Abstract
The role of Epstein Barr virus (EBV) in the onset of Hodgkin’s lymphoma has been a subject of ongoing research. However, confirmation of EBV oncogenic involvement was not possible due to the small number of neoplastic cells characteristic for this type of tumor. Presence of EBV infection in neoplastic and non-neoplastic cells was analyzed in 81 cases of Hodgkin’s lymphoma. In neoplastic cells, using an immunohistochemical method, latent membrane protein 1 (LMP1) was found in 33,3% of cases, while in situ hybridization results demonstrated the presence of EBER RNA in 48,1% of the cases. EBER RNA was found in non-neoplastic lymphocytes in 38,3% of cases. EBV is most frequently associated with Hodgkin’s lymphoma in the first and seventh decade of life, specifically the nodular sclerosis subtype. No apparent difference was observed in the association of Hodgin’s lymphoma with EBV between genders, or in relation to clinical stage of the disease and average age of the patient. However, association with childhood age is significantly greater in comparison to adults. EBV associated disease shows a significantly greater prevalence in T lymphocytes. Slightly more abundant are cytotoxic T lymphocytes, which are also more frequently in contact with Reed-Sternberg cells, although there is no difference in number and positioning of histiocytes. Variations between the data on the association of EBV with Hodgkin’s lymphoma among studies from different parts of the world suggest that factors of age, gender, ethnic background and social status might present biological modifiers of EBV influence on the pathogenesis of this neoplasm. The differences in non-neoplastic infiltrate EBV+ and EBV-lymphoma indicate the effect of the virus on the immune interaction of tumor and host in this disease.
Keywords: Hodgkin’s lymphoma, immunohistochemistry, Epstein-Barr virus, LMP-1, EBER RNK, in situ hybridization
INTRODUCTION
Infectious agents as a cause of Hodgkin’s lymphoma were extensively studied, mainly due to the clinical symptoms such as cycling fever and night sweats. Factors such as, morphological appearance of reactive tissue surrounding neoplastic cells, epidemiological findings of bimodular curve of age incidence, geographic diversity of incidence in younger population, and differences in risk factors in children of different social classes are also taken in consideration (1). The role of Epstein Barr virus (EBV) in the onset of Hodgkin’s lymphoma was discussed in the past, however the research on this subject was difficult to perform due to a small number of neoplastic cells characteristic for this type of tumor. Following the development of sensitive DNA methods, association of EBV with this neoplasm was confirmed, along with the monoclonal presence of EBV genome (2,3). The association of EBV with specific types of Hodgkin’s disease varies. A mixed cellularity type is EBV positive in up to 96% of cases, whereas nodular scleorsis is less frequently associated with this infection (4). Lymphocyte depletion is rarely associated with EBV, while lymphocyte predominance type is practically never positive. There are significant regional differences in respect to this association. Higher rates of EBV associated Hodgkin’s lymphoma were found in Peru and Honduras (over 90%), in comparison with those found in western Europe and USA (up to 50%) (4,5,6,7). Higher incidence of EBV associated Hodgkin’s lymphoma was found in younger patients population (under 15 years of age) as well as older patients population (over 50 years of age) (8). In AIDS patients with progressing Hodgkin’s lymphoma, EBV is always present in Reed-Sternberg cells (9). Some authors describe EBV receptor expression (CR2, CD21) on the surface of atypical cells in EBV positive cases of Hodgkin’s lymphoma, thus explaining viral presence in the cells (10,11). Hodgkin’s lymphoma associated with EBV infection shows expression of EBV gene in the form of latency type II. Reed-Sternberg cells and their variants exhibit strong expression of latent membrane protein 1 (LMP1), although expression of Epstein Barr viral nuclear antigen 2 (EBNA2) is absent (4). Northern and Western blots of Hodgkin’s lymphoma samples frequently reveal presence of latent membrane protein 2A (LMP2A) and latent membrane protein 2B (LMP2B) (12). LMP1 is known to be a target for the attack by cytotoxic T lymphocytes in healthy individuals. High expression of LMP1, LMP2A, -2B in tumor cells of Hodgkin’s lymphoma is an indicator of some specific immunological deficiencies in patients with this disease. It is known that patients with Hodgkin’s lymphoma have impaired immune response (13). With regards to prognosis, variation among EBV positive and EBV negative cases was not found (14). Numerous international studies done on this subject suggest that age, gender, ethnic background and social status factors might represent biological modifiers of EBV effect on the pathogen-esis of this neoplasm. Differences found among studies indicate epidemiologically complex relation of the virus and this disease, which can only partly be contributed to small samples and demographic diversity. Neoplastic cells of Hodgkin’s lymphoma survive in the surroundings rich in inflammatory cells which main role is prevention of their growth and proliferation. Even though they are resistant to endogenous apoptosis, neoplas-tic cells of Hodgkin lymphoma are mainly sensitive to therapeutic treatments that lead to cell death. It is not known which factors influence the balance between apoptotic and antiapoptotic mechanisms in untreated disease, or the predominance of apoptotic signals during treatment. EBV presence in neoplastic cells of Hodgkin disease might influence cytotoxic T cells’ mechanism which main role is to destroy cells carrying pathogens, hence indirectly controlling programmed cell death. For better understanding of this enigmatic lymphoma it is necessary to identify factors involved in its emergence, and frequent association with EBV infection.
OBJECTIVE
The objective of this study is to determine the presence of EBV infection in neoplastic cells of Hodgkin lymphoma and non-neoplastic inflammatory infiltrate. The study will classify EBV positive and negative cases of lymphoma according to the age, gender and clinical stage of the disease. The cell structure of a non-neoplas-tic inflammatory infiltrate and involvement of CD8+ cytotoxic T lymphocytes in EBV positive and negative cases of Hodgkin’s lymphoma will be analyzed. Association level of Hodgkin’s lymphoma with EBV infection will be compared with the results of similar studies.
MATERIALS AND METHODS
The study involved 81 cases of Hodgkin’s lymphoma diagnosed at the Department of Pathology at the University Clinics Center in Tuzla, in the period from 1985 to 1999. Immunohistochemical method of three-step immunoperoxidase with streptavidin was used in the diagnostics. For the purpose of phenotyping neoplas-tic and background cells primary antibodies were used for CD3(PC3/188A), CD8(C8/144B), CD15(C3D-1), CD20cy(L26), CD30(Ber-H2), CD45(T29/33) and CD68(PG-M1). EBV infection detection was done using primary antibody for latent membrane protein LMP(CS 1-4) (DAKO, Glostrup, Denmark). Deparaffinized, 5 μιη thick tissue slices, formalin fixed and in paraffin embedded Hodgkin’s lymphoma tissue samples were incubated with primary antibodies for 1 hour. Prior to the incubation endogenous peroxidase activity was inhibited, and antigen de-masking performed, by cooking for 15 minutes in buffer pH 6,0. Incubation with biotin labeled secondary antibodies and streptavidine labeled peroxi-dase lasted 30 minutes. Chromogen used was 3,3 diami-nobensidine hydrochloride, while contrast staining was done with hematoxilin. Epstein Barr virus ribonucleic acid (EBER RNA) detection was done with an in situ hybridization method, using FITC labeled peptide nucleic acid probes (PNA) (Dako, Glostrup, Denmark). Following standard procedure of deparaffinization and rehydra-tion, pretreatment of tissue slices was performed with proteinase K for 20 minutes. Next step was hybridization with PNA probe for 90 minutes at 55°C, and incubation with secondary anti-FITC antibody labeled with alkaline phosphatase for 30 minutes. Visualization was done with BCIP/NBT/levamisol substrate system for 60 minutes, followed by hematoxiline contrast staining. Statistical analysis included calculation of mean value, standard deviation, proportions testing, and hi-square test.
RESULTS
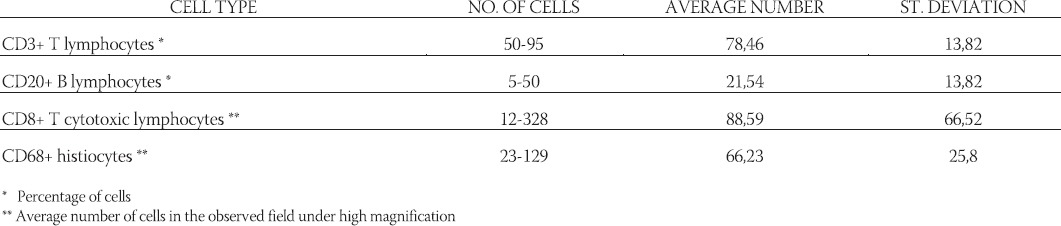
Neoplastic Hodgkin’s lymphoma cells were immunohis-tochemically positive for latent membrane protein 1 in 27 (33,3%) cases (Figure 1). In situ hybridization results demonstrated positive EBER RNA in Reed-Sternberg, Hodgkin’s and lacunar cells in 39 (48,1%) cases (Figure 2). Positive non-neoplastic lymphoid background cells were found in 31 (38,3%) cases. Out of 39 cases of Hodgkin lymphoma, for which in situ hybridization confirmed the association with EBV infection, 21 (53,8%) cases were of male, and 18 (46,2%) female gender. EBV associated Hodgkin’s lymphoma in one (2,6%) case was of lymphocyte predominance type, in 26 (66,7%) cases nodular sclerosis type, in 10 (25,6%) cases of mixed cellularity type, and in 2 (5,1%) cases of lymphocyte depletion type. The youngest patient was 4, and the oldest 69 years old. The average age was 38,5 years, with standard deviation of 21,2 years. Distribution of EBV positive cases according to the gender and age is shown in Figure 3. Incidence of known specific clinical stages according to the Ann Arbor classification, for EBV associated Hodgkin’s disease is presented in Table 1. In the group of subjects in which in situ hybridization demonstrated the presence of EBER RNA in neoplas-tic cells, 27 (69,2%) cases were positive for EBV LMP1 with immunohistochemical method. Lymphocytes of the background infiltrate in the EBV associated group of subjects showed no presence of EBV LMP1 immu-nohistochemically, while in situ hybridization showed the presence of EBER RNA in lymphocytes of 28 (71,8%) cases. Neoplastic cells in this group were positive for CD15 in 33 (84,6%) cases. CD20 was positive in neoplas-tic cells of 6 (15,4%) cases in this group, while positive CD3 was not found. CD45 was positive in one (2,6%) case. CD30 in Hodgkin’s cells was positive in 38 (97,4%) cases of this group. Table 2 presents the presence of CD3 positive T lymphocytes, CD20 positive B lymphocytes, CD8 positive T cytotoxic lymphocytes and CD68 positive histiocytes in the background infiltrate of the EBER RNA positive Hodgkin’s lymphoma cases. Cytotoxic CD8 positive T lymphocytes were found in close proximity of neoplastic cells of EBER RNA positive Hodgkin’s lymphoma in 35 (89,7%) cases. Histio-cytes positive for CD68 antigen were found next to Hodgkin’s cells in 28 (71,8%) cases. Out of 42 cases of Hodgkin lymphoma, not associated with EBV infection, 24 (57,1%) patients were male, and 18 (42,9%) of female gender. EBV negative Hodgkin lymphoma in three (7,1%) cases was of a lymphocyte predominance type, in 31 (73,8%) cases of nodular sclerosis type, in 7 (16,7%) cases of mixed cellularity type and in one (2,4%) case of lymphocytic depletion type. The youngest patient was 15 years old, the oldest 73 years old, average age was 37,6 years, with standard deviation of 17,7 years. Incidence of known specific clinical stages according to the Ann Arbor classification, for EBV negative Hodgkin’s lymphoma is shown in Table 1. Lymphocytes of the background infiltrate in EBV negative group of subjects, did not show presence of EBV LMP1 by immunohistochemis-try, while in situ hybridization confirmed the presence of EBER RNA in lymphocytes of 3 (7,1%) cases. Neoplastic cells of this group were positive for CD15 in 38 (90,5%) cases. CD20 was positive in neoplastic cells in 8 (19,0%) cases of this group, positive CD3 was not found, while CD45 was positive in one (2,4%) case. CD30 was positive in Hodgkin’s cells in 39 (92,9%) cases of this group.
FIGURE 1.
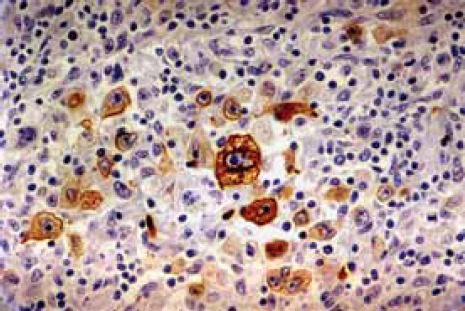
Neoplastic Hodgkin’s lymphoma cells positive for LMP1
FIGURE 2.
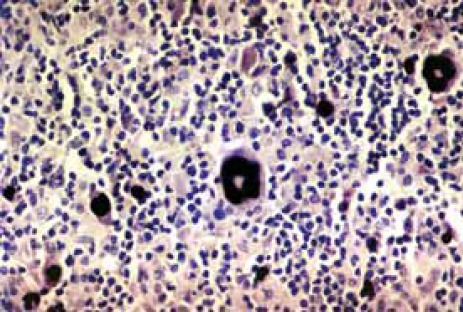
EBER RNA positive Reed-Sternberg and Hodgkin’s cells
FIGURE 3.
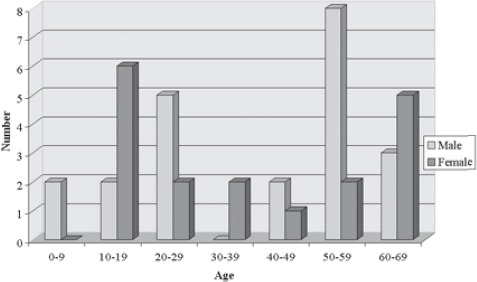
Distribution of EBV positive Hodgkin’s lymphoma according to the age and gender
TABLE 1.
Incidence of clinical stage for EBV associated Hodgkin’s lymphoma according to the Ann Arbor classification

TABLE 2.
Presence of cell types in the background infiltrates in EBER RNA positive Hodgkin’s lymphoma
DISCUSSION
Numerous findings indicate an etiological role of Epstein Barr virus, widely spread herpes virus, in numerous lymphoid and epithelial malignancies. Epidemiological studies have shown three times greater risk of acquiring Hodgkin’s lymphoma in individuals who were diagnosed with infectious mononucleosis. Higher antibody titer for EBV antigens was found in patients with Hodg-kin’s lymphoma when compared with the control group (15). The fact that EBV makes human B lymphocytes immortal, and in mouse neoplastic, that expression of EBV gene in immortalized cell lines of rodents leads to transformation in vitro and in vivo, as well as the tu-morigenic effect of EBV in some primates, have led to biological basis of EBV association with Hodgkin lymphoma (16,18,19,20). The role of EBV in pathogenesis of Hodgkin’s lymphoma is further supported by findings of latent EBV infection in some of these neoplasms, using Southern blot DNA hybridization, in situ hybridization and antigen detection, as well as polymerase chain reaction (2,3,5,21). Lower association with EBV infection in comparison to present study was presented by the authors of study in Philippines (42,9%), Costa Rica (40%), France (33% and 29%), India (31%). Statistically significantly lower association was found in studies done in Poland (33%)(p<0,05), Israel (30%)(p<0,05), and Sweden (27%)(p<0,01) (22,23,24,25,26,27,28,29). Higher association of EBV with Hodgkin’s lymphoma was found in fifteen studies: in Turkey (55%), Japan (59% and 64%), China (60,7%), Malaysia (61%), Taiwan (62,9%), and Algeria (66%). Statistically significant increase in second study done in Algeria (72%)(p<0,05), Korea (69% and 76%)(p<0,01; p<0,05), Mexico (70%)(p<0,05), Kenya (79% and 92%)(p<0,05), and studies from Peru and Honduras showed association with EBV infection higher than 90% (7,24,25,30,31,32,33,34,35,36,37,38,39,40). The association with EBV infection in 48% cases, similar to the present study was found in one study conducted in Italy (41). With regards to the association of a particular Hodgkin’s lymphoma subtypes with EBV infection, considerable increase in the frequency of EBV association with mixed cellularity subtype was determined by the authors of studies done in Poland (61,8%), Philippines (66.6), Great Britain (68%), Taiwan (69,2%), international studies from 1546 instances of Hodgkin’s disease (70,4%), USA (75%), Korea (75% and 85%), France (80%), Mexico (81%), Japan (84%), Malaysia (86%), Costa Rica (86%), China (90,9%) and Hong Kong (100%) (1,22,23,25,27,32,33,34,35,36,37,38,42,43,44). Less frequent association of EBV and this lymphoma subtype in relation to the present study group was found in studies from Sweden (38,0%), Turkey (40,0%) and Israel (45,0%) (28,29,30). Statistically significant decrease in association (p<0,05) was found by authors from India with 21,7% EBV positive cases of this subtype (26). Comparison of the association of a nodular sclerosis subtype with EBV infection between present study group and the above mentioned studies, demonstrated increased frequency of an EBV infection in nodular sclerosis subtype presented in studies from Mexico (50,0%), Hong Kong (56,0%), Korea (59,0%), Turkey (62,5%), and Taiwan (63,9%), even though none of them showed statistically significant difference (30,34,36,37,44). Association frequency of a lymphocyte depletion subtype with EBV in the above listed studies spans from 0% in the study done in China, to 100% in the studies done in Hong Kong, India, Taiwan and Sweden (26,29,32,34,44). The studies on the association of lymphocyte predominance with EBV differ in findings; in the studies done in India, Great Britain, Poland and Malaysia association was not found, while the study from Taiwan shows association in 16,7% cases, Sweden 20%, Turkey 40%, and international study of 1546 cases of Hodgkin’s disease 54,9% (1,26,27,29,30,33,34,42). In relation to the present study group statistically significant difference was not found. In the distribution of EBV associated cases according to the age in the present study group, the highest frequency of these cases is found in the first (100,0%) and seventh (72,7%) decade of life. Statistically significant increase in the frequency of EBV positive Hodgkin’s lymphoma (p<0,05) is found in the seventh in comparison to fourth decade of life. A frequency of EBV infection in the present study group was only slightly higher among female (50,0%) than male gender (46,6%). Distribution of the association of a particular lymphoma subtypes with EBV infection regarding patients gender did not show any considerable differences. Distribution of the association in a particular Hodgkin’s lymphoma subtypes with EBV infection in regard to the patient gender and age, showed the highest association with EBV nodular sclerosis subtype (57,1%) for males in the third decade of life. Half of the cases with nodular sclerosis subtype in the fifth, sixth and seventh decade of life were associated with EBV infection. Mixed cellularity in the sixth decade of life in male was associated with EBV in 75,0% cases, and in the seventh decade in 66,6% cases. All cases of the lymphocyte depletion in males of the second and third decade of life were EBV associated. In males in the fourth decade of life association of Hodgkin’s lymphoma with EBV infection was not found. In females, the subtype of nodular sclerosis associated with EBV infection in second decade was found in 71,4% cases, and in the seventh decade in 100,0% cases. In the EBV associated case of mixed cellularity for females in the second, third and seventh decade one case was found in each group, contributing to 50,0%, 100,0%, or more precisely 50,0% cases of this subtype were found in those decades. Absence of EBV associated Hodgkin’s lymphoma in the eight decade of life is not possible to interpret due to small number of cases found in that age group. According to the data presented above, male population showed frequent association with EBV infection and nodular sclerosis subtype in the second decade, and mixed cellularity in the sixth and seventh decade of life. In female population nodular sclerosis subtype in the second and seventh decade of life showed the highest association with EBV infection. Analyzed with respect to the clinical stage of the disease, cases associated with EBV infection did not show statistically significant difference. Subtype of Hodgkin’s lymphoma is usually considered as a basic factor of risk for the association of this disease with EBV infection. In the study of 1546 cases of Hodgkin’s disease it was found that 75,0% cases of mixed cellularity and 25,0% cases of nodular sclerosis are associated with EBV (1). The differences between the subtypes of mixed cel-lularity and nodular sclerosis in the frequency and forms of association with EBV infection, along with clinical and other epidemiological indicators, are considered as a basis for the hypothesis that these two subtypes represent distinct entities (45). The absence of statistically significant differences in the frequency of EBV associated cases of mixed cellularity and nodular sclerosis (58,8% in comparison to 45,6%) in the present study group, does not support the above mentioned hypothesis. While the authors of the mentioned study found that the risk for development of Hodgkin’s lymphoma associated with EBV is two times lower for young females than that for males of the same age, the present studied group for males of ages from 15 to 39 years resulted in 31,6% cases, and in females 33,3% cases associated with EBV infections (1). Nodular sclerosis subtype was found to be rarely associated with EBV infection in young females in the above mentioned study, although the present study group found 31,3% of cases associated with this virus. Even though the association of Hodgkin lymphoma with EBV infection in young females of the present study group is larger in relation to the data of discussed studies, it is significantly lower in respect to the association in childhood and in patients over 50 years of age. This could be in accordance with already assumed preventive effects in the reproductive age of a female (46). Hodgkin lymphoma in children of present study group showed statistically significant increase in the frequency of association with EBV infection (p<0,05) in comparison to adult group. In the age group up to 13, all of the six found cases showed EBV presence in the neoplastic cells. In a particular subtypes of Hodgkin’s lymphoma in children, the frequency of association is found in 100% (1/1) cases of lymphocyte depletion, 83,3% (5/6) cases of nodular sclerosis and 66,6% (2/3) cases of mixed cellu-larity. All the male children had EBV positive neoplastic cells, while in 66,6% of female children Hodgkin’s lymphoma was associated with the EBV infection. According to these data Hodgkin’s lymphoma in children of the present study group is pronouncedly associated with an EBV infection, almost regularly in children up to 13 years of age, more often in male children, with association to the subtypes of lymphocyte depletion and nodular sclerosis. In the present study of children group Hodgkin’s lymphoma showed greater association with EBV infection, hence contradicting the results of numerous other studies. In the study done in Australia 70% of EBV associated Hodgkin’s lymphoma was found in children, in South Africa 68% and 50%, in United Arab Emirates 60%, in USA 58%, in Brazil 58%, in Greece 57%, in France 54%, in Argentina, Great Britain, Egypt and Jordan each 50% of EBV positive cases (47,48,49,50,51,52). Statistically significant decrease in the association of this neoplasm with EBV infection in children, in comparison to present study, was found by the authors of two studies done in USA with 38,6%(p<0,05) and 33,3%(p<0,01) of these cases (7,43). Greater association of Hodgkin’s lymphoma with EBV infection in children in comparison to the present study, was found in the studies done in Costa Rica (81%), China (81,7%), Honduras (100%) and Kenya (100%) (47,53,54). On the other hand, high frequency association of Hodgkin’s lymphoma with EBV in children of the present study group, corresponds to the type of the disease also found in developing countries. Most of the authors presented association of a mixed cellularity subtype, and partly lymphocyte depletion with EBV in childhood (1,43,50,52,53,54). Mean age of the patients with Hodgkin’s lymphoma associated with EBV infection did not show statistically significant difference from the mean age of patients suffering from the disease not associated with this infection. A slightly higher mean age in patients with Hodgkin’s lymphoma associated with EBV is a consequence of a significant number of cases found in patients 50 years old or older. On the other hand, high frequency association of this disease with EBV in children (80%), could not have a significant influence on the decrease of mean age in patients with EBV associated disease. This is due to a small number of children with this disease (12,3%) in presently analyzed group. Characteristics of the inflammatory infiltrate in the group of patients with established association with EBV infection, in comparison to the group in which the association was not found, statistically significant higher abundance of T cells is noticed in the first (78,4%) when compared to the second group (71,3%). B lymphoid cells were significantly prevalent (p<0,05) in infiltrate of the EBV negative when compared to EBV positive Hodgkin’s lymphoma. Histiocytes positive for CD68 antigen were found in the infiltrate of EBV positive disease in almost the same number as in EBV negative disease. Examination of cell structure in the inflammatory infiltrate lead to the conclusion that presence of EBV in neoplastic cells of Hodgkin’s lymphoma has significant influence on the presence of CD3 positive T cells and CD20 positive B cells. However, a significant influence on the number of CD68 positive histiocytes in background infiltrate was not found. The use of in situ hybridization method in the studies of association of EBV with Hodgkin lymphoma demonstrated presence of the virus in certain number of non-neoplastic lym-phoid cells (5,55). Presence of EBER RNA was found in non-neoplastic lymphocytes of the studied group in 31 (38,3%) cases. Considerable majority (p<0,0001) of these cases (90,3%) showed presence of EBER RNA in neo-plastic cells as well, while in 9,7% cases of neoplastic cases neoplastic cells were negative for EBV. In distribution of cases with EBER RNA positive non-neoplastic lymphocytes according to subtype of Hodgkin’s lymphoma statistically significant difference was not found. They are more often associated with subtype of lymphocyte depletion (66,6%), followed by mixed cellularity (41,2%) and nodular sclerosis (38,6%), while they are not found in the lymphocyte predominance type. Interestingly, in the group with EBV negative Hodgkin’s lymphoma we found all three cases with EBV positive non-neoplastic lymphocytes to be of nodular sclerosis subtype. The presence of EBV in neoplastic and non-neoplastic cells of Hodgkin lymphoma could indicate coexistence of a clonal proliferation of EBV infected Reed Sternberg and Hodgkin’s cells and nonspecific infection of non-neo-plastic lymphocytes. The finding that speaks in favor to this hypothesis is that EBV positive neoplastic cells and non-neoplastic lymphocytes of Hodgkin lymphoma are infected with different strains of EBV, contributing to different oncogene abilities of this virus (56). Viral infection of non-neoplastic lymphocytes could affect their function, thus impairing their defense mechanism against neoplastic tissue.
CONCLUSION
The presence of latent membrane protein 1, using an immunohistochemical method was determined in 27 (33,3%) cases, while in situ hybridization results demonstrated presence of EBER1 and EBER2 RNK in 39 (48,1%) studied cases of Hodgkin’s lymphoma. EBV is associated with 66,6% cases of lymphocyte depletion subtype, 58,8% cases of mixed cellularity, 45,6% cases of nodular sclerosis and 25,0% cases of lymphocyte predominance. All cases of Hodgkin’s lymphoma diagnosed in the first decade of life are EBV positive, as well as 72,7% cases found in the seventh decade of life. In the eight decade, association with EBV infection has not been found, while it is 22,2% in the fourth decade. The frequency of Hodgkin’s lymphoma association with EBV infection in children is 80%. All male children and 66,6% of female children have EBV associated disease. Association of this neoplasm with EBV infection does not show significant difference in respect to the clinical stage of disease, gender and mean age of patients. Variations between data on the association of EBV with Hodgkin’s lymphoma among studies from different parts of the world, suggests that factors of age, gender, ethnic background and social status may present biological modifiers of EBV influence on pathogenesis of this neoplasm. The differences in the non-neoplastic infiltrate between EBV+ and EBV-lymphomas suggest the effect of the virus on the immune interaction of tumor and host in this disease.
REFERENCES
- 1.Glaser S.L, Lin R.J, Stewart S.L, et al. Epstein-Barr virus-Associated Hodgkin’s disease: Epidemiologic characteristics in international data. Int. J. Cancer. 1997;70:375–382. doi: 10.1002/(sici)1097-0215(19970207)70:4<375::aid-ijc1>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 2.Weiss L.M, Mohaved L.A, Butler A.E, Swanson S.A, Frierson F.H, Cooper P.H. Analysis of lymphoepitehelioma and lympho-epithelioma-like carcinomas for Epstein-Barr virus by in situ hybridization. Am. J. Surg. Pathol. 1989;13:625–631. doi: 10.1097/00000478-198908000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Anagnostopoulos I, Herbst H, Niedobitek G, Stein H. Demonstration of monoclonal EBV genomes in Hodgkin’s disease and Ki-1positive anaplastic large cell lymphoma by combined Southern and in situ hybridization. Blood. 1989;74:810–816. [PubMed] [Google Scholar]
- 4.Pallesen G, Hamilton-Dutoit S.J, Rowe M, et al. Expression of Epstein-Barr virus replicative proteins in AIDS-related non-Hodgkin’s lymphoma cells. J. Pathol. 1991;165:289–299. doi: 10.1002/path.1711650404. [DOI] [PubMed] [Google Scholar]
- 5.Weiss L.M, Chen Y.Y, Liu X.F, Shibata D. Epstein-Barr virus and Hodgkin’s disease. A correlative in situ hybridization and poly-merase chain reaction study. Am. J. Pathol. 1991;139:1259–1265. [PMC free article] [PubMed] [Google Scholar]
- 6.Chang K.L, Arber D.A, Weiss L.M. CD30: a review. Appl. Immunohistochem. 1993;1:244–255. [Google Scholar]
- 7.Ambinder R.F, Browning P.J, Lorenzana I, et al. Epstein-Barr virus and Hodgkin’s disease in Honduras and the United States. Blood. 1993;81:462–467. [PubMed] [Google Scholar]
- 8.Jarrett R.F, Gallagher A, Jones D.B. Detection of Epstein-Barr virus genomes in Hodgkin’s disease: relation to age. J. Clin. Pathol. 1991;44:844–848. doi: 10.1136/jcp.44.10.844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Audouin J, Diebold J, Pallesen G. Frequent expression of Epstein-Barr virus latent membrane protein-1 in tumor cells of Hodgkin’s disease in HIV-positive patients. J. Pathol. 1992;167:381–384. doi: 10.1002/path.1711670406. [DOI] [PubMed] [Google Scholar]
- 10.Jiwa N.M, Van der Valk P, Mullink H, Vos W, Harstman A, Maurice M.M. Epstein-Barr virus DNA in Reed-Sternberg cells of Hodgkin’s disease is frequently associated with CR2 (EBV receptor) expression. Histopathol. 1992;21:51–57. doi: 10.1111/j.1365-2559.1992.tb00342.x. [DOI] [PubMed] [Google Scholar]
- 11.Delsol G, Meggetto F, Brousset M.N. Relation of follicular dendritic reticulum cells to Reed-Sternberg cells of Hodgkin’s disease with emphasis on the expression of CD21 antigen. Am. J. Pathol. 1993;142:1729–1738. [PMC free article] [PubMed] [Google Scholar]
- 12.Deacon E.M, Pallesen G, Niedobitek G, et al. Epstein-Barr virus and Hodgkin’s disease: Transcriptional analysis of viral latency in the malignant cells. J. Exp Med. 1993;177:339–349. doi: 10.1084/jem.177.2.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kumar R.K, Penny R. Cell-mediated immune deficiency in Hodgkin’s disease. Immunol. Today. 1982;3:269–273. doi: 10.1016/0167-5699(82)90082-2. [DOI] [PubMed] [Google Scholar]
- 14.Vestlev P.M, Pallesen G, Sandvey K, Hamilton-Dutoit S.J. Prognosis of Hodgkin’s disease is not influenced by Epstein-Barr virus latent protein. Int. J. Cancer. 1992;50:670–671. doi: 10.1002/ijc.2910500432. [DOI] [PubMed] [Google Scholar]
- 15.Mueller N. Epidemiologic studies assessing the role of the Epstein-Barr virus in Hodgkin’s disease. Yale J Biol. Med. 1987;60:321–327. [PMC free article] [PubMed] [Google Scholar]
- 16.Wang D, Liebowitz D, Kieff E. An EBV membrane protein expressed in immortalized lymphocytes transforms established rodent cells. Cell. 1985;43:831–840. doi: 10.1016/0092-8674(85)90256-9. [DOI] [PubMed] [Google Scholar]
- 17.Moorthy R.K, Thorley-Dawson D.A. Biochemical, genetic, and functional analyses of the phosphorylation sites on the EBV-en-coded oncogenic latent membrane protein LMP-1. J. Virol. 1993;67:2637–2645. doi: 10.1128/jvi.67.5.2637-2645.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang D, Liebowitz D, Wang F, et al. Epstein-Barr virus latent membrane protein (LMP) alters lymphocyte morphology, adhesion, and growth: deletion of the amino terminus abolishes activity. J. Virol. 1988;62:4173–4184. doi: 10.1128/jvi.62.11.4173-4184.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaye K.M, Izumi K.M, Kieff E. Epstein-Barr virus latent membrane protein-1 is essential for B-lymphocyte growth transformation. Proc. Nat. Acad. Sci. 1993;90:9150–9154. doi: 10.1073/pnas.90.19.9150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cleary M.L, Epstein M.A, Finerty S, et al. Individual tumors of multifocal EB virus-induced malignant lymphomas in tamarinds arise from different B-cell clones. Science. 1985;228:722–724. doi: 10.1126/science.2986287. [DOI] [PubMed] [Google Scholar]
- 21.Herbst H, Dallenbach F, Hummel M. Epstein-Barr virus latent membrane protein expression in Hodgkin and Reed-Sternberg cells. Proc. Nat. Acad. Sci. USA. 1991;88:4766–4770. doi: 10.1073/pnas.88.11.4766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paulino A.F, Paulino-Cabrera E, Weiss L.M, Medeiros L.J. Hodg-kin’s disease in the Philippines. Mod. Pathol. 1996;9:115–119. [PubMed] [Google Scholar]
- 23.Monterroso V, Zhou Y, Koo S, Glackin C, Bujan W, Medeiros L.J. Hodgkin’s disease in Costa Rica: a report of 40 cases analyzed for Epstein-Barr virus. Am. J. Clin. Pathol. 1998;109:618–624. doi: 10.1093/ajcp/109.5.618. [DOI] [PubMed] [Google Scholar]
- 24.Belkaid M.I, Briere J, Djebbara Z, Beldjord K, Andrieu J.M, Colonna p. Comparison of Epstein-Barr virus markers in Reed-Stern-berg cells in adult Hodgkin’s disease tissues from an industrialized and developing country. Leuk. Lymphoma. 1995;17:163–168. doi: 10.3109/10428199509051717. [DOI] [PubMed] [Google Scholar]
- 25.Belkaid M.I, Zerhouni F, Beljord K, et al. Association of Epstein-Barr virus and Hodgkin’s disease: comparison between Algerian and French patients. Bull. Cancer. 1995;85:357–363. [PubMed] [Google Scholar]
- 26.Ratha K, Shanthi P, Madhavan M, Senthamarai A. Study of association of Epstein-Barr virus with Hodgkin’s disease. Ind. J. Pathol. Microbiol. 1997;40:351–354. [PubMed] [Google Scholar]
- 27.Kordek R, Jesionek-Kupnicka D, Biernat W, Wozniak L. Expression of the latent membrane protein of Epstein-Barr virus in Hodgkin’s disease. Age and subtype distribution in Polish patients. Acta. Haematol. Pol. 1996;27:15–20. [PubMed] [Google Scholar]
- 28.Ben-Arush M, Rosenthal J, Peretz-Nahum M, et al. Hodgkin’s disease in childhood-northern Israel cancer center experience 1971-1990. Harefuah. 1993;125:333–337. [PubMed] [Google Scholar]
- 29.Enblad G, Sandvey K, Sundstrom C, Pallesen G, Glimelius B. Epstein-Barr virus distribution in Hodgkin’s disease in an un-selected Swedish population. Acta Oncol. 1999;38:425–429. doi: 10.1080/028418699431942. [DOI] [PubMed] [Google Scholar]
- 30.Durmaz R, Aydin A, Koroglu M, et al. Detection and genotyp-ing of Epstein-Barr virus by polymerase chain reaction in tissues obtained from cases with Hodgkin’s disease in Turkey. Acta Virol. 1998;42:375–381. [PubMed] [Google Scholar]
- 31.Kusuda M, Toriyama K, Kamidigo N.O, Itakura H. A comparison of epidemiological, histological, and virologic studies on Hodgkin’s disease in western Kenya and Nagasaki, Japan. Am. J. Trop Med. Hyg. 1998;59:801–807. doi: 10.4269/ajtmh.1998.59.801. [DOI] [PubMed] [Google Scholar]
- 32.Zhou X.G, Hamilton-Dutoit S, Yan O.H, Pallesen G. The association between Epstein-Barr virus and Chinese Hodgkin’s disease. Int. J. Cancer. 1993;55:359–363. doi: 10.1002/ijc.2910550303. [DOI] [PubMed] [Google Scholar]
- 33.Peh S.C, Looi L.M, Pallesen G. Epstein-Barr virus (EBV) and Hodgkin’s disease in a multiethnic population in Malaysia. Histopathology. 1997;30:227–233. doi: 10.1046/j.1365-2559.1997.d01-594.x. [DOI] [PubMed] [Google Scholar]
- 34.Liu S.M, Chow K.C, Chiu C.F, Tzeng C.H. Expression of Ep-stein-Barr virus in patients with Hodgkin’s disease in Taiwan. Cancer. 1998;83:367–371. doi: 10.1002/(sici)1097-0142(19980715)83:2<367::aid-cncr22>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 35.Tomita Y, Ohsawa M, Kanno H, et al. Epstein-Barr virus in Hodgkin’s disease patients in Japan. Cancer. 1996;77:186–192. doi: 10.1002/(SICI)1097-0142(19960101)77:1<186::AID-CNCR30>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 36.Huh J, Park C, Juhng S, Kim C.E, Poppema S, Kim C. A pathologic study of Hodgkin’s disease in Korea and its association with Epstein-Barr virus infection. Cancer. 1996;77:949–955. doi: 10.1002/(sici)1097-0142(19960301)77:5<949::aid-cncr22>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 37.Quintanilla-Martinez L, Gamboa-Dominguez A, Gamez-Ledes-ma I, Angeles-Angeles A, Mohar A. Association of Epstein-Barr virus latent membrane protein and Hodgkin’s disease in Mexico. Mod. Pathol. 1996;8:675–679. [PubMed] [Google Scholar]
- 38.Park C.S, Juhng S.W, Brigati D.J, Montone K.T. Analysis of Ep-stein-Barr virus in Hodgkin’s disease: experience of a single university hospital in Korea. J. Clin. Lab. Anal. 1994;8:412–417. doi: 10.1002/jcla.1860080612. [DOI] [PubMed] [Google Scholar]
- 39.Leoncini L, Spina D, Nyong’o A, et al. Neoplastic cells of Hodg-kin’s disease show differences in EBV expression between Kenya and Italy. Int. J. Cancer. 1996;65:781–784. doi: 10.1002/(SICI)1097-0215(19960315)65:6<781::AID-IJC13>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 40.Chang K.L, Albujar P.F, Chen Y.Y, Johnson R.M, Weiss L.M. High prevalence of Epstein-Barr virus in the Reed-Sternberg cells of Hodgkin’s disease occurring in Peru. Blood. 1993;81:496–501. [PubMed] [Google Scholar]
- 41.Leoncini L, Spina D, Nyong’o A, et al. Neoplastic cells of Hodg-kin’s disease show differences in EBV expression between Kenya and Italy. Int J Cancer. 1996;65:781–784. doi: 10.1002/(SICI)1097-0215(19960315)65:6<781::AID-IJC13>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 42.Khan G, Norton A.J, Slavin G. Epstein-Barr virus in Hodgkin’s Disease. Relation to age and subtype. Cancer. 1993;71:3124–3129. doi: 10.1002/1097-0142(19930515)71:10<3124::aid-cncr2820711038>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 43.Andriko J.A, Aguilera N.S, Nandedkar M.A, Abbondanzo S.L. Childhood Hodgkin’s disease in the United States: an analysis of histological subtypes and association with Epstein-Barr virus. Mod. Pathol. 1997;10:366–371. [PubMed] [Google Scholar]
- 44.Chan J.K.C, Yip T.T.C, Tsang W.Y.W, Lau W.H, Wong G.S.C, Ma V.W.S. Detection of Epstein-Barr virus in Hodgkin’s disease occurring in an Oriental population. Hum. Pathol. 1995;26:314–318. doi: 10.1016/0046-8177(95)90064-0. [DOI] [PubMed] [Google Scholar]
- 45.Cozen W, Katz J, Mack TM. Risk patterns of Hodgkin’s disease in Los Angeles vary by cell type. Cancer Epidemiol. Bio. Prev. 1992;1:261–268. [PubMed] [Google Scholar]
- 46.Glaser R, Kutz L.A, MacCallum R.C, Malarkey W.B. Hormonal modulation of Epstein-Barr virus replication. Neuroendocrinology. 1995;62:356–361. doi: 10.1159/000127025. [DOI] [PubMed] [Google Scholar]
- 47.Weinreb M, Day P.J, Niggli F. The role of Epstein-Barr virus in Hodgkin’s disease from defferent geografical areas. Arch. Dis. Child. 1996;74:27–31. doi: 10.1136/adc.74.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Engel M, Essop M.F, Close P, Hartley P, Pallesen G, SinclairSmith C. Improved prognosis of Epstein-Barr virus associated childhood Hodgkin’s lymphoma: study of 47 South African cases. J. Clin. Pathol. 2000;53:182–186. doi: 10.1136/jcp.53.3.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Razzouk B.I, Gan Y.J, Mendonca C. Epstein-Barr virus in pediatric Hodgkin’s disease: age and histiotype are more predictive than geographic region. Med. Pediatr. Oncol. 1997;28:248–254. doi: 10.1002/(sici)1096-911x(199704)28:4<248::aid-mpo2>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 50.Kanavaros P, Sakalidou A, Gaulard P, et al. Expression of the Epstein-Barr virus encoded latent membrane protein in tumor cells of Hodgkin’s disease occurring in childhood. Acta Morphol. Hung. 1992;40:223–230. [PubMed] [Google Scholar]
- 51.Brousset P, Rochaix P, Chittal S, Rubie H, Robert A, Delsol G. High incidence of Epstein-Barr virus detection in Hodgkin’s disease and absence of detection in anaplastic large cell lymphoma in children. Histopathology. 1993;23:189–191. doi: 10.1111/j.1365-2559.1993.tb00480.x. [DOI] [PubMed] [Google Scholar]
- 52.Preciado M.V, Diez B, Grinstein S. Epstein Barr virus in Argentine pediatric Hodgkin’s disease. Leuk. Lymphoma. 1997;24:283–290. doi: 10.3109/10428199709039015. [DOI] [PubMed] [Google Scholar]
- 53.Li P.J, Zhou X.G, Liu S.R. The association of Epstein-Barr virus with Hodgkin’s lymphoma in childhood. Chung. Hua. Ping. Li. Hsueh. Tsa. Chih. 1994;23:224–226. [PubMed] [Google Scholar]
- 54.Weinreb M, Day P.J, Niggli F, et al. The consistent association between Epstein-Barr virus and Hodgkin’s disease in children in Kenya. Blood. 1996;87:3828–3836. [PubMed] [Google Scholar]
- 55.Valente G, Negro F, Pacchioni D, Palestro G. Infection by Ep-stein-Barr virus in Hodgkin’s disease is not restricted to the ReedSternberg cells. Br. J. Hematol. 1994;86:405–406. doi: 10.1111/j.1365-2141.1994.tb04753.x. [DOI] [PubMed] [Google Scholar]
- 56.Meggetto F, Brousset P, Selves J, Delsol G, Mariame B. Reed-Strenberg cells and “bystender” lymphocytes in lymph nodes affected by Hodgkin’s disease are infected with different strains of Epstein-Barr virus. J. Virol. 1997;71:2547–2549. doi: 10.1128/jvi.71.3.2547-2549.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]


