Kang Q, Zhao Y, Hu X, Li N. Lats2 modulates adipocyte proliferation and differentiation via hippo signaling. PLoS One. 2013;8:e72042. doi: 10.1371/journal.pone.0072042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seo E, Basu-Roy U, Gunaratne PH, Coarfa C, Lim DS, Basilico C, Mansukhani A. SOX2 regulates YAP1 to maintain stemness and determine cell fate in the osteo-adipo lineage. Cell Rep. 2013;3:2075–2087. doi: 10.1016/j.celrep.2013.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen SN, Gurha P, Lombardi R, Ruggiero A, Willerson JT, Marian AJ. The hippo pathway is activated and is a causal mechanism for adipogenesis in arrhythmogenic cardiomyopathy. Circ Res. 2014;114:454–468. doi: 10.1161/CIRCRESAHA.114.302810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ali AT, Hochfeld WE, Myburgh R, Pepper MS. Adipocyte and adipogenesis. Eur J Cell Biol. 2013;92:229–236. doi: 10.1016/j.ejcb.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 14.Russell-Jones D. Molecular, pharmacological and clinical aspects of liraglutide, a once-daily human GLP-1 analogue. Mol Cell Endocrinol. 2009;297:137–140. doi: 10.1016/j.mce.2008.11.018. [DOI] [PubMed] [Google Scholar]
- 15.Challa TD, Beaton N, Arnold M, Rudofsky G, Langhans W, Wolfrum C. Regulation of adipocyte formation by GLP-1/GLP-1R signaling. J Biol Chem. 2012;287:6421–6430. doi: 10.1074/jbc.M111.310342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang J, Ren J, Song J, Liu F, Wu C, Wang X, Gong L, Li W, Xiao F, Yan F, et al. Glucagon-like peptide 1 regulates adipogenesis in 3T3-L1 preadipocytes. Int J Mol Med. 2013;31:1429–1435. doi: 10.3892/ijmm.2013.1350. [DOI] [PubMed] [Google Scholar]
- 17.Liu R, Li N, Lin Y, Wang M, Peng Y, Lewi K, Wang Q. Glucagon like peptide-1 promotes adipocyte differentiation via the Wnt4 mediated sequestering of beta-catenin. PLoS One. 2016;11:e0160212. doi: 10.1371/journal.pone.0160212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhu E, Yang Y, Zhang J, Li Y, Li C, Chen L, Sun B. Liraglutide suppresses obesity and induces brown fat-like phenotype via Soluble Guanylyl Cyclase mediated pathway in vivo and in vitro. Oncotarget. 2016;7:81077–81089. doi: 10.18632/oncotarget.13189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arsenijevic T, Grégoire F, Delforge V, Delporte C, Perret J. Murine 3T3-L1 adipocyte cell differentiation model: Validated reference genes for qPCR gene expression analysis. PLoS One. 2012;7:e37517. doi: 10.1371/journal.pone.0037517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 21.Zhu E, Wang X, Zheng B, Wang Q, Hao J, Chen S, Zhao Q, Zhao L, Wu Z, Yin Z. miR-20b suppresses Th17 differentiation and the pathogenesis of experimental autoimmune encephalomyelitis by targeting RORγt and STAT3. J Immunol. 2014;192:5599–5609. doi: 10.4049/jimmunol.1303488. [DOI] [PubMed] [Google Scholar]
- 22.Astrup A, Rössner S, Van Gaal L, Rissanen A, Niskanen L, Al Hakim M, Madsen J, Rasmussen MF, Lean ME, NN8022-1807 Study Group Effects of liraglutide in the treatment of obesity: A randomised, double-blind, placebo-controlled study. Lancet. 2009;374:1606–1616. doi: 10.1016/S0140-6736(09)61375-1. [DOI] [PubMed] [Google Scholar]
- 23.Ng SY, Wilding JP. Liraglutide in the treatment of obesity. Expert Opin Biol Ther. 2014;14:1215–1224. doi: 10.1517/14712598.2014.925870. [DOI] [PubMed] [Google Scholar]
- 24.Lefterova MI, Lazar MA. New developments in adipogenesis. Trends Endocrinol Metab. 2009;20:107–114. doi: 10.1016/j.tem.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 25.Gumbiner BM, Kim NG. The Hippo-YAP signaling pathway and contact inhibition of growth. J Cell Sci. 2014;127:709–717. doi: 10.1242/jcs.140103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park BH, Kim DS, Won GW, Jeon HJ, Oh BC, Lee Y, Kim EG, Lee YH. Mammalian ste20-like kinase and SAV1 promote 3T3-L1 adipocyte differentiation by activation of PPARγ. PLoS One. 2012;7:e30983. doi: 10.1371/journal.pone.0030983. [DOI] [PMC free article] [PubMed] [Google Scholar]