Abstract
Purpose:
Few studies of robotic gastric gastrointestinal stromal tumors (GISTs) resection have been conducted. This study was aimed to evaluate the robotic gastrotomy with intracorporeal suture for patients with GISTs located at cardia and subcardiac region.
Materials and Methods:
From January 2014 to August 2016, 11 patients with GISTs located at cardia and subcardiac region underwent robotic gastrotomy with intracorporeal suture. Data of these patients were collected.
Results:
The mean operative time was 82.7 minutes and the mean blood loss was 30.0 mL. No complication was reported. The postoperative length of stay was 3.3 days. On postoperative day 14, inflammation recovered to preoperative level. On postoperative month 6, the nutritional status was similar to that before the surgery. After 25.5 months follow-up, all patients survived with no recurrence or metastasis.
Conclusions:
Robotic gastrotomy with intracorporeal suture for patients with GISTs located at cardia and subcardiac region is safe and feasible.
Key Words: robotic surgery, gastric gastrointestinal stromal tumor, cardia, gastrotomy, intracorporeal suture
Gastrointestinal stromal tumors (GISTs) are the most common stromal tumors of the digestive tract. The morbidity of GISTs is 20/1 million individuals, and ∼60% of tumors develop in the stomach.1 Complete R0 resection without lymph node dissection is the optimal therapeutic regimen for locally removable gastric GISTs because these tumors rarely exhibit lymph node metastasis.1,2
In many centers, laparoscopy is extensively applied for the surgical treatment of gastric GISTs. GISTs located at the fundus of the stomach, greater curvature of the stomach and anterior wall of the gastric body were easily removed with laparoscopic wedge resection (LWR), especially for GISTs <2 cm.3 While guaranteeing surgical quality and tumor safety, laparoscopy reduces intraoperative bleeding, decreases postoperative complications and accelerates patients’ recovery.4–8 However, GISTs located at cardia and subcardiac region are hardly treated with laparoscopy due to its technical limitations, such as the limited instrument range of motion and poor camera lens stability.8–10
The Da Vinci Surgical Robot has a range of motion in 7 directions, high-definition 3D field of view and software-eliminated tremor, making it more convenient to expose specific sites and remove gastric GISTs at these sites.11–14 However, studies on robotic gastric GISTs resection was few. Therefore, this study was designed to evaluate the robotic gastrotomy with intracorporeal suture for patients with GISTs located at cardia and subcardiac region.
MATERIALS AND METHODS
Patients
From January 2014 to August 2016, 11 patients with GISTs located at cardia and subcardiac region underwent robotic gastrotomy with intracorporeal suture in our center. The patients were confirmed to have gastric submucosal space-occupying lesions preoperatively by ultrasound gastroscopy and computed tomographic examination and were confirmed to have gastric GISTs by a postoperative pathological biopsy. After signing an informed consent, the patients received robotic gastrotomy with intracorporeal suture. Data of these patients were recorded. The procedure was approved by the Institutional Review Board of Nanjing University (NCT03238820).
Surgical Procedures
Patients were placed in the Trendelenburg position. Pneumoperitoneum was accomplished after general anesthesia, with a pneumoperitoneum pressure of 12 mm Hg. One 12-mm trocar was placed below the umbilicus, through which the robotic camera lens entered the abdominal cavity. One 8-mm trocar placed in the upper left quadrant of the abdomen and two 8-mm trocars placed in the upper right quadrant of the abdomen were manipulated by the surgeon. One 12-mm trocar was placed in the left upper quadrant of the abdomen, which was manipulated by the assistant. The installation method of the robotic system was similar to that used in our previous reports15 (Fig. 1). Intraoperatively, the gastric wall was opened with the ultrasound knife, and the tumor was removed according to the tumor margin, followed by intracorporeal robotic gastric wall suture with 3-0 absorbable suture (Covidien, Mansfield, MA) under robotic visualization (Fig. 2). The specimens, which were packed in specimen bags, were removed from the periumbilical or lower abdominal incisions. The placement of surgical incisions was showed in Figure 3.
FIGURE 1.
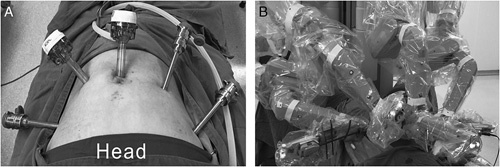
The installation method of the robotic system. Placement of trocars (A); installed robotic system for robotic gastrotomy with intracorporeal suture (B).
FIGURE 2.
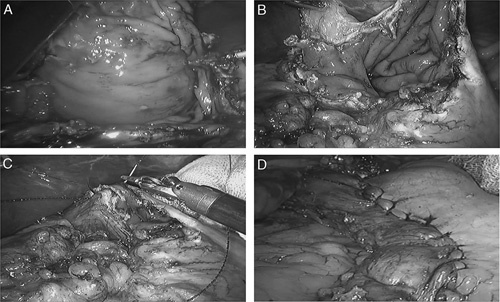
Robotic gastrotomy with intracorporeal suture. The gastric wall was opened with the ultrasound knife (A); the tumor was removed (B); the gastric wall was sutured under robotic visualization (C); the gastric wall was sutured completely (D).
FIGURE 3.
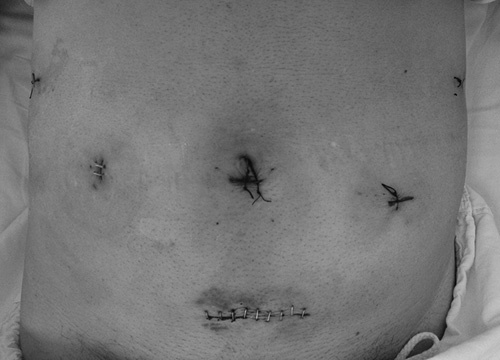
Placement of postoperative surgical incisions.
Follow-up
The discharged patients were followed up through telephone calls or outpatient visits until April 2017; the follow-up data included survival conditions, whether received imatinib treatment, recurrence, and metastasis.
Outcomes
The observational indexes included patient demographics, tumor data, operative time, blood loss, postoperative length of stay, complications, inflammation, nutritional status, and long-term survival. The risk classification of the National Institutes of Health was used to evaluate the risk of postoperative recurrence.16
Statistical Analysis
The data were presented as the mean±SD or mean (range) as appropriate for continuous variables. The differences of nutritional status and inflammation before and after the surgery were calculated by using paired t test. Statistical significance was set at the 5% level. All statistical analyses were performed by using SPSS 16.0 (SPSS, Chicago, IL).
RESULTS
Patient Demographics
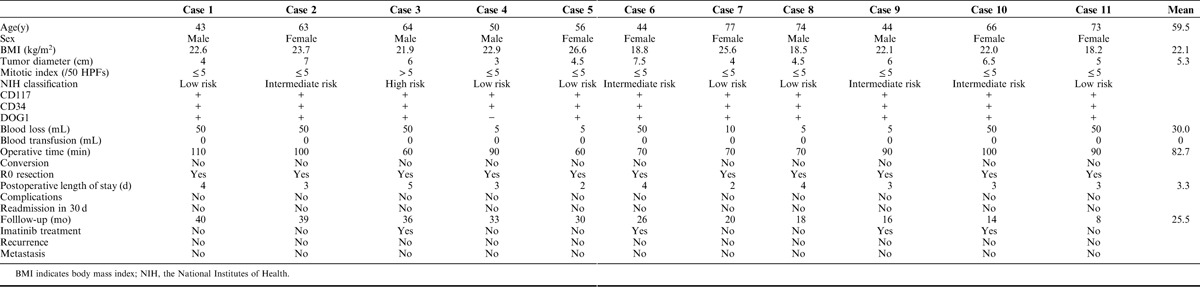
From January 2014 to August 2016, a total of 11 patients with GISTs located at cardia and subcardiac region received robotic gastrotomy with intracorporeal suture. The mean age was 59.5 (range, 43 to 77) years old, and the mean body mass index (BMI) was 22.1 (range, 18.2 to 26.6) kg/m2 (Table 1). Five patients were male and 6 patients were female. The mean tumor diameter was 5.3 (range, 3 to 7.5) cm. Mitotic index of case 3 was >5/50 HPFs, and others’ mitotic indexes were less than 5/50 HPFs. Six cases were at low risks for aggressive behavior, 4 cases were at intermediate risks, and 1 case showed high risk. CD117 and CD34 of all cases were positive. DOG1 of case 4 was negative and DOG1 of others were positive.
TABLE 1.
Basic Information of Patients With Gastric Gastrointestinal Stromal Tumors Located at Cardia and Subcardiac Region Who Underwent Robotic Gastrotomy Combined With Manual Suture
Perioperative Outcomes
The mean blood loss was 30.0 (range, 5 to 50) mL and the mean operative time was 82.7 (range, 60 to 110) minutes (Table 1). All cases underwent robotic gastrotomy with intracorporeal suture. No case was converted to open surgery. R0 resections were achieved in all patients. The mean postoperative length of stay was 3.3 (range, 2 to 5) days. No complications were reported within the hospital stay and 30 days after discharge from the hospital. Thus, nobody needed readmission.
Follow-up
The mean follow-up period was 25.5 (range, 8 to 40) months and all patients survived with no GISTs recurrence or metastasis (Table 1). Case 3, 6, 9,10 received imatinib treatment after the surgery.
Inflammation
Inflammation were evaluated based on white blood cell count, neutrophil percent, interleukin-6 (IL-6) and C-reactive protein (Fig. 4). The mean white blood cell count increased from preoperative 6.5±1.7 ×109/L to 11.9±3.3 ×109/L on postoperative day (POD) 1 (P<0.001), 12.6±3.7×109/L on POD 2 (P<0.001), 9.6±3.4×109/L on POD 3 (P=0.001), and recovered to preoperative level on POD 7 (6.6±1.1×109/L, P=0.714). The mean neutrophil percent fluctuated from preoperative 65.4±8.6% to 87.8±4.5% on POD 1 (P<0.001), then down back to 82.0±4.8% on POD 2 (P<0.001), 75.2±6.0% on POD 3 (P=0.002), 66.6±4.2% on POD 7 (P=0.660), and 64.1±4.9% on POD 14 (P=0.552). Before the surgery, the mean IL-6 was 7.1±3.0 ng/L. The mean IL-6 were 22.4±22.2 ng/L on POD 1 (P=0.028), 26.6±23.7 ng/L on POD 2 (P=0.022), 36.2±36.5 ng/L on POD 3 (P=0.024), 25.0±20.0 ng/L on POD 7 (P=0.014), and 7.7±3.0 ng/L on POD 14 (P=0.587), respectively. Similar to IL-6, the mean C-reactive protein raised from preoperative 1.4±3.0 to 51.0±27.6 mg/L on POD 2 (P<0.001), and then reduced to the preoperative level on POD 14 (P=0.818).
FIGURE 4.
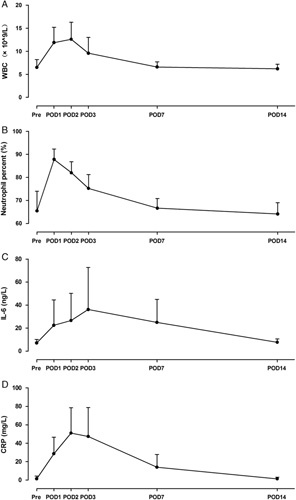
Inflammation of patients with gastric gastrointestinal stromal tumors located at cardia and cardiac region underwent robotic gastrotomy with intracorporeal suture. A, Changes of white blood cell count (WBC) before and after the surgery. WBC on postoperative day 1 (POD 1), POD 2, and POD 3 were higher than that before the surgery (pre) significantly. B, Changes of neutrophil percent before and after the surgery. Compared with preoperative level, neutrophil percent on POD 1, POD 2, and POD 3 increased significantly. C, Changes of interleukin-6 (IL-6) before and after the surgery. The IL-6 on POD1, POD 2, POD 3, and POD 7 were higher than that before the surgery, and then down back to preoperative level on POD 14. D, Changes of C-reactive protein (CRP) before and after the surgery. On POD 1, POD 2, POD 3, and POD 7, CRP rose significantly. On POD 14, CRP dropped down to preoperative level. The error bars represent SD.
Nutritional Status
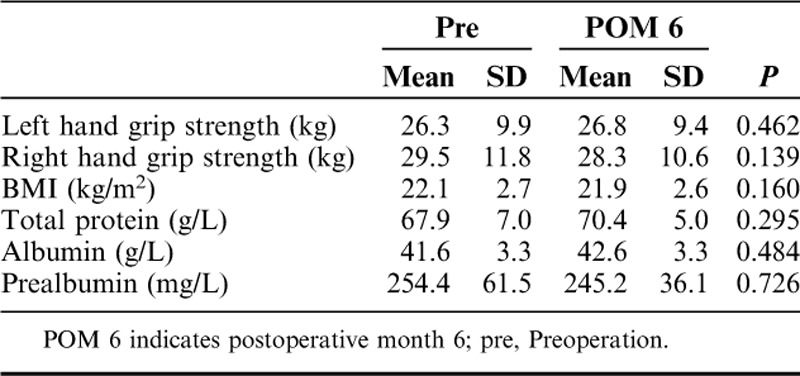
Before the surgery, the mean hand grip strengths were 26.3±9.9 kg on the left and 29.5±11.8 on the right. After 6 months, both hand grip strengths were similar to those before the surgery (Table 2). Compared with preoperative nutritional status, no significant change was reported on postoperative month 6 (POM 6) in the BMI (22.1 to 21.9 kg/m2, P=0.160), total protein (67.9 to 70.4 g/L, P=0.295), albumin (41.6 to 42.6 g/L, P=0.484), and prealbumin (254.4 to 245.2 mg/L, P=0.726), which suggested nutritional status recovered fast after the gastrotomy.
TABLE 2.
Nutritional Status Before and After the Surgery
DISCUSSION
Approximately 60% of GISTs occur in the stomach.1 R0 resection without lymph node dissection is the optimal therapeutic regimen for locally removable gastric GISTs.1,2,17 As we know, laparoscopy, which reduces intraoperative bleeding and accelerates the postoperative recovery safely, has been extensively used to treat gastric GISTs.4–8 However, gastric GISTs in specific sites are difficult to treat. Some scholars who have attempted to apply the robot for gastric GISTs resection have discovered that the robot has some advantages for treating gastric GISTs at specific sites, such as the cardia, pylorus.11,18,19 In this study, we found robotic gastrotomy with intracorporeal suture for patients with GISTs located at cardia and subcardiac region was safe and feasible. In addition, the inflammation of these patients was slight and nutritional status recovered fast postoperatively.
Because lymphatic metastasis is rare in gastric GISTs,9 LWR is often used.6–8,20 However, the simple LWR cannot be used to accurately determine the tumor location and boundary during the surgery. Furthermore, this will lead to excessive gastric wall resection, which may cause postoperative complications such as stenosis when the tumor is located at the cardia.11,21 Consequently, some surgeons have proposed combined use of a laparoscope and gastroscope for gastric GISTs resection,21–23 which allows the determination of the tumor boundary and the preservation of more normal gastric wall intraoperatively. However, Komatsu et al21 found that the combination of the laparoscope with gastroscope is time-consuming, and it was only suitable for small gastric GISTs. In this study, we found that patients who had GISTs located at cardia and subcardiac region had good results in terms of perioperative outcomes. The advantages of the robot lie in its flexible operation arm and high-definition field of view, which make it possible to open the gastric wall for accurate resection based on tumor boundary and complete suture in vivo intraoperatively. On the basis of all of these advantages, we found robotic gastrotomy with intracorporeal suture was safe and feasible for GISTs located at cardia and subcardiac region in this study. Furthermore, we found the inflammation was slight after the surgery, which resulted from mild surgical trauma of robotic surgery.
In this study, the mean tumor diameter was 5.3 cm, and these gastric GISTs were safely and effectively removed by the robot with good short-term and long-term results. Resection of large gastric GISTs remains controversial. Although it was found safe to remove large gastric GISTs with laporoscopy in some studies,24,25 irreparable outcomes may occur in the case of intraoperative tumor breakage or overflow.26 Furthermore, Severino et al27 found that laparoscopic resection of large gastric GISTs should be completed by experienced surgeons; thus, laparoscopy has not been recommended in removing large gastric GISTs by guidelines released by the National Comprehensive Cancer Network and the Asian Expert Consensus.1,9 Studies on robotic gastric GISTs resection were few, and whether the robot is suitable for large gastric GISTs resection need more studies to verify.
In most studies,1,10,28,29 R0 resection should be achieved in gastric GISTs resection and intraoperative tumor breakage or tumor cell overflow should not occur. As verified in a recent study, opening the gastric wall for tumor resection will not cause recurrence induced by tumor cell overflow30; meanwhile, another study on gastric cancer confirmed that using the robot for intracorporeal anastomosis was safe and feasible.15 These provided strong evidence for opening the gastric wall for tumor resection with robot and performing digestive tract suture in vivo. In this study, we found that it is convenient and less time-consuming to use intracorporeal suture with robot. The advantages of robotic suture in vivo were also mentioned in some case reports.31,32
The incidence of complications after gastric GISTs resection is ∼1.8% to 22.7%22,27,30,33; these complications mainly include pulmonary infection, gastrointestinal bleeding, anastomotic stenosis, gastro-esophageal reflux, anastomotic fistula, and delayed gastric emptying. In this study, no postoperative pulmonary infection was observed, which may be related to no placement of nasogastric tube during the perioperative period. In addition, no anastomotic stenosis and gastro-esophageal reflux was observed. We think it was related to the use of gastrotomy with intracorporeal suture, which avoided excessive gastric wall resection.
Hu et al34 recently published a multicenter study, the result showed that the 5-year survival rates for patients undergoing laparoscopic and open gastric GISTs resection were 87.3% and 77.8%, respectively, which was similar to that of a European multicenter study.25 As we know, the factors that influence the prognosis mainly include tumor size and mitotic index. That is to say the postoperative recurrence rate remains high for high-risk patients, even their tumors have been completely removed.35 Therefore, though the mean follow-up period was 25.5 months and we did not find recurrence and metastasis in this study, whether the robot is beneficial to long-term survival of gastric GISTs requires further study. In addition, evaluation of postoperative recurrence risk is crucial for the prognosis and subsequent imatinib adjuvant treatment.
This study has 2 limitations. First, the sample size is small and only one patient was at high risk. Although the good results were found in this study, the conclusion need further verification in the future. We are going to conduct a randomized controlled trial on robotic gastrotomy with intracorporeal suture for GISTs located at cardia. Second, data collection of this study was imperfect, and some data of the body composition lost. We had intended to evaluate the nutritional status with the body composition. However, only BMI, total protein, albumin, and prealbumin could be used to evaluate the nutritional status and changes within six months after the surgery were not be recorded.
In conclusion, robotic gastrotomy with intracorporeal suture for patients with GISTs located at cardia and subcardiac region is safe and feasible. Postoperative inflammation of these patients may be slight and nutritional status may recover fast after the surgery.
Footnotes
Supported by the National Natural Science Foundation of China (Grant number: 81500417) and the Social Development Fund of Jiangsu Province, China (Grant number: BE2015687).
The authors declare no conflicts of interest.
REFERENCES
- 1.Koo DH, Ryu MH, Kim KM, et al. Asian consensus guidelines for the diagnosis and management of gastrointestinal stromal tumor. Cancer Res Treat. 2016;48:1155–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Casali PG, Blay JY. Experts ECECPo. Gastrointestinal stromal tumours: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21(suppl 5):v98–v102. [DOI] [PubMed] [Google Scholar]
- 3.Demetri GD, Benjamin RS, Blanke CD, et al. NCCN Task Force report: management of patients with gastrointestinal stromal tumor (GIST)—update of the NCCN clinical practice guidelines. J Natl Compr Canc Netw. 2007;5(suppl 2):S1–S29. Quiz S30. [PubMed] [Google Scholar]
- 4.Koh YX, Chok AY, Zheng HL, et al. A systematic review and meta-analysis comparing laparoscopic versus open gastric resections for gastrointestinal stromal tumors of the stomach. Ann Surg Oncol. 2013;20:3549–3560. [DOI] [PubMed] [Google Scholar]
- 5.De Vogelaere K, Hoorens A, Haentjens P, et al. Laparoscopic versus open resection of gastrointestinal stromal tumors of the stomach. Surg Endosc. 2013;27:1546–1554. [DOI] [PubMed] [Google Scholar]
- 6.Goh BK, Goh YC, Eng AK, et al. Outcome after laparoscopic versus open wedge resection for suspected gastric gastrointestinal stromal tumors: a matched-pair case-control study. Eur J Surg Oncol. 2015;41:905–910. [DOI] [PubMed] [Google Scholar]
- 7.Karakousis GC, Singer S, Zheng J, et al. Laparoscopic versus open gastric resections for primary gastrointestinal stromal tumors (GISTs): a size-matched comparison. Ann Surg Oncol. 2011;18:1599–1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Novitsky YW, Kercher KW, Sing RF, et al. Long-term outcomes of laparoscopic resection of gastric gastrointestinal stromal tumors. Ann Surg. 2006;243:738–745; discussion 745–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Demetri GD, von Mehren M, Antonescu CR, et al. NCCN Task Force report: update on the management of patients with gastrointestinal stromal tumors. J Natl Compr Canc Netw. 2010;8(suppl 2):S1–S41. Quiz S42–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nishimura J, Nakajima K, Omori T, et al. Surgical strategy for gastric gastrointestinal stromal tumors: laparoscopic vs. open resection. Surg Endosc. 2007;21:875–878. [DOI] [PubMed] [Google Scholar]
- 11.Vicente E, Quijano Y, Ielpo B, et al. Robot-assisted resection of gastrointestinal stromal tumors (GIST): a single center case series and literature review. Int J Med Robot. 2016;12:718–723. [DOI] [PubMed] [Google Scholar]
- 12.Giulianotti PC, Coratti A, Angelini M, et al. Robotics in general surgery: personal experience in a large community hospital. Arch Surg. 2003;138:777–784. [DOI] [PubMed] [Google Scholar]
- 13.Diana M, Marescaux J. Robotic surgery. Br J Surg. 2015;102:e15–e28. [DOI] [PubMed] [Google Scholar]
- 14.Lanfranco AR, Castellanos AE, Desai JP, et al. Robotic surgery: a current perspective. Ann Surg. 2004;239:14–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang G, Jiang Z, Zhao J, et al. Assessing the safety and efficacy of full robotic gastrectomy with intracorporeal robot-sewn anastomosis for gastric cancer: a randomized clinical trial. J Surg Oncol. 2016;113:397–404. [DOI] [PubMed] [Google Scholar]
- 16.Fletcher CD, Berman JJ, Corless C, et al. Diagnosis of gastrointestinal stromal tumors: a consensus approach. Hum Pathol. 2002;33:459–465. [DOI] [PubMed] [Google Scholar]
- 17.Matthews BD, Walsh RM, Kercher KW, et al. Laparoscopic vs open resection of gastric stromal tumors. Surg Endosc. 2002;16:803–807. [DOI] [PubMed] [Google Scholar]
- 18.Buchs NC, Bucher P, Pugin F, et al. Robot-assisted oncologic resection for large gastric gastrointestinal stromal tumor: a preliminary case series. J Laparoendosc Adv Surg Tech A. 2010;20:411–415. [DOI] [PubMed] [Google Scholar]
- 19.Desiderio J, Trastulli S, Cirocchi R, et al. Robotic gastric resection of large gastrointestinal stromal tumors. Int J Surg. 2013;11:191–196. [DOI] [PubMed] [Google Scholar]
- 20.Choi SM, Kim MC, Jung GJ, et al. Laparoscopic wedge resection for gastric GIST: long-term follow-up results. Eur J Surg Oncol. 2007;33:444–447. [DOI] [PubMed] [Google Scholar]
- 21.Komatsu S, Ichikawa D, Kosuga T, et al. Clinical impact of laparoscopy and endoscopy cooperative surgery (LECS) on gastric submucosal tumor after its standardization. Anticancer Res. 2016;36:3041–3047. [PubMed] [Google Scholar]
- 22.Balde AI, Chen T, Hu Y, et al. Safety analysis of laparoscopic endoscopic cooperative surgery versus endoscopic submucosal dissection for selected gastric gastrointestinal stromal tumors: a propensity score-matched study. Surg Endosc. 2017;31:843–851. [DOI] [PubMed] [Google Scholar]
- 23.Hiki N, Yamamoto Y, Fukunaga T, et al. Laparoscopic and endoscopic cooperative surgery for gastrointestinal stromal tumor dissection. Surg Endosc. 2008;22:1729–1735. [DOI] [PubMed] [Google Scholar]
- 24.Khoo CY, Goh BK, Eng AK, et al. Laparoscopic wedge resection for suspected large (>/=5 cm) gastric gastrointestinal stromal tumors. Surg Endosc. 2017;31:2271–2279. [DOI] [PubMed] [Google Scholar]
- 25.Piessen G, Lefevre JH, Cabau M, et al. Laparoscopic versus open surgery for gastric gastrointestinal stromal tumors: what is the impact on postoperative outcome and oncologic results? Ann Surg. 2015;262:831–839; discussion 829–840. [DOI] [PubMed] [Google Scholar]
- 26.Hohenberger P, Ronellenfitsch U, Oladeji O, et al. Pattern of recurrence in patients with ruptured primary gastrointestinal stromal tumour. Br J Surg. 2010;97:1854–1859. [DOI] [PubMed] [Google Scholar]
- 27.Severino BU, Fuks D, Lainas P, et al. Large gastrointestinal stromal tumours of the stomach: Is laparoscopy reasonable? J Minim Access Surg. 2016;12:148–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Joensuu H. Gastrointestinal stromal tumor (GIST). Ann Oncol. 2006;17 (suppl 10):x280–x286. [DOI] [PubMed] [Google Scholar]
- 29.Kang YK, Kang HJ, Kim KM, et al. Clinical practice guideline for accurate diagnosis and effective treatment of gastrointestinal stromal tumor in Korea. Cancer Res Treat. 2012;44:85–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee S, Kim YN, Son T, et al. Oncologic safety of laparoscopic wedge resection with gastrotomy for gastric gastrointestinal stromal tumor: comparison with conventional laparoscopic wedge resection. J Gastric Cancer. 2015;15:231–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moriyama H, Ishikawa N, Kawaguchi M, et al. Robot-assisted laparoscopic resection for gastric gastrointestinal stromal tumor. Surg Laparosc Endosc Percutan Tech. 2012;22:e155–e156. [DOI] [PubMed] [Google Scholar]
- 32.Ortiz-Oshiro E, Exposito PB, Sierra JM, et al. Laparoscopic and robotic distal gastrectomy for gastrointestinal stromal tumour: case report. Int J Med Robot. 2012;8:491–495. [DOI] [PubMed] [Google Scholar]
- 33.Wan P, Yan C, Li C, et al. Choices of surgical approaches for gastrointestinal stromal tumors of the stomach: laparoscopic versus open resection. Dig Surg. 2012;29:243–250. [DOI] [PubMed] [Google Scholar]
- 34.Hu J, Or BH, Hu K, et al. Comparison of the post-operative outcomes and survival of laparoscopic versus open resections for gastric gastrointestinal stromal tumors: a multi-center prospective cohort study. Int J Surg. 2016;33 (pt A):65–71. [DOI] [PubMed] [Google Scholar]
- 35.Tokumaru T, Okabayashi T, Shima Y, et al. Surgical management in patients with gastrointestinal stromal tumors: a single-center experience. Oncology. 2016;90:273–279. [DOI] [PubMed] [Google Scholar]


