Abstract
It is known that oxidative stress produced by proinflammatory myeloid cells plays an important role in demyelination and neuronal injury in progressive multiple sclerosis (MS). Myeloperoxidase (MPO) is a pro-oxidative enzyme released from myeloid cells during inflammation. It has been shown that MPO-dependent oxidative stress plays important roles in inducing tissue injury in many inflammatory diseases. In this report, we treated NOD experimental autoimmune encephalomyelitis (EAE) mice, a murine model of progressive MS, with N-acetyl lysyltyrosylcysteine amide (KYC), a novel specific MPO inhibitor. Our data showed that KYC treatment not only attenuated MPO-mediated oxidative stress but also reduced demyelination and axonal injury in NOD EAE mice. More importantly, we found that KYC treatment increased oligodendrocyte regeneration and neurogenesis in NOD EAE mice. Taken together, our data suggests that targeting MPO should be a good therapeutic approach for reducing oxidative injury and preserving neuronal function in progressive MS patients.
Keywords: experimental autoimmune encephalomyelitis, multiple sclerosis, myeloperoxidase, neurogenesis, oligodendrocyte regeneration, oxidative stress
Introduction
Multiple sclerosis (MS) is a neuroinflammatory autoimmune disease. In MS, inflammation, demyelination, and axonal damage in both brain and spinal cord impair physical and cognitive abilities, eventually resulting in death of patients 1. Accumulated evidence strongly supports the idea that oxidative stress plays a major role in demyelination and axonal damage 2–4. During the progression of disease, activated microglial cells and macrophages produce a wide variety of oxidants that oxidize lipids, proteins, and DNA to increase tissue injury 5. Recent studies have suggested that microglia/macrophage-dependent oxidative stress is one of the key driving forces to cause demyelination and neurodegeneration in progressive MS 6–8. Despite such progress, few, if any, studies have been designed to determine how oxidative stress increases central nervous system (CNS) injury in progressive MS.
Myeloperoxidase (MPO) is one of the most potent pro-oxidative enzymes from myeloid cells 9,10 and capable of generating a wide variety of oxidants 11 for oxidizing biomolecules and inducing cellular injury 10,12. Over the last 20 years, accumulated evidence has demonstrated that MPO indeed plays an active role in inflammation and demyelination in MS 13–19. Inhibition of MPO activity in experimental autoimmune encephalomyelitis (EAE) models of MS reduced disease scores in mice 19,20, suggesting that MPO plays an important role in MS. We found that N-acetyl lysyltyrosylcysteine amide (KYC), a novel specific inhibitor for MPO, effectively attenuated disease severity in different EAE models when administered both prophylactically and therapeutically 20. KYC markedly reduced MPO protein levels and activity, the number of myeloid cells, and blood–brain barrier leakage in the CNS of EAE mice. Our data suggested that inhibition of MPO could be an effective approach to reduce inflammation and oxidative injury in MS patients.
Previously we found KYC effectively reduced the EAE disease score in a monophasic C57BL/6 model 20. As oxidative stress produced by proinflammatory myeloid cells plays an important role in demyelination and neuronal injury in progressive MS, we treated NOD EAE mice, a model for progressive MS 21, with KYC. We found that KYC treatment not only attenuated MPO-mediated oxidative stress but also reduced demyelination and axonal injury in NOD EAE mice. More importantly, we discovered that KYC treatment increased oligodendrocyte regeneration and neurogenesis in NOD EAE mice. Taken together, we suggest that targeting MPO should be a good therapeutic approach for reducing the oxidative injury and preserving neuronal functions in progressive MS patients.
Materials and methods
Experimental autoimmune encephalomyelitis induction
Female NOD/ShiLtJ mice (8–10 weeks old) were purchased from Jackson Laboratory (Bar Harbor, Maine, USA), and housed in the Medical College of Wisconsin Biomedical Resource Center. All animal procedures were approved by the Institutional Animal Care and Use Committee. EAE was induced by myelin oligodendrocyte glycoprotein as described previously 22. Briefly, mice were anaesthetized with 2% isoflurane, and immunized with 200 µg myelin oligodendrocyte glycoprotein35–55 peptide emulsified in complete Freund’s adjuvant (Chondrex, Redmond, Washington, USA) containing 4 mg/ml Mycobacterium (subcutaneous injection, 50 µl/site, total 200 µl/mouse), followed by intraperitoneal injection of 200 ng pertussis toxin (List Biological Laboratories, Campbell, California, USA) on days 0 and 2.
Experimental autoimmune encephalomyelitis disease severity score
Mice were scored daily as follows: 0, no disease; 1, limp tail; 1.5, hind limb ataxia; 2, hind limb paresis; 2.5, partial hind limb paralysis; 3, total hind limb paralysis; 4, hind and fore limb paralysis; and 5, death. Mice were monitored for neurological disease by daily inspection and mice reaching score 4 were euthanized immediately. Wet food was placed in the cage once mice reached score 3. The duration of the experiment was 70 days 22. A total of 40 mice were used for all experiments, of which only one mouse reached endpoint and was euthanized. No animal was found dead.
Drug administration
Groups of mice were administered intraperitoneally either PBS or KYC (Biomatik, Wilmington, Delaware, USA) 3.0 mg/kg once daily or 15 mg/kg twice daily from day 10 after postimmunization (EAE onset). KYC doses used were on the basis of our previous publication 20.
Immunohistochemistry
EAE mice were perfused with 4% paraformaldehyde on day 70 postimmunization. Spinal cords were removed and fixed in 4% paraformaldehyde overnight, then transferred to 20 and 30% sucrose for 1 day, respectively. Longitudinal spinal cord sections were cut by cryostat (CM1900; Leica, Wetzlar, Germany); 10-μm sections were used for immunohistochemistry. The following protocol was used to determine demyelination, neurofilament, MPO-mediated oxidant production, oligodendrocyte regeneration, and neuroregeneration in the spinal cords of mice. Frozen sections were incubated with 5% goat or donkey serum in 0.01 M PBS for 1 h. The sections were incubated with the following primary antibodies at 4°C overnight: goat anti-myelin basic protein (MBP) (sc-13914, 1 : 50; Santa Cruz, Dallas, Texas, USA), mouse ant-SMI31 (801601, 1 : 1000; Biolegend Inc., San Diego, California, USA), rabbit anti-chlorotyrosine (ClTyr) (HP5002, 1 : 50; Hycult Biotech, Plymouth Meeting, Pennsylvania, USA), mouse anti-MPO (HM1051, 1 : 50; Hycult Biotech), rabbit anti-NG2 (sc-20162, 1 : 50; Santa Cruz), mouse anti-O4 (MAB3450, 1 : 500; EMD Millipore, Billerica, Massachusetts, USA), mouse anti-CNPase (sc-166063, 1 : 50; Santa Cruz). The sections were rinsed and incubated with goat or donkey corresponding secondary antibodies conjugated with Alexa Fluor 488 or 568 (1 : 200) for 1 h at room temperature. Finally, the sections were counterstained with 4′,6-diamidino-2-phenylindole to visualize cell nuclei. Comparable spinal cord sections from the thoracic region in mice from normal control, PBS-treated and KYC-treated groups were selected for analysis. Images of three areas were captured at random using a fluorescence microscope (DP71; Olympus America Inc., Center Valley, Pennsylvania, USA). Counting of the immunostained positive cells in each area was determined using NIH ImageJ (NIH; Bethesda, Marryland, USA) and presented as counts per square millimeter by a single ‘blind’ investigator, who had no knowledge of assignment of treatment groups. Similarly, the mean gray value of immunofluorescence was quantified using NIH ImageJ and normalized by the level of normal control group.
Statistical analysis
Data were presented as mean±SEM. EAE disease scores were analyzed using repeated measure two-way analysis of variance. Other statistical analyses were performed using one-way analysis of variance with the appropriate post-hoc test for multiple comparisons. A P value that is less than 0.05 was considered statistically significant.
Results and discussion
It is known that irreversible demyelination and axonal injury are major hallmarks for progressive MS. Up to date, no therapeutic tools are available to halt or even slow down this process. We investigated if KYC treatment can slow down the progress of EAE in NOD mice. We induced EAE in NOD mice and treated NOD EAE mice with PBS or KYC (3 mg/kg/day or 15 mg/kg/twice per day, intraperitoneal) (Fig. 1). We found that KYC significantly reduced disease scores in NOD EAE mice even at the dose of 3 mg/kg/day [PBS (n=20) vs. KYC (n=10), P<0.05]. Increasing the KYC dose to 15 mg/kg/twice per day further decreased the disease scores of NOD EAE mice [PBS (n=20) vs. KYC (n=10), P<0.001]. EAE in NOD mice is progressive 21 and the severity of the disease increases during the course of the disease. It might require more KYC for treatment than monophasic C57 EAE mice 20. Nevertheless, our data suggested that MPO oxidation damages neuronal functions in progressive MS.
Fig. 1.
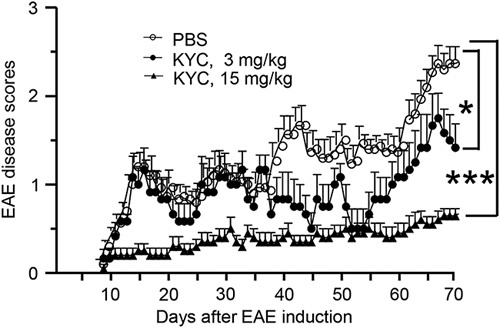
Effect of N-acetyl lysyltyrosylcysteine amide (KYC) on disease progression in progressive experimental autoimmune encephalomyelitis (EAE). Induction of chronic progressive EAE in NOD/ShiLtJ mice and KYC treatment was described in the Materials and methods section. The disease scores were evaluated daily starting at day 8 after EAE induction. n=20 for PBS, n=10 for KYC 3 mg/kg, or 15 mg/kg groups. Statistical analysis: KYC vs. PBS, repeated-measure two-way analysis of variance, *P<0.05, ***P<0.001.
Next, we determined the changes of MPO and its oxidation products in the spinal cords of NOD EAE mice 70 days after EAE induction. Our data showed that EAE significantly increased MPO in the spinal cords of NOD EAE mice (n=5, P<0.001) (Fig. 2a and b). Treatment with KYC markedly reduced the amount of MPO in the spinal cords of NOD EAE mice (n=5, P<0.01) (Fig. 2a and b). To confirm that inhibition of MPO activity and reduction of MPO level leads to the reduction of MPO-mediated oxidation, we analyzed the levels of ClTyr, a specific marker for MPO-oxidation in the spinal cord sections. We found that ClTyr was increased in NOD EAE mice compared with normal controls, which was significantly reduced by KYC treatment (n=5, P<0.01) (Fig. 2c and d). Previous studies have shown that activation of microglia and neutrophils results in the increase of MPO in MS 17,19,20. Inhibition of MPO not only reduced the level of oxidative stress but also the number of myeloid cells in the CNS of EAE mice 19,20. In agreement with previous reports 17,19,20, NOD EAE mice also have an increased number of microglia that leads to the increase of MPO-mediated oxidative stress. Inhibition of MPO activity reduces MPO-dependent oxidative stress, and consequentially, reduces inflammation and microglia activation.
Fig. 2.
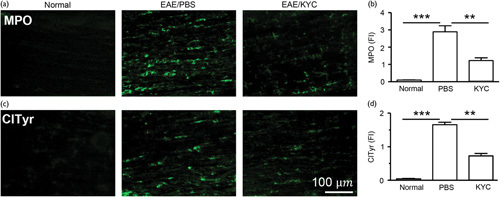
Effect of N-acetyl lysyltyrosylcysteine amide (KYC) on oxidative stress in NOD experimental autoimmune encephalomyelitis (EAE) mice. Frozen sections of spinal cords of NOD mice 70 days after EAE induction were immunostained with an antibody against myeloperoxidase (MPO) (a), chlorotyrosine (ClTyr) (c). The bar graphs (b, d) are the results of fluorescence intensity (FI) analysis of (a) and (c), respectively. Data are expressed as mean±SEM. One-way analysis of variance with Bonferroni’s test, **P<0.01, ***P<0.001, n=5/group.
Demyelination and axonal injury are the hallmarks of MS. We determined the effect of KYC on both demyelination and axonal injury in NOD EAE mice. Inducing EAE in NOD mice reduced MBP (n=5, P<0.01), a marker for myelination, and phosphorylated neurofilament H for axon (n=5, P<0.01) in the spinal cords of NOD EAE mice (Fig. 3a–d). KYC treatment noticeably reduced the loss of MBP (KYC vs. PBS, n=5, P<0.05) and phosphorylated neurofilament H (KYC vs. PBS, n=5, P<0.05). Although the detailed mechanism of how inhibition of MPO protects myelin membrane and neuronal axon is still unknown, inhibition of MPO could attenuate the direct oxidative injury to myelin and neuronal axon or reduce the overall inflammation, such as decreasing microglia activation.
Fig. 3.
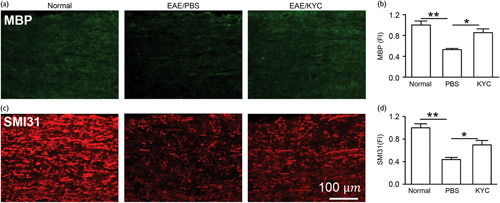
Effects of N-acetyl lysyltyrosylcysteine amide (KYC) on demyelination and axonal injury in NOD experimental autoimmune encephalomyelitis (EAE) mice. The change of demyelination and axonal injury in the spinal cord sections of NOD mice 70 days after EAE induction were probed with antibodies against myelin basic protein (MBP) (a, b), SMI31 (phosphorylated neurofilament H) (c, d). (a) and (c) are the images of immunofluorescent staining of spinal cord sections of EAE mice. (b) and (d) are the respective intensity (FI) analyses. Fluorescence intensity (FI) of normal groups was normalized to 1. One-way analysis of variance with Bonferroni’s test, *P<0.05, **P<0.01, n=5/group.
Finally, we investigated if KYC treatment can promote oligodendrocyte regeneration and neurogenesis in NOD EAE mice. To determine if inhibition of MPO activity could increase oligodendrocyte regeneration, we immunostained NG2+ cells for oligodendrocyte progenitor cells and O4+ cells for early oligodendrocyte progenitor cells 23. Our results showed that KYC significantly increased the number of NG2+ (Fig. 4a and b, P<0.05) and O4+ cells (Fig. 4c and d, P<0.01). Moreover, staining spinal cords from NOD EAE mice with CNPase antibody, a marker for oligodendrocytes forming myelin, showed that KYC significantly increases CNPase in the CNS of EAE mice (Fig. 4e and f, P<0.01). These results indicate that MPO plays a deleterious role in oligodendrocyte regeneration, and KYC, through inhibiting MPO activity, protects and promotes the oligodendrocyte regeneration in NOD EAE mice. We also evaluated the changes in neural stem cells (NSC) in the spinal cords of NOD EAE mice. SOX2 is a transcription factor critical in maintaining NSC 24. EAE increased SOX2+ NSC but KYC treatment further augmented SOX2+ NSC in NOD EAE mice (Fig. 5a and b, P<0.001), which agreed with a previous study of stroke 25. Moreover, KYC also increased the number of doublecortin+ cells, an indicator for immature neurons (Fig. 5c and d, P<0.01). Our data demonstrated for the first time that inhibition of MPO could protect and promote oligodendrocyte regeneration and neurogenesis in progressive MS. Previously, a study showed that inhibition of MPO increased neurogeneration in a murine model of stroke 25. Here, we present data clearly suggesting that inhibition of MPO could be an effective strategy for increasing the repair of myelin and axon in progressive MS patients.
Fig. 4.
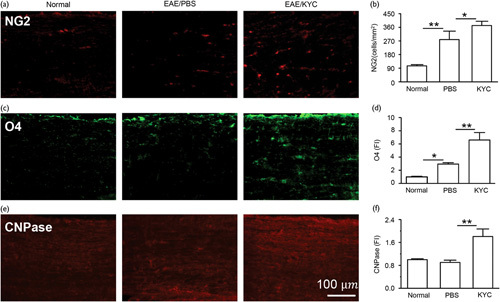
Effect of N-acetyl lysyltyrosylcysteine amide (KYC) on oligodendrocyte regeneration in NOD experimental autoimmune encephalomyelitis (EAE) mice. Immunostaining of NG2+ cells (a), O4+ cells (c), and CNPase (e). (b), (d), and (f) were cell counts (cells/mm2) or fluorescence intensity (FI) of (a), (c), and (e), respectively. Normal control FI was normalized to 1. One-way analysis of variance with Bonferroni’s test, *P<0.05, **P<0.01, n=5/group.
Fig. 5.
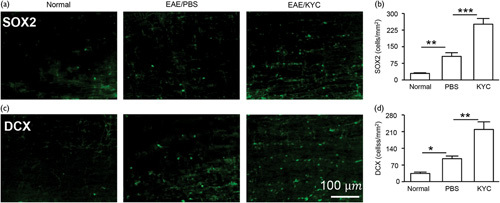
Effect of N-acetyl lysyltyrosylcysteine amide (KYC) on neurogenesis in NOD experimental autoimmune encephalomyelitis (EAE) mice. Immunostaining of SOX2 (a) and doublecortin (DCX) (c). (b) and (d) were cell counts (cells/mm2) of SOX2+ and DCX+ cells, respectively. One-way analysis of variance with Bonferroni’s test, *P<0.05, **P<0.01, ***P<0.001, n=5/group.
Conclusion
Our data strongly suggests that MPO plays a major role in progressive MS oxidative injury, thus inhibition of MPO could be an important therapeutic tool for progressive MS patients.
Acknowledgements
This work was supported by National MS Society grant RG 4975A1/2 (H.Z.).
Conflicts of interest
There are no conflicts of interest.
References
- 1.Goldenberg MM. Multiple sclerosis review. P T 2012; 37:175–184. [PMC free article] [PubMed] [Google Scholar]
- 2.Lassmann H, van Horssen J. Oxidative stress and its impact on neurons and glia in multiple sclerosis lesions. Biochim Biophys Acta 2016; 1862:506–510. [DOI] [PubMed] [Google Scholar]
- 3.Ljubisavljevic S. Oxidative stress and neurobiology of demyelination. Mol Neurobiol 2016; 53:744–758. [DOI] [PubMed] [Google Scholar]
- 4.Frischer JM, Bramow S, Dal-Bianco A, Lucchinetti CF, Rauschka H, Schmidbauer M, et al. The relation between inflammation and neurodegeneration in multiple sclerosis brains. Brain 2009; 132 (Pt 5):1175–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Correale J. The role of microglial activation in disease progression. Mult Scler 2014; 20:1288–1295. [DOI] [PubMed] [Google Scholar]
- 6.Geissmann F, Gordon S, Hume DA, Mowat AM, Randolph GJ. Unravelling mononuclear phagocyte heterogeneity. Nat Rev Immunol 2010; 10:453–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pérez-Cerdá F, Sánchez-Gómez MV, Matute C. The link of inflammation and neurodegeneration in progressive multiple sclerosis. Mult Scler Demyelinating Disord 2016; 1:9. [Google Scholar]
- 8.Abdelhak A, Weber MS, Tumani H. Primary progressive multiple sclerosis: putting together the puzzle. Front Neurol 2017; 8:234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang H, Jing X, Shi Y, Xu H, Du J, Guan T, et al. N-acetyl lysyltyrosylcysteine amide inhibits myeloperoxidase, a novel tripeptide inhibitor. J Lipid Res 2013; 54:3016–3029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davies MJ, Hawkins CL, Pattison DI, Rees MD. Mammalian heme peroxidases: from molecular mechanisms to health implications. Antioxid Redox Signal 2008; 10:1199–1234. [DOI] [PubMed] [Google Scholar]
- 11.Arnhold J, Flemmig J. Human myeloperoxidase in innate and acquired immunity. Arch Biochem Biophys 2010; 500:92–106. [DOI] [PubMed] [Google Scholar]
- 12.Van der Veen BS, de Winther MP, Heeringa P. Myeloperoxidase: molecular mechanisms of action and their relevance to human health and disease. Antioxid Redox Signal 2009; 11:2899–2937. [DOI] [PubMed] [Google Scholar]
- 13.Nagra RM, Becher B, Tourtellotte WW, Antel JP, Gold D, Paladino T, et al. Immunohistochemical and genetic evidence of myeloperoxidase involvement in multiple sclerosis. J Neuroimmunol 1997; 78:97–107. [DOI] [PubMed] [Google Scholar]
- 14.Gray E, Thomas TL, Betmouni S, Scolding N, Love S. Elevated myeloperoxidase activity in white matter in multiple sclerosis. Neurosci Lett 2008; 444:195–198. [DOI] [PubMed] [Google Scholar]
- 15.Gray E, Thomas TL, Betmouni S, Scolding N, Love S. Elevated activity and microglial expression of myeloperoxidase in demyelinated cerebral cortex in multiple sclerosis. Brain Pathol 2008; 18:86–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chataway J, Sawcer S, Feakes R, Coraddu F, Broadley S, Jones HB, et al. A screen of candidates from peaks of linkage: evidence for the involvement of myeloperoxidase in multiple sclerosis. J Neuroimmunol 1999; 98:208–213. [DOI] [PubMed] [Google Scholar]
- 17.Lefkowitz DL, Lefkowitz SS. Microglia and myeloperoxidase: a deadly partnership in neurodegenerative disease. Free Radic Biol Med 2008; 45:726–731. [DOI] [PubMed] [Google Scholar]
- 18.Chen JW, Breckwoldt MO, Aikawa E, Chiang G, Weissleder R. Myeloperoxidase-targeted imaging of active inflammatory lesions in murine experimental autoimmune encephalomyelitis. Brain 2008; 131 (Pt 4): 1123–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Forghani R, Wojtkiewicz GR, Zhang Y, Seeburg D, Bautz BR, Pulli B, et al. Demyelinating diseases: myeloperoxidase as an imaging biomarker and therapeutic target. Radiology 2012; 263:451–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang H, Ray A, Miller NM, Hartwig D, Pritchard KA, Jr, Dittel BN. Inhibition of Myeloperoxidase at the peak of experimental autoimmune encephalomyelitis restores blood–brain-barrier integrity and ameliorates disease severity. J Neurochem 2016; 136:826–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anderson AC, Chandwaskar R, Lee DH, Sullivan JM, Solomon A, Rodriguez-Manzanet R, et al. A transgenic model of central nervous system autoimmunity mediated by CD4+ and CD8+ T and B cells. J Immunol 2012; 188:2084–2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Basso AS, Frenkel D, Quintana FJ, Costa-Pinto FA, Petrovic-Stojkovic S, Puckett L, et al. Reversal of axonal loss and disability in a mouse model of progressive multiple sclerosis. J Clin Invest 2008; 118:1532–1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dawson MR, Levine JM, Reynolds R. NG2-expressing cells in the central nervous system: are they oligodendroglial progenitors? J Neurosci Res 2000; 61:471–479. [DOI] [PubMed] [Google Scholar]
- 24.Hsieh J. Orchestrating transcriptional control of adult neurogenesis. Genes Dev 2012; 26:1010–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim H, Wei Y, Lee JY, Wu Y, Zheng Y, Moskowitz MA, Chen JW. Myeloperoxidase inhibition increases neurogenesis after ischemic stroke. J Pharmacol Exp Ther 2016; 359:262–272. [DOI] [PMC free article] [PubMed] [Google Scholar]


