Abstract
Through retrospective review of consecutive charts, we compare the short-term and long-term clinical outcomes after robotic-assisted right colectomy with intracorporeal anastomosis (RIA) (n=89) and laparoscopic right colectomy with extracorporeal anastomosis (LEA) (n=135). Cohorts were similar in demographic characteristics, comorbidities, pathology, and perioperative outcomes (conversion, days to flatus and bowel movement, and length of hospitalization). The RIA cohort experienced statistically significant: less blood loss, shorter incision lengths, and longer specimen lengths than the LEA cohort. Operative times were significantly longer for the RIA group. No incisional hernias occurred in the RIA group, whereas the LEA group had 5 incisional hernias; mean follow-up was 33 and 30 months, respectively. RIA is effective and safe and provides some clinical advantages. Future studies may show that, in obese and other technically challenging patients, RIA facilitates resection of a longer, consistent specimen with less mesentery trauma that can be extracted through smaller incisions.
Key Words: robotics, laparoscopy, colorectal surgery, minimally invasive surgery, intracorporeal anastomosis, extracorporeal anastomosis
Laparoscopic right colectomy is associated with earlier return to normal bowel function, shorter length of hospital stay, fewer wound complications, and similar oncological outcomes as can be achieved through the conventional open approach.1 Construction of the ileocolic anastomosis can be accomplished with an extracorporeal or intracorporeal technique. However, the intracorporeal anastomosis (IA) has not yet become the standard of care for minimally invasive surgery, primarily because it is more technically challenging. Consequently, extracorporeal anastomosis (EA) continues to be more widely adopted.2 Nevertheless, there is an increasing body of literature describing the benefits of IA in right hemicolectomy, namely the ability to mobilize the bowel under vision without extending traction on the mesentery.3,4 Potential intraoperative benefits of the IA technique include less mobilization—especially in patients with high body mass index, reduced manipulation of bowel, smaller incisions, and freedom to choose the extraction site.4–7 Whereas the EA extraction incision is midline, the IA extraction incision can be placed off the midline in a transverse, paramedian, or oblique position and potentially lower the risk of incisional hernia.4,8,9 Postoperative benefits include reduced narcotic use, faster time to flatus, shorter length of hospital stay, and reduced hernia rates.4,5,9,10
Robotic-assisted right colectomy with intracorporeal anastomosis (RIA) (da Vinci Surgical System™; Intuitive Surgical Inc., Sunnyvale, CA) is a relatively new means to overcome some of the technical challenges of the laparoscopic approach, and early studies have reported its feasibility and safety, improved early postoperative outcomes, and similar rates of anastomotic leak.10,11 In a meta-analysis of trials comparing robotic right colectomy with laparoscopic right colectomy—both with intracorporeal and extracorporeal anastomotic techniques—Pettruciani et al12 reported the feasibility and safety of robotic right colectomy but the lack of significant differences in short-term outcomes, complications, or conversion rates.
The primary objective of the current study is to compare retrospectively the short-term clinical outcomes after RIA and laparoscopic right colectomy with extracorporeal anastomosis (LEA). The secondary objective is to compare the incidence of hernia at long-term follow-up (>1 y) based on the location of the extraction site incision.
MATERIALS AND METHODS
This was a retrospective comparative chart review of deidentified operative and postoperative data that were collected prospectively and in parallel from all consecutive and eligible patients, who underwent elective right colectomy with either the RIA or the LEA approach from January 1, 2009 to March 14, 2015. The surgical techniques for both the LEA and the RIA approaches have been described in detail.8,13
Briefly, for the LEA approach, a total of 4 ports were placed: one 12-mm umbilical and three 5-mm ports for the trocars. The right colon was completely mobilized and an Echelon linear cutter was used to transect the ileocolic pedicle and the terminal ileum intracorporeally. Securing the specimen with a grasper, the surgeon extended the umbilical incision laterally to form the extraction site. The specimen was delivered and transected with a linear cutter, and an extracorporeal functional end-to-end anastomosis was constructed using the linear stapler. The common enterotomy was closed with a reloadable linear stapler, and the extraction site was closed in 1 layer in the normal manner.
For the RIA approach, both the da Vinci Si and Xi Systems were used. With the Si system, the majority of procedures were performed with 3 robot arms; the exceptions were the selective use of 4 arms for obese patients. For the Xi system, 4 arms of the da Vinci robot were consistently used. The lesion was localized using a 5-mm laparoscope before docking the robot. After positioning the operating table to allow the small intestine to fall away from the midline and allow optimal visualization of the ileocolic pedicle, the robot was docked from the patient’s right side. The robotic camera was inserted through an 8- or 8.5- mm periumbilical port (depending on the da Vinci System used), and the assistant introduced the laparoscopic instruments through the lateral 12-mm port. After complete intracorporeal resection of the specimen using a medial-to-lateral dissection, the terminal ileum and transverse colon were placed side-by-side in isoperistaltic manner for the IA. A stay suture was placed in the terminal ileum and transverse colon and then placed through the abdominal wall to provide tension, lift, and alignment of the 2 limbs of the bowel. Hot shears were used to create the colotomy and ileotomy, through which the jaws of the endoscopic linear stapler were introduced to form a common channel. The remaining common enterotomy was closed in 2 layers using robotic suturing techniques. The stay suture was cut and a toothed grasper introduced through the 12-mm left lateral port to secure the specimen. The robot was then undocked, and the incision was enlarged and the specimen extracted. The abdominal wall was closed in 2 layers and port incisions were closed in the usual manner.
We collected demographic and clinical perioperative surgical information from each patient’s chart as well as hernia incidence through postoperative follow-up; any patient who was followed beyond 12 months or who reported the development of an incisional hernia before 12 months was included. Half of the patients in each group (RIA, 51.9%; LEA, 49.4%) were available for follow-up at or beyond 12 months after surgery. These patients provided information regarding long-term incidence of incisional hernia. All personal identifiers were stripped from the archival data (hospital and/or office charts); thus, there was no active patient recruitment. When possible, the principal study surgeon collected information on perioperative dates including surgery dates, discharge dates, and readmission dates; the Central IRB (WIRB) approved the data collection. Cases were reviewed for eligibility, including the evaluation of available clinical findings preoperatively and postoperatively in the outpatient setting. All patients signed informed consent for their prospective follow-up data collection, and a study-specific informed consent waiver was obtained for any retrospective data collection. The participating surgeons were board-certified colon and rectal surgeons.
Consecutive cases were eligible from those patients who were above 18 years of age and who underwent full robotic or hybrid robotic right colectomy on an elective basis with IA and from those patients who underwent elective traditional LEA. Patients were excluded from both cohorts if they had perforated, obstructing, or locally invasive neoplasms; if they had emergency procedures; or if they had right colectomies associated with other major surgical procedures, such as hepatectomy or other intestinal resections. Conversions for IA were defined by the use of the extraction site for any portion of the dissection other than for the extraction of the specimen.8 Conversions in the EA procedures were characterized by lengthening the planned extraction site incision to complete the procedure.
Standard univariate and multivariate techniques were used to describe the study endpoints. Methods appropriate for rates and proportions as well as means, SDs, and confidence intervals for continuous variables were also used. Analyses were based only on available data with missing data excluded. The 2 cohorts were compared using standard statistical tests appropriate for data types. All continuous variables were compared between the robotic and laparoscopic cohorts using Student t test, whereas discrete variables were compared using χ2 test. A P-value of <0.05 was considered statistically significant. All analyses were performed in SAS 9.4 (SAS Institute Inc., Cary, NC).
RESULTS
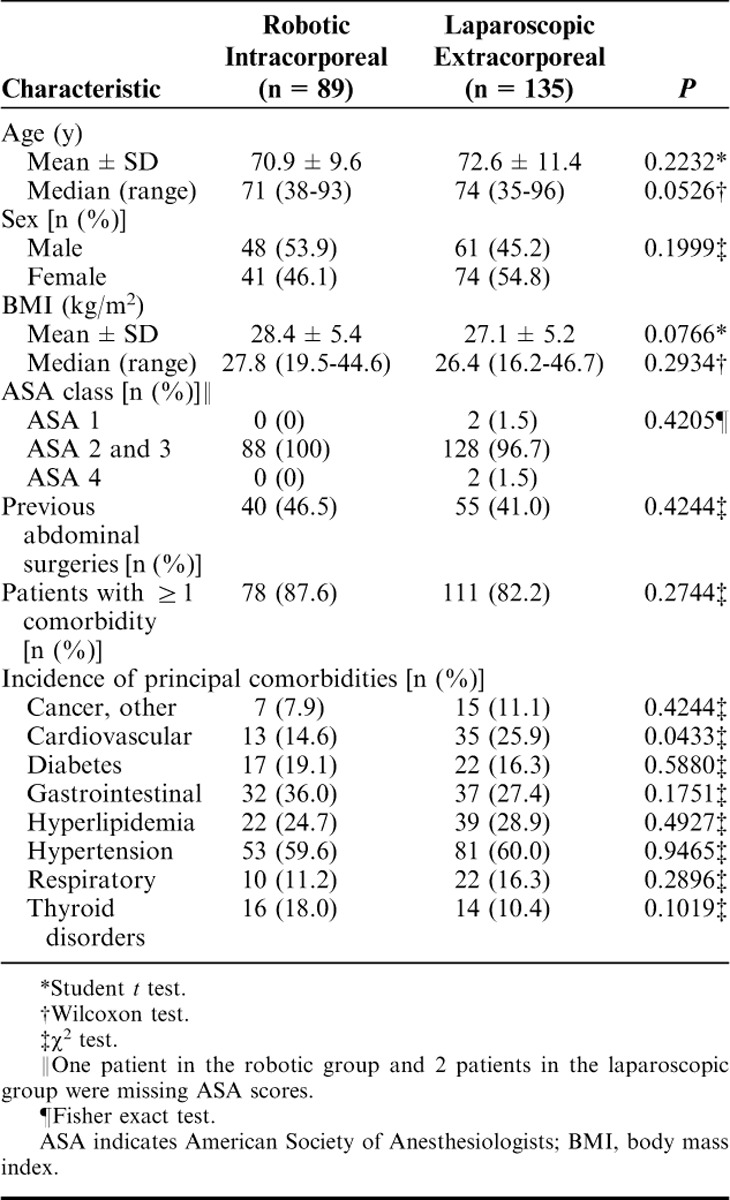
A total study population of 224 (elective RIA, n=89; elective LEA, n=135) consecutive patients was reviewed. Patient characteristics, including demographics and comorbidities, were statistically balanced and are presented in Table 1. Most patients in both cohorts presented with at least 1 comorbidity, and nearly half in each group had previous abdominal surgery. All of the patients in the RIA cohort were classified as ASA 2 and 3, and all but 4 patients in the LEA cohort were likewise classified as ASA 2 and 3. Twelve patients (13.5%) in the RIA group and 23 (17.0%) in the LEA group had concomitant procedures including, umbilical hernia repair, ventral hernia repair, cholecystectomy, and colonoscopy.
TABLE 1.
Patient Demographics and Comorbidities
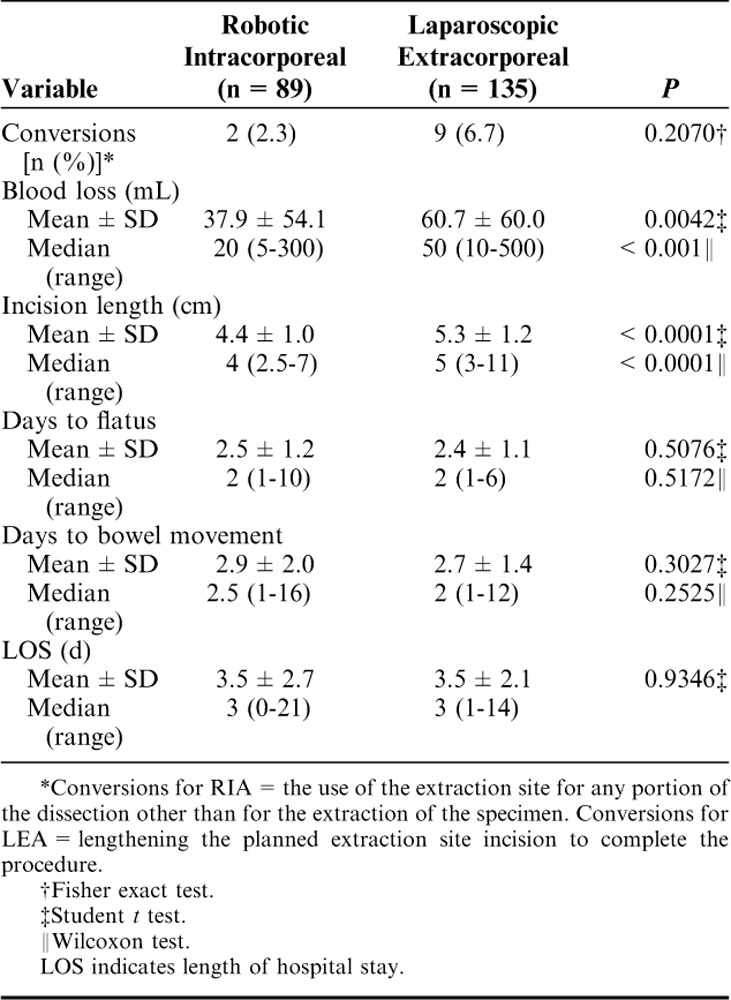
Few cases required conversion; although the LEA group was associated with a higher conversion rate (6.7%, n=9) than occurred in the RIA group (2.3%, n=2) (Table 2). Incision size was significantly smaller for the RIA group [median=4 cm (range, 2.5 to 7 cm)] than for the LEA group [median=5 cm (range, 3 to 11 cm)] (P<0.0001). Also, significantly less blood loss occurred in the RIA group [median=20 mL (range, 5 to 300 mL)] than in the LEA group [median=50 mL (range, 10 to 500 mL] (P<0.001). Days to flatus and to bowel movement as well as hospital length of stay were similar between groups. In addition, mean operative time (skin-to-skin time) was significantly longer for the RIA group than for the LEA group: 190.2±40.7 versus 98.8±44.3 minutes, respectively (P<0.0001).
TABLE 2.
Intraoperative and Perioperative Outcomes
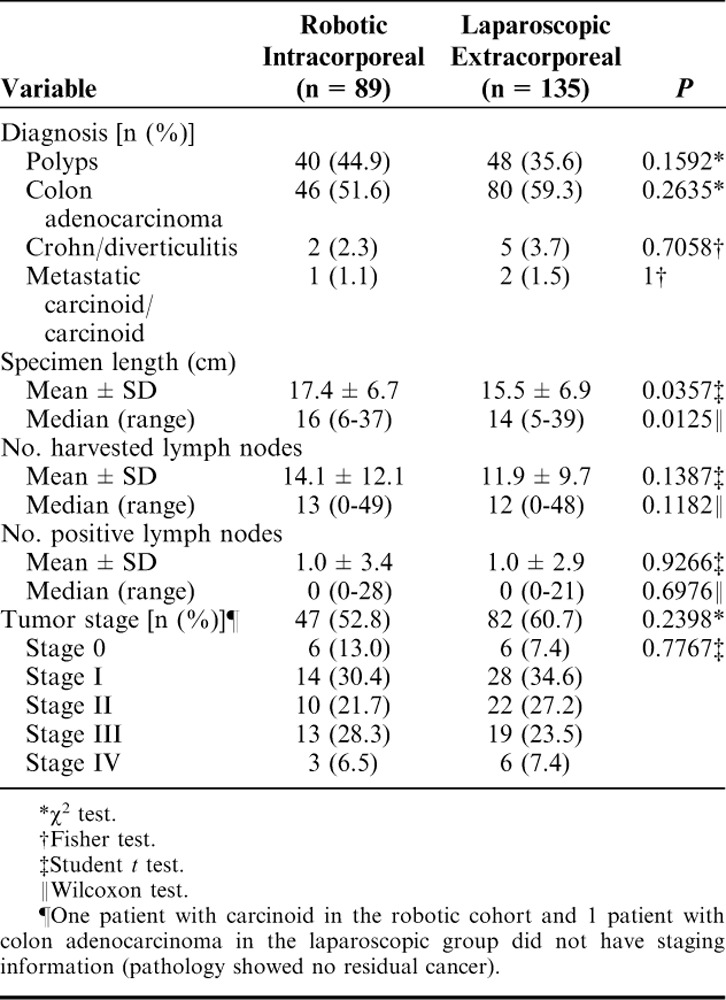
The primary diagnoses based on pathologic analysis were adenomatous polyps and colon adenocarcinoma (Table 3). Both mean and median extracted specimen lengths were statistically greater for those patients in the RIA group versus for those in the LEA group: 17.4±6.7 cm versus 15.5±6.9 cm (P=0.0357) and 16 cm (range, 6 to 37 cm) versus 14 cm (range, 5 to 39 cm) (P=0.0125). In addition, the RIA group was associated with a greater number of harvested lymph nodes, although the difference between the 2 groups was not significant. Tumor staging also was similar between the 2 groups.
TABLE 3.
Pathology Details
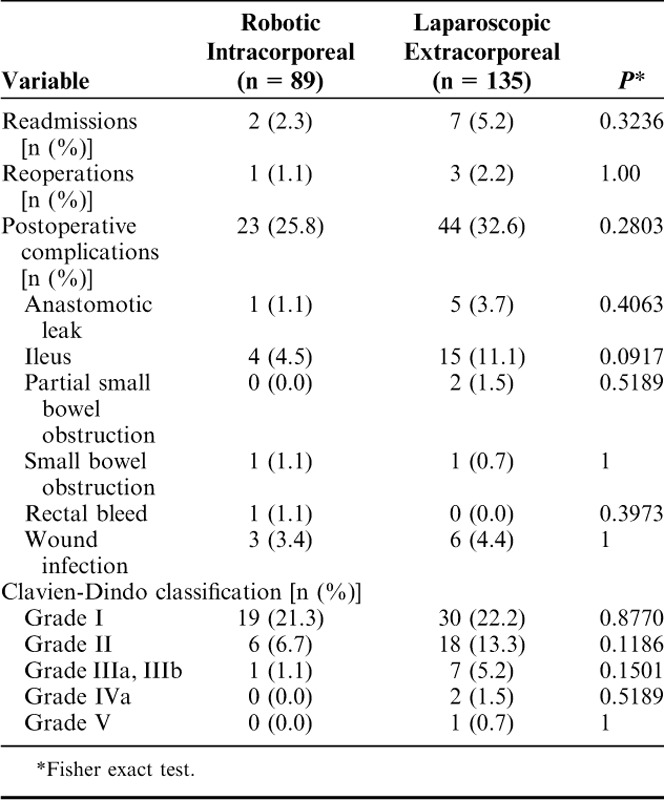
Although rates of readmissions, reoperations, and major postoperative complications (Clavien-Dindo morbidity grade>II) within 30 days of the procedures were not statistically significant between the cohorts, they trended to be less for the RIA group than for the LEA group (Table 4). There were 2 readmissions in the RIA group: 1 for partial small bowel obstruction (1.1%) and 1 for anastomotic leak (1.1%). The patient with the partial small bowel obstruction was successfully treated medically, and the patient with the anastomotic leak underwent reoperation with ileostomy and revision of the anastomosis followed by ileostomy closure 3 months later. In the LEA group, there were 7 readmissions and 3 reoperations. Of the 7 readmissions, 4 patients were observed and treated medically and 3 underwent surgery for small bowel obstruction with anastomotic leak and abscess (n=1), extraction site hernia repair (n=1), and anastomotic leak with colocutaneous fistula and resultant re-resection of the anastomosis (n=1).
TABLE 4.
Readmissions, Reoperations, and Postoperative Complications With 30 Days of the Procedure
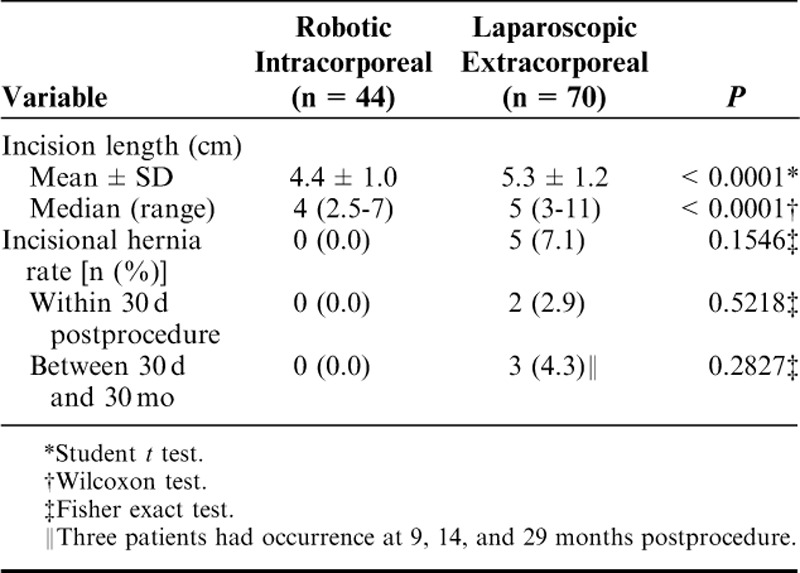
Rates of incisional hernia for the 2 groups are presented in Table 5. There were no reports of incisional hernias within the RIA group of patients, who were followed to a mean of 33 months. Five patients in the LEA group had incisional hernias: 2 developed within 30 days of their procedures and 3 patients developed incisional hernias at a mean of 30 months for an overall rate of 7.1%. The follow-up rate at or beyond 12 months after surgery was 51.9% (70/135) in the RIA group and 49.4% (44/89) in the LEA group.
TABLE 5.
Incision Length and Incisional Hernia Rate
There were 4 deaths in the LEA group; 3 occurred within 30 days of surgery (one 80-y-old male died within 2 d of his surgery during postoperative recovery due to complications from an anastomotic leak, one 96-y-old female died within 14 d of her surgery and her cause of death was believed to be age-related, and one 75-y-old male with severe comorbidities died 28 d after surgery). The fourth death was of a 70-y-old male who died at 5 to 6 months after surgery due to disease recurrence. There were no deaths in the RIA group.
DISCUSSION
Most surgeons performing laparoscopic right colectomy use the EA approach. In comparison, IA is considered more technically challenging and difficult in terms of laparoscopic suturing.14 However, robotic-assisted IA during right colectomy is gaining interest among experienced colorectal surgeons, several of whom have reported their experiences.10,11,13,15,16 This renewed interest in IA seems to correlate with increased use of robotics in colorectal resections. We believe that the dexterity and precision provided by robotic instrumentation that allows 7 df, motion scaling and tremor reduction as well as the surgeon-controlled 3D high-definition visualization have facilitated the adoption of more complex tasks like IA. Thus, we were interested in reviewing our data to determine if there were benefits to RIA compared with LEA.
Our study involved the collection of intraoperative, near-term postoperative, and long-term data from January 2009 to March 2015. The study populations were similar in demographic characteristics, incidences of comorbidities, pathology, and early postoperative results (days to flatus and to bowel movement and length of hospitalization). Statistically significant advantages of RIA were: less blood loss, shorter incision lengths, and longer specimen lengths. Operative times were significantly longer in the robotic cohort; however, our operative times were within the mean range reported in the literature (189 to 270 min).8,17–21 The robotic technique is relatively new and continuously undergoes rapid advancements and refinements as the robotic technology changes (Si to Xi, vessel sealer, Endowrist stapler, etc.) These advancements will likely impact efficiencies and operative times.
Longer specimen lengths can be achieved due to the totally intracorporeal resection of the specimen. Longer specimen length likely accounts for the higher lymph node yield in the RIA group compared with that achieved in the LEA group, although the difference in yield did not reach statistical significance due to limited patient sample size. We feel that a more consistent and standardized resection can be realized with the RIA technique. For example, proximal and distal margins are clearly visualized and do not depend on extent of dissection or mobilization, abdominal wall thickness, or the lack of adequate exposure through a small extraction site as is true with the EA technique.
Smaller extraction site incisions can be achieved with IA than can be achieved with EA, as has been reported in the literature.8,22 Our study results reflect smaller incisions with RIA and no incisional hernias with 30 months of follow-up. The ability to choose between extraction sites is possible with RIA and provides the benefit of keeping the extraction site off the midline. Most extraction sites for the LEA group were midline and incorporated the umbilicus. Samia et al9 reported lower hernia rates with off-midline incisions as well as fewer wound complications.
The RIA group also trended toward lower rates of conversion (2.25% vs. 6.7%) despite both study groups having similar rates of previous abdominal surgeries (46.5% vs. 41.0%, respectively), and the RIA group had a higher percentage of patients with body mass index >30 kg/m2 (33.7% vs. 23.7%). Our low conversion rate was similar to that reported by D’Annibale et al17 in a group of patients who underwent RIA for malignant disease (n=50, conversion=0.0%). In our robotic group, the 2 cases were converted to EA. Trastulli et al,23 in their recent systematic review and meta-analysis, analyzed the literature describing intraoperative and postoperative results after robotic and laparoscopic right colectomy: the weighted rate of conversion to open surgery was lower (although it did not reach statistical significance) for the robotic cases (4.3%) than for the laparoscopic patients (7.1%).
The robotic group experienced lower rates of readmissions, postoperative complications, and incisional hernias than the LEA group—although these differences were not statistically significant. The tendency toward lower rates of postoperative complications and mortality indicates the safety and efficacy of the robotic-assisted procedure.
The limitations of our study are its retrospective design and the comparison of groups that were dissimilar in anastomotic technique (EA vs. IA) and in minimally invasive approach (laparoscopic vs. robotic). An ideal retrospective chart review would compare laparoscopic IA with robotic IA. In our experience, surgeon comfort with intracorporeal anastomosis was achieved only with the robotic approach; however, we did not systematically evaluate surgeon benefits and ergonomics. If future prospective controlled comparative studies confirm the advantages of IA, the role of robotic right colectomy may gain importance.
Strengths of the study were the use of a standardized technique for all of the robotic-assisted IA surgeries, a standardized technique for all of the laparoscopic EA surgeries, and the standardized prospective collection and rigorous analysis of extensive data on large groups of consecutive cases. Both patient cohorts were similar in demographics, and our results showed for the most part statistical equivalence between RIA and LEA. Results were similar to those found in systematic reviews and meta-analyses.23–26
LEA provides acceptable results with few complications. In our experience, robotic right colectomy with IA provided similar efficacy and safety with some key clinical advantages—but the procedure is longer. Future studies may show that, in obese and other technically challenging patients, RIA facilitates resection of a longer, consistent specimen with less mesentery trauma that can be extracted through smaller incisions. Furthermore, the anastomosis can be created in a more reliable, reproducible, and easier manner. The advantages inherent to the robot, such as better ergonomics, surgical dexterity, and improved stable 3D high-definition visualization, may make this possible.
ACKNOWLEDGMENTS
The authors thank Manuel Viamonte III, MD and Rene F. Hartmann, MD for their contributions in reviewing the manuscript drafts. The authors also thank Wainwright Medical Communications (Los Gatos, CA) and Monica Shah, MD (Intuitive Surgical, Clinical Affairs) for editorial support.
Footnotes
H.J.L. received a research grant from Intuitive Surgical (Sunnyvale, CA). D.H. received support from a grant from the Foundation for Surgical Fellowships. The other authors declare no conflicts of interest.
REFERENCES
- 1.Rondelli FM, Trastulli S, Avenia N, et al. Is laparoscopic right colectomy more effective than open resection? A meta-analysis of randomized and nonrandomized studies. Colorectal Dis. 2012;14:e447–e469. [DOI] [PubMed] [Google Scholar]
- 2.Jamali FR, Soweid AM, Dimassi H, et al. Evaluating the degree of difficulty of laparoscopic colorectal surgery. Arch Surg. 2008;143:762–767. [DOI] [PubMed] [Google Scholar]
- 3.Milone M, Elmore U, Di Salvo E, et al. Intracorporeal versus extracorporeal anastomosis. Results for a multicenter comparative study on 512 right-sided colorectal cancers. Surg Endosc. 2015;29:2314–2320. [DOI] [PubMed] [Google Scholar]
- 4.Shapiro R, Keler U, Segev L, et al. Laparoscopic right hemicolectomy with intracorporeal anastomosis: short- and long-term benefits in comparison with extracorporeal anastomosis. Surg Endosc. 2015;30:3823–3829. [DOI] [PubMed] [Google Scholar]
- 5.Grams J, Tong W, Greenstein AJ, et al. Comparison of intracorporeal versus extracorporeal anastomosis in laparoscopic-assisted hemicolectomy. Surg Endosc. 2010;24:1886–1891. [DOI] [PubMed] [Google Scholar]
- 6.Hellan M, Anderson C, Pigazzi A. Extracorporeal versus intracorporeal anastomosis for laparoscopic right hemicolectomy. JSLS. 2009;13:312–317. [PMC free article] [PubMed] [Google Scholar]
- 7.Raftopoulos I, Courcoulas AP, Blumberg D. Should completely intracorporeal anastomosis be considered in obese patients who undergo laparoscopic colectomy for benign or malignant disease of the colon? Surgery. 2006;140:675–682. [DOI] [PubMed] [Google Scholar]
- 8.Lujan HJ, Molano A, Burgos A, et al. Robotic right colectomy with intracorporeal anastomosis: experience with 52 consecutive cases. J Laparoendosc Adv Surg Tech A. 2015;25:117–122. [DOI] [PubMed] [Google Scholar]
- 9.Samia H, Lawrence J, Nobel T, et al. Extraction site location and incisional hernias after laparoscopic colorectal surgery: should we be avoiding the midline? Am J Surg. 2013;205:264–268. [DOI] [PubMed] [Google Scholar]
- 10.Trastulli S, Coratti A, Guarino S, et al. Robotic right colectomy with intracorporeal anastomosis compared with laparoscopic right colectomy with extracorporeal and intracorporeal anastomosis: a retrospective multicentre study. Surg Endosc. 2015;29:1512–1521. [DOI] [PubMed] [Google Scholar]
- 11.Morpurgo E, Contardo T, Molaro R, et al. Robotic-assisted intracorporeal anastomosis versus extracorporeal anastomosis in laparoscopic right hemicolectomy for cancer: a case control study. J Laparoendosc Adv Surg Tech A. 2013;23:414–417. [DOI] [PubMed] [Google Scholar]
- 12.Pettruciani N, Sirimarco D, Nigri GR, et al. Robotic right colectomy: a worthwhile procedure? Results of a meta-analysis of trials comparing robotic versus laparoscopic right colectomy. J Min Access Surg. 2015;11:22–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lujan HJ, Maciel VH, Romero R, et al. Laparoscopic versus robotic right colectomy: a single surgeon’s experience. J Robotic Surg. 2013;7:95–102. [DOI] [PubMed] [Google Scholar]
- 14.Vignali A, Bissolati M, De Nardi P, et al. Extracorporeal vs. intracorporeal ileocolic stapled anastomoses in laparoscopic right colectomy: an interim analysis of a randomized clinical trial. J Laparoendosc Adv Surg Tech. 2016;26:1–6. [DOI] [PubMed] [Google Scholar]
- 15.Huettner F, Pacheco PE, Doubet JL, et al. One hundred and two consecutive robotic-assisted minimally invasive colectomies—an outcome and technical update. J Gastrointest Surg. 2011;15:1195–1204. [DOI] [PubMed] [Google Scholar]
- 16.Fung AK, Aly EH. Robotic colonic surgery: is it advisable to commence a new learning curve? Dis Colon Rectum. 2013;56:786–796. [DOI] [PubMed] [Google Scholar]
- 17.D’Annibale A, Pernazza G, Morpurgo E, et al. Robotic right colon resection: evaluation of first 50 consecutive cases for malignant disease. Ann Surg Oncol. 2010;17:2856–2862. [DOI] [PubMed] [Google Scholar]
- 18.Rawlings AL, Woodland JH, Vegunta RK, et al. Robotic versus laparoscopic colectomy. Surg Endosc. 2007;21:1701–1708. [DOI] [PubMed] [Google Scholar]
- 19.Antoniou SA, Antoniou GA, Kock OO, et al. Robot-assisted laparoscopic surgery of the colon and rectum. Surg Endosc. 2012;26:1–11. [DOI] [PubMed] [Google Scholar]
- 20.Buchs NC, Pugin F, Bucher P, et al. Totally robotic right colectomy: a preliminary case series and an overview of the literature. Int J Med Robot. 2011;7:348–352. [DOI] [PubMed] [Google Scholar]
- 21.DeSouza AL, Prasad LM, Park JJ, et al. Robotic assistance in right hemicolectomy: is there a role? Dis Colon Rectum. 2010;53:1000–1006. [DOI] [PubMed] [Google Scholar]
- 22.Hellan M, Stein H, Pigazzi A. Totally robotic low anterior resection with total mesorectal excision and splenic flexure mobilization. Surg Endosc. 2009;23:447–451. [DOI] [PubMed] [Google Scholar]
- 23.Trastulli S, Cirocchi R, Desiderio J, et al. Robotic versus laparoscopic approach in colonic resections for cancer and benign diseases: systematic review and meta-analysis. PLoS One. 2015;10:e0134062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cirocchi R, Trastulli S, Farinella E, et al. Intracorporeal versus extracorporeal anastomosis during laparoscopic right hemicolectomy—systematic review and meta-analysis. Surg Oncol. 2013;22:1–13. [DOI] [PubMed] [Google Scholar]
- 25.Feroci F, Lenzi E, Garzi A, et al. Intracorporeal versus extracorporeal anastomosis after laparoscopic right hemicolectomy for cancer: a systematic review and meta-analysis. Int J Colorectal Dis. 2013;28:1177–1186. [DOI] [PubMed] [Google Scholar]
- 26.Carnuccio P, Jimeno J, Parés D. Laparoscopic right colectomy: a systematic review and meta-analysis of observational studies comparing two types of anastomosis. Tech Coloproctol. 2014;18:5–12. [DOI] [PubMed] [Google Scholar]


