Abstract
BACKGROUND
Remote ischemic preconditioning (RIPC) can inhibit recurrent ischemic events effectively in patients with acute or chronic cerebral ischemia. However, it is still unclear whether RIPC can impede ischemic injury after carotid artery stenting (CAS) in patients with severe carotid artery stenosis.
METHODS
Subjects with severe carotid artery stenosis were recruited in this randomized controlled study, and assigned to RIPC, sham, and no intervention (control) groups. All subjects received standard medical therapy. Subjects in the RIPC and sham groups underwent RIPC and sham RIPC twice daily, respectively, for 2 weeks before CAS. Plasma neuron-specific enolase and S-100B were used to evaluate safety, hypersensitive C-reactive protein, and new ischemic diffusion-weighted imaging lesions were used to determine treatment efficacy. The primary outcomes were the presence of ≥1 newly ischemic brain lesions on diffusion-weighted imaging within 48 hours after stenting and clinical events within 6 months after stenting.
RESULTS
We randomly assigned 189 subjects in this study (63 subjects in each group). Both RIPC and sham RIPC procedures were well tolerated and completed with high compliance (98.41% and 95.24%, respectively). Neither plasma neuron-specific enolase levels nor S-100B levels changed significantly before and after treatment. No severe adverse event was attributed to RIPC and sham RIPC procedures. The incidence of new diffusion-weighted imaging lesions in the RIPC group (15.87%) was significantly lower than in the sham group (36.51%; relative risk, 0.44; 96% confidence interval, 0.20–0.91; P<0.01) and the control group (41.27%; relative risk, 0.39; 96% confidence interval, 0.21–0.82; P<0.01). The volumes of lesions were smaller in the RIPC group than in the control and sham groups (P<0.01 each). Ischemic events that occurred after CAS were 1 transient ischemic attack in the RIPC group, 2 strokes in the control group, and 2 strokes and 1 transient ischemic attack in the sham group, but these results were not significantly different among the 3 groups (P=0.597).
CONCLUSIONS
RIPC is safe in patients undergoing CAS, which may be able to decrease ischemic brain injury secondary to CAS. However, the mechanisms and effects of RIPC on clinical outcomes in this cohort of patients need further investigation.
CLINICAL TRIAL REGISTRATION
URL: http://www.clinicaltrials.gov. Unique identifier: NCT01654666
Keywords: carotid stenosis, cerebral embolism, ischemic preconditioning, stents
Carotid artery stenting (CAS) is a widely used procedure for treating carotid artery disease, and embolization is a common perioperative complication.1,2 Embolization can cause ischemic cerebrovascular events that may lead to neurological or cognitive disability and negate the therapeutic benefits.3,4 Several strategies, including dual antiplatelet therapy, intraoperative anticoagulation, and embolic protection device placement, are implemented to reduce the risk of embolization; however, new brain lesions on cerebral diffusion-weighted imaging (DWI) and their clinical consequences (ie, stroke or transient ischemic attack) remain high.5–7 Therefore, alternative strategies are urgently needed.
Remote ischemic preconditioning (RIPC) is a protective systemic strategy by which ≥1 cycles of brief, nonlethal limb ischemia confer protection against subsequent prolonged, severe ischemia in distant organs.8–10 The mechanisms involved in providing RIPC-induced distant organ protection are quite complex and interlinked, but their effect on inflammatory responses may be one of the most important ones.11,12 Recently, several studies have demonstrated that RIPC is a promising strategy to reduce the deleterious effects of embolization associated with percutaneous coronary interventions.13,14 A phase 2 study by our group showed that RIPC twice daily for 300 days may reduce stroke recurrence by improving cerebral perfusion in patients with symptomatic atherosclerotic intracranial artery stenosis.10 Although another study suggested that 4 sessions of RIPC for 35 minutes in the prehospital phase was neutral, post hoc analysis suggests there might be a neuroprotective effect.15 In addition, another study also showed that RIPC could reduce plasma inflammatory markers in octo- and nonagenarians with symptomatic atherosclerotic intracranial artery stenosis.16 However, whether RIPC is safe and effective for patients undergoing CAS is still unknown.
Among patients with carotid stenosis, plasma hypersensitive C-reactive protein (hs-CRP) has been a sensitive biomarker of inflammatory response, which reflects the stability of plaques and the injury of vascular endothelium.17 Impairment of blood-brain barrier integrity and neuronal damage can be detected by the elevation of plasma neuron-specific enolase (NSE) and S-100B.18,19 In this proof-of-concept, randomized, 3-arm (RIPC, sham, control group) clinical trial, we tested whether RIPC was safe and effective to reduce ischemic brain lesions on DWI after a CAS procedure, improve clinical outcomes at 6 months, and decrease plasma hs-CRP levels in subjects who underwent CAS. In addition, we examined plasma NSE and S-100B to determine the effects of RIPC on brain injury.
METHODS
Subjects
Subjects were eligible for enrollment if they had symptomatic or asymptomatic atheromatous carotid artery stenosis measured as ≥70% by digital subtraction angiography or by other noninvasive methods, such as duplex ultrasound, computed tomography angiography, or magnetic resonance angiography. The rate of stenosis was calculated based on the NASCET study (North American Symptomatic Carotid Endarterectomy Trial) criteria.20 Symptomatic carotid artery stenosis was defined as the occurrence of a transient ischemic attack (TIA),21 amaurosis fugax,22 and ischemic stroke23 attributed to a proximal and corresponding carotid artery lesion, which occurred within 6 months before randomization. Subjects with eligible carotid stenosis but who did not meet the definition of symptomatic cases were considered as asymptomatic. Additional inclusion criteria included (1) ≥18 years of age; (2) tolerance to any of the necessary medications, including clopidogrel, aspirin, and statins; (3) ability to complete a brain MRI examination; (4) a negative pregnancy test within 7 days before randomization for any woman with childbearing potential; (5) stable vital signs, and normal renal and hepatic functions; and (6) subject or his or her legally authorized representative was able to provide an informed consent.
Subjects who met any of the following exclusion criteria were excluded: (1) evolving stroke; (2) prior ipsilateral stroke with residual deficits; (3) severe dementia at enrollment; (4) bleeding disorder; (5) chronic atrial fibrillation; (6) myocardial infarction within previous 30 days; (7) uncontrolled hypertension (defined as systolic blood pressure ≥200 mm Hg despite medications at enrollment); (8) participation in another device or drug trial simultaneously; (9) any condition that hampers proper angiographic assessment or made percutaneous arterial access unsafe; (10) high-risk candidate as defined by the CREST study (Carotid Revascularization Endarterectomy versus Stenting Trial)24; (11) any vascular, soft tissue, or orthopedic injury (eg, superficial wounds and fractures of the arm) that contraindicated bilateral arm ischemic preconditioning; and (12) peripheral vascular disease (especially subclavian arterial and upper limb artery stenosis or occlusion).
Study Design
This was a proof-of-concept, phase 2, assessor-blinded, randomized controlled clinical trial. To eliminate the influence of psychological factors, a sham RIPC group was included in which the intracuff pressure was only inflated to 60 mm Hg. To determine whether intracuff pressure of 60 mm Hg on arms, which does not block blood perfusion but causes an oppressive feeling, could produce neuroprotective effects; a no-intervention (control) group was also designed in the present study. All enrolled subjects were randomly assigned in a 1:1:1 ratio to the RIPC group, the control group, and the sham group, and followed for 6 months after CAS procedures. Randomization was performed by opaque envelopes that concealed the group allocation. This study was approved by the Ethics Committee of Xuanwu Hospital of Capital Medical University. All subjects or their legally authorized representative provided informed consent before enrollment. The trial was registered at www.ClinicalTrial.gov (Unique identifier: NCT01654666)
Although there was a profound difference between the pressure point used in the RIPC group and that used in the sham group (200 versus 60 mm Hg), subjects were not made aware of either the exact values or what it took to obtain an optimal ischemic event. Interventionists were responsible for CAS procedures, and investigators were responsible for evaluating the results of MRI and clinical events; they were all blinded to the treatment assignment.
Interventions
All subjects received standard medical therapy including modifiable risk factor management, antiplatelets, and statins. Administration of antihypertensive agents and antidiabetic agents were elective at the discretion of the treating physician. In addition, after the exclusion of subjects with intravascular thrombosis and unstable plaques in blood vessels of both arms, detected by vascular ultrasound, subjects in the RIPC group and the sham group underwent RIPC and sham RIPC, respectively, twice daily for 2 weeks before CAS. The RIPC consisted of 5 cycles of simultaneous bilateral upper arm ischemia for 5 minutes followed by reperfusion for another 5 minutes. The procedure was performed by using an electric autocontrol device with cuffs that inflated to a pressure of 200 mm Hg during the ischemic period (Patent No. CN200820123637.X, China).10,16 This was done with assistance from a hospital-based nurse or a caregiver at home. The device recorded and documented each RIPC cycle, and the patient’s heart rate and blood pressure, as well, in real time. The RIPC process could be stopped at any time if the subject experienced any major discomfort. Subjects in the sham group (n=63) underwent a sham RIPC procedure during which bilateral upper arm cuffs were inflated to a pressure of 60 mm Hg for 5 minutes, followed by 5 minutes of relaxation of the cuffs, for a total of 5 cycles twice daily until the day before CAS.10,16 The same device was used in this study with different cuff pressure settings, one for active and the other one for sham.
All subjects received standard medication treatment including atorvastatin 20 mg daily, aspirin 100 mg daily for 2 weeks, and clopidogrel 75 mg daily for 4 days before the CAS procedure. After CAS, all subjects continued this standard statin and dual antiplatelet therapy for at least 3 months, and then changed to aspirin 100 mg daily and atorvastatin 20 mg daily continuously. Interventionists who had conducted at least 300 successful CAS were allowed to perform the procedures for this study. Local anesthesia was used in all subjects, and stenting was performed via the transfemoral approach with self-expanding stents. A cerebral protection device was mandatory during procedures. The type and size of stents, protective devices, and other devices, and the strategies of intervention were left to the discretion of the interventionists. In the beginning, angiograms of the target carotid lesion and intracranial arteries were performed to evaluate the severity and morphology of the target stenosis and intracranial branches. Angiograms were performed again at the end of the procedure to reevaluate the stenosis and compared with prestenting. Intravenous heparin to maintain the activated clotting time from 250 to 300 s during the procedure was mandatory.
Imaging
All subjects were scanned within 72 hours before and within 48 hours after CAS by a 3T MRI scanner with a dedicated 4-channel phased-array head coil (Trio system; Siemens Magnetom scanner). The pre- and posttreatment MRI sequences are: axial spin-echo T1-weighted, fast-spin T2-weighted, fluid-attenuated inversion recovery, DWI, and apparent diffusion coefficient. DWI was acquired with an echo-planar sequence. An isotropic sequence was used, with b value of 0, 500, and 1000 s/mm2, repetition time 3000 ms, echo time 80 ms, number of excitation 4, slice thickness 5 mm, gap 1.5 mm, 160×160 matrix, and 240 mm×240 mm field of view. A new brain lesion was diagnosed if increased signal intensity was visible on DWI with correspondent decreased signal on apparent diffusion coefficient, and if such lesion was not seen on the pretreatment scan. On each scan, the number of new DWI lesions, volume of single lesions, and volume of all lesions were measured on DWI. Lesions were considered separate if there was no continuity between them on adjacent slices or on the same slice.7 Volumes of distinct lesions were calculated by manually tracing the lesions with the internal measuring function of the MRI scanner and multiplying the area by slice thickness. A neuroradiologist and a neurologist, both masked to the treatment assignment, analyzed the image data separately. Disagreement was resolved by reaching a consensus between the 2 of them, or, if no consensus could be reached, another reviewer had the final decision.
Blood Samples and Plasma Testing
Blood samples were drawn from the cubital vein; the points of measurement included baseline, pre-CAS, right 1 hour and 24 hours post-CAS. These samples were centrifuged immediately after collection, and serum hs-CRP and NSE levels were examined in fresh plasma samples, whereas plasma S-100B levels were tested in samples stored at −80°C until batch evaluation.
Outcomes Assessment
Safety Outcomes Assessment
The following adverse events were defined as safety outcomes16: (1) elevation of plasma NSE and S-100B levels beyond normal limits after RIPC and sham RIPC procedure; (2) inability to tolerate RIPC or sham RIPC procedure that leads to the discontinuation from the study; (3) objective signs of tissue or neurovascular injury resulting from RIPC and sham RIPC procedure. An inspection that was done by staffs blinded to the study protocol included palpation of distal radial pulses, visual inspection for local edema, erythema and skin lesions, and palpation for tenderness.
These safety outcomes were evaluated by observers blind to the treatment assignment, and any suspicious adverse event associated with RIPC or sham RIPC procedure was reported to the investigators.
Efficacy Outcomes Assessment
The primary outcome was the presence of ≥1 new brain lesions on DWI within 48 hours after CAS and the incidence of clinical events (ie, ischemic stroke, TIA, acute myocardial infarction, hemorrhagic stroke, hyperperfusion syndrome, and death) within 6 months after CAS. Ischemic stroke was defined as a clinical episode of neurological dysfunction caused by focal cerebral, spinal, or retinal infarction on DWI.23 TIA was defined as a transient episode of neurological dysfunction caused by focal brain, spinal cord, or retinal ischemia, without corresponding acute infarction on DWI.21
The secondary outcomes were the number and volume of new brain lesions on DWI within 48 hours; the changes in plasma hs-CRP levels, NSE levels, and S-100B levels; and adverse events within 6 months.
All efficacy outcomes were assessed by 2 observers blind to the treatment assignment; any disagreement was resolved by reaching a consensus between the 2 of them, or if no consensus could be reached, another observer had the final decision.
Statistical Analysis
All statistical comparisons were made among 3 groups, and multiple comparisons were made if an overall significant difference was detected. Categorical variables were presented as counts and percentages, and analyzed with the χ2 test or continuity correction where appropriate. Continuous variables were presented as mean and standard deviation or median and interquartile range, and compared among 3 groups with analysis of variance or Kruskal-Wallis test. For the primary outcome measure, missing data were regarded as no clinical event happened or no new brain lesion on DWI scans for primary outcome data; for continuous outcome data, missing data were imputed by mean or median of the nonmissing values.
Two coprimary efficacy outcomes were used in this study. The primary outcomes were analyzed by both the intention-to-treat and per-protocol analysis. When assessing the primary outcomes, we compared the incidences of stroke and TIA between groups via continuity correction and new brain lesions between groups via χ2 tests. These coprimary outcomes were analyzed via a modified Hochberg procedure to ensure noninflation of the overall 5% type I error rate.25 In comparison with clinical events that were more likely subjected to other confounding factors, the new brain lesions on post-CAS scans were considered to be a more sensitive method to assess the efficacy of RIPC, whose biological effects are considered to be limited to 48 to 72 hours after the procedures.26 Therefore, the overall 5% type I error rate was divided into 2 parts: (1) a 4% significance level would be used for the analysis of new brain lesions on post-CAS scans within 48 hours, and (2) a 1% significance level would be used for the analysis of the clinical events within 6 months after CAS. Relative risk (RR) and 96% confidence interval (CI) were calculated with the incidences of new brain lesions, with RR <1 indicating a treatment effect favoring RIPC, and the upper limit of 96% CI <1 was considered statistical significance. We used the Kruskal-Wallis test to compare the number and volumes of new brain lesions among 3 groups. Plasma hs-CRP levels were presented as median and interquartile range, and were compared by using the Kruskal-Wallis test. Plasma NSE and S-100B were presented as mean and standard deviation, and compared by using analysis of variance. To test the difference of plasma biomarkers of different measure points in the same group, paired t tests were used for NSE and S-100B, and Wilcoxon signed-rank test was used for hs-CRP. For new brain lesions, a P value of <0.04 was considered a statistically significant difference. For clinical events, a P value of <0.01 was considered a statistically significant difference. For other outcomes, a P value of <0.05 was considered a statistically significant difference. If there were statistically significant differences among 3 groups, multiple comparisons would be performed and the P values were adjusted via the Bonferroni method. The statistical analyses were conducted with SPSS statistics software for Windows version 19.0 (IBM, Inc).
Sample size and power were calculated based on previous studies6,7,10,15,16 and new brain lesions on post-CAS scans, which were much better to indicate the efficacy of RIPC. We expected ≈20% of subjects in the RIPC group and 50% of subjects in the other 2 groups would have new brain lesions on post-CAS DWI scans. The intended target sample size was 189 subjects allowing 15% loss to follow-up and with 90% power and an α of 0.04 (2-sided) of significance.
RESULTS
From August 1, 2012, to December 20, 2014, 320 subjects were screened in Xuanwu Hospital of Capital Medical University, and 236 subjects met the inclusion criteria. Thirty-five subjects were excluded because of exclusion criteria. Of the 201 subjects invited, 12 subjects refused consent. Last, 189 (59.06%) subjects were randomly assigned equally to the RIPC group (RIPC plus standard medical therapy), the sham group (sham RIPC plus standard medical therapy), and the control group (standard medical therapy alone) (Figure 1).
Figure 1. Enrollment and randomization.
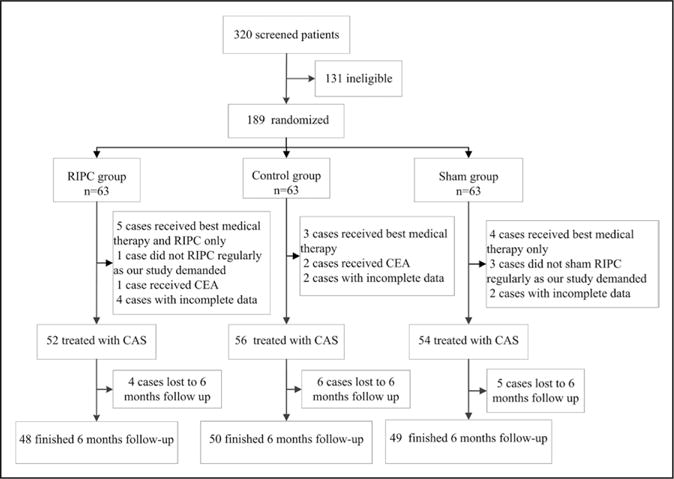
CAS indicates carotid artery stenting; CEA, carotid endarterectomy; and RIPC, remote ischemic preconditioning.
Baseline Characteristics
Baseline characteristics of 189 subjects were summarized in Table 1. Age, concomitant risk factors, clinical manifestation, type of aortic arch,27 and coexistence of intracranial and extracranial artery stenosis or occlusion, did not differ significantly among the 3 groups, with the exception that the plasma high-density lipoprotein levels were significantly lower in the RIPC group than in the sham group. The type of stents and embolic protection devices and their sizes were presented in the online-only Data Supplement, both closed-cell stents (ie, Carotid Wallstent, Boston Scientific Corp) and open-cell stents (ie, Precise, Cordis; Protégé EV3; and Acculink, Abbott Vascular) and 4 types of embolic protection devices (ie, Spider EV3; FilterWire, Boston Scientific Corp; Accunet, Abbott Vascular; Angioguard, Cordis) were used for CAS procedures. The types and sizes of these materials had no significant differences among 3 groups (online-only Data Supplement Tables I and II).
Table 1.
Baseline Characteristics
| RIPC Group n=63 | Control Group n=63 | Sham Group n=63 | P Value | |
|---|---|---|---|---|
| Age (in years) | 67.5±8.6 | 66.5±8.6 | 65.7±8.2 | 0.511 |
| Male (%) | 46 (73.0) | 45 (71.4) | 44 (69.8) | 0.925 |
| Serum glucose, mmol/L | 5.68±1.44 | 5.58±1.23 | 5.31±1.20 | 0.243 |
| TG, mmol/L | 3.64±0.91 | 3.51±0.93 | 3.52±1.04 | 0.691 |
| LDL, mmol/L | 2.11±0.75 | 2.06±0.75 | 2.16±0.85 | 0.779 |
| HDL, mmol/L | 1.34±0.33 | 1.28±0.38 | 1.47±0.42 | 0.022 |
| Cholesterol, mmol/L | 1.65±0.88 | 1.46±0.83 | 1.44±0.66 | 0.268 |
| Homocysteine | 14.94±4.77 | 16.46±7.88 | 16.93±6.04 | 0.187 |
| Vascular risk factors | ||||
| Hypertension | 42 (66.7) | 39 (61.9) | 45 (71.4) | 0.526 |
| Diabetes mellitus | 20 (31.7) | 21 (33.3) | 24 (38.1) | 0.737 |
| Hypercholesterolemia | 22 (34.9) | 24 (38.1) | 19 (30.2) | 0.640 |
| Smoking (past and present) | 33 (52.4) | 30 (47.6) | 29 (46.0) | 0.759 |
| Coronary heart disease | 9 (14.3) | 7 (11.1) | 5 (7.9) | 0.526 |
| Systolic blood pressure at randomization (mm Hg) | 133.6±13.3 | 133.3±16.5 | 132.0±16.5 | 0.823 |
| Carotid disease | ||||
| Symptomatic | 41(65.1) | 45(71.4) | 42 (66.7) | 0.730 |
| Asymptomatic | 22(34.9) | 18(28.6) | 21 (33.3) | 0.730 |
| Modified Rankin Scale at randomization | ||||
| 0 | 51 (81.0) | 50 (79.4) | 49 (77.8) | 0.908 |
| 1 | 10 (15.9) | 11 (17.5) | 11 (17.5) | 0.963 |
| 2 | 2 (3.18) | 1 (1.59) | 2 (3.18) | — |
| 3 | 0 (0) | 1 (1.59) | 1 (1.59) | — |
| Type of aortic arch27 | ||||
| Type I | 26 (41.3) | 28 (44.4) | 31 (49.2) | 0.666 |
| Type II | 20 (31.7) | 24 (38.1) | 16 (25.4) | 0.310 |
| Type III | 6 (9.5) | 4 (6.3) | 7 (11.1) | 0.636 |
| Location of target stenosis* | ||||
| L-ICA | 30 (47.6) | 28 (44.4) | 34 (54.0) | 0.553 |
| R-ICA | 33 (52.4) | 35 (55.6) | 29 (46.0) | 0.553 |
| Coexistence of stenosis† | ||||
| Intracranial artery | 2 (3.2) | 1 (1.6) | 4 (6.3) | 0.354 |
| Extracranial artery | 22 (34.9) | 19 (30.1) | 20 (31.7) | 0.844 |
| Coexistence of occlusion† | ||||
| Intracranial artery | 4 (6.3) | 2 (3.2) | 3 (4.8) | 0.705 |
| Extracranial artery | 11 (17.5) | 9 (14.3) | 13 (20.6) | 0.644 |
| Interval between randomization and treatment | 19 (16.0–22.0) | 18.5 (15.3–22.7) | 18 (15.0–21.3) | 0.536 |
| Interval between CAS and post-CAS scan, d | 1 (1–2) | 1 (1–2) | 1 (1–2) | 0.531 |
| DWI lesion on pretreatment scan‡ | 2 (3.2) | 2 (3.2) | 1 (1.6) | 0.814 |
Data are mean±SD, n (%), or median (IQR). CAS indicates carotid artery stenting; DWI, diffuse-weighted imaging; HDL, high-density lipoprotein; ICA, internal carotid artery; IQR, interquartile range; L, left; LDL, low-density lipoprotein; R, right; RIPC, remote ischemic preconditioning; and TG, triglyceride.
The degrees of stenosis are calculated according to the NASCET (North American Symptomatic Carotid Endarterectomy Trial) method.
Intracranial artery including intracranial segments of internal carotid artery and vertebral artery, basilar artery and main stems of anterior cerebral artery, middle cerebral artery, and posterior cerebral artery; extracranial artery including common carotid artery, subclavian artery, extracranial segment of vertebral artery, and innominate artery.
To reduce the risk of hemorrhage after CAS, the scans were evaluated by interventionists to determine whether the operation should be postponed.
Safety Outcomes
Compliance and Adverse Events
Both the RIPC and sham procedures were well tolerated. Only 1 subject in the RIPC group and 3 subjects in the sham group did not undergo the procedure per the treatment protocol. Both the RIPC and sham RIPC procedures were completed with a high compliance rate (98.41% and 95.24%, respectively). Six subjects in the RIPC group experienced arm skin petechiae from repeated pressure cuff applications, and these petechiae disappeared 2 weeks after stopping the RIPC procedure. No ecchymosis, tenderness to palpation, edema, skin breakage, or other skin lesions were observed.10,16
During the RIPC procedure, no subjects in the RIPC group experienced a stroke in comparison with the other 2 groups, and no subjects experienced cardiovascular events or died before the CAS procedure.
Plasma NSE and S-100B
At baseline, plasma NSE and S-100B levels were not significantly different among the 3 groups (online-only Data Supplement Tables III and IV, P>0.05 each). At 1 and 24 hours after CAS, the plasma NSE and S-100B levels changed nonsignificantly (P>0.05 each) as well.
Efficacy Outcomes
Imaging Results
The interval between the CAS procedure and post-CAS scans was similar among the 3 groups (median 1 day and interquartile range 1–2 days in each group, P=0.531). Fifty-nine (31.22%) of 189 subjects had ≥1 new DWI lesions on posttreatment scans. Within this population, 15.87% of subjects in the RIPC group had at least 1 new DWI lesion versus 41.27% of subjects in the control group (RR, 0.39; 96% CI, 0.21–0.82; P=0.002) and 36.51% of subjects in the sham group (RR, 0.44; 96% CI, 0.20–0.91; P=0.008), but the incidence was not significant between the control and sham group (RR, 1.13; 96% CI, 0.72–1.93; P=0.584) (Table 2 and Figure 2). Per-protocol analysis of the incidences of new DWI lesions was listed in the online-only Data Supplement and online-only Data Supplement Table V.
Table 2.
Primary Outcomes and Other Clinical Outcomes
| RIPC Group n=63 | Control Group n=63 | Sham Group n=63 | RR (96% CI) | P | |
|---|---|---|---|---|---|
| At least 1 new lesion* | 10 (15.87) | 26 (41.27) | 23 (36.51) | 0.39 (0.21–0.82)† 0.44 (0.20–0.91)‡ 1.13 (0.72–1.93)§ |
0.005 |
| Stroke/TIA | 1 (1.59) | 2 (3.17) | 3 (4.76) | ||
| Any ischemic stroke | 0 | 2 (3.17) | 2 (3.17) | ||
| TIA | 1 (1.59) | 0 | 1 (1.59) | ||
| Hemorrhage or hyperperfusion | 0 | 1 (1.59) | 0 | ||
| All-cause death and ischemic cardiovascular events | 0 | 0 | 0 |
Data are n (%). CI indicates confidence interval; DWI, diffuse-weighted imaging; RIPC, remote ischemic preconditioning; RR, relative risk; and TIA, transient ischemic attack.
New lesion was only diagnosed if (1) increased signal intensity was visible in DWI and corresponding decreased signal intensity on apparent diffusion coefficient and (2) if the lesion was not seen on pretreatment scan.
Comparison between the RIPC group and the control group.
Comparison between the RIPC group and the sham group.
Comparison between the sham group and the control group.
Figure 2. Comparisons of new brain lesions on posttreatment scans.
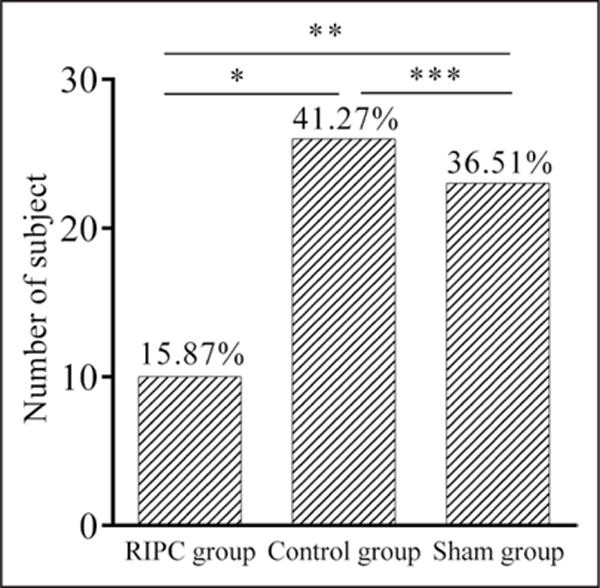
RIPC indicates remote ischemic preconditioning. *Comparison between the RIPC group and control group, P=0.002. **Comparison between the RIPC group and sham group, P=0.008. ***Comparison between the control group and sham group, P=0.584.
The median numbers of new lesions were 1.5 (1.0–3.0) in the RIPC group, 2.0 (1.0–5.5) in the control group, and 2.0 (1.0–4.0) in the sham group. Overall, no significant differences in the number of new DWI lesions among 3 groups (P=0.380) were detected. The volume of single lesions was 0.03 mL (0.02–0.05) in the RIPC group in comparison with 0.07 mL (0.05–0.10) in the control group (P<0.001) and 0.08 mL (0.06–0.12) in the sham group (P<0.001). In the RIPC group, volume of all lesions was 0.06 mL (0.03–0.11) in comparison with 0.17 mL (0.10–0.56) in the control group (P=0.002) and 0.16 mL (0.07–0.42) in the sham group (P=0.012). Overall, subjects treated by RIPC in the RIPC group had smaller lesions per person than did subjects in the other 2 groups. In addition, the volume of single lesions and total lesions between the control group and the sham group had no significant difference (P=0.054 and P=0.326, respectively) (Table 3).
Table 3.
Number and Volume of New Brain Lesions
| RIPC Group n=63 |
Control Group n=63 |
Sham Group n=63 |
P Value* | |
|---|---|---|---|---|
| Number of lesions | 1.5 (1.0–3.0) | 2.0 (1.0–5.5) | 2.0 (1.0–4.0) | 0.380 |
| Single lesion volume (mL)† | 0.03 (0.02–0.05) | 0.07 (0.05–0.10) | 0.08 (0.06–0.12) |
P1<0.001 P2<0.001 |
| Total lesions volume (mL)‡ | 0.06 (0.03–0.11) | 0.17 (0.10–0.56) | 0.16 (0.07–0.42) |
P1=0.002 P2=0.012 |
Data are medians (IQR). IQR indicates interquartile range; and RIPC, remote ischemic preconditioning.
P1 indicates the comparison between the RIPC group and the control group. P2 is the comparison between the RIPC group and the sham group.
The volume of single lesions.
The volume of all new lesions in each subject.
Clinical Events
Forty-eight subjects in the RIPC group, 50 subjects in the control group, and 49 subjects in the sham group were followed up completely over 6 months (Figure 1). Six (3.17%) of 189 subjects experienced ischemic cerebrovascular events. One subject in the RIPC group had a TIA, in comparison with 2 subjects having ischemic stroke in the control group (P=1.00) and 3 subjects having ischemic events (2 strokes and 1 TIA) in the sham group (P=0.611). The direction of effect favored RIPC treatment, but the between-group differences were not significant because of the small number of events. No significant difference was detected between the control group and the sham group as well (P=1.00) (Table 2). Per-protocol analysis of clinical events was listed in the online-only Data Supplement and online-only Data Supplement Table VI.
No subject died of or experienced ischemic cardiovascular events and hemorrhagic stroke within 6 months after CAS. However, 1 patient without a history of epilepsy in the control group had a seizure on day 16 after CAS.
Plasma hs-CRP Results
The plasma hs-CRP levels were not significantly different among 3 groups at baseline (online-only Data Supplement Table VII, P=0.893). At the other measurement points, although the plasma hs-CRP levels in the RIPC group were lower than in the other 2 groups, no statistically significant difference was found (online-only Data Supplement Table VII, P>0.10 each). The plasma hs-CRP increased significantly at 24 hours after CAS in comparison with 1 hour after CAS (P<0.001) in all groups.
DISCUSSION
In this randomized sham-control and blank-control clinical trial, it was safe to perform RIPC in patients undergoing CAS, and RIPC was associated with a significant reduction in new brain lesions and lesion sizes on MRI early after CAS, but did not significantly reduce the occurrence of TIAs/strokes at 6 months.
With the exception that 6 subjects in the RIPC group experienced cuff extrusion–related petechiae on local arms, which similarly occurred in several previous studies,10,16,28 we failed to observe any other severe local or systematic adverse event associated with RIPC procedures. There was no significant difference in the plasma S-100B and NSE levels between baseline and pre-CAS, suggesting that RIPC does not induce any brain injury and damage to the integrity of the blood-brain barrier. In addition, the majority of subjects tolerated the RIPC or sham RIPC procedure. RIPC appears to be a safe, low-cost, and easy-to-use strategy that can be used in patients undergoing CAS.
We found that both the volumes of single lesions and those of multiple lesions combined in each subject were smaller in the RIPC group than in the other 2 groups, and the incidence of new DWI lesions was significantly lower in the RIPC group. However, when lesions were present, the number of new DWI lesions in RIPC group was not significantly different than that in the sham and control groups. Similar findings of reduced lesional volumes but not reduced number of lesions were found by other investigators in testing with other putative protective agents (reloaded statins).29 The underlying causes for this discrepancy are not entirely clear; a possible explanation is that the brain-protective effect from RIPC is adequate to shrink the volume of the embolic infarction but not to eliminate the embolism. Given that a nonsignificant reduction in the number of lesions between RIPC, sham, and control groups was seen (1.5 versus 2 versus 2), another possibility is that the study was underpowered to detect a statistically significant difference.
The clinical outcome analysis indicates that RIPC may have potential effects on reducing the risk of ischemic stroke and TIA, even though the protective effect was not statistically significant, insufficient sample size may be one of the reasons explaining the lack of statistical significance.7,30 In addition, the low rate of events and the nature of short protective duration might also be other reasons for the lack of a statistically significant difference in the observed clinical events.26 Therefore, our preliminary findings need a confirmation from an adequately powered multicenter phase 3 clinical trial.
In this study, ≈40% subjects in the control and sham group had new DWI lesions. As reported in other studies,6,7,31 most of the new brain lesions were tiny and silent. Although these silent lesions generally do not cause immediate disabling neurological deficits, these subjects with silent lesions are at a greater risk of developing cognitive decline and dementia late in life.6,32 In addition, new brain lesions can impair the neural circuits and brain connectivity and increase the risk of various psychiatric (eg, depression) or neurological conditions (eg, gait unsteadiness, urinary incontinence).33,34 Therefore, strategy to reduce these CAS-related brain lesions can be beneficial in the reduction of insidious or subtle clinical complications. In this study, RIPC can reduce the incidence of brain lesions, and the volume of single lesions and total lesions, as well; it may have potential to reduce the cognitive impairment in patients undergoing CAS. However, in a pilot study, RIPC did not improve cognition function after off-pump coronary artery bypass graft surgery.35 Whether RIPC is a viable strategy to prevent cognitive decline after major surgery needs to be further investigated.
In this study, we found that the plasma hs-CRP levels of pre-CAS were lower than baseline in all groups; this is likely attributed to standard statin management and the relatively better-controlled cerebrovascular risk factors.36 In this study population, however, we found that RIPC cannot significantly decrease plasma hs-CRP levels. One possible explanation is that statins and dual antiplatelet therapy may already reduce plasma hs-CRP levels. Another possible explanation is that, during the CAS procedure, many factors (including large-artery endothelial damage, complications of femoral arterial puncture) can increase plasma hs-CRP levels,37 and this may offset the protective effects of RIPC.
Elevation of plasma NSE levels could be detected several hours after brain injury and reached their peaks 24 hours later, and the plasma S-100B levels increased significantly 2 hours after CAS with a gradual decline over the next hours.38 Besides, there is a tight correlation between the volume of infarct tissue and serum NSE and S100B levels.38,39 However, in the present study, blood samples were drawn 1 hour and 24 hours after CAS, and the majority of cerebral ischemic lesions caused by CAS were tiny. Therefore, it is not surprising to find that the plasma NSE and S-100B levels had no significant difference, even though DWI detected many brain infarction lesions.
Several trials showed that 3 or 4 cycles of 5-minute ischemia and 5-minute reperfusion of upper limb before cardiac surgery or percutaneous coronary intervention was an effective strategy to improve both short- and long-term clinical outcomes, with the exception of stroke or TIA.13,40–43 However, the other 2 trials did show that 5 cycles of 5-minute ischemia and 5-minute reperfusion of bilateral upper limbs for a longer period (180 days or 300 days) can reduce ischemic cerebrovascular events.10,16 We still do not know the optimal RIPC protocol for humans. These disparate results from clinical trials may be attributable to the various RIPC protocols ranging from “several short-lived bouts of ischemic/reperfusion” to “multiple and more prolonged ischemic interventions.”44 In this study, although we found that 5 cycles of 5-minute ischemia and 5-minute reperfusion of bilateral arms for 2 weeks were effective to protect patients undergoing CAS, the optimal dose still needs further investigations.
There are several limitations in this study. First, the RIPC dose tested in this study is rather pragmatic and tailored to the CAS procedure, but it may not be the optimal one. Second, our study was not powered to detect the differences of clinical outcomes, such as stroke/TIA, and the event rate turned out to be lower than we expected. Third, although we found that RIPC can reduce new vascular brain injury on neuroimaging, the underlying mechanisms of such neuroprotection were not well investigated.
CONCLUSION
RIPC, a noninvasive therapy, if done twice daily for 2 weeks before CAS, appears to be a safe, feasible, and effective strategy to reduce the incidence of new brain lesions on MRI early after CAS. The results of this trial warrant a large multicenter randomized controlled phase 3 trial to confirm the efficacy of the RIPC.
Supplementary Material
Clinical Perspective.
What Is New?
Remote ischemic preconditioning is a protective systemic strategy by which cycles of bilateral limbs ischemia are applied briefly to confer protection from subsequent severe ischemia in distant organs.
In this single-center prospective randomized controlled trial, we assessed whether remote ischemic preconditioning is safe and effective in attenuating ischemic injury related to carotid artery stenting (CAS).
We discover, for the first time, that daily remote ischemic preconditioning for 2 weeks before CAS is feasible, safe, and well tolerated, and may effectively attenuate secondary brain injury as evidenced by a decreased incidence and reduced volumes of new ischemic lesions on MRI performed within 48 hours postoperation.
What Are the Clinical Implications?
The clinical implications derived from the findings of this study are that, if results are confirmed by future larger studies, remote ischemic preconditioning can evolve as an emerging nonpharmacological neuroprotectant method for inhibiting CAS-related cerebral ischemic events, which could be incorporated into clinical treatment paradigms during the preoperative period of CAS in the future, to enlarge the benefits and decrease the complication of CAS.
Acknowledgments
We would like to thank all the subjects who agreed to participate in this study. We also thank Dr Xiaoyan Yan from Peking University Clinical Research Institute for providing statistical assistance during preparing the manuscript.
SOURCES OF FUNDING
This study was funded by The National Science Fund for Distinguished Young Scholars (No. 81325007), Chang Jiang Scholars Program (No.T2014251), and Beijing Municipal Science and Technology Commission (No. Z141107001514006).
Footnotes
DISCLOSURES
Dr Ji received research funding (paid to his institution) from the National Science Fund for Distinguished Young Scholars, Chang Jiang Scholars Program, and Beijing Municipal Science & Technology Commission. Dr Ji is one of the inventors of the electric autocontrol device that has been patented in China (CN200820123637.X). The other authors report no conflicts.
The online-only Data Supplement is available with this article at http://circ.ahajournals.org/lookup/suppl/doi:10.1161/CIRCULATIONAHA.116.024807/-/DC1.
References
- 1.Tulip HH, Rosero EB, Higuera AJ, Ilarraza A, Valentine RJ, Timaran CH. Cerebral embolization in asymptomatic versus symptomatic patients after carotid stenting. J Vasc Surg. 2012;56:1579–1584. doi: 10.1016/j.jvs.2012.06.074. discussion 1584. [DOI] [PubMed] [Google Scholar]
- 2.Rosenfield K, Matsumura JS, Chaturvedi S, Riles T, Ansel GM, Metzger DC, Wechsler L, Jaff MR, Gray W, Investigators AI Randomized trial of stent versus surgery for asymptomatic carotid stenosis. N Engl J Med. 2016;374:1011–1020. doi: 10.1056/NEJMoa1515706. [DOI] [PubMed] [Google Scholar]
- 3.Brott TG, Hobson RW, 2nd, Howard G, Roubin GS, Clark WM, Brooks W, Mackey A, Hill MD, Leimgruber PP, Sheffet AJ, Howard VJ, Moore WS, Voeks JH, Hopkins LN, Cutlip DE, Cohen DJ, Popma JJ, Ferguson RD, Cohen SN, Blackshear JL, Silver FL, Mohr JP, Lal BK, Meschia JF, CREST Investigators Stenting versus endarterectomy for treatment of carotid-artery stenosis. N Engl J Med. 2010;363:11–23. doi: 10.1056/NEJMoa0912321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bonati LH, Dobson J, Algra A, Branchereau A, Chatellier G, Fraedrich G, Mali WP, Zeumer H, Brown MM, Mas JL, Ringleb PA. Short-term outcome after stenting versus endarterectomy for symptomatic carotid stenosis: a preplanned meta-analysis of individual patient data. Lancet. 2010;376:1062–1073. doi: 10.1016/S0140-6736(10)61009-4. [DOI] [PubMed] [Google Scholar]
- 5.Bonati LH, Lyrer P, Ederle J, Featherstone R, Brown MM. Percutaneous transluminal balloon angioplasty and stenting for carotid artery stenosis. Cochrane Database Syst Rev. 2012;9:CD000515. doi: 10.1002/14651858.CD000515.pub4. [DOI] [PubMed] [Google Scholar]
- 6.Maggio P, Altamura C, Landi D, Migliore S, Lupoi D, Moffa F, Quintiliani L, Vollaro S, Palazzo P, Altavilla R, Pasqualetti P, Errante Y, Quattrocchi CC, Tibuzzi F, Passarelli F, Arpesani R, di Giambattista G, Grasso FR, Luppi G, Vernieri F. Diffusion-weighted lesions after carotid artery stenting are associated with cognitive impairment. J Neurol Sci. 2013;328:58–63. doi: 10.1016/j.jns.2013.02.019. [DOI] [PubMed] [Google Scholar]
- 7.Bonati LH, Jongen LM, Haller S, Flach HZ, Dobson J, Nederkoorn PJ, Macdonald S, Gaines PA, Waaijer A, Waajier A, Stierli P, Jäger HR, Lyrer PA, Kappelle LJ, Wetzel SG, van der Lugt A, Mali WP, Brown MM, van der Worp HB, Engelter ST, ICSS-MRI study group New ischaemic brain lesions on MRI after stenting or endarterectomy for symptomatic carotid stenosis: a substudy of the International Carotid Stenting Study (ICSS) Lancet Neurol. 2010;9:353–362. doi: 10.1016/S1474-4422(10)70057-0. [DOI] [PubMed] [Google Scholar]
- 8.Hausenloy DJ, Candilio L, Evans R, Ariti C, Jenkins DP, Kolvekar S, Knight R, Kunst G, Laing C, Nicholas J, Pepper J, Robertson S, Xenou M, Clayton T, Yellon DM, ERICCA Trial Investigators Remote ischemic preconditioning and outcomes of cardiac surgery. N Engl J Med. 2015;373:1408–1417. doi: 10.1056/NEJMoa1413534. [DOI] [PubMed] [Google Scholar]
- 9.Meybohm P, Bein B, Brosteanu O, Cremer J, Gruenewald M, Stoppe C, Coburn M, Schaelte G, Böning A, Niemann B, Roesner J, Kletzin F, Strouhal U, Reyher C, Laufenberg-Feldmann R, Ferner M, Brandes IF, Bauer M, Stehr SN, Kortgen A, Wittmann M, Baumgarten G, Meyer-Treschan T, Kienbaum P, Heringlake M, Schön J, Sander M, Treskatsch S, Smul T, Wolwender E, Schilling T, Fuernau G, Hasenclever D, Zacharowski K, RIPHeart Study Collaborators A multicenter trial of remote ischemic preconditioning for heart surgery. N Engl J Med. 2015;373:1397–1407. doi: 10.1056/NEJMoa1413579. [DOI] [PubMed] [Google Scholar]
- 10.Meng R, Asmaro K, Meng L, Liu Y, Ma C, Xi C, Li G, Ren C, Luo Y, Ling F, Jia J, Hua Y, Wang X, Ding Y, Lo EH, Ji X. Upper limb ischemic preconditioning prevents recurrent stroke in intracranial arterial stenosis. Neurology. 2012;79:1853–1861. doi: 10.1212/WNL.0b013e318271f76a. [DOI] [PubMed] [Google Scholar]
- 11.Hausenloy DJ, Yellon DM. Remote ischaemic preconditioning: underlying mechanisms and clinical application. Cardiovasc Res. 2008;79:377–386. doi: 10.1093/cvr/cvn114. [DOI] [PubMed] [Google Scholar]
- 12.Randhawa PK, Bali A, Jaggi AS. RIPC for multiorgan salvage in clinical settings: evolution of concept, evidences and mechanisms. Eur J Pharmacol. 2015;746:317–332. doi: 10.1016/j.ejphar.2014.08.016. [DOI] [PubMed] [Google Scholar]
- 13.Hoole SP, Heck PM, Sharples L, Khan SN, Duehmke R, Densem CG, Clarke SC, Shapiro LM, Schofield PM, O’Sullivan M, Dutka DP. Cardiac Remote Ischemic Preconditioning in Coronary Stenting (CRISP Stent) Study: a prospective, randomized control trial. Circulation. 2009;119:820–827. doi: 10.1161/CIRCULA-TIONAHA.108.809723. [DOI] [PubMed] [Google Scholar]
- 14.Dezfulian C, Garrett M, Gonzalez NR. Clinical application of preconditioning and postconditioning to achieve neuroprotection. Transl Stroke Res. 2013;4:19–24. doi: 10.1007/s12975-012-0224-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hougaard KD, Hjort N, Zeidler D, Sørensen L, Nørgaard A, Hansen TM, von Weitzel-Mudersbach P, Simonsen CZ, Damgaard D, Gottrup H, Svendsen K, Rasmussen PV, Ribe LR, Mikkelsen IK, Nagenthiraja K, Cho TH, Redington AN, Bøtker HE, Østergaard L, Mouridsen K, Andersen G. Remote ischemic perconditioning as an adjunct therapy to thrombolysis in patients with acute ischemic stroke: a randomized trial. Stroke. 2014;45:159–167. doi: 10.1161/STROKEAHA.113.001346. [DOI] [PubMed] [Google Scholar]
- 16.Meng R, Ding Y, Asmaro K, Brogan D, Meng L, Sui M, Shi J, Duan Y, Sun Z, Yu Y, Jia J, Ji X. Ischemic conditioning is safe and effective for octo- and nonagenarians in stroke prevention and treatment. Neurotherapeutics. 2015;12:667–677. doi: 10.1007/s13311-015-0358-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kelly CR, Weisz G, Maehara A, Mintz GS, Mehran R, Lansky AJ, Parise H, de Bruyne B, Serruys PW, Stone GW. Relation of C-reactive protein levels to instability of untreated vulnerable coronary plaques (from the PROSPECT Study) Am J Cardiol. 2014;114:376–383. doi: 10.1016/j.amjcard.2014.04.048. [DOI] [PubMed] [Google Scholar]
- 18.Sulaj M, Saniova B, Drobna E, Schudichova J. Serum neuron specific enolase and malondialdehyde in patients after out-of-hospital cardiac arrest. Cell Mol Neurobiol. 2009;29:807–810. doi: 10.1007/s10571-009-9361-y. [DOI] [PubMed] [Google Scholar]
- 19.Elting JW, de Jager AE, Teelken AW, Schaaf MJ, Maurits NM, van der Naalt J, Sibinga CT, Sulter GA, De Keyser J. Comparison of serum S-100 protein levels following stroke and traumatic brain injury. J Neurol Sci. 2000;181:104–110. doi: 10.1016/s0022-510x(00)00442-1. [DOI] [PubMed] [Google Scholar]
- 20.Barnett HJ, Taylor DW, Eliasziw M, Fox AJ, Ferguson GG, Haynes RB, Rankin RN, Clagett GP, Hachinski VC, Sackett DL, Thorpe KE, Meldrum HE, Spence JD. Benefit of carotid endarterectomy in patients with symptomatic moderate or severe stenosis. North American Symptomatic Carotid Endarterectomy Trial Collaborators. N Engl J Med. 1998;339:1415–1425. doi: 10.1056/NEJM199811123392002. [DOI] [PubMed] [Google Scholar]
- 21.Easton JD, Saver JL, Albers GW, Alberts MJ, Chaturvedi S, Feldmann E, Hatsukami TS, Higashida RT, Johnston SC, Kidwell CS, Lutsep HL, Miller E, Sacco RL, American Heart A, American Stroke Association Stroke C, Council on Cardiovascular S, Anesthesia, Council on Cardiovascular R, Intervention, Council on Cardiovascular N and Interdisciplinary Council on Peripheral Vascular D Definition and evaluation of transient ischemic attack. Stroke. 2009;40:2276–2293. doi: 10.1161/STROKEAHA.108.192218. [DOI] [PubMed] [Google Scholar]
- 22.Current management of amaurosis fugax. The Amaurosis Fugax Study Group. Stroke. 1990;21:201–208. doi: 10.1161/STROKEAHA.108.192218. [DOI] [PubMed] [Google Scholar]
- 23.Sacco RL, Kasner SE, Broderick JP, Caplan LR, Connors JJ, Culebras A, Elkind MS, George MG, Hamdan AD, Higashida RT, Hoh BL, Janis LS, Kase CS, Kleindorfer DO, Lee JM, Moseley ME, Peterson ED, Turan TN, Valderrama AL, Vinters HV, American Heart Association Stroke Council CoCS, Anesthesia, Council on Cardiovascular R, Intervention, Council on C, Stroke N, Council on E, Prevention, Council on Peripheral Vascular D, Council on Nutrition PA and Metabolism An updated definition of stroke for the 21st century: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44:2064–2089. doi: 10.1161/STR.0b013e318296aeca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sheffet AJ, Roubin G, Howard G, Howard V, Moore W, Meschia JF, Hobson RW, 2nd, Brott TG. Design of the Carotid Revascularization Endarterectomy vs. Stenting Trial (CREST) Int J Stroke. 2010;5:40–46. doi: 10.1111/j.1747-4949.2009.00405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hochberg Y. A sharper Bonferroni procedure for multiple tests of significance. Biometrika. 1988;75:800–802. doi: 10.1093/biomet/75.4.800. [DOI] [Google Scholar]
- 26.Hausenloy DJ, Yellon DM. Ischaemic conditioning and reperfusion injury. Nat Rev Cardiol. 2016;13:193–209. doi: 10.1038/nrcardio.2016.5. [DOI] [PubMed] [Google Scholar]
- 27.Madhwal S, Rajagopal V, Bhatt DL, Bajzer CT, Whitlow P, Kapadia SR. Predictors of difficult carotid stenting as determined by aortic arch angiography. J Invasive Cardiol. 2008;20:200–204. [PubMed] [Google Scholar]
- 28.Koch S, Katsnelson M, Dong C, Perez-Pinzon M. Remote ischemic limb preconditioning after subarachnoid hemorrhage: a phase Ib study of safety and feasibility. Stroke. 2011;42:1387–1391. doi: 10.1161/STROKEAHA.110.605840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Patti G, Tomai F, Melfi R, Ricottini E, Macrì M, Sedati P, Giardina A, Aurigemma C, Leporace M, D’Ambrosio A, Di Sciascio G. Strategies of clopidogrel load and atorvastatin reload to prevent ischemic cerebral events in patients undergoing protected carotid stenting. Results of the randomized ARMYDA-9 CAROTID (Clopidogrel and Atorvastatin Treatment During Carotid Artery Stenting) study. J Am Coll Cardiol. 2013;61:1379–1387. doi: 10.1016/j.jacc.2013.01.015. [DOI] [PubMed] [Google Scholar]
- 30.Zhang L, Zhao Z, Ouyang Y, Bao J, Lu Q, Feng R, Zhou J, Jing Z. Systematic review and meta-analysis of carotid artery stenting versus endarterectomy for carotid stenosis: a chronological and worldwide study. Medicine. 2015;94:e1060. doi: 10.1097/MD.0000000000001060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roh HG, Byun HS, Ryoo JW, Na DG, Moon WJ, Lee BB, Kim DI. Prospective analysis of cerebral infarction after carotid endarterectomy and carotid artery stent placement by using diffusion-weighted imaging. AJNR Am J Neuroradiol. 2005;26:376–384. [PMC free article] [PubMed] [Google Scholar]
- 32.Vermeer SE, Prins ND, den Heijer T, Hofman A, Koudstaal PJ, Breteler MM. Silent brain infarcts and the risk of dementia and cognitive decline. N Engl J Med. 2003;348:1215–1222. doi: 10.1056/NEJMoa022066. [DOI] [PubMed] [Google Scholar]
- 33.van Meer MP, Otte WM, van der Marel K, Nijboer CH, Kavelaars A, van der Sprenkel JW, Viergever MA, Dijkhuizen RM. Extent of bilateral neuronal network reorganization and functional recovery in relation to stroke severity. J Neurosci. 2012;32:4495–4507. doi: 10.1523/JNEUROSCI.3662-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang Z, Liao W, Chen H, Mantini D, Ding JR, Xu Q, Wang Z, Yuan C, Chen G, Jiao Q, Lu G. Altered functional-structural coupling of large-scale brain networks in idiopathic generalized epilepsy. Brain. 2011;134(pt 10):2912–2928. doi: 10.1093/brain/awr223. [DOI] [PubMed] [Google Scholar]
- 35.Joung KW, Rhim JH, Chin JH, Kim WJ, Choi DK, Lee EH, Hahm KD, Sim JY, Choi IC. Effect of remote ischemic preconditioning on cognitive function after off-pump coronary artery bypass graft: a pilot study. Korean J Anesthesiol. 2013;65:418–424. doi: 10.4097/kjae.2013.65.5.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ridker PM, Cannon CP, Morrow D, Rifai N, Rose LM, Mccabe CH, Pfeffer MA, Braunwald E. C-reactive protein levels and outcomes after statin therapy. N Engl J Med. 2005;352:20–28. doi: 10.1056/NEJMoa042378. [DOI] [PubMed] [Google Scholar]
- 37.Xia ZY, Yang H, Qu HQ, Cheng WD, Wang LX. Impact of carotid artery stenting on plasma interleukin-6, tumor necrosis factor-α and C-reactive protein. Int Angiol. 2012;31:28–32. [PubMed] [Google Scholar]
- 38.Mattusch C, Diederich KW, Schmidt A, Scheinert D, Thiele H, Schuler G, Desch S. Effect of carotid artery stenting on the release of S-100B and neurone-specific enolase. Angiology. 2011;62:376–380. doi: 10.1177/0003319710387920. [DOI] [PubMed] [Google Scholar]
- 39.Foerch C, Singer OC, Neumann-Haefelin T, de Rochemont RDM, Steinmetz H, Sitzer M. Evaluation of serum S100B as a surrogate marker for long-term outcome and infarct volume in acute middle cerebral artery infarction. Arch Neurol. 2005;62:1130–1134. doi: 10.1001/archneur.62.7.1130. [DOI] [PubMed] [Google Scholar]
- 40.Davies WR, Brown AJ, Watson W, McCormick LM, West NE, Dutka DP, Hoole SP. Remote ischemic preconditioning improves outcome at 6 years after elective percutaneous coronary intervention: the CRISP stent trial long-term follow-up. Circ Cardiovasc Interv. 2013;6:246–251. doi: 10.1161/CIRCINTERVENTIONS.112.000184. [DOI] [PubMed] [Google Scholar]
- 41.Zarbock A, Schmidt C, Van Aken H, Wempe C, Martens S, Zahn PK, Wolf B, Goebel U, Schwer CI, Rosenberger P, Haeberle H, Görlich D, Kellum JA, Meersch M, RenalRIPC Investigators Effect of remote ischemic preconditioning on kidney injury among high-risk patients undergoing cardiac surgery: a randomized clinical trial. JAMA. 2015;313:2133–2141. doi: 10.1001/jama.2015.4189. [DOI] [PubMed] [Google Scholar]
- 42.Hong DM, Lee EH, Kim HJ, Min JJ, Chin JH, Choi DK, Bahk JH, Sim JY, Choi IC, Jeon Y. Does remote ischaemic preconditioning with postconditioning improve clinical outcomes of patients undergoing cardiac surgery? Remote Ischaemic Preconditioning with Postconditioning Outcome Trial. Eur Heart J. 2014;35:176–183. doi: 10.1093/eurheartj/eht346. [DOI] [PubMed] [Google Scholar]
- 43.Sloth AD, Schmidt MR, Munk K, Kharbanda RK, Redington AN, Schmidt M, Pedersen L, Sørensen HT, Bøtker HE, CONDI Investigators Improved long-term clinical outcomes in patients with ST-elevation myocardial infarction undergoing remote ischaemic conditioning as an adjunct to primary percutaneous coronary intervention. Eur Heart J. 2014;35:168–175. doi: 10.1093/eur-heartj/eht369. [DOI] [PubMed] [Google Scholar]
- 44.Skyschally A, van Caster P, Iliodromitis EK, Schulz R, Kremastinos DT, Heusch G. Ischemic postconditioning: experimental models and protocol algorithms. Basic Res Cardiol. 2009;104:469–483. doi: 10.1007/s00395-009-0040-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


