Abstract
The toll-like receptors (TLRs) mediate the recognition of Helicobacter pylori and initiate the innate immune response to infection. We hypothesized those genetic polymorphisms in the TLR1, TLR2, TLR4, and TLR10 influence bacterial infection, affecting susceptibility H. pylori to disease outcomes. Genomic DNA was extracted and genotypes of TLR1 (rs4833095), TLR2 (rs3804099 and rs3804100), TLR4 (rs10759932), and TLR10 (rs10004195) polymorphism were detected by the TagMan single-nucleotide epolymorphisms genotyping assay using the real-time PCR hybridization probe method. The TLR1 (rs4833095), C allele and the TLR10 (rs10004195), A allele frequency was significantly increased risk in the H. pylori infection group (odds ratio=1.76, 95% confidence interval=1.84–2.15, P=0.01 and odds ratio=1.81, 95% confidence interval=1.18–3.26, P=0.04, respectively). The TLR1 (rs4833095), C allele and TLR10 (rs10004195), A allele are susceptible TLRs polymorphisms in the Thai population. These findings suggest that TLR1 rs4833095 and TLR10 rs10004195 may play crucial roles in H. pylori susceptibility and gastric pathogenesis.
Keywords: gastritis, Helicobacter pylori, TLR1, TLR10, TLR2, TLR4, toll-like receptor polymorphisms
Introduction
Helicobacter pylori (H. pylori) is a gram-negative bacterium that colonizes the stomach. The global prevalence rate of H. pylori infection is as high as 90% in some developing countries (Goh et al., 2011) and an estimated 20–30% of infected individuals subsequently develop disease, including peptic ulcers, gastric cancer, or mucosal-associated lymphoid tissue lymphomas (Basso et al., 2010). The risk of different clinical outcome expression of H. pylori infection is believed to rely on interactions between host genetic factors and bacterial factors (Bagheri et al., 2014). Toll-like receptors (TLRs) are pathogen-associated molecular pattern receptors, mediators of innate immunity that are essential for the recognition of microbial antigens by the immune cells. The gastric epithelial cell signaling response to H. pylori infection. TLR1, TLR2, TLR4, TLR5, TLR6, and TLR10 on the cell surface or TLR9 in intracellular vesicles interact with H. pylori virulence factor causes the host immune responses and lead sing to the activation of NF-kB and subsequent proinflammatory cytokine release (Smith, 2014). The TLRs polymorphisms can confer host susceptibility to H. pylori infection and likely influence association with H. pylori-related diseases by modulating the release of proinflammatory or antiproinflammatory cytokines, which underlie gastric inflammation and the immune response to H. pylori. TLR1 polymorphisms have been reported to confer a high risk of H. pylori infection. The TLR1 rs4833095 CT genotype or the T allele has been shown to be correlated with a decreased risk of H. pylori infection and reduced risks of chronic atrophic gastritis and intestinal metaplasia in Chinese patients (Yang et al., 2013). The polymorphisms of TLR2 are associated with susceptibility to gastric cancer among various ethnic groups such as in Japanese, Chinese, and Caucasian populations. TLR4 are more frequent in relation to gastritis, and gastric cancer has been found worldwide including Europe, Asia, and America (Ishihara S et al., 2004 Huang H et al., 2010; Castano-Rodriguez et al., 2014a, 2014b). Especially in the Chinese population, Castano-Rodriguez et al.(2014a, 2014b) reported that six TLR4 polymorphisms (rs11536889, rs10759931, rs1927911, rs10116253, rs10759932, and rs2149356) were associated significantly with gastric cancer. However, there is no association between TLR4 polymorphisms and H. pylori related gastritisc diseases in some studied populations such as in an ethnic Kashmiri population (Qadri et al., 2014) or a weak association revealed very rare in Japanese population (Tahara et al., 2007; Hishida et al., 2011). Polymorphisms in TLR10 have a known direct association with H. pylori susceptibility in European and Chinese populations. The TLR10 rs10004195, T allele decreased the risk of chronic atrophic gastritis and haplotype TT had a protective effect on H. pylori infection or precancerous gastric lesions (Tang et al., 2015). Ravishankar Ram et al. (2015) found that individuals with the rs10004195 T allele were not protected against the development of GC in Malaysian patients whereas haplotype AA of rs10004195 was associated with an increase in GC irrespective of H. pylori infection. Among different populations, the association between the TLR(s) polymorphism and H. pylori infection-related diseases is inconsistent, but the impact of TLR polymorphisms on the H. pylori-related pathologic process has not yet been established in Thailand patients. The investigation of polymorphisms in Thai populations could provide novel insights into targeted treatment in genetically susceptible individuals and improve the prevention of H. pylori-related GC. Therefore, we hypothesized that specific genetic polymorphisms in TLR1, TLR2, TLR4, and TLR10 genes are likely associated with H. pylori-related gastric diseases and outcomes by affecting the H. pylori susceptibility of the hosts. It can be expected that the association of genetic polymorphisms in the host and clinical outcomes of H. pylori-related gastritis will be uncovered with advancing technologies and, in the near future, there is every prospect of defining full genetic risk profiles.
Patients and methods
Patients
Four hundred patients undergoing esophagogastroduodenoscopy for investigation of chronic abdominal pain participated in this study from December 2014 to March 2016. The following exclusion criteria were applied: previous H. pylori eradication treatment in the previous 2 months, significant medical illnesses, history of previous gastric surgery, and the use of antimicrobials or gastrointestinal medications such as proton-pump inhibitor or bismuth compounds within the previous 2 months. The study was carried out in accordance with good clinical practice and the guidelines of the Declaration of Helsinki. All patients provided written informed consent and the study protocol was approved by the Ethics Committee for Research Involving Human Subjects, Suranaree University of Technology (EC-58-58 and EC-58-59).
Diagnosis of H. pylori-associated gastritis
A diagnosis of H. Pylori-associated gastritis was made if H. pylori were seen on histopathological examination and the rapid urease test was positive. Finally, we proved bacterial infection by PCR.
Biopsy specimens
The esophagogastroduodenoscopy procedures were performed using an upper GI video endoscope (Olympus EVIS EXERA III, CV-190, Japan). The entire stomach was examined first by conventional endoscopy and then biopsies were performed using the ‘Site Specific Biopsy’ technique (Tongtawee et al., 2015a, 2015b). Gastric tissue specimens for histological analysis were sent to the pathologist. The hematoxylin and eosin stain, and Giemsa stain were used for the identification of H. pylori.
DNA preparation
Genomic DNA was extracted from formalin-fixed, paraffin-embedded tissue of 400 gastric patients using the QIAamp DNA formalin-fixed, paraffin-embedded tissue kit (Qiagen, Duesseldorf, Germany). The DNA extraction was performed according to the manufacturer’s instructions. Briefly, deparaffinize the paraffin-embedded tissues in xylene and hydrate in 100% ethanol.
Single-nucleotide polymorphisms selection and genotyping
Four common single-nucleotide polymorphisms (SNPs) in TLR1, TLR2 TLR4, and TLR10 genes have been reported with functional effects or association with gastric cancer. TLR1 rs4833095 (C>T), TLR2 rs3804099 (T>C), rs3804100 (T>C), TLR4 rs10759932 (T>C), and TLR10 rs10004195 (T>A) were selected according to the SNP database of the National Center for Biotechnology Information. The genotype of TLR(s) polymorphism was determined by TagMan allelic discrimination using a predesigned Custom TagMan SNP Genotyping Assay by real-time PCR (Roche diagnostics, Neuilly sur Seine, France). Forward and reverse primers were used along with wild-type probe VIC and probes FAM used for the variant allele. Primers and probes were supplied by Applied Biosystems (Foster city, California, USA). The real-time PCR system was used according to the manufacturer’s instructions (LightCycler 480 II instrument; Roche Diagnostics, Neuilly sur Seine, France). Briefly, the PCR conditions were as follows: 95°C for 10 min, 55 cycles of 95°C for 15 s, and 60°C for 1 min. The success rate of genotyping for each SNP was more than 94%. Negative controls and duplicate samples were used to check the accuracy of genotyping and initially analyzed using LightCycler 480 Software 1.5 (Roche Applied Science, Mannheim, Germany).
Statistical analysis
The patients’ demographic data, patients’ symptom, endoscopic findings, and TLRs were examined through univariate analysis. Backward stepwise procedures were used to carry out the multivariate analysis; the final model included only those variables that were found to be statistically significant in the univariate analysis. The associations were expressed as odds ratios (OR) with their confidence intervals (95% CI). The data were analyzed using the SPSS software (IBM SPSS Statistics for Windows, version 20, Armonk, New York, USA). Significance was set at P less than 0.05.
Results
Characteristics of study population
A total of 400 patients, 204 H. Pylori-positive cases and 196 H. Pylori-negative cases, were matched by sex, age, patient symptoms, and endoscopic findings (Table 1). In the Thai population, the mean age was 46±1.5 years (18–70 years) for the H. Pylori-infected patients and 42±2.5 years (19–68 years) for the H. Pylori-infected patients. Most of the gastritis was chronic dyspepsia (74.3 or 71.8%). Nonulcer gastritis/duodinitis was found to be the predominant endoscopic finding identified, 140 in H. Pylori-positive cases and 128 in H. Pylori-negative cases, but there were no statistically significant differences in the distribution of sex, age, patient symptoms, and endoscopic findings between H. Pylori-infected patients and uninfected patients.
Table 1.
Association between demographic data, patients’ symptoms, and endoscopic findings H. pylori infection (univariate analysis)
Association between toll-like receptors polymorphism and H. pylori infection
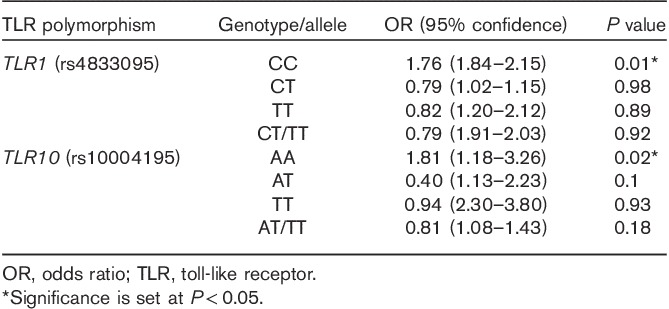
Figure 1 presents the genotype distribution of all SNPs. Among all patients, the distribution of TLR2 rs3804099, rs3804100, and TLR4 rs10759932 showed a higher frequency of the TT genotype than the TC/TT genotypes, whereas TLR1 rs4833095 showed a lower frequency of the CC genotype than the CT/TT genotypes. Similarly, the TLR10 rs10004195 AA genotype showed a lower frequency than the CT/TT genotypes. Genotypes of the TLR1, TLR2, TLR4, and TLR10 polymorphisms were differentiated using the TagMan SNP genotyping assay by real-time PCR (Fig. 2). We evaluated the association between TLR1, TLR2, TLR4, and TLR10 polymorphisms and the risk of H. pylori infection. Interestingly, TLR1 rs4833095 showed a significant association with H. pylori infection (P=0.01); frequencies of CC, CT, and TT genotypes were 60, 2, and 38% in H. pylori-positive patients in contrast to 7, 93, and 0% in H. pylori-negative patients, respectively. TLR10 rs10004195 also showed a significant association with H. pylori infection (P=0.04); frequencies of AA, AT, and TT genotypes were 29, 5, and 66% in H. pylori-positive patients and 1, 3, and 26% in H. pylori-negative patients, respectively (Table 2). In the multivariate analysis model, we detected a significantly increased risk of TLR1 rs4833095 for H. pylori infection with the homozygous CC (OR=1.76, 95% CI=1.84–2.15, P=0.01). In addition, compared with TLR10 rs1004195, we found that the AA genotype led to a significantly increased risk for H. pylori-related infection (OR=1.81, 95% CI=1.18–3.26, P=0.02) as shown in Table 3.
Fig. 1.
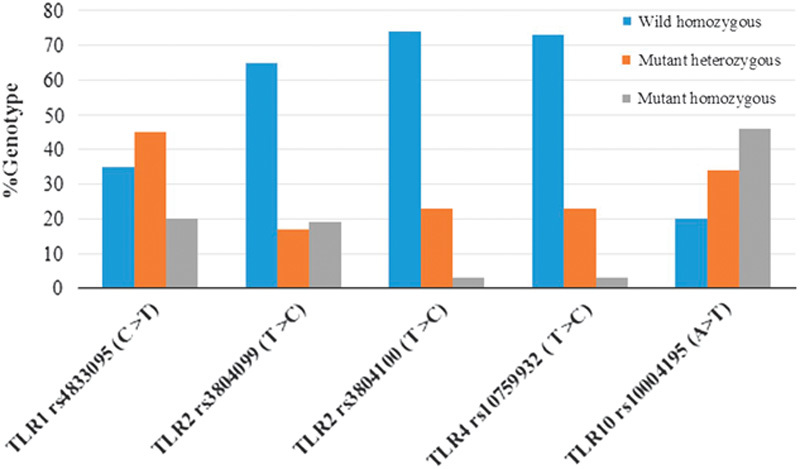
Genotype (heterozygous and homozygous) distributions of Toll-like receptor polymorphisms -TLR1 rs4833095, TLR2 rs3804099 and rs3804100, TLR4 rs10759932 and TLR10 rs10004195.
Fig. 2.
TaqMan SNP Genotyping Assay resulting in single indistinguishable cluster in scatter plot. TLR1 rs4833095 (a), TLR2 rs4804099 (b), TLR2 rs4804100 (c), TLR4 rs10759932 (d) and TLR10 rs10004195 (E).
Table 2.
Association between toll-like receptors genotypes and H. pylori infection (univariate analysis)
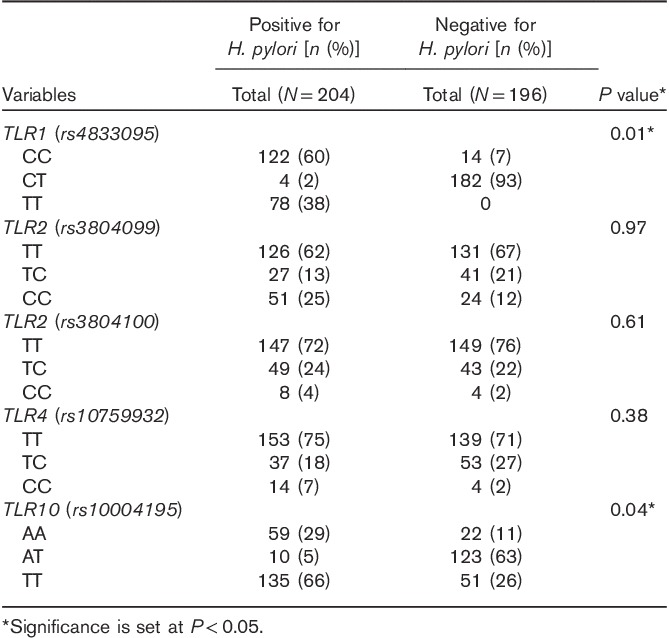
Table 3.
Predictive value of toll-like receptors for H. pylori infection (multivariate analysis)
Discussion
This study indicated that TLR1 rs4833095 and TLR10 rs10004195 were associated with H. pylori susceptibility in the Thai population. The TLR1 rs4803395, C allele was associated with a significantly increased risk for H. pylori infection and the TLR10 rs10004195, A allele conferred a high risk of H. pylori infection. Furthermore, the CT genotype or the T allele of TLR1 rs4803395 conferred protection against H. pylori infection, whereas the AT genotype or the T allele of TLR10 rs10004195 exerted a protective effect on H. pylori infection; however, this was not statistically significant (Table 2). This finding further supports that TLR1 and TLR10 genetic polymorphisms have an impact on the susceptibility to H. pylori infection by the modulation of the signaling pathway in the inflammatory response initiated by interactions between the host receptor and bacterial antigens such as lipopolysaccharides. The different outcomes of the inflammatory response, including the development of precancerous lesions of gastric mucosa, are likely mediated by the downstream expression of the key inflammatory cytokines, especially interleukin-1β (IL-1β), IL-1α, IL-6, IL-8, IL-10, tumor necrosis factor-α, and interferons, which have been shown to be varied among different genetic polymorphisms of TLR genes. In line with this hypothesis, some SNPs of these genes were reported to be closely associated with susceptibility to gastric cancer in the Chinese population (Tang et al., 2015). H. pylori infection plays an important role in gastric cancer, but there is a low incidence of gastric cancer in the Thai population (Uchida et al., 2015). In this study, the TLR1 and TLR10 genotype distributions were identified and variant genotypes (heterozygous and homozygous) were predominantly found in Thai patients. The percentage of CC alleles was 65% in TLR1 rs4833095 and the frequency of the A allele was 80% in TLR10 rs10001495 (Fig. 1). Variant genotypes of TLR1 rs4833095 and TLR10 rs10001495 in Thai population may suggest that these genotypes of TLR1 rs4833095 and TLR10 rs10001495 in Thai population can impair signaling to promote the H. pylori infection through mediating host–cell interactions. Therefore, some SNPs of TLR1 and TLR10 were proposed to play a role in modifying a risk for the development of gastric cancer or confer a protective effect for certain human cancers including gastric cancer in Thai patients, resulting in the so-called phenomenon ‘Thailand enigma’. This study also allows the detection of the SNPs of TLR2 rs3804099, rs3804100, and TLR4 rs10759932 and shows a pattern allelic distribution of these SNPs in Thai patients. Although the polymorphism of TLR2 rs3804099 was shown to be associated with susceptibility to gastric cancer in the Chinese population (Zeng et al., 2011) and TLR2 rs3804100 was reported to be commonly associated with an increased risk of H. pylori infection-related gastric cancer (Castano-Rodriguez et al., 2014a, 2014b), the actual distribution of each genotype-related disease is unclear. The TLR4 rs10759932, C allele was shown to be associated with a significantly decreased risk of H. pylori infection (Castano-Rodriguez et al., 2014a, 2014b), but there was no association between TLR4 rs10759932 and H. pylori infection in Thai patients. TLR4 rs10759932 might not play a direct role in the susceptibility to H. pylori infection or there are some other alternative SNPs located in the TLR4 gene that contribute toward H. pylori-related gastric disease as an analogous list for the TLR4 gene polymorphisms with the potential for oncogenomic studies (Kutikhin, 2011a, 2011b). Similarly, it was found that TLR2 and TLR5, but not TLR4, are required for H. pylori-induced NF-kB activation and chemokine expression by epithelial cells (Smith et al., 2003). In addition, our recent studies in genetic polymorphism in another pathway such as MDM2 in the Thai population showed an association with H. pylori infection (Tongtawee et al., 2015a, 2015b) and the association of MDM2 with more severe inflammation in H. pylori-associated gastritis (Tongtawee et al., 2015a, 2015b). These could provide the supportive evidences that the outcome of the infection mainly depends on the immune response of the host.
Conclusion
Through TLR1, TLR2, TLR4, and TLR10 polymorphism analysis by a prospective cross-sectional study in Thailand, it was found that the TLR1 (rs4833095)-C allele and the TLR10 (rs10004195)-A allele are H. pylori infection-susceptible TLRs polymorphisms in the Thai population. The different genotypes of the TLRs polymorphism indicated that the outcome of H. pylori infection depends on the immune status of the host. However, larger multicenter studies are needed with higher H. pylori-positive cases to test this hypothesis. Further evaluation of TLR polymorphisms associated with gastric cancer could provide an understanding of the underlying factors attributable to the clinical consequences of H. pylori infection.
Acknowledgements
The authors would like to acknowledge a grant from SUT Research and Development Fund.
Conflicts of interest
There are no conflicts of interest.
References
- Bagheri N, Azadegan-Dehkordi F, Sanei H, Taghikhani A, Rahimian G, Salimzadeh L, et al. (2014). Associations of a TLR4 single-nucleotide polymorphism with H. pylori associated gastric diseases in Iranian patients. Clin Res Hepatol Gastroenterol 38:366–371. [DOI] [PubMed] [Google Scholar]
- Basso D, Plebani M, Kusters JG. (2010). Pathogenesis of Helicobacter pylori infection. Helicobacter 15:14–20. [DOI] [PubMed] [Google Scholar]
- Castano-Rodriguez N, Kaakoush NO, Pardo AL, Goh KL, Fock KM, Mitchell HM. (2014a). Genetic polymorphisms in the Toll-like receptor signalling pathway in Helicobacter pylori infection and related gastric cancer. Hum Immunol 75:808–815. [DOI] [PubMed] [Google Scholar]
- Castano-Rodriguez N, Kaakoush NO, Mitchell HM. (2014b). Pattern-recognition receptors and gastric cancer. Front Immunol 5:336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figura N, Marano L, Moretti E, Ponzetto A. (2016). Helicobacter pylori infection and gastric carcinoma: not all the strains and patients are alike. World J Gastrointest Oncol 8:40–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goh KL, Chan WK, Shiota S, Yamaoka Y. (2011). Epidemiology of Helicobacter pylori infection and public health implications. Helicobacter 16 (Suppl 1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hishida A, Matsuo K, Goto Y, Naito M, Wakai K, Tajima K, et al. (2011). No associations of Toll-like receptor 2 (TLR2) −196 to −174del polymorphism with the risk of Helicobacter pylori seropositivity, gastric atrophy, and gastric cancer in Japanese. Gastric Cancer 13:251–257. [DOI] [PubMed] [Google Scholar]
- Huang H, Wu J, Jin G, Zhang H, Ding Y, Hua Z, et al. (2010). A 5′-flanking region polymorphism in toll-like receptor 4 is associated with gastric cancer in a Chinese population. J Biomed Res 24:100–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishihara S, Rumi MA, Kadowaki Y, Ortega-Cava CF, Yuki T, Yoshino N, et al. (2004). Essential role of MD-2 in TLR4-dependent signaling during Helicobacter pylori-associated gastritis. J Immunol 173:1406–1416. [DOI] [PubMed] [Google Scholar]
- Kutikhin AG. (2011a). Impact of Toll-like receptor 4 polymorphisms on risk of cancer. Hum Immunol 72:193–206. [DOI] [PubMed] [Google Scholar]
- Kutikhin AG. (2011b). Association of polymorphisms in TLR genes and in genes of the Toll-like receptor signaling pathway with cancer risk. Hum Immunol 72:1095–1116. [DOI] [PubMed] [Google Scholar]
- Qadri Q, Rasool R, Afroze D, Naqash S, Gulzar GM, Yousuf A, et al. (2014). Study of TLR4 and IL-8 gene polymorphisms in H. pylori-induced inflammation in gastric cancer in an ethnic Kashmiri population. Immunol Invest 43:324–336. [DOI] [PubMed] [Google Scholar]
- Ravishankar Ram M, Goh KL, Leow AH, Poh BH, Loke MF, Harrison R, et al. (2015). Polymorphisms at locus 4p14 of Toll-like receptors TLR-1 and TLR-10 confer susceptibility to gastric carcinoma in Helicobacter pylori infection. PLoS One 10:e0141865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM. (2014). Role of Toll-like receptors in Helicobacter pylori infection and immunity. World J Gastrointest Pathophysiol 5:133–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MF, Jr, Mitchell A, Li G, Ding S, Fitzmaurice AM, Ryan K, et al. (2003). Toll-like receptor (TLR) 2 and TLR5, but not TLR4, are required for Helicobacter pylori-induced NF-kappa B activation and chemokine expression by epithelial cells. J Biol Chem 278:32552–32560. [DOI] [PubMed] [Google Scholar]
- Tahara T, Arisawa T, Shibata T, Hirata I, Nakano H. (2007). Absence of common polymorphisms of Toll like receptor 4 (TLR4): Asp299Gly, Thr399Ile in patients with gastroduodenal diseases in Japan. J Clin Biochem Nutr 40:62–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang FB, Li ZX, Wang YM, Zhang L, Ma JL, Zhou T, et al. (2015). Toll-like receptor 1 and 10 polymorphisms, Helicobacter pylori susceptibility and risk of gastric lesions in a high-risk Chinese population. Infect Genet Evol 31:263–269. [DOI] [PubMed] [Google Scholar]
- Tongtawee T, Dechsukhum C, Leeanansaksiri W, Kaewpitoon S, Kaewpitoon N, Loyd RA, et al. (2015a). Genetic polymorphism of MDM2 SNP309 in patients with Helicobacter Pylori-associated gastritis. Asian Pac J Cancer Prev 16:7049–7052. [DOI] [PubMed] [Google Scholar]
- Tongtawee T, Dechsukhum C, Talabnin K, Leeanansaksiri W, Kaewpitoon S, Kaewpitoon N, et al. (2015b). Correlation between patterns of Mdm2 SNIP 309 and histopathological severity of Helicobacter pylori associated gastritis in Thailand. Asian Pac J Cancer Prev 16:7781–7784. [DOI] [PubMed] [Google Scholar]
- Uchida T, Miftahussurur M, Pittayanon R, Vilaichone RK, Wisedopas N, Ratanachu-Ek T, et al. (2015). Helicobacter pylori infection in thailand: a nationwide study of the CagA phenotype. PLoS One 10:e0136775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang CA, Scheibenbogen C, Bauer S, Kleinle C, Wex T, Bornschein J, et al. (2013). A frequent Toll-like receptor 1 gene polymorphism affects NK- and T-cell IFN-gamma production and is associated with Helicobacter pylori-induced gastric disease. Helicobacter 18:13–21. [DOI] [PubMed] [Google Scholar]
- Zeng HM, Pan KF, Zhang Y, Zhang L, Ma JL, Zhou T, et al. (2011). The correlation between polymorphisms of Toll-like receptor 2 and Toll-like receptor 9 and susceptibility to gastric cancer. Zhonghua Yu Fang Yi Xue Za Zhi 45:588–592. [PubMed] [Google Scholar]


