Abstract
Background: Anaplastic thyroid cancer (ATC) is one of the most lethal forms of cancer with a high mortality rate. Current guidelines support surgery for resectable ATC followed by external beam radiation therapy (EBRT) with or without chemotherapy. Treatment for those who are unresectable is palliative. Our goal was to examine first-line therapies as well as the role of genomic profiling in an effort better understand how to approach ATC.
Methods: This is a retrospective study of ATC patients who were seen at our institution from January 2013 to October 2015. Median overall survival (OS) and time to treatment failure (TTF) were calculated by the Kaplan-Meier method.
Results: Fifty-four patients were included. Median age at diagnosis was 63 years and 29/54 (54%) were women. The majority had stage IVC disease at diagnosis (50%), followed by IVB (32%), and IVA (18%). Approximately 93% had somatic gene testing. Initial treatment was surgery in 23 patients, EBRT with or without radiosensitizing chemotherapy in 29 patients, and systemic chemotherapy in 2 patients. Nineteen patients had all three treatment modalities. For the entire cohort, median OS was 11.9 months with 39% survival at 1 year and median TTF was 3.8 months. The majority of patients (74%) developed new distant metastasis or progression of existing metastatic disease. Patients who received trimodal therapy consisting of surgery, EBRT, and chemotherapy had a median OS of 22.1 months versus 6.5 months in those who received dual therapy with EBRT and chemotherapy (p = 0.0008). The TTF was the same in the two groups (7.0 and 6.5 months, respectively). Men were three times more likely to die from ATC than women (p = 0.0024). No differences in OS or TTF were noted based on tumor size (5 cm cutoff), age (60 years cutoff), or presence of any mutation. There was a trend toward shorter TTF in patients with somatic mutations in TP53.
Conclusion: Patients with ATC amenable to aggressive tri-modal therapy demonstrate improved survival. The short TTF, due primarily to distant metastatic disease, highlights the potential opportunity for improved outcomes with earlier initiation of systemic therapy including adjuvant or neoadjuvant therapy.
Keywords: : anaplastic thyroid cancer, undifferentiated, thyroid surgery, chemotherapy, radiation, BRAF, p53
Introduction
Anaplastic thyroid cancer (ATC) is a rare cancer. It comprises less than 2% of all thyroid cancers but more than 50% of annual thyroid related mortality (1,2). Although ATC can often arise spontaneously, nearly half of all cases are thought to be derived from more well-differentiated thyroid cancer (DTC), since the driver mutations (early mutational events), BRAF and RAS, commonly found in DTC, are also present in ATC. ATC is characterized by a greater mutation burden than DTC due to acquisition of late event mutations in TP53, TERT, and PIK3CA (3). Clinically, most patients are elderly, have lower performance status, and present with locally advanced disease and distant metastases (4,5). All ATCs are staged by the American Joint Committee on Cancer as stage IV at presentation because of the high mortality rate. The median overall survival, from the time of diagnosis, of ATC patients has been reported to be 3–5 months with a one year survival rate of 20% (5).
After comprehensive staging, treatment options should be based on a multidisciplinary consensus (1,6,7). Complete surgical resection of the primary tumor is considered in those with localized disease followed by high dose external beam radiation (EBRT) with or without concurrent radiosensitizing chemotherapy for curative intent. Patients with unresectable disease can be offered nonsurgical palliative treatment consisting of EBRT with or without concurrent chemotherapy. Despite this comprehensive approach, the disease process often continues to progress and patients die early, indicating that our current options are largely ineffective. Cytotoxic chemotherapy (combination or single agent taxanes and platinum, or anthracyclines) is the primary treatment strategy for metastatic disease, but this approach has shown very low response rates with unacceptable toxicity (1). Since most ATC patients either present with distant metastasis or go on to develop distant metastasis following presentation, addressing the disease via more efficacious systemic therapies is an important clinical focus. Several clinical trials with targeted therapy and immunotherapy are ongoing and may hold promise (NCT01236547, NCT02244463, NCT02726503, NCT02034110, and NCT02688608). Our goal was to identify the patterns of failure of our first-line therapies in ATC and to understand the influence of the tumor's genomic profile, in an effort to rethink how we approach this devastating disease, especially in the era of molecularly targeted therapy.
Materials and Methods
Patient population, data collection, and definitions
Following approval by the institutional review board, all new referrals for pathologically proven ATC (either previously treated or newly diagnosed) who were seen at our institution between January 1, 2013, and October 1, 2015, were evaluated. Those with microfoci ATC in the setting of DTC were also included as they are considered stage IVA at diagnosis. The diagnosis of ATC was made based on surgical pathology or cytology from fine needle aspiration and confirmed by an experienced head and neck pathologist.
Stage was determined by the American Joint Committee on Cancer staging system based on pathology and radiographic extent (8). Under this system, nodal involvement did not influence stage. Stage IVA disease was defined as limited to the thyroid gland but may have had minimal extrathyroidal extension. Stage IVB was defined as having gross extrathyroidal extension, including invasion to local structures, such as carotid, trachea, or esophagus. Stage IVC disease was defined as the presence of distant metastasis at diagnosis. Evidence of transformation was also documented, defined as a new diagnosis of ATC after a known prior diagnosis of treated DTC.
A head and neck surgeon reviewed each surgical case and operative report in order to determine the extent of the procedure. The extent of resection was based on the operative report and post-operative imaging. Only complete tumor resection (R0) or microscopic residual (R1) surgeries were counted as a surgery; debulking or incomplete surgeries that left gross residual disease (R2) were not defined as surgery in our series, because they have not been shown to provide a therapeutic benefit (9).
Systemic therapy included conventional cytotoxic chemotherapy (platinum, taxanes, or anthracyclines), targeted agents, or immunotherapy (checkpoint inhibitors). For the purpose of this study, in order to distinguish the type and timing of chemotherapy given, “radiosensitizing chemotherapy” refers to cytotoxic agents given to patients during EBRT [as described in the American Thyroid Association guidelines for management of patients with anaplastic thyroid cancer (1)], while “systemic chemotherapy” refers to cytotoxic agents administered as single treatment modality. Targeted therapy included multikinase inhibitors or selective BRAF and MEK inhibitors.
In addition to baseline tumor and patient characteristics, we also documented somatic mutations found in the tumor using a Next Generation Sequencing platform in our CLIA-certified molecular pathology laboratory. Ion AmpliSeq™ Cancer Hotspot Panel (Life Technologies, Carlsbad, CA) for detecting point mutations, short insertions and deletion, as well as high level of amplification in the coding sequence of a total of 50 genes was utilized.
Study objectives
The primary study outcomes measures were time to treatment failure (TTF) and location of progressive disease (locoregional vs. distant metastatic progression). TTF was defined as the time from the first treatment start until progression of disease or death (whichever occurred first). Progression was defined as recurrence, worsening of known disease, or appearance of new lesions, if no evidence of disease was noted after the first treatment. This was based on radiology reports and the treating physician's documentation. The secondary objective was assessment of overall survival (OS), defined as the time from first treatment start until death or last follow-up.
Statistical methods
Descriptive statistics were used to summarize all key study variables. The Kaplan-Meier estimation was used to graphically display OS and TTF distributions and comparisons between subgroups. Statistical comparisons between subgroups were made using a log-rank test. Cox proportional hazards regression analysis was used to calculate hazard ratio, confidence intervals [CI] and p-values; p < 0.05 was considered statistically significant.
Results
Patient and tumor characteristics
A total of 70 new ATC patients were seen at our institution between January 2013 and October 2015. Sixteen patients were excluded because they either died or were lost to follow-up prior to initiation of treatment. This left 54 patients for analysis.
Table 1 shows the baseline patient characteristics. The patient population was equal in sex, nearly 70% of the patients were over 60 years old, and approximately 78% were non-Hispanic Caucasian. Median tumor size was 5 cm (range 1.3–12 cm, and 2 patients had an incidental small focus of ATC in the background of DTC). The pathologic cellular pattern (i.e., spindle, squamous, pleomorphic, or giant cell) was often mixed. About 30% had documented coexisting DTC or lymph node involvement based on surgical pathology. Ten patients were found to have ATC transformation: nine of them were previously diagnosed with papillary thyroid cancer (PTC) and one with follicular thyroid cancer. Half of the patients had stage IVC disease at diagnosis. Twenty-six of the 27 (96%) patients who presented with distant metastasis at diagnosis had disease in their lung. One patient with stage IVC disease had a single focus confirmed as ATC in his humerus. This patient had a previous history of well-differentiated PTC and had been treated at least 2 years before with total thyroidectomy and radioactive iodine. There were 2 patients who presented with multi-site metastasis (scalp, bone, and pancreas) in addition to lung at diagnosis.
Table 1.
Baseline Characteristics
| Characteristic | No. of patients (%) N = 54 |
|---|---|
| Sex | |
| Female | 29 (53.7) |
| Male | 25 (46.3) |
| Age (median 63) | |
| <60 years | 17 (31.5) |
| ≥60 | 37 (68.5) |
| Ethnicity | |
| Caucasian | 42 (77.8) |
| Hispanic | 5 (9.3) |
| Black | 3 (5.6) |
| Asian | 4 (7.4) |
| Tumor size (range 1.3–12 cm) | |
| Median: <5 cm | 25 (46.3) |
| ≥5 cm | 29 (53.7) |
| Stage | |
| IVA | 10 (18.5) |
| IVB | 17 (31.5) |
| IVC | 27 (50) |
| ATC transformationa | 10 (18.5) |
| Coexisting DTCb | 31 (57.4) |
| Lymph node involvement | 33 (61.1) |
| Histologic pattern (often mixed) | |
| Spindle | 18 (33.3) |
| Squamous | 10 (18.5) |
| Giant cell | 3 (5.6) |
| Pleomorphic | 6 (11.1) |
| Epithelioid | 10 (18.5) |
| Leukocytosisc | 9 (16.7) |
Transformation is defined as prior history of differentiated thyroid cancer, and only later developed anaplastic thyroid cancer.
Coexisting DTC is defined as presence of DTC at the time of ATC diagnosis.
Defined as greater than 10,000 white blood cells per microliter.
ATC, anaplastic thyroid cancer; DTC, differentiated thyroid cancer.
Treatment characteristics
First-line treatments included: surgery, EBRT, and/or systemic therapy (either radiosensitizing chemotherapy, systemic chemotherapy, targeted therapy, or immunotherapy). Eighty-five percent of the patients (46/54) received a combination of EBRT and radiosensitizing chemotherapy with or without surgery. In total, 39% (21/54) of patients during the study period received treatment within the context of a clinical trial.
Surgery
There were 23 patients who underwent R0 or R1 surgery. Of the 5 patients who underwent R0 surgery, there was 1 patient with stage IVA disease, 3 with stage IVB disease, and 1 with stage IVC disease. Tumor size ranged from 3.5 cm to 10 cm, and 4 out of 5 patients had lymph node involvement. One patient had pure ATC, and the remaining 4 patients had coexisting DTC, though the extent of pathologic DTC varied from minor to dominant. Only 2 out of 23 patients had confirmed diagnosis of ATC prior to their surgery. The remaining 21 patients (91% of those who underwent surgery) had biopsy or fine needle aspiration that suggested a more differentiated thyroid cancer preoperatively, primarily PTC or poorly differentiated thyroid cancer (PDTC) that was deemed operable, but post-operatively were diagnosed as ATC. These patients were also more likely to have a coexisting differentiated component and less advanced/extensive disease, compared to those who did not. One surgery consisted of complete resection of a humerus bone metastasis that revealed ATC (transformed from PTC). All other surgeries consisted of thyroidectomy with or without central and lateral compartment dissection. Of 23 operations, 9 were performed at our institution (including the 2 known ATC patients prior to surgery), and the remaining 14 were performed at outside institutions. All but one of the patients who underwent surgery also received adjuvant EBRT.
Radiation therapy
EBRT was administered to 48 patients. Twenty-seven patients (50%) received EBRT with radiosensitizing chemotherapy as the first-line treatment to gross tumor. Adjuvant EBRT with radiosensitizing chemotherapy was given to 19 (35%) patients after surgery, and 2 patients received EBRT alone after R0/R1 resection. One patient had an R0 surgery but weeks later became ill due to other comorbidities and her performance status was not suitable for further radiation or systemic therapy.
Eight of 48 patients received EBRT less than 4500 cGy (low dose). Low dose EBRT was administered for palliative intent in 5 of these 8 patients. In 2 patients, only low dose was achievable because they could not complete the intended full dose. One patient received EBRT to the humerus with a cumulative dose of 3600 cGy. Ten patients received additional radiation therapy to metastatic regions outside the neck, including whole brain radiation after the initial course of radiation.
First-line systemic therapy
Forty-six patients (85%) received radiosensitizing therapy with EBRT consisting primarily of platinum or taxane-based agents as their first exposure to systemic therapy. Three patients received systemic chemotherapy as first-line treatment, again with a platinum or taxane. Two patients were started on targeted therapy as their first systemic agent(s) (one received lenvatinib and the other was treated with the dabrafenib/trametinib combination). One patient was given immunotherapy. Administration of first-line systemic therapy was given in the context of a clinical trial in nine patients.
Systemic therapy administered in second line or beyond
Second-line systemic chemotherapy was given to 18 patients for progressive disease occurring after completion of EBRT/radiosensitizing chemotherapy. After receiving prior systemic therapy, 12 patients were treated on clinical trials involving either targeted therapy or immunotherapy. The outcomes associated with the interventions given under the clinical trial protocols are not yet published and will not be addressed in our study.
Clinical outcomes
Outcomes for the entire cohort
The median TTF for the entire cohort was 3.8 months [CI 3–6.5] and median OS was 11.9 months [CI 8.4–24.9], with a median follow up time of 17.8 months. Twenty-one out of 54 patients (39%) were still alive at the end of the study period. First-line therapy failed in 44/54 (81%) of patients, of which 40/54 (74%) occurred at distant sites (either new metastasis or progression of existing disease). Median time to distant failure was 4 months (CI 3–7.7), with the probability of distant disease of 84% within 12 months in the entire cohort. Twenty-eight of 54 (52%) patients had locoregional failure after first-line treatment. A summary of failure sites based on stage and treatment is shown in Table 2.
Table 2.
Sites of Failure Based on Stage and Initial Treatments Received
| First-line treatment | Overall local control | Overall failure | Sites of failure | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Stage | Surgerya | EBRT | Systemicb | n | n (%) | n (%) | Distant only (n) | Local only (n) | Local and distant (n) |
| IVA N = 10 |
x | x | x | 6 | 4 (67%) | 3 (50%) | 1 | 0 | 2 |
| x | x | 2 | 1 (50%) | 1 (50%) | 0 | 0 | 1 | ||
| x | x | 2 | 0 (0%) | 2 (100%) | 0 | 0 | 2 | ||
| IVB N = 17 |
x | x | x | 7 | 4 (57%) | 4 (57%) | 1 | 0 | 3 |
| x | x | 9 | 5 (56%) | 9 (100%) | 5 | 1 | 3 | ||
| x | x | 1 | 1 (100%) | 1 (100%) | 1 | 0 | 0 | ||
| IVC N = 27 |
x | x | x | 6 | 3 (50%) | 5 (83%) | 2 | 0 | 3 |
| x | x | 16 | 7 (44%) | 16 (94%) | 6 | 1 | 8 | ||
| x | 2 | 0 (0%) | 2 (100%) | 0 | 0 | 2 | |||
| x | 2 | 0 (0%) | 2 (100%) | 0 | 1 | 1 | |||
| xc | 1 | 1 (100%) | 0 (0%) | 0 | 0 | 0 | |||
Surgery refers to R0 or R1 resections.
Systemic refers to concurrent cytotoxic chemotherapy with EBRT, single cytotoxic chemotherapy, or targeted therapy.
Short follow-up time in this patient.
EBRT, external beam radiation therapy.
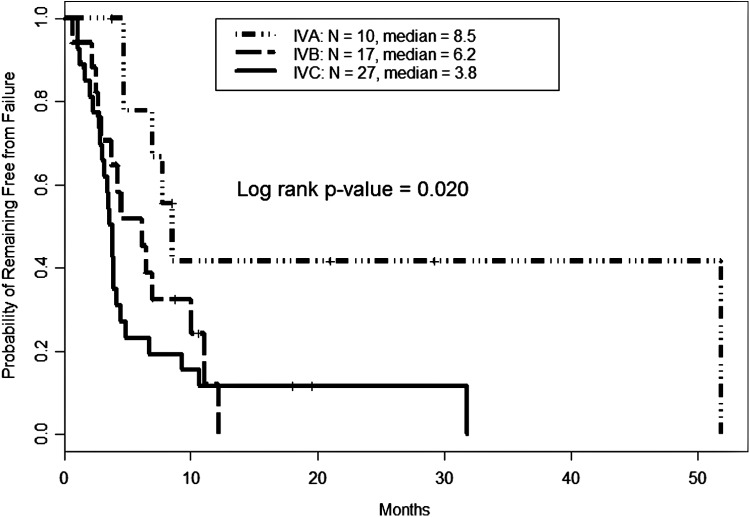
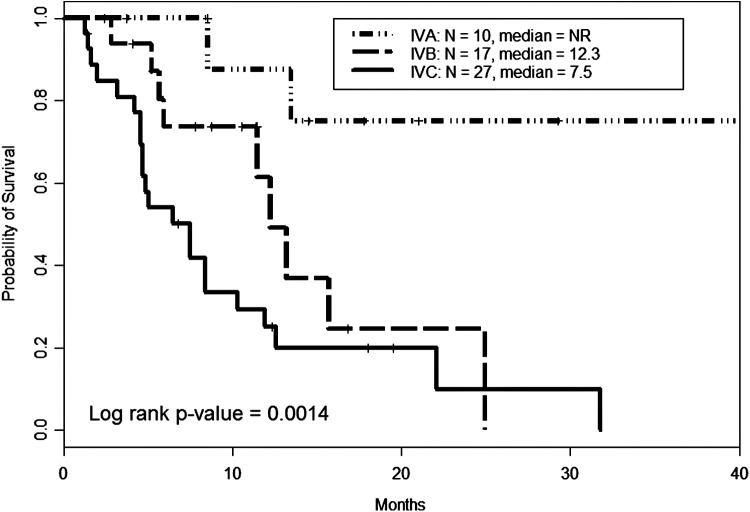
Median TTF by stage at diagnosis was 8.5, 6.2, and 3.8 months for stages IVA, IVB, and IVC, respectively (Fig. 1). Median OS by stage was not reached for the 10 stage IVA patients. In stage IVB and IVC patients, median OS was 12.3 [CI 11.5–NR] and 7.5 [CI 4.7–11.9] months, respectively (Fig. 2). The univariate analysis of baseline characteristics is shown in Table 3. Male sex significantly impacted both TTF and OS. Multivariate analysis revealed advanced stage, male sex, and pleomorphic/giant cell pattern negatively influenced both failure of treatment(s) and survival (Table 4).
FIG. 1.
Time to failure in anaplastic thyroid carcinoma by stage. Failure (defined as development of new disease, progressive disease, or death) after first treatment was calculated using the Kaplan-Meier method. Those with more advanced disease failed earlier; however, even those with stage IVA (or disease limited to the thyroid) still experienced evidence of failure at a median of 8.5 months.
FIG. 2.
Overall survival in anaplastic thyroid carcinoma by stage. Median overall survival for the entire cohort was about 12 months. When separated by stage, median survival was not reached for the IVA group. Median overall survival in IVB and IVC patients was 12.3 and 7.5 months, respectively.
Table 3.
Patient and Tumor Characteristics and Impact on Survival and Failure
| Characteristic (N) | Median failure (months) | Median survival (months) | Survival p-value (HR) | Failure p-value (HR) |
|---|---|---|---|---|
| Sex | ||||
| Female (29) | 6.8 | 22.1 | 0.0024 (3.2) | 0.03 (2.0) |
| Male (25) | 3.8 | 7.5 | ||
| Age, years (median = 63) | 0.23 (1.6) | 0.23 (1.5) | ||
| <60 (17) | 7 | 15.7 | ||
| ≥60 (37) | 4.1 | 10.3 | ||
| Ethnicity | ||||
| Caucasian, (42) | 4.1 | 8.5 (4.1) | 0.062 (2.5) | 0.024 (2.4) |
| Hispanic, (5) | – | – | ||
| Black, (3) | – | – | ||
| Asian, (4) | – | – | ||
| Tumor size (range 1.3–12 cm) | 0.27 (1.5) | 0.43 (1.3) | ||
| Median <5 cm (25) | 6.8 | 12.3 | ||
| ≥5 cm (29) | 3.9 | 8.4 | ||
| Stage | 0.0014 | 0.02 | ||
| IVA (10) | 8.5 | NR | ||
| IVB (17) | 6.2 | 12.3 | ||
| IVC (27) | 3.8 | 7.5 | ||
| Transformationa (10) | 3.9 | 11.5 | 0.96 (1.0) | 0.48 (0.7) |
| No transformation (44) | 4.5 | 12.3 | ||
| Coexisting DTCb(31) | 6.5 | 13.2 | 0.078 (0.5) | 0.37 (0.8) |
| No coexisting DTC (23) | 3.9 | |||
| Lymph node involvement (33) | 4.1 | 8.4 | 1.4 (0.41) | 1.4 (0.37) |
| No lymph node involvement (21) | 4.5 | 15.7 | ||
| Histologic pattern (often mixed) | ||||
| Spindle (18) | 5.4 | 11.9 | 0.95 (1.0) | 0.86 (0.9) |
| Squamous (10) | 7.1 | 11.9 | 0.98 (1.0) | 0.46 (0.8) |
| Giant cell (3) | 3.8 | 5 | – | – |
| Pleomorphic (6) | 2.8 | 5.6 | 0.044 (3.2) | 0.14 (2.2) |
| Epithelioid (10) | 4.0 | 10.3 | 0.87 (0.9) | 0.95 (1.0) |
| Leukocytosisc (9) | 3.2 | 4.7 | 0.081 (2.3) | 0.58 (1.3) |
| Normal WBC (45) | 4.7 | 12.3 | ||
Transformation is defined as prior history of differentiated thyroid cancer, and only later developed anaplastic thyroid cancer.
Coexisting DTC is defined as presence of DTC at the time of ATC diagnosis.
Leukocytosis is defined as >10,000 white blood cells per microliter.
HR, hazard ratio; NR, not reached; WBC, white blood cell count.
Table 4.
Multivariate Analysis of Baseline Characteristics Compared to Survival and Failure
| Overall survival | Failure | |||
|---|---|---|---|---|
| Variable | HR [CI] | p-Value | HR [CI] | p-Value |
| Tumor Size: 5 cm | 0.9 (0.4–1.9) | 0.75 | 1.0 (0.5–2.0) | 0.99 |
| Sex (M vs. F) | 2.2 (1.0–5.0) | 0.049 | 2.0 (1–3.9) | 0.038 |
| Age: 60 years | 1.3 (0.6–2.8) | 0.50 | 1.0 (0.5–2.1) | 0.92 |
| Stage: IVB vs IVA | 4.0 (0.8–20) | 0.088 | 2.7 (0.9–8.0) | 0.081 |
| Stage: IVC vs IVA | 5.9 (1.3–27) | 0.023 | 3.8 (1.4–10) | 0.0095 |
| Histology: Pleomorphic/Giant Cell vs others | 2.7 (1.1–6.8) | 0.036 | 2.9 (1.2–7.0) | 0.019 |
CI, confidence interval; M, male; F, female.
Outcomes by treatment modality
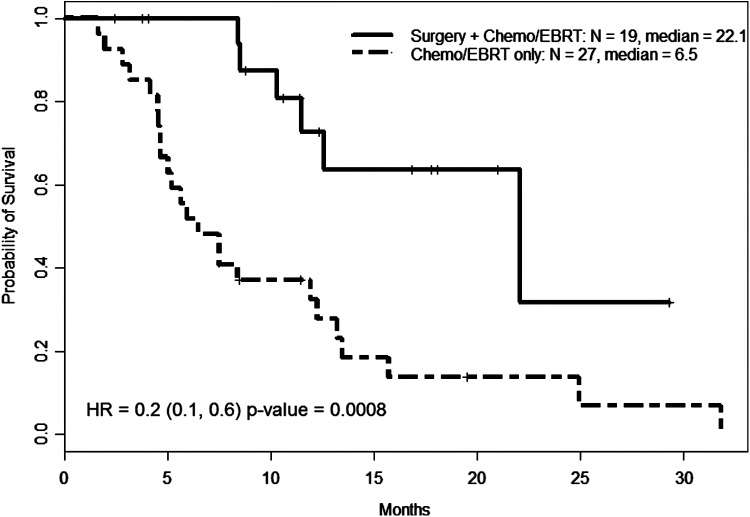
Table 2 details the location of recurring/progressing disease based on stage and treatment. Due to small numbers, Kaplan-Meier curves could only be compared between patients who either had tri-modal therapy consisting of surgery (R0/R1), EBRT, and radiosensitizing chemotherapy (n = 19) and those who had dual therapy with EBRT and radiosensitizing chemotherapy (n = 27) without surgery (including those who had R2 resections). The median OS between tri-modal and dual therapy was 22.1 months and 6.5 months, respectively (p = 0.0008, hazard ratio 0.2) (Fig. 3). This was statistically significant on both univariate and multivariate analyses. However, there was no statistically significant difference in median TTF between tri-modal and dual therapy (7.0 and 6.5 months, respectively), which was primarily driven by distant metastasis. Of note, there was no difference in the mean tumor size between those who received surgery versus those who did not. There were more advanced-stage cases in the dual therapy group (16 IVC, 9 IVB, and 2 IVA) versus those in trimodal therapy group (6 IVC, 7 IVB, and 6 IVA), which may have contributed for the best outcomes seen in the latter group.
FIG. 3.
Overall survival by treatment modality in select patients. Nineteen patients underwent complete surgery followed by external beam radiation therapy (EBRT) and radiosensitizing chemotherapy (chemo/EBRT). Twenty-eight patients only underwent chemo/EBRT without prior surgery. Those who underwent R0/R1 surgeries survived longer (22.1 months) versus those who did not (only 6.5 months). HR, hazard ratio.
Specifically in those patients with stage IVA/IVB disease and who received EBRT (combined with other treatment modalities), 50% had progression or development of new metastases at distant sites (distant failure). In fact, 6 of the 10 (60%) stage IVA patients had evidence of distant failure after initial treatment.
Although the numbers were small, we examined the 17 IVB patients separately to evaluate the utility of surgery in this group. Those who had surgery plus any other treatment modality were more likely to be alive and had fewer failures at the end of the study period (7/8, 87.5% alive) versus those who received dual therapy that did not include surgery (1/9 or 11.1% alive); due to small numbers, statistical significance could not be calculated.
Impact of genomic events on clinical outcomes
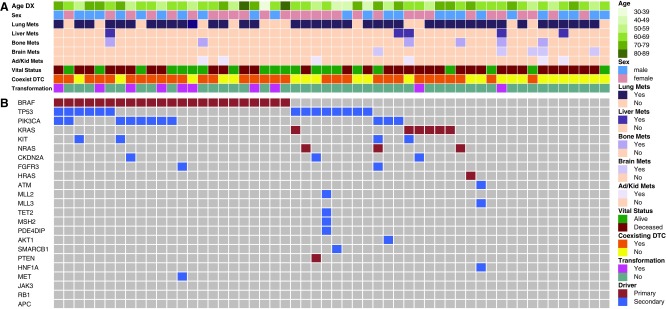
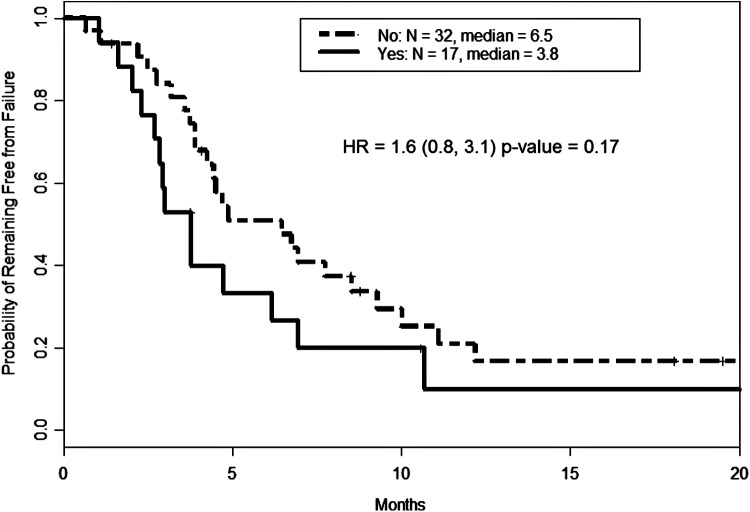
Genomic testing was performed in 50/54 (92.5%) of patients. The most common mutation seen was BRAFV600E (48% among those tested) followed by mutations in TP53 (34.7%), PIK3CA (27.7%) and RAS (combined K-, N-, H-RAS at 21.3%). These results along with corresponding patient and tumor characteristics are shown in Figure 4. As expected, RAS and BRAF did not coexist in any one tumor. In patients with ATC transformation from a prior history of PTC, a mutation in BRAF was found in 89% (8/9) of patients. The presence or number of mutations did not statistically influence outcomes, however there was a trend toward shorter TTF in patients with a TP53 mutation (Fig. 5).
FIG. 4.
Oncoprint and baseline characteristics of patients with anaplastic thyroid carcinoma. Each column represents one patient, and compared baseline patient and tumor characteristics to presence of mutations and outcomes. (A) Clinicopathologic features, including age, gender, coexisting differentiated thyroid carcinoma, vital status, history of transformation, and sites of distant metastatic disease. Color key is shown to the right. (B) Oncoprint of anaplastic thyroid cancer patients showing the presence of genetic alteration. Maroon and blue colors represent primary (driver) and secondary mutations, respectively. DTC, differentiated thyroid cancer; DX, diagnosis.
FIG. 5.
Time to failure based on presence or absence of TP53 mutation. Though not statistically significant, the presence of a TP53 mutation (indicated as “yes”) trended toward likelihood of failure.
Discussion
Previous retrospective studies of ATC have focused mainly on prognostic factors and survival with different treatment modalities and in large part did not include genetic analysis of tumors. Our study is unique in that we documented the time to and location of progressive disease after initial treatment in addition to tumor genetic analysis. In our patient population, median OS was nearly one year for all stages. Median TTF was 3.8 months, with the majority of the patients experiencing new or progressing distant disease, regardless of initial disease stage. These data suggest that ATC is largely a systemic disease and should be approached as such.
Similar to other reports (1,10–14), we show that patients who had complete surgery (R0/R1) combined with EBRT and radiosensitizing chemotherapy (trimodal therapy) survived longer (median OS 22 months) than those treated with EBRT and radiosensitizing chemotherapy (dual therapy, median OS 6.5 months). Complete surgical resection, as the distinguishing intervention, when combined with adjuvant therapy, significantly improved survival, particularly in the stage IVA and selected IVB patients. However, there are several areas of bias inherent in our study. The tri-modal group had less advanced disease compared to the dual therapy group (6 IVA and 6 IVC vs. 2 IVA and 16 IVC, respectively). Additionally, patients of older age and multiple comorbidities at diagnosis were presumably not considered good surgical candidates, although we were not able to extract this information. On the other hand, the overwhelming majority of patients (91%) who had R0/R1 surgeries were not known to have ATC prior to their operation (“incidental ATC” phenomenon), suggesting that surgeons were more willing to undertake a complicated surgery because the diagnosis was of a more differentiated thyroid cancer. The tumor characteristics were also very similar in terms of size, as were the number of stage IVB patients in both groups. This suggests that the ability of the surgeon to perform a complete surgery (R0/R1) is the most important determinant of survival in suitable candidates. Others have shown this in a prospective manner with outcomes consistent with ours. In a prospective pilot study, Kumar et al. showed a median OS of 22 months (identical to our findings) in patients undergoing R0/R1/R2 surgery, EBRT with radiosensitizing chemotherapy, followed by adjuvant chemotherapy (15). Their group of patients were highly selected and consisted of mostly stage IVB (20/29, 69%) patients suitable for aggressive multimodal therapy (16). Consistent with our finding, most patients died of distant metastases.
Although surgery plus EBRT/radiosensitizing chemotherapy improved OS in our series, in terms of TTF, the difference was negligible (7 months after surgery versus 6.5 months without surgery). The pattern of treatment failure was primarily at distant sites, suggesting that a systemic approach to ATC is critical. Unfortunately, most patients do not tolerate systemic cytotoxic chemotherapy after completion of EBRT. Hence considering a systemic approach early to reduce the risk of failure may be necessary. Uruno et al. investigated initiating 4 to 8 doses of weekly paclitaxel upfront in ATC patients with an appropriate performance status, no matter the stage, followed by reclassifying whether the patients were considered surgical candidates (17). Surgery was performed in the appropriate subgroup or considered for further courses of chemotherapy. EBRT was the last intervention performed. Although the number of patients who completed all aspects of treatment (paclitaxel, followed by surgery, another 4 to 8 doses of paclitaxel, and finally local EBRT) was small (16 out of the initial 45 patients), the median OS of this group was 16 months. It is important to note that 12 of these 16 patients were stage IVB, and who may not have otherwise been surgical candidates at initial diagnosis. Also the number of new distant metastasis during the treatment courses was zero in this subgroup (17). While systemic chemotherapy has potential morbidity and mortality, the concept of neoadjuvant therapy as novel approach to ATC could potentially limit or delay development of distant metastases and allows some patients to become potential candidates for surgical resection.
In terms of tumor genetics, our study revealed a prevalence of BRAFV600E mutation in 48% of those who were tested. The prevalence of BRAFV600E mutation was similar to those of a recent genomic profiling of 33 ATC patients published by Landa et al. (18). Not surprisingly, in our cohort, 89% of patients who had a previous diagnosis of PTC (i.e., ATC transformation) had BRAF-mutated ATC. Thus, a prior diagnosis of PTC should raise the suspicion of a BRAFV600E mutation. This is particularly relevant given that there are clinical trials with BRAF inhibitors for BRAF-mutated ATC with promising results (19), and there are ongoing studies with this class of drug (NCT02034110, NCT01596140).
The prevalence of TP53 in the study by Landa et al. exceeded 70%, compared with nearly 35% in our cohort; however, their group used whole-exome sequencing, which would have identified more mutations. While not statistically significant, we did note a trend toward a shorter median TTF of 3.8 months in those with a TP53 mutation versus TP53 wild type (6.5 months). Additionally, Landa et al. also commented on the prevalence of a mutation in the TERT promoter region in 73% of their ATC patients (18). Mutations in the TERT promoter region have been known to be associated with worse prognostic outcomes and generally a more aggressive disease in thyroid cancer (20,21). Landa et al. further demonstrated a greater mutation burden compared to poorly differentiated thyroid cancer, with a median number of mutations of 6 versus 2, respectively (18). Though we might have expected the number of mutations to correspond with worse outcomes, there was no relation seen. This could have been due to the fact that our genomic analysis did not capture all mutations, including TERT promoter mutations, or simply that the high mutation burden and prevalence in ATC is a marker of more aggressive pathology compared to DTC or PDTC.
Advanced age and disease stage, larger tumor size, and elevated white blood cell count have been shown to be negative prognostic factors for ATC outcomes (5,22,23). Using age of 60 years (though our median was age 63), and tumor size of 5 cm as our cutoff points for comparison of outcomes as used in other studies, these factors were not found to be significantly associated with worse outcomes in our cohort. However, the stage at diagnosis, and interestingly male sex, were significantly associated with both shorter TTF and OS. Male sex has been shown to be associated with worse prognosis, not only in differentiated thyroid cancer (10, 24), but also in ATC (5). Although women have a higher incidence of thyroid cancer, men have shorter disease-free survival and higher mortality (25,26). Recent studies have shown differences in the expression of estrogen receptor subtypes in thyroid cells and their response to estrogen in ATC versus more differentiated thyroid cancer (27,28). This could pathologically explain the sex disparity in outcomes. However, what determines the tumor-specific sex hormone receptor expression is unclear. Other possible reasons is that in general, the age-specific incidence of thyroid cancer is generally at a younger age in women than in men, which was also true in our cohort, where most of the patients diagnosed under the age of 60 were female, and the majority are still alive.
Our study is the first to document a significant impact on both failure and survival based on the specific morphologic pattern seen on ATC. The combined pleomorphic/giant cell pattern was statistically associated with likelihood of failure and mortality. Though our numbers are small, one hypothesis could be a higher mitotic index typically associated with giant cell patterns (29), however in our series we did not specifically capture this value. Prior studies have shown no predictive value with outcome based on histologic categories (i.e., spindle, squamous, and giant cell) (30).
With any retrospective review, there are known limitations. One important limitation of this study is that some patients had treatment elsewhere prior to presentation at our institution, and therefore it is possible that their treatment could have been different than what would have been done had they presented to our institution as a new patient without previous treatment. Another limitation of this study is the likely heterogeneity related to the imaging schedule. Though we did not use RECIST criteria, in a disease as aggressive as ATC, most patients had clear evidence of progression.
Conclusion
ATC is a devastating and rapidly morbid disease and we are now at a critical point where implementing new therapeutic strategy is necessary. Surgical intervention, in the appropriate setting, may extend survival and should be strongly considered in patients who can achieve locoregional control. Though the initial standard treatment involves early EBRT with or without radiosensitizing chemotherapy, our study suggests that rethinking this strategy may be warranted. Clinical trial designs with initial, highly effective systemic (likely targeted) therapy to control local disease and at the same time to either prevent or control distant metastasis should be considered in the future. With the increasing number of clinical trials available for this rare condition, especially in the era of targeted therapy, this is an exciting time to further investigate newer systemic treatment strategies in the adjuvant and neoadjuvant setting in an effort to optimize the patient's chance of surgical resection with the ultimate goal to improve quality of life and overall survival.
Acknowledgments
This study was supported in part through The University of Texas MD Anderson Cancer Center's Cancer Center Support Grant CA16672.
Author Disclosure Statement
Dr. Cabanillas has received research funding from Roche and Eisai. For all other authors, no competing financial interests exist.
References
- 1.Smallridge RC, Ain KB, Asa SL, Bible KC, Brierley JD, Burman KD, Kebebew E, Lee NY, Nikiforov YE, Rosenthal MS, Shah MH, Shaha AR, Tuttle RM, American Thyroid Association Anaplastic Thyroid Cancer Guidelines Taskforce 2012 American Thyroid Association guidelines for management of patients with anaplastic thyroid cancer. Thyroid 22:1104–1139 [DOI] [PubMed] [Google Scholar]
- 2.Giuffrida D, Gharib H. 2000. Anaplastic thyroid carcinoma: current diagnosis and treatment. Ann Oncol 11:1083–1089 [DOI] [PubMed] [Google Scholar]
- 3.Cabanillas ME, Zafereo M, Gunn GB, Ferrarotto R. 2016. Anaplastic thyroid carcinoma: treatment in the age of molecular targeted therapy. J Oncol Pract 12:511–518 [DOI] [PubMed] [Google Scholar]
- 4.Akaishi J, Sugino K, Kitagawa W, Nagahama M, Kameyama K, Shimizu K, Ito K, Ito K. 2011. Prognostic factors and treatment outcomes of 100 cases of anaplastic thyroid carcinoma. Thyroid 21:1183–1189 [DOI] [PubMed] [Google Scholar]
- 5.Kebebew E, Greenspan FS, Clark OH, Woeber KA, McMillan A. 2005. Anaplastic thyroid carcinoma. Treatment outcome and prognostic factors. Cancer 103:1330–1335 [DOI] [PubMed] [Google Scholar]
- 6.Keutgen XM, Sadowski SM, Kebebew E. 2015. Management of anaplastic thyroid cancer. Gland Surg 4:44–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aslan ZA, Granados-Garcia M, Luna-Ortiz K, Guerrero-Huerta FJ, Gomez-Pedraza A, Namendys-Silva SA, Meneses-Garcia A, Ordonez-Mosquera JM. 2014. Anaplastic thyroid cancer: multimodal treatment results. Ecancermedicalscience 8:449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Greene FL, Page DL, Fleming ID, Fritz AG, Balch CM, Haller DG, MOrrow M. 2002. AJCC cancer staging manual, 6th edition. Springer, New York [Google Scholar]
- 9.Brignardello E, Palestini N, Felicetti F, Castiglione A, Piovesan A, Gallo M, Freddi M, Ricardi U, Gasparri G, Ciccone G, Arvat E, Boccuzzi G. 2014. Early surgery and survival of patients with anaplastic thyroid carcinoma: analysis of a case series referred to a single institution between 1999 and 2012. Thyroid 24:1600–1606 [DOI] [PubMed] [Google Scholar]
- 10.McIver B, Hay ID, Giuffrida DF, Dvorak CE, Grant CS, Thompson GB, van Heerden JA, Goellner JR. 2001. Anaplastic thyroid carcinoma: a 50-year experience at a single institution. Surgery 130:1028–1034 [DOI] [PubMed] [Google Scholar]
- 11.Hunt JL, Tometsko M, LiVolsi VA, Swalsky P, Finkelstein SD, Barnes EL. 2003. Molecular evidence of anaplastic transformation in coexisting well-differentiated and anaplastic carcinomas of the thyroid. Am J Surg Pathol 27:1559–1564 [DOI] [PubMed] [Google Scholar]
- 12.Haigh PI, Ituarte PH, Wu HS, Treseler PA, Posner MD, Quivey JM, Duh QY, Clark OH. 2001. Completely resected anaplastic thyroid carcinoma combined with adjuvant chemotherapy and irradiation is associated with prolonged survival. Cancer 91:2335–2342 [PubMed] [Google Scholar]
- 13.Swaak-Kragten AT, de Wilt JH, Schmitz PI, Bontenbal M, Levendag PC. 2009. Multimodality treatment for anaplastic thyroid carcinoma–treatment outcome in 75 patients. Radiother Oncol 92:100–104 [DOI] [PubMed] [Google Scholar]
- 14.Yoshida A, Sugino K, Sugitani I, Miyauchi A. 2014. Anaplastic thyroid carcinomas incidentally found on postoperative pathological examination. World J Surg 38:2311–2316 [DOI] [PubMed] [Google Scholar]
- 15.Foote RL, Molina JR, Kasperbauer JL, Lloyd RV, McIver B, Morris JC, Grant CS, Thompson GB, Richards ML, Hay ID, Smallridge RC, Bible KC. 2011. Enhanced survival in locoregionally confined anaplastic thyroid carcinoma: a single-institution experience using aggressive multimodal therapy. Thyroid 21:25–30 [DOI] [PubMed] [Google Scholar]
- 16.Kumar A, Prasongsook N, Kasperbauer J, Smallridge RC, Molina JR, Morris JC, Hay I, Thompson G, Grant C, Richards M, Fatourechi V, McIver B, Suman VJ, Lloyd RV, Sebo T, Foote RL, Bible KC. 2015. Aggressive Multimodal Therapy in Anaplastic Thyroid Cancer: The Mayo Clinic Experience 15th International Thyroid Congress, Orlando, FL [Google Scholar]
- 17.Uruno T, Ogimi Y, Saito F, Masaki C, Akaishi J, Tomoda C, Matsuzu K, Suzuki A, Ohkuwa K, Shibuya H, Kitagawa W, Nagahama M, Sugino K, Ito K. 2015. Proposal of a New Staging System and Treatment Algorithm for ATC by Induction Weekly Paclitaxel International Thyroid Conference, Orlando, FL [Google Scholar]
- 18.Landa I, Ibrahimpasic T, Boucai L, Sinha R, Knauf JA, Shah RH, Dogan S, Ricarte-Filho JC, Krishnamoorthy GP, Xu B, Schultz N, Berger MF, Sander C, Taylor BS, Ghossein R, Ganly I, Fagin JA. 2016. Genomic and transcriptomic hallmarks of poorly differentiated and anaplastic thyroid cancers. J Clin Invest 126:1052–1066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hyman DM, Puzanov I, Subbiah V, Faris JE, Chau I, Blay JY, Wolf J, Raje NS, Diamond EL, Hollebecque A, Gervais R, Elez-Fernandez ME, Italiano A, Hofheinz RD, Hidalgo M, Chan E, Schuler M, Lasserre SF, Makrutzki M, Sirzen F, Veronese ML, Tabernero J, Baselga J. 2015. Vemurafenib in multiple nonmelanoma cancers with BRAF V600 mutations. N Engl J Med 373:726–736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu R, Xing M. 2016. TERT promoter mutations in thyroid cancer. Endocr Relat Cancer 23:R143–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.George JR, Henderson YC, Williams MD, Roberts DB, Hei H, Lai SY, Clayman GL. 2015. Association of TERT promoter mutation, but not BRAF mutation, with increased mortality in PTC. J Clin Endocrinol Metab 100:E1550–1559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim TY, Kim KW, Jung TS, Kim JM, Kim SW, Chung KW, Kim EY, Gong G, Oh YL, Cho SY, Yi KH, Kim WB, Park DJ, Chung JH, Cho BY, Shong YK. 2007. Prognostic factors for Korean patients with anaplastic thyroid carcinoma. Head Neck 29:765–772 [DOI] [PubMed] [Google Scholar]
- 23.Sugitani I, Miyauchi A, Sugino K, Okamoto T, Yoshida A, Suzuki S. 2012. Prognostic factors and treatment outcomes for anaplastic thyroid carcinoma: ATC Research Consortium of Japan cohort study of 677 patients. World J Surg 36:1247–1254 [DOI] [PubMed] [Google Scholar]
- 24.Shi RL, Qu N, Liao T, Wei WJ, Wang YL, Ji QH. 2016. The trend of age-group effect on prognosis in differentiated thyroid cancer. Sci Rep 6:27086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gilliland FD, Hunt WC, Morris DM, Key CR. 1997. Prognostic factors for thyroid carcinoma. A population-based study of 15,698 cases from the Surveillance, Epidemiology and End Results (SEER) program 1973–1991. Cancer 79:564–573 [DOI] [PubMed] [Google Scholar]
- 26.Kilfoy BA, Devesa SS, Ward MH, Zhang Y, Rosenberg PS, Holford TR, Anderson WF. 2009. Gender is an age-specific effect modifier for papillary cancers of the thyroid gland. Cancer Epidemiol Biomarkers Prev 18:1092–1100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zeng Q, Chen G, Vlantis A, Tse G, van Hasselt C. 2008. The contributions of oestrogen receptor isoforms to the development of papillary and anaplastic thyroid carcinomas. J Pathol 214:425–433 [DOI] [PubMed] [Google Scholar]
- 28.Zeng Q, Chen GG, Vlantis AC, van Hasselt CA. 2007. Oestrogen mediates the growth of human thyroid carcinoma cells via an oestrogen receptor-ERK pathway. Cell Prolif 40:921–935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carcangiu ML, Steeper T, Zampi G, Rosai J. 1985. Anaplastic thyroid carcinoma. A study of 70 cases. Am J Clin Pathol 83:135–158 [DOI] [PubMed] [Google Scholar]
- 30.Ragazzi M, Ciarrocchi A, Sancisi V, Gandolfi G, Bisagni A, Piana S. 2014. Update on anaplastic thyroid carcinoma: morphological, molecular, and genetic features of the most aggressive thyroid cancer. Int J Endocrinol 2014:790834. [DOI] [PMC free article] [PubMed] [Google Scholar]