Abstract
Tissue engineering is a promising therapeutic strategy to regenerate skeletal muscle. However, ex vivo cultivation methods typically result in a low differentiation efficiency of stem cells as well as grafts that resemble the native tissues morphologically, but lack contractile function. The application of biomimetic tensile strain provides a potent stimulus for enhancing myogenic differentiation and engineering functional skeletal muscle grafts. We reviewed integrin-dependent mechanisms that potentially link mechanotransduction pathways to the upregulation of myogenic genes. Yet, gaps in our understanding make it challenging to use these pathways to theoretically determine optimal ex vivo strain regimens. A multitude of strain protocols have been applied to in vitro cultures for the cultivation of myogenic progenitors (adipose- and bone marrow-derived stem cells and satellite cells) and transformed murine myoblasts, C2C12s. Strain regimens are characterized by orientation, amplitude, and time-dependent factors (effective frequency, duration, and the rest period between successive strain cycles). Analysis of published data has identified possible minimum/maximum values for these parameters and suggests that uniaxial strains may be more potent than biaxial strains, possibly because they more closely mimic physiologic strain profiles. The application of these biophysical stimuli for engineering 3D skeletal muscle grafts is nontrivial and typically requires custom-designed bioreactors used in combination with biomaterial scaffolds. Consideration of the physical properties of these scaffolds is critical for effective transmission of the applied strains to encapsulated cells. Taken together, these studies demonstrate that biomimetic tensile strain generally results in improved myogenic outcomes in myogenic progenitors and differentiated myoblasts. However, for 3D systems, the optimization of the strain regimen may require the entire system including cells, biomaterials, and bioreactor, to be considered in tandem.
Keywords: : skeletal muscle, tensile strain, biophysical cues, mechanotransduction, bioreactors
Introduction
Skeletal muscle is a mechanically active tissue, acting to stabilize and move the bony skeleton. When skeletal muscle undergoes small amounts of damage, for example the small tears that may occur due to physical activity, the native adult stem cell population, satellite cells, is able to repair the damage through proliferation and fusion with the damaged myofibers.1 However, in cases where large volumes of skeletal muscle tissue are lost, either due to tumor resection or trauma-induced volumetric muscle loss (VML),2 satellite cells are unable to naturally repair the deficit.3 The current clinical strategy to treat VML is autografting. However, there are many shortcomings of this approach, including donor site morbidity and loss of function at the donor and recipient sites, hence the need for engineered skeletal muscle. The traditional tissue engineering strategy incorporates a combination of cells, scaffolds, and growth factors for tissue-specific differentiation and function.4 However, employing these approaches to engineer skeletal muscle has resulted in tissues that show histologic and morphologic similarities to native muscle, but do not exhibit functional contractility within an order of magnitude of the force generated by native muscle.5
Mechanical stimulation is essential for the maintenance of skeletal muscle structure: skeletal muscle atrophies with lack of mechanical stimulation and hypertrophies with rigorous exercise. Providing biomimetic stimulation has been demonstrably advantageous for engineering other tissue types, including cartilage,6,7 ligament,8 bone,9,10 and cardiac muscle.11 Therefore, to enhance the contractility of engineered skeletal muscle grafts, mechanical stimulation can be applied during in vitro culture. Strain regimens can differ vastly and significant consideration must be given to determine the appropriate application of biophysical stimuli such as static versus cyclic, continuous versus intermittent, low amplitude versus high amplitude, and other parameters.
Due to limited mechanistic insight, it is a significant challenge to determine an optimized strain regimen a priori to maximize the functionality of engineered skeletal muscle. Tensile strain is the most commonly applied biophysical stimulus used to mimic the native muscle environment: while each individual muscle belly shortens during movement, the contraction of individual myofibers against two ends that are tethered to the rigid bone induces tensile strains in the tissues as they are contracting. It has been shown that under proper biochemical conditions, applied biaxial12 and uniaxial strains13 can improve the myogenic phenotype of myoblast-like cells in 2D culture. Similarly, static14 and cyclic5 uniaxial strain increased the myogenic outcomes of myoblast-like cells in 3D culture. While other biophysical cues such as alignment, topography,15–17 substrate modulus,18–20 and electrical stimulation21–23 are important to consider while engineering skeletal muscle constructs (see recent review by Bursac et al24), this review focuses on the use of tensile strain to enhance differentiation and tissue maturation of engineered muscle.
This review summarizes the major findings from studies in which tensile strain has been applied to enhance differentiation into myoblasts and muscle morphogenesis. We will not discuss the role of vascularization and innervation in muscle regeneration. We examine the mechanistic basis by which applied strains are mechanotransduced into an increased myogenic gene expression and report on the observed effects of strain parameters such as frequency, amplitude, and duration on both progenitor and terminally differentiated cells. Our review encompasses studies of monolayer (2D) and biomaterial-based (3D) systems, touching future applications for translatable therapies and addressing potential future research directions in the field.
Mechanotransduction and Myogenesis
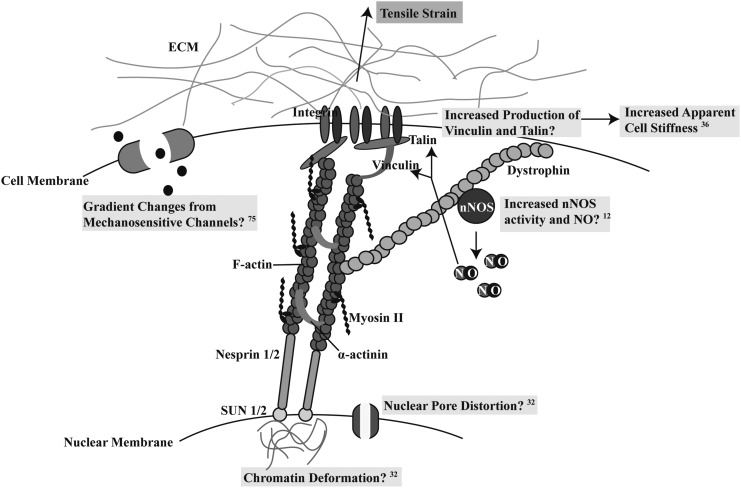
While the intersection of myogenic and mechanotransduction pathways has yet to be fully understood, there are known mechanisms that can help explain how these cellular phenomena coexist in muscle development and regeneration. Integrin receptors and mechanosensitive channels, as illustrated in Figure 1, are among the cellular hardware through which strain-generated signaling can be propagated within the cell. Integrin-mediated adhesions enable cells to detect mechanical stresses applied to the extracellular matrix (ECM). Signaling can be propagated inside the cell through integrin-connected proteins such as focal adhesion kinase (FAK). In this regard, Quach et al. have shown that the FAK activity regulates myoblast fusion; FAK inhibition impairs fusion, leaving myogenic factors unchanged.25 The Rho-Rock pathway has also been shown to affect myogenesis.26 The expression of RhoA protein first rapidly increases and then decreases after 2–3 days. Continuous upregulation of RhoA is an obstacle to further differentiation and fusion. Doherty et al. have described the mechanism of molecular regulation governing this time dependence of RhoA.27 Skeletal muscle differentiation involves several transcription factors such as myoD and Myf5 followed by myogenin and MRF4, which results in the activation of the thick filament motor protein, myosin heavy chain (MHC). It has been hypothesized that the strain effect on myogenesis may occur through strain-generated signaling that targets intracellular transcription factors such as myoD and affects their transcriptional activity.28
FIG. 1.
Proposed mechanisms of myogenic differentiation under tensile strain. Mechanotransduction is required for the upregulation of myogenic genes following tensile strain. Various mechanisms are highlighted in the text boxes. Direct tethering of the nucleus to the ECM through the cytoskeleton is likely the primary means by which forces upregulate myogenic gene expression as several signaling pathways (e.g., nuclear pore distortion,32 chromatin deformation,32 cell stiffening,36 and NO signaling12) rely on physical connections. Mechanosensitive ion channels embedded within the cell membrane may also be responsible for the upregulation of myogenic genes.75 ECM, extracellular matrix.
Integrin signaling proceeding through FAK also regulates stem cell fate decision by changes in cytoskeletal tension.29 This is potentially mediated through mechanosensitive channels tethered to the cytoskeleton that regulate Ca2+ transport. However, another mechanism can be the direct mechanochemical transmission of the applied strains to the cell nucleus. This transmission is structurally supported by the integrin connections to the cytoskeleton through adapter proteins (vinculin, paxilin, talin, and so on) and the cytoskeletal connection to the nucleus by Nesprin 1/2 and SUN 1/2. This direct tethering of the nucleus to the ECM through the cytoskeleton is depicted in Figure 1. A study by Maniotis et al. demonstrated how the mechanical perturbation introduced at the integrin resulted in the mechanical changes in the nucleus.30 Moreover, Thomas et al. showed that changes in cell and nuclear shape resulted in changes in gene expression and protein synthesis.31 There are two hypotheses that describe how mechanical strains that have been transmitted to the nucleus cause perturbations of the nuclear content: (1) transport of biochemical factors through the nuclear pores can change on a timescale of ∼25 s for diffusion over 50 μm, and (2) the chromatin content can deform due to 3D deformation of the nucleus as a whole on a timescale of ∼2 μs as the stress wave propagates through the cytoskelton.32 The latter mechanism has been computationally simulated in the case of the original integrin perturbation caused by changes in the cell–substrate adhesion area.33
In addition to these pathways, there is some evidence that the neuronal nitric oxide synthase (nNOS) pathway is involved in the translation of mechanical cues to phenotypic changes in the cell. The application of Nω-Nitro-L-arginine methyl ester hydrochloride (L-NAME), an NOS blocking agent, to a culture of C2C12 myoblasts under biaxial cyclic strain eliminated the increase in myogenic outcomes that was seen with strain alone.12 Strain has also been shown to increase the nNOS expression in skeletal muscle, which associates with the cytodystrophin complex and increases the release of nitric oxide (NO).34,35 This chain of events increased the apparent Young's Modulus of the cells through higher production of talin and vinculin36 (Fig. 1) and could lead to a positive feedback loop for amplification of the strain signal. AKT activation through phosphorylation is also nNOS dependent.12 AKT has many important downstream targets, including phosphorylation of GSK-3β, making it inactive and unable to translocate to the nucleus to turn on atrophy genes and preventing muscular atrophy.37 Likely, several of these mechanotransduction pathways might be simultaneously involved in the translation of applied strain to myogenic phenotypic changes in myoblast-like cells.
Physiological Basis of Strain
Although difficult to measure directly in vivo, there is considerable indirect evidence indicating that skeletal muscle begins to generate contractile forces early in embryogenesis. Sharir et al. demonstrated that such early skeletal muscle contractile force is necessary to ensure the proper shaping of long bones.38 Using a mouse model of muscular dysgenesis (mdg), in which the mice develop skeletal muscle normally, but without the ability to contract due to an excitation–contraction coupling defect, this group demonstrated that the loss of muscle contractility resulted in the loss of characteristic circumferential shapes of long bones. Using previously described values of force per unit area of embryonic skeletal muscle, Nowlan et al. mathematically modeled these muscle forces, during both flexion and extension, in the humerus and femur of E13.5–15.5 mice. They observed a peak force of ∼480 μN during extension of the humerus in the highest developmental stage observed, nearly triple the force exerted only 2 days earlier in embryonic development.39 The importance of these contractile muscular forces for proper development of the musculoskeletal system extends well beyond mice, having also been observed in zebrafish40 as well as higher mammalian species, including humans.41,42
Understanding the stresses and strains generated by skeletal muscle in adult mammalian organisms becomes far more complex, in large part, owing to the inherent hierarchical organization of skeletal muscle itself, which has evolved to maximize function. Single fibers can be differentiated into type I, type IIa, type IIb, or type IIx based on MHC expression (humans and larger mammals do not express type IIb), which are well known to have characteristic differences in force production and shortening velocity; type II fibers contract more quickly and with greater force than type I fibers, while type I fibers appear more resistant to fatigue.43 The significance of such differences in fiber-type force generation is dramatically reduced when not in isolation, as muscle groups in mammals are generally a heterogeneous mixture of fiber types, with muscle group length, pennation, and neuromuscular activation also contributing to the overall force generating capacity of any given muscle group.44 While this heterogeneity makes it difficult to mimic physiological strain levels in adult human skeletal muscle, other parameters such as duration and frequency can be mimicked in vitro. For example, a frequency of 1 Hz is similar to the natural stride frequency,45 mimicking the strain cycle of muscles of locomotion. However, it could also be advantageous to mimic weight lifting, as this is a common way to increase muscle mass. Rat weight lifting studies have shown increased muscle mass following a lifting regimen that had the rats lifting weights at a frequency of 0.05 Hz. This study was performed over a 16-week period,46 possibly motivating a lower frequency, longer duration application of strain in vitro for myogenesis.
2D Tensile Strain Regimens
Despite the insight provided from analyzing development and physiological processes of skeletal muscle, there remains a knowledge gap regarding the optimization of strain regimens for in vitro skeletal muscle development. A vast majority of these studies have been performed on flexible membranes on which cells were grown in monolayers. These studies are summarized in Table 1. The first nine of the 11 studies detailed in Table 1 utilized the commercially available Flexcell® tension system to apply strain to a flexible silicone membrane. It is notable that variability in the strain field of both the uniaxial and biaxial systems is a limitation of the Flexcell design47,48 and should be considered in reviewing the data from these studies. The remaining two studies used stepper motor systems to stretch silicone49,50 substrates. Aside from this, the strain parameters considered in these studies were orientation (uniaxial vs. biaxial), time-dependent effects, and amplitude.
Table 1.
2D Strain Regimens and Outcomes of Their Application
| Strain mode | Cells | Amplitud e (%) | Effective frequency | Regimen | Major findings | Refs. |
|---|---|---|---|---|---|---|
| Biaxial, Cyclic | Bovine Satellite Cells | 10 | 0.25 Hz (2 s strain, 2 s rest) | 1 h/day for 5 days | • ↓ MRF expression | 54 |
| • ↓ Fusion | ||||||
| C2C12 | 10 | 0.25 Hz (2 s strain, 2 s rest) | 1 h/day for 5 days | • ↑ Proliferation | 53 | |
| • ↓ MRF expression and fusion | ||||||
| 20 | 0.1 Hz (2 s strain, 4 s rest) | 24 h | • Alignment | 51 | ||
| • ↓ MyoD and MNF-α | ||||||
| 17 | 1 Hz (0.5 s strain, 0.5 s rest) | 1 h/day for 5 days | • ↑ Proliferation | 52 | ||
| • ↓ MHC-positive cells | ||||||
| 12 | 0.7 Hz | 1 h/day for 5 days | • ↑ myotube area and diameter; Increased block in the presence of L-NAME | 12 | ||
| 15 | 0.5 Hz (1 s strain, 1 s rest) | 48 h →3-day rest | • No Alignment | 13 | ||
| • ↑ myotube formation right after strain, which is lost after 3-day rest | ||||||
| Uniaxial, Cyclic | C2C12 | 15 | 0.5 Hz (1 s strain, 1 s rest) | 48 h → 3-day rest | • Alignment perpendicular to strain | 13 |
| • Increase in myotube formation and MHC expression persisting after rest period | ||||||
| C2C12/Human ASC | 12 | 0.5 Hz (1 s strain, 1 s rest) | 48 h → 5-day rest | • ↑ Proliferation | 56 | |
| • No impact on differentiation | ||||||
| • ASCs in coculture with C2C12s expressed sarcomeric proteins | ||||||
| • No MRFs irrespective of strain | ||||||
| Human ASC | 11 | 0.5 Hz | 1 h/day for 18 days | • Alignment 45° to strain | 55 | |
| • Multinucleation | ||||||
| • ↑ Desmin, MyoD, and MHC with strain and biochemical factors | ||||||
| Rat ASC | 10 | 1 Hz | 24 h | • Alignment perpendicular to strain direction | 49 | |
| • ↑ MyoD, Myh2, MyoG with strain and biochemical factors | ||||||
| Mouse BMSC | 10 | 0.08–0.5 Hz | 3–7 days | • Parallel alignment to applied strain | 50 | |
| • ↑ Multinucleation | ||||||
| • ↑ Expression of Myf5 and MRF4 | ||||||
| C2C12 | • Perpendicular alignment to applied strain | |||||
| • ↑ myosin + myotubes | ||||||
| • ↑ Myf5, MyoD, and Mrf4 |
MHC, myosin heavy chain.
Uniaxial versus biaxial strains
Researchers have applied both uniaxial and biaxial strains. All the studies employing biaxial strain used the Flexcell system with plates that allow for equibiaxial strains in the radial and circumferential directions. One key study compared 15% biaxial and uniaxial strains. In both cases, a 0.5 Hz effective frequency was employed, which comprised one second of loading and unloading to 15% strain followed by 1 s of rest. The strain regimen was applied for 2 days. It was found that both systems induced an increased myotube formation over the 2 days of strain application, but only in the uniaxial case did the increase in myotube diameter persist for greater than 3 days of rest.13 This corroborated the findings of Soltow et al. who found that improved C2C12 myotube formation in response to biaxial strain was lost when stretching ceased.12
Several studies reported downregulation of myogenic genes in C2C12s under biaxial strain.51–54 These negative results contrasted with the upregulation of myogenic genes observed with the application of uniaxial strains13,49,50 may indicate that the uniaxial strain is preferable for the increased myogenesis and could be due to the fact that uniaxial strain more closely mimics the unidirectional contraction of individual muscle fibers. In addition, uniaxial strain induces cellular alignment,13,49,50,55 which could be helpful in cell fusion. Alignment was not always seen in biaxial experiments.13
Time dependence
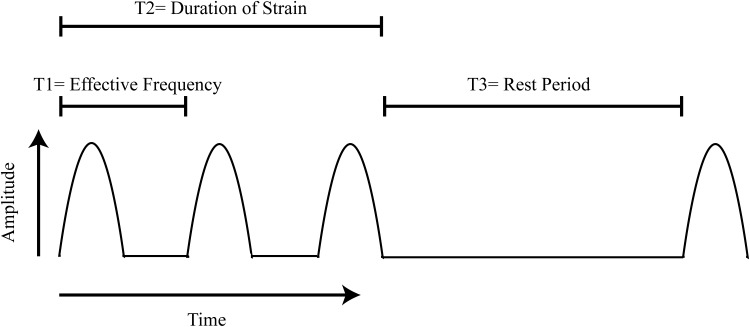
We have defined three timing factors (T1–T3) to describe a strain regimen: effective frequency (T1), duration (T2), and rest (T3) (Fig. 2). In combination, T1–T3 may have a significant effect on the myogenic outcome of applied strain. The effective frequency of the applied strain is defined as 1/(total time of each strain cycle) and includes any delays between successive strains. T1 ranged from 0.0850 to 1 Hz49 and often mimicked the pattern of contraction followed by a brief delay.13,51,56 One study that reported a negative impact of strain on C2C12 myogenesis also notably used one of the lowest effective frequencies, 0.1 Hz.51 This may be below the threshold for improved outcomes and is in agreement with previous studies that have shown that cells will not align under strain at low frequencies.57
FIG. 2.
Time-dependent factors characterizing strain regimen. Three time-dependent parameters impact the myogenic outcomes of straining protocols: T1: the effective frequency of strain application [1/(total time of each strain cycle)], T2: the duration of strain application, and T3: the rest period between applications.
Signaling changes in response to biophysical stimulation occur over a period of seconds.32 However, this time scale may be way below the minimum T2 period needed to stimulate myogenic responses. In a 3D study, Moon et al. applied strain (T1 = 0.05 Hz; T2 = 5 min; T3 = 55 min) and repeated that cycle up to 3 weeks. They reported measurable increases in contractility of the engineered tissue relative to static controls.5 This suggests that T2 may be as low as 5 min although most studies utilize much longer periods ranging from 1 h to several days (Table 1). Whether T2 is short or long, it is likely the entire cycle has to be repeated to elicit a sustained improvement in contractility. That is, if the T3 is too long, the benefits of strain may be lost. For example, some studies utilized a T2 period that spanned from 24 to 48 h and followed this by a T3 period of several days before they evaluated myogenic markers.13,56 In both of these cases, the impact of the strain was lost after several days of rest. Other groups sought to mimic a long-term exercise regimen (T2 = 1 h; T3 = 23 h) over a multiple-day study.12,55 Neither of these had long periods without stimulation following their study before assessing myogenesis; so there is no evidence that these regimen would have sustained effects in the absence of strain. Overall, more rigorous studies that compare the effects of T1, T2, and T3 might prove beneficial in advancing our understanding of the time-dependent effects of strain.
Strain amplitude
Native muscle fibers have been shown to reach strains of 33% in vivo in the tibialis anterior during 45° plantar flexion58 and can be strained beyond 100% ex vivo.59 All studies in Table 1 applied strain amplitudes between 10% and 20% as this is within the typical physiological range. Soltow et al. explored a range of strain amplitudes from 6% to 18% and saw a linear increase in NO production with increasing strain amplitude.12 Despite this, they primarily studied 12% strain fearing that 18% strain would lead to inflammatory pathways and an increased expression of inducible nitric oxide synthase (iNOS).60,61 In fact, an upper limit of strain amplitude for positive myogenic outcomes could be a possible explanation for the decrease in MyoD and myocyte nuclear factor-α (MNF-α) seen under 20% strain.51 However, satellite cells have exhibited an increased proliferation following 25% strain, although the study did not explore myogenic outcomes in the strained satellite cells.62 It is also likely that there is a threshold of strain amplitude below which no positive myogenic outcomes are possible, as it has been shown that alignment caused by strain does not occur at <3% strain.63 Table 1 demonstrates the wide range of strain regimens applied to 2D in vitro cultures. Many of the studies had positive myogenic outcomes associated with strain application, but the variation of regimens and cell types makes it very difficult to elucidate an “optimal” myogenic strain regimen.
Cellular Phenotype and Strain-Induced Myogenesis
The cell type being cultured may also dictate the efficacy of a particular strain regimen. Strain has been used as a myogenic cue for several different cell types, including multipotent adipose-derived stem cells (ASCs) and bone marrow-derived mesenchymal stem cells (MSCs), unipotent satellite cells, and terminally differentiated transformed murine myoblasts, C2C12 s. Satellite cells differ from myoblasts in that satellite cells are in vivo stem cells present in adults and myoblasts are muscle progenitors that fuse to form myotubes during embryogenesis in vivo, and can be cultured in vitro. It is possible that different cell types respond differently to identical cues as some of the cell types are more stem-like, while others are already committed to the myogenic lineage.
Adipose-derived stem cells and mesenchymal stem cells
The most frequently used stem cells for skeletal muscle tissue engineering are ASCs and MSCs. Both MSCs64 and ASCs65 have been shown to differentiate down the myogenic lineage. However, in a direct comparison of bone marrow MSCs to ASCs, ASCs had higher proliferation rates and expression of stem cell markers, while MSCs had higher expression of the myogenic marker, myogenin.66 However, both cell types exhibit low myogenic differentiation efficiency and many groups have applied biophysical cues, including strain, to cultures of ASCs or MSCs in an attempt to improve the efficiency of cells differentiating toward skeletal muscle. Two different groups have shown an increased expression of myogenic factors under strain when in combination with biochemical factors in human55 and rat49 ASCs. Alternatively, Dugan et al. showed that ASCs in coculture with C2C12s were able to express sarcomeric proteins, but no MRFs regardless of whether or not they were strained.56 Cyclic, uniaxial tensile strain has also been shown to increase multinucleation and expression of Myf5 and MRF4 in mouse BMSCs.50 Interestingly, the same study showed that, while the mouse BMSCs aligned in the direction of the applied strain, rat BMSCs, C2C12s, and human fibroblasts all aligned perpendicular to the applied strain,50 indicating that even individual myogenic and promyogenic cell populations may respond differently to biophysical cues.
Satellite cells
Satellite cells, which are the resident in vivo stem cells of adult skeletal muscle, have also been shown to be responsive to mechanical strain. Satellite cells are activated in vitro by the application of 25% cyclic strain as measured by a BrdU proliferation assay.62 This is closely related to studies that have shown that mechanical stretch and the resulting MGF upregulation activate satellite cells in vivo.67 Satellite cells have also been shown to proliferate and activate following exercise in vivo, and form myotubes better ex vivo following exercise.68
C2C12s
C2C12s are transformed mouse myoblasts committed to the myogenic lineage and are often used to test the impact of different biochemical factors and mechanical parameters on myogenesis. There has been rigorous debate regarding C2C12s and the impact of applied strain. One group has shown that there is no sustained effect of biaxial strain on C2C12s compared to increased myogenic outcomes when C2C12s are cultured under uniaxial strain conditions.13 Akimoto et al. showed a decrease in myogenic outcomes under 20% cyclic, biaxial strain.51 This was consistent with other studies that showed decreases in myogenic outcomes of C2C12s under biaxial strain.52,53
Biophysical Strain of 3D TE Skeletal Muscle Constructs
Cell-ECM interactions vary greatly between 2D and 3D cultures,69 suggesting that cell straining may have varied outcomes in 2D and 3D. Bioreactor systems are needed to characterize the effects of strain on 3D cell constructs. However, 3D strain systems are not without challenges: the scaffolds must be reproducibly made for a global strain to be applied uniformly to all cells within the construct and the bioreactor system should allow multiple constructs to be strained at a time for high-throughput experiments that can provide statistical power.
Bioreactor design
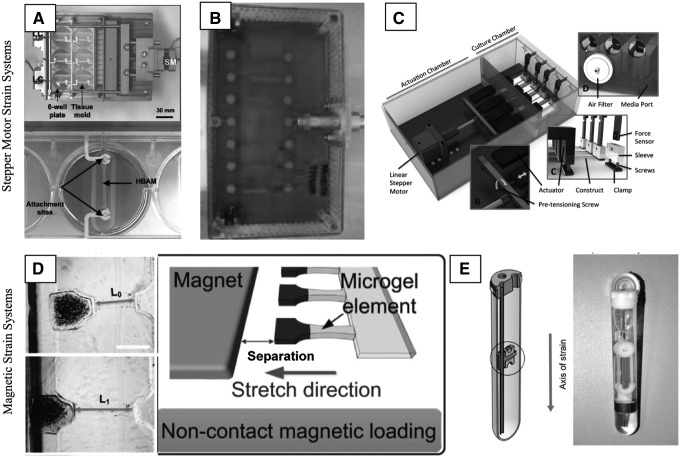
There are a few commercially available bioreactors that allow researchers to apply strain in 3D, including the Flexcell Tissue Train system, which allows for application of uniaxial strain to a restrained gel 3D construct, the Cellscale Mechanoculture T6, which can apply uniaxial strain to 3D structures fixed between two clamps, and the Cellscale Mechanoculture B1, which can apply biaxial strain to a 3D circular construct. However, these systems have restrictions in terms of the types of constructs they can accommodate, the range of strain regimens they can apply, and their imaging compatibility. Alternatively, many groups have custom designed their own bioreactor systems (Fig. 3). Custom-designed systems that have been reported in the literature have been classified into those that apply strain through the controlled movement of a stepper motor (Fig. 3A–C) and systems that use magnets to control the applied strain (Fig. 3D, E). The systems are distinguished by four primary capabilities: (1) the ability to accurately prestrain the constructs,70 (2) the ability to accommodate one (MechanoCulture B1) or multiple5,14,70–72 samples, (3) the capacity to measure active and passive forces70,71 in real time, and (4) imaging compatibility with a fluorescent microscope.70,72
FIG. 3.
Custom bioreactor systems for strain of 3D constructs. (A) Application of uniaxial strain to six cell-laden collagen hydrogels or Matrigel® with 50 μm resolution. System capable of measuring passive force.71 (B) This system holds up to 10 naturally derived decellularized collagen constructs.5 (C) The application of uniaxial strain to four electrospun fibrin constructs. The system controls for zero pretensioning of the fiber, and can measure active contractile forces produced in response to electrical stimulation. The system is compatible with use of a confocal microscope.70 (D) Gelatin methacrylate substrate with embedded magnetic particles at one end to apply strain to the microgel, which is the cell-containing region of the construct. This system allows the application of large static strains up to 60%.72 (E) The system uses a combination of magnetic elements and a stepper motor to apply strain to the cell-seeded fibrin gel construct molded into a ring structure. The bottom hook has a magnetic element that is moved by an external plate, allowing for contactless strain. The bottom magnetic plate is controlled by a stepper motor. This system can hold up to 36 constructs.14
Biomaterial scaffolds
The main goal of a bioreactor system is the transmission of the external (biochemical and biophysical) stimuli to the stem cells to provide their optimal differentiation. In this study, the material properties of the scaffolds play a critical role: the hydrogel or porous scaffold normally has solid and liquid components. They can be anisotropic (fibrous), isotropic (hydrogel), elastic, or viscoelastic (exhibiting creep and stress relaxation). Therefore, changes in scaffold structure, pore orientation, and fiber size directly impact the mechanical properties of the substrate as well as whether the strains applied by the bioreactor system would be faithfully and uniformly transmitted to the cells embedded in the pores or encapsulated within the hydrogel material. The interplay of these parameters combined with changes in cell-ECM interactions makes it challenging to study mechanotransduction in 3D and/or to directly apply findings from 2D studies to 3D systems. The various biomaterial scaffolds used are included in Table 2. Collagen is a component of native muscle tissues and is commonly used in scaffolds.5,71 Fibrin is another naturally occurring, commonly used scaffold material in 3D skeletal muscle constructs14 and can be electrospun to form constructs that provide alignment cues.70
Table 2.
Recent Studies on Strain Applied for Engineering 3D Skeletal Muscle Constructs
| Cells | Scaffold | Frequency, amplitude, duration | Advantages | Disadvantages | Outcomes | Refs. | |
|---|---|---|---|---|---|---|---|
| Stepper Motor | Primary human myoblasts | Collagen/Matrigel | Increase 3.5 μm/10 min up to 10% strain (4 days) | • Measured passive force | • No pretension control | • ↑ Myofiber diameter | 71 |
| 10% static (4 days) | • 50 μm resolution | • Isotropic substrate | • ↑ Gel area covered by myofibers | ||||
| 5% cyclic (2 days) | • Complex strain regimen | ||||||
| 10% cyclic (2 days) | |||||||
| 15% cyclic (4 days) | |||||||
| Acellular Collagen Matrix | • 10% cyclic strain | • Biological cues from matrix | • Unable to measure force | • ↑ Contractility | 5 | ||
| • 0.05 Hz | • Holds up to 10 constructs | • No pretension control | • ↑ Alignment | ||||
| • 5 min/h for 5–21 days | • ↑ Organization | ||||||
| Magnetic Strain | C2C12 | Gelatin Metha-crylate | • 10, 20, 40, and 60% static strain | • Elastic response of substrate up to 60% strain | • Unable to provide cyclic strain | • Highest myogenic gene expression at 40% strain. | 72 |
| • 10 h/day for 10 days | • High cell density | • Complex manu-facturing process | • ↑ Myofiber diameter at 40% strain | ||||
| Fibrin | • 10% static strain for 6 h → 3% static strain for 18 h/day | • 10 μm resolution | • Unable to measure force | • ↑ In multinucleation | 14 | ||
| • 7 days | • Encapsulation of cells may increase density of construct | • No pretension control | • ↑ Alignment | ||||
| • ↑ Expression of myogenic markers |
Myogenic outcomes
The application of tensile strain to engineer 3D skeletal muscle grafts is still a relatively nascent field. An optimized strain regimen for 3D myogenic constructs is likely to be specific to the cell phenotype and the biomaterial substrate. 3D studies tend to be longer in duration than monolayer studies with the ultimate goal being a functional, contractile skeletal muscle graft. Of the four studies summarized in Table 2, two used static strain,14,72 one employed cyclic strain exclusively,5 and the final study used a combination of static and cyclic strains,71 proving that the field is far from a consensus on the optimal strain regimen. However, Heher et al. compared static and cyclic strains in a preliminary study and found that cyclic strain delayed the onset of myogenesis, suggesting that static strain may be more favorable in a 3D environment.14 The strain amplitudes ranged from 10% to 60%. In one study of static strain, Li et al. found that 40% strain increased the expression of myogenic genes compared to 20% or 60% strain.72 This is in contrast to what has been reported in 2D studies and possibly confirms the fact that the strain applied by the system may not be the strains experienced by individual cells.
Despite the variable strain regimens applied in the studies outlined in Table 2, all the groups reported positive myogenic outcomes following strain application. Most groups indicated an increased myotube diameter,71,72 coverage,71 and alignment5,14 under strain. Moon et al. showed forcible contraction with the application of potassium chloride of a strained sample after 3 weeks. They implanted their constructs in vivo (murine latissimus dorsi VML model) and measured the force of contraction of the engineered construct in response to electrical stimulation following explantation from the mice. After 4 weeks in vivo, the electrically stimulated maximum tetanic force of contraction per unit area of the engineered construct was only about 1% of that of native muscle.5 This group has shown improved outcomes with their mechanically preconditioned construct in a VML model in subsequent studies.73,74 Powell et al. also measured the passive tension of their constructs under strain, but did not implant the constructs in vivo.71 All the 3D systems point toward strain as a positive myogenic cue, yet to date, very few studies have generated measurable contraction and none of the studies have contraction forces on an order of magnitude similar to that of native skeletal muscle.
Future Perspectives/Conclusion
Tensile strain is the key biophysical stimulus used to enhance the contractility of engineered skeletal muscle grafts. The application of tensile strain has been shown to enhance myogenic differentiation of cells in 2D and 3D systems. While a number of putative mechanotransduction pathways may govern the myogenic effect of the strain stimulus, increased understanding of the underlying mechanism can help to optimize strain regimens applied in vitro. Current strain regimens are adopted empirically or intuitively following the biomimetic paradigm (i.e., mimicking native physiology). In this review, we have defined strain regimens comprising orientation, strain amplitude, and three time-dependent factors: T1—effective frequency, T2—duration, and T3—rest period. To date, various studies in the literature have used different combinations of T1, T2, and T3 in addition to different cellular phenotypes, making it challenging to draw a consensus on which conditions work best for inducing myogenesis. Despite the lack of standardization, it may be possible to elicit certain rules governing minimum values of T1 and T2 and a maximum value of T3 that may result in sustained myogenic outcomes. Future studies in engineering functional 3D constructs for application to the clinic may also require in-depth consideration for the biomaterial substrates capable of effectively transmitting the applied strain to the cells and the use of advanced bioreactor systems capable of administering precise strains to the tissue.
Disclosure Statement
No competing financial interest exists.
References
- 1.Grounds M.D. Age-associated changes in the response of skeletal muscle cells to exercise and regeneration. Ann N Y Acad Sci 854, 78, 1998 [DOI] [PubMed] [Google Scholar]
- 2.Grogan B.F., and Hsu J.R. Volumetric muscle loss. J Am Acad Orthop Surg 19 Suppl 1, S35, 2011 [DOI] [PubMed] [Google Scholar]
- 3.Mauro A. Satellite cell of skeletal muscle fibers. J Biophys Biochem Cytol 9, 493, 1961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vacanti J.P., and Langer R. Tissue engineering: the design and fabrication of living replacement devices for surgical reconstruction and transplantation. Lancet 354, SI32, 1999 [DOI] [PubMed] [Google Scholar]
- 5.Moon du G., Christ G., Stitzel J.D., Atala A., and Yoo J.J. Cyclic mechanical preconditioning improves engineered muscle contraction. Tissue Eng Part A 14, 473, 2008 [DOI] [PubMed] [Google Scholar]
- 6.Mauck R.L., Soltz M a, Wang C.C., Wong D.D., Chao P.H., Valhmu W.B., et al. Functional tissue engineering of articular cartilage through dynamic loading of chondrocyte-seeded agarose gels. J Biomech Eng 122, 252, 2000 [DOI] [PubMed] [Google Scholar]
- 7.Hung C.T., Mauck R.L., Wang C.C.B., Lima E.G., and Ateshian G.A. A paradigm for functional tissue engineering of articular cartilage via applied physiologic deformational loading. Ann Biomed Eng 32, 35, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Altman G., Horan R., Martin I., Farhadi J., Stark P., Volloch V., et al. Cell Differentiation by mechanical stress. FASEB J 16, 270, 2002 [DOI] [PubMed] [Google Scholar]
- 9.Li R., Liang L., Dou Y., Huang Z., Mo H., Wang Y., et al. Mechanical strain regulates osteogenic and adipogenic differentiation of bone marrow mesenchymal stem cells. Biomed Res Int 2015, 873251, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Donahue S.W., Jacobs C.R., and Donahue H.J. Flow-induced calcium oscillations in rat osteoblasts are age, loading frequency, and shear stress dependent. Am J Physiol Cell Physiol 281, C1635, 2001 [DOI] [PubMed] [Google Scholar]
- 11.Akhyari P., Fedak P.W.M., Weisel R.D., Lee T.-Y.J., Verma S., Mickle DA, et al. Mechanical stretch regimen enhances the formation of bioengineered autologous cardiac muscle grafts. Circulation 106, I137, 2002 [PubMed] [Google Scholar]
- 12.Soltow Q.A., Zeanah E.H., Lira V.A., and Criswell D.S. Cessation of cyclic stretch induces atrophy of C2C12 myotubes. Biochem Biophys Res Commun 434, 316, 2013 [DOI] [PubMed] [Google Scholar]
- 13.Pennisi C.P., Olesen C.G., de Zee, M., Rasmussen J., and Zachar V. Uniaxial cyclic strain drives assembly and differentiation of skeletal myocytes. Tissue Eng Part A 17, 2543, 2011 [DOI] [PubMed] [Google Scholar]
- 14.Heher P., Maleiner B., Prüller J., Teuschl A.H., Kollmitzer J., Monforte X., et al. A novel bioreactor for the generation of highly aligned 3D skeletal muscle-like constructs through orientation of fibrin via application of static strain. Acta Biomater 1, 2015 [DOI] [PubMed] [Google Scholar]
- 15.Choi J.S., Lee S.J., Christ G.J., Atala A., and Yoo J.J. The influence of electrospun aligned poly(??-caprolactone)/collagen nanofiber meshes on the formation of self-aligned skeletal muscle myotubes. Biomaterials 29, 2899, 2008 [DOI] [PubMed] [Google Scholar]
- 16.Chen M.-C., Sun Y.-C., and Chen Y.-H. Electrically conductive nanofibers with highly oriented structures and their potential application in skeletal muscle tissue engineering. Acta Biomater 9, 5562, 2013 [DOI] [PubMed] [Google Scholar]
- 17.Lam M.T., Sim S., Zhu X., and Takayama S. The effect of continuous wavy micropatterns on silicone substrates on the alignment of skeletal muscle myoblasts and myotubes. Biomaterials 27, 4340, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Engler A.J., Griffin M.A., Sen S., Bönnemann C.G., Sweeney H.L., and Discher D.E. Myotubes differentiate optimally on substrates with tissue-like stiffness: pathological implications for soft or stiff microenvironments. J Cell Biol 166, 877, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gilbert P.M., Havenstrite K.L., Magnusson K.E.G., Sacco A., Leonardi N.A., Nguyen N.K., et al. Substrate elasticity regulates skeletal muscle stem cell self-renewal in culture. Science 329, 1078, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Serena E., Zatti S., Reghelin E., Pasut A., Cimetta E., and Elvassore N. Soft substrates drive optimal differentiation of human healthy and dystrophic myotubes. Integr Biol (Camb) 2, 193, 2010 [DOI] [PubMed] [Google Scholar]
- 21.Liao I.-C., Liu J.B., Bursac N., and Leong K.W. Effect of electromechanical stimulation on the maturation of myotubes on aligned electrospun fibers. Exp Cell Res 1, 133, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rangarajan S., Madden L., and Bursac N. Use of flow, electrical, and mechanical stimulation to promote engineering of striated muscles. Ann Biomed Eng 42, 1391, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wehrle U., Dsterhft S., and Pette D. Effects of chronic electrical stimulation on myosin heavy chain expression in satellite cell cultures derived from rat muscles of different fibertype composition. Differentiation 58, 37, 1994 [DOI] [PubMed] [Google Scholar]
- 24.Bursac N., Juhas M., and Rando T.A. Synergizing engineering and biology to treat and model skeletal muscle injury and disease. Annu Rev Biomed Eng 17, 217, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Quach N.L., Biressi S., Reichardt L.F., Keller C., and Rando T.A. Focal Adhesion kinase signaling regulates the expression of caveolin 3 and? 1 integrin, genes essential for normal myoblast fusion. Mol Biol Cell 20, 3422, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Charrasse S., Comunale F., Gumbach Y., Poulat F., Blangy A., and Gauthier-Rouviere C. RhoA GTPase regulates M-cadherin activity and myoblast fusion. Mol Biol Cell 17, 749, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Doherty J.T., Lenhart K.C., Cameron M V., Mack C.P., Conlon F.L., and Taylor J.M. Skeletal muscle differentiation and fusion are regulated by the BAR-containing Rho-GTPase-activating protein (Rho-GAP), GRAF. J Biol Chem 286, 25903, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Deshpande R.S., and Spector A.A. Modeling Stem Cell Myogenic Differentiation. Sci Rep 7, 40639, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sun Y., Chen C.S., and Fu J. Forcing stem cells to behave: a biophysical perspective of the cellular microenvironment. Annu Rev Biophys 41, 519, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maniotis A.J., Chen C.S., and Ingber D.E. Demonstration of Mechanical Connections between integrins, cytoskeletal filaments, and nucleoplasm that stabilize nuclear structure. Proc Natl Acad Sci U S A 94, 849, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thomas C.H., Collier J.H., Sfeir C.S., and Healy K.E. Engineering gene expression and protein synthesis by modulation of nuclear shape. Proc Natl Acad Sci U S A 99, 1972, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang N., Tytell J.D., and Ingber D.E. Mechanotransduction at a distance: mechanically coupling the extracellular matrix with the nucleus. Nat Rev Mol Cell Biol 10, 75, 2009 [DOI] [PubMed] [Google Scholar]
- 33.Jean R.P., Chen C.S., and Spector A.A. Finite-element analysis of the adhesion-cytoskeleton-nucleus mechanotransduction pathway during endothelial cell rounding: axisymmetric model. J Biomech Eng 127, 594, 2005 [DOI] [PubMed] [Google Scholar]
- 34.Tidball J.G., Lavergne E., Lau K.S., Spencer M.J., Stull J.T., and Wehling M. Mechanical loading regulates NOS expression and activity in developing and adult skeletal muscle. Am J Physiol 275, C260, 1998 [DOI] [PubMed] [Google Scholar]
- 35.Wakayama Y., Inoue M., Kojima H., Murahashi M., Shibuya S., and Oniki H. Localization of sarcoglycan, neuronal nitric oxide synthase,??-dystroglycan, and dystrophin molecules in normal skeletal myofiber: triple immunogold labeling electron microscopy. Microsc Res Tech 55, 154, 2001 [DOI] [PubMed] [Google Scholar]
- 36.Zhang J.S., Kraus W.E., and Truskey G.A. Stretch-induced nitric oxide modulates mechanical properties of skeletal muscle cells. Am J Physiol Cell Physiol 287, C292, 2004 [DOI] [PubMed] [Google Scholar]
- 37.Bodine S.C., Stitt T.N., Gonzalez M., Kline W.O., Stover G.L., Bauerlein R., et al. Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat Cell Biol 3, 1014, 2001 [DOI] [PubMed] [Google Scholar]
- 38.Sharir A., Stern T., Rot C., Shahar R., and Zelzer E. Muscle force regulates bone shaping for optimal load-bearing capacity during embryogenesis. Development 138, 3247, 2011 [DOI] [PubMed] [Google Scholar]
- 39.Nowlan N.C., Dumas G., Tajbakhsh S., Prendergast P.J., and Murphy P. Biophysical stimuli induced by passive movements compensate for lack of skeletal muscle during embryonic skeletogenesis. Biomech Model Mechanobiol 11, 207, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shwartz Y., Farkas Z., Stern T., Aszódi A., and Zelzer E. Muscle contraction controls skeletal morphogenesis through regulation of chondrocyte convergent extension. Dev Biol 370, 154, 2012 [DOI] [PubMed] [Google Scholar]
- 41.Rodríguez J.I., Palacios J., García-Alix A., Pastor I., and Paniagua R. Effects of immobilization on fetal bone development. A morphometric study in newborns with congenital neuromuscular diseases with intrauterine onset. Calcif Tissue Int 43, 335, 1988 [DOI] [PubMed] [Google Scholar]
- 42.Rodriguez J.I., Garcia-Alix A., Palacios J., and Paniagua R. Changes fetal immobility in the long caused due to disease by neuromuscular. J Bone Joint Surg Am 70, 1052, 1988 [PubMed] [Google Scholar]
- 43.Greising S.M., Gransee H.M., Mantilla C.B., and Sieck G.C. Systems biology of skeletal muscle: fiber type as an organizing principle. Wiley Interdiscip Rev Syst Biol Med 4, 1, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Choi S.J. Differential susceptibility on myosin heavy chain isoform following eccentric-induced muscle damage. J Exerc Rehabil 10, 344, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Danion F., Varraine E., Bonnard M., and Pailhous J. Stride variability in human gait: the effect of stride frequency and stride length. Gait Posture 18, 69, 2003 [DOI] [PubMed] [Google Scholar]
- 46.Wong T.S., and Booth F.W. Skeletal muscle enlargement with weight-lifting exercise by rats. J Appl Physiol 65, 950, 1988 [DOI] [PubMed] [Google Scholar]
- 47.Vande Geest J.P., Di Martino E.S., and Vorp D.A. An analysis of the complete strain field within Flexercell™ membranes. J Biomech 37, 1923, 2004 [DOI] [PubMed] [Google Scholar]
- 48.Colombo A., Cahill P.A., and Lally C. An analysis of the strain field in biaxial Flexcell membranes for different waveforms and frequencies. Proc Inst Mech Eng H 222, 1235, 2008 [DOI] [PubMed] [Google Scholar]
- 49.Bayati V., Sadeghi Y., Shokrgozar M.A., Haghighipour N., Azadmanesh K., Amanzadeh A., et al. The evaluation of cyclic uniaxial strain on myogenic differentiation of adipose-derived stem cells. Tissue Cell 43, 359, 2011 [DOI] [PubMed] [Google Scholar]
- 50.Egusa H., Kobayashi M., Matsumoto T., Sasaki J-I, Uraguchi S., and Yatani H. Application of cyclic strain for accelerated skeletal myogenic differentiation of mouse bone marrow-derived mesenchymal stromal cells with cell alignment. Tissue Eng Part A 19, 770, 2013 [DOI] [PubMed] [Google Scholar]
- 51.Akimoto T., Ushida T., Miyaki S., Tateishi T., and Fukubayashi T. Mechanical stretch is a down-regulatory signal for differentiation of C2C12 myogenic cells. Mater Sci Eng C 17, 75, 2001 [Google Scholar]
- 52.Kumar A., Murphy R., Robinson P., Wei L., and Boriek A.M. Cyclic mechanical strain inhibits skeletal myogenesis through activation of focal adhesion kinase, Rac-1 GTPase, and NF-kappaB transcription factor. FASEB J 18, 1524, 2004 [DOI] [PubMed] [Google Scholar]
- 53.Kook S.-H., Lee H.-J., Chung W.-T., Hwang I.-H., Lee S.-A., Kim B.-S., et al. Cyclic mechanical stretch stimulates the proliferation of C2C12 myoblasts and inhibits their differentiation via prolonged activation of p38 MAPK. Mol Cells 25, 479, 2008 [PubMed] [Google Scholar]
- 54.Kook S.-H., Son Y.-O., Choi K.-C., Lee H.-J., Chung W.-T., Hwang I.-H., et al. Cyclic mechanical stress suppresses myogenic differentiation of adult bovine satellite cells through activation of extracellular signal-regulated kinase. Mol Cell Biochem 309, 133, 2008 [DOI] [PubMed] [Google Scholar]
- 55.Yilgor Huri, P., Cook C.A., Hutton D.L., Goh B.C., Gimble J.M., DiGirolamo D.J., et al. Biophysical cues enhance myogenesis of human adipose derived stem/stromal cells. Biochem Biophys Res Commun 438, 180, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dugan J.M., Cartmell S.H., and Gough J.E. Uniaxial cyclic strain of human adipose-derived mesenchymal stem cells and C2C12 myoblasts in coculture. J Tissue Eng 5, 2041731414530138, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tondon A., Hsu H.J., and Kaunas R. Dependence of cyclic stretch-induced stress fiber reorientation on stretch waveform. J Biomech 45, 728, 2012 [DOI] [PubMed] [Google Scholar]
- 58.Maganaris C.N. Force-length characteristics of in vivo human skeletal muscle. Acta Physiol Scand 172, 279, 2001 [DOI] [PubMed] [Google Scholar]
- 59.Bobbert M.F., Ettema G.C., and Huijing P.A. The force-length relationship of a muscle-tendon complex: experimental results and model calculations. Eur J Appl Physiol Occup Physiol 61, 323, 1990 [DOI] [PubMed] [Google Scholar]
- 60.Agarwal S., Deschner J., Long P., Verma A., Hofman C., Evans C.H., et al. Role of NF-κB transcription factors in antiinflammatory and proinflammatory actions of mechanical signals. Arthritis Rheum 50, 3541, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Agarwal S., Long P., Seyedain A., Piesco N., Shree A., and Gassner R. A central role for nuclear factor-κB pathway in the antiinflammatory and proinflammatory actions of mechanical strain. FASEB J 17, 899, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tatsumi R., Sheehan S.M., Iwasaki H., Hattori a, and Allen R.E. Mechanical stretch induces activation of skeletal muscle satellite cells in vitro. Exp Cell Res 267, 107, 2001 [DOI] [PubMed] [Google Scholar]
- 63.Kaunas R., Nguyen P., Usami S., and Chien S. Cooperative effects of Rho and mechanical stretch on stress fiber organization. Proc Natl Acad Sci USA 102, 15895, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wakitani S., Saito T., and Caplan A.I. Myogenic cells derived from rat bone marrow mesenchymal stem cells exposed to 5-azacytidine. Muscle Nerve 18, 1417, 1995 [DOI] [PubMed] [Google Scholar]
- 65.Mizuno H., Zuk P.A., Ph D., Zhu M., Lorenz H.P., Benhaim P., et al. Experimental Myogenic Differentiation by Human Processed Lipoaspirate Cells. Plast Reconstr Surg 109, 199, 2001 [DOI] [PubMed] [Google Scholar]
- 66.Meligy F.Y., Shigemura K., Behnsawy H.M., Fujisawa M., Kawabata M., and Shirakawa T. The efficiency of in vitro isolation and myogenic differentiation of MSCs derived from adipose connective tissue, bone marrow, and skeletal muscle tissue. In Vitro Cell Dev Biol Anim 48, 203, 2012 [DOI] [PubMed] [Google Scholar]
- 67.Hill M., Wernig A., and Goldspink G. Muscle satellite (stem) cell activation during local tissue injury and repair. J Anat 203, 89, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cisterna B., Giagnacovo M., Costanzo M., Fattoretti P., Zancanaro C., Pellicciari C., et al. Adapted physical exercise enhances activation and differentiation potential of satellite cells in the skeletal muscle of old mice. J Anat 228, 771, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cukierman E., Pankov R., Stevens D.R., and Yamada K.M. Taking cell-matrix adhesions to the third dimension. Science 294, 1708, 2001 [DOI] [PubMed] [Google Scholar]
- 70.Cook C.A., Huri P.Y., Ginn B.P., Gilbert-Honick J., Somers S.M., Temple J.P., et al. Characterization of a novel bioreactor system for 3D cellular mechanobiology studies. Biotechnol Bioeng 113, 1825, 2016 [DOI] [PubMed] [Google Scholar]
- 71.Powell C. a., Smiley, B.L., Mills, J., and Vandenburgh, H.H. Mechanical stimulation improves tissue-engineered human skeletal muscle. Am J Physiol Cell Physiol 283, C1557, 2002 [DOI] [PubMed] [Google Scholar]
- 72.Li Y., Huang G., Gao B., Li M., Genin G.M., Lu T.J., et al. Magnetically actuated cell-laden microscale hydrogels for probing strain-induced cell responses in three dimensions. NPG Asia Mater 8, e238, 2016 [Google Scholar]
- 73.Machingal M.A., Corona B.T., Walters T.J., Kesireddy V., Koval C.N., Dannahower A., et al. A tissue-engineered muscle repair construct for functional restoration of an irrecoverable muscle injury in a murine model. Tissue Eng Part A 17, 2291, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Corona B.T., Machingal M.A., Criswell T., Vadhavkar M., Dannahower A.C., Bergman C., et al. Further development of a tissue engineered muscle repair construct in vitro for enhanced functional recovery following implantation in vivo in a murine model of volumetric muscle loss injury. Tissue Eng Part A 18, 1213, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Orr A.W., Helmke B.P., Blackman B.R., and Schwartz M.A. Mechanisms of mechanotransduction. Dev Cell 10, 11, 2006 [DOI] [PubMed] [Google Scholar]