Abstract
High-grade gliomas (World Health Organization grade III–IV) are highly lethal primary brain tumors. Imaging modalities, including MRI and FDG PET, provide a limited ability to differentiate treatment effects (such as radiation necrosis) from recurrent or residual tumor. As the first step in validating the applicability of prostate-specific membrane antigen (PSMA)– targeted imaging in high-grade gliomas, we evaluated the ability of the PSMA-targeted small molecule [18F]DCFPyL (2-(3-(1carboxy-5-(6-[18F] fluoro-pyridine-3-carbonyl)-amino]-pentyl)-ureido)-pentanedioic acid) to image high-grade gliomas in a series of 3 prospectively recruited patients. We found [18F]DCFPyL binds PSMA in the neovasculature of glioblastoma multiforme and tumor cells of anaplastic astrocytoma.
Keywords: glioblastoma, gliomas, MRI, PET/CT, primary brain tumor, prostate-specific membrane antigen, PSMA
FIGURE 1.
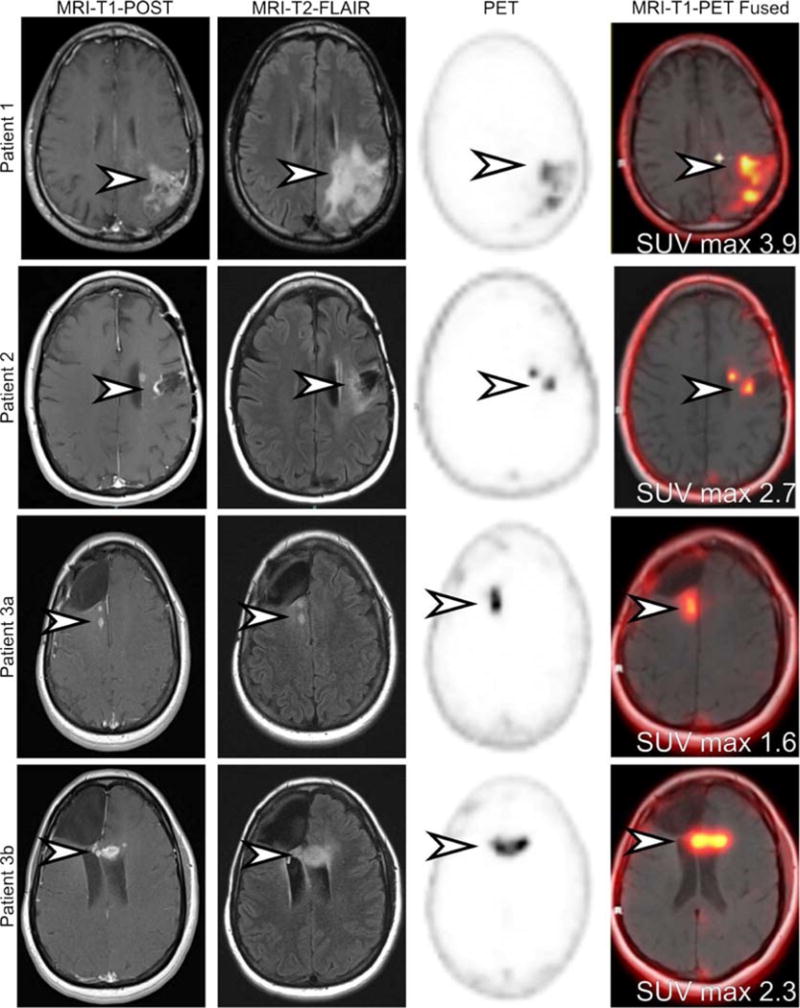
MRI and 18F-DCFPyL PET images demonstrate areas of presumed tumor involvement and normal brain. Three patients with findings on MRI highly suggestive of glioblastoma multiforme (GBM) were prospectively recruited and imaged with [18F]DCFPyL.1,2 All patients initially had low-grade gliomas that progressed to high-grade disease and had undergone a variety of treatment regimens. Patient 1 demonstrated an area of parenchymal edema (MRI-T2-FLAIR) and MRI contrast enhancement in the frontoparietal region (arrowhead). The area demonstrated increased [18F]DCFPyL (arrowhead). Patient 2 demonstrated increased nodular frontal lobe contrast enhancement deep to a prior tumor resection cavity (arrowhead). There was associated surrounding parenchymal edema (arrowhead). The area demonstrated corresponding increased [18F]DCFPyL activity (arrowhead). Patient 3 had 2 areas of increased contrast-enhancing nodularity and parenchymal edema surrounding a prior resection cavity (arrowhead). The first lesion (3a) is located in the right periventricular white matter, whereas the second lesion (3b) is located in the genu of the left corpus callosum. Both of these lesions demonstrated increased [18F]DCFPyL activity (arrowhead). No uptake was seen in normal brain parenchyma.
FIGURE 2.
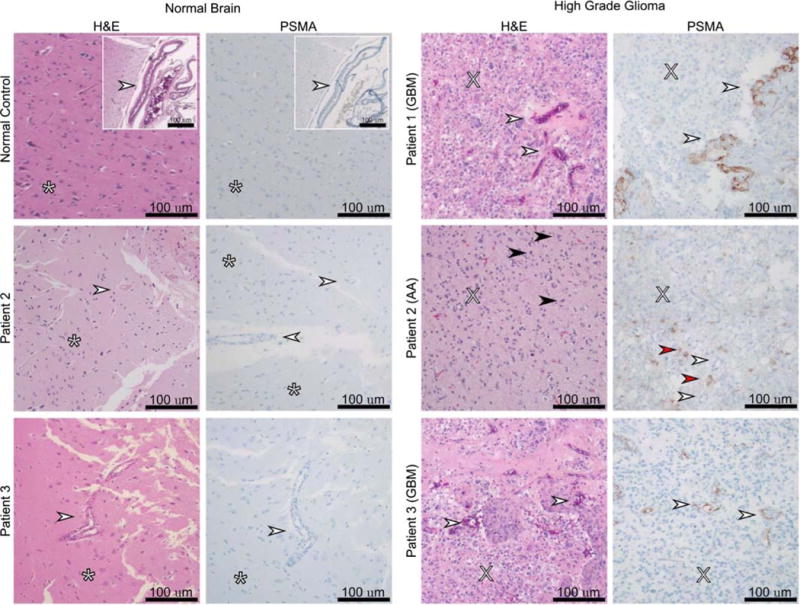
Prostate-specific membrane antigen (PSMA) expression in tumor samples and normal brain tissue. Biopsy of the suspicious lesions, described above, demonstrated antibody staining correlating with 18F-DCFPyL uptake. Normal brain tissue samples were used as a negative control. Staining was localized to tumor neovasculature in patients with GBM (patients 1 and 3) or tumor cells in anaplastic astrocytoma (patient 2). No staining was observed in normal brain or vessels. White arrow: blood vessels, asterisk: normal brain, X: anaplasia and high cellularity, black arrow: mitosis, red arrow: PSMA staining of anaplastic astrocytoma tumor cells. This is the first time a PSMA agent has imaged an anaplastic astrocytoma and the first time a GBM has been imaged with an 18F-PSMA agent. Overall, the tested gliomas demonstrated increased radiotracer uptake when compared with background, as has been previously documented with other PSMA agents.3,4 Radiotracer uptakes for our patients were lower than what has been reported with 68Ga-PSMA were SUV max ranged from 5.8 to 13.5.4 The difference in uptake is nonconclusive as the biology of the tumors may be different or, less likely, based on our experience with other tumors, could be ascribed to the imaging agent. Regardless, the tumor-to-normal tissue uptake ratio was superb for a diagnostic imaging modality. Our histological findings also correlate to previously published data where PSMA expression has been seen in tumor cells of lower-grade gliomas,5 whereas the expression for GBM has been mostly in the tumor neovasculature5–7 with an exception.8 As a conclusion, we found [18F]DCFPyL binds PSMA in the neovasculature of GBM and in tumor cells of anaplastic astrocytoma. Additional prospective studies to validate the use of PSMA-targeted imaging in GBM and to address specific clinical questions such as the differentiation of radiation necrosis from recurrent or residual tumor may be in order.
Acknowledgments
STUDY FUNDING
The authors thank Judy Buchanan for her intellectual contributions.
Sources of funding: The authors acknowledge the Yousem Family research award, CA134675, CA184288, CA103175, and CA183031 for financial support. M.H. has an uncompensated advisory role with Cavion Pharmaceutical Company (Charlottesville, VA). M.G.P. is the coinventor on a US patent covering 18F-DCFPyL and as such is entitled to a portion of any licensing fees and royalties generated by this technology.
Footnotes
Conflicts of interest: This arrangement has been reviewed and approved by the Johns Hopkins University in accordance with its conflict of interest policies. The other authors have none declared.
Author contributions: R.A.S.F. contributed to drafting and revising the manuscript for content, study concept and design, acquisition and interpretation of data, statistical analysis, and study coordination. J.R.M. participated in revising the manuscript and acquisition and analysis of data. M.H. participated in revising the manuscript, acquisition of data, and study concept. C.F. participated in acquisition of data and study coordination. J.J.L. participated in revising the manuscript, acquisition of data, and study concept. L.B.S. participated in revising the manuscript, acquisition and analysis of data, and study concept. M.S.J. participated in revising the manuscript, acquisition and analysis of data, and study concept. Z.S. participated in revising the manuscript, acquisition and analysis of data, study supervision, and study concept. M.G.P. participated in providing vital reagents and patents, revising the manuscript, acquisition and analysis of data, study supervision, and study concept. S.P.R. contributed to drafting and revising the manuscript for content, study concept and design, acquisition and interpretation of data, and study coordination.
References
- 1.Chen Y, Pullambhatla M, Foss CA, et al. 2-(3-{1-Carboxy-5-[(6-[18F]fluoro-pyridine-3-carbonyl)-amino]-pentyl}-ureido)-pentanedioic acid, [18F] DCFPyL, a PSMA-based PET imaging agent for prostate cancer. Clin Cancer Res. 2011;17:7645–7653. doi: 10.1158/1078-0432.CCR-11-1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Szabo Z, Mena E, Rowe SP, et al. Initial evaluation of [(18)F]DCFPyL for prostate-specific membrane antigen (PSMA)–targeted PET imaging of prostate cancer. Mol Imaging Biol. 2015;17:565–574. doi: 10.1007/s11307-015-0850-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schwenck J, Tabatabai G, Skardelly M, et al. In vivo visualization of prostate-specific membrane antigen in glioblastoma. Eur J Nucl Med Mol Imaging. 2015;42:170–171. doi: 10.1007/s00259-014-2921-5. [DOI] [PubMed] [Google Scholar]
- 4.Sasikumar A, Joy A, Pillai MR, et al. Diagnostic value of 68Ga PSMA-11 PET/CT imaging of brain tumors—preliminary analysis. Clin Nucl Med. 2017;42:e41–e48. doi: 10.1097/RLU.0000000000001451. [DOI] [PubMed] [Google Scholar]
- 5.Nomura N, Pastorino S, Jiang P, et al. Prostate specific membrane antigen (PSMA) expression in primary gliomas and breast cancer brain metastases. Cancer Cell Int. 2014;14:26. doi: 10.1186/1475-2867-14-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wernicke AG, Edgar MA, Lavi E, et al. Prostate-specific membrane antigen as a potential novel vascular target for treatment of glioblastoma multiforme. Arch Pathol Lab Med. 2011;135:1486–1489. doi: 10.5858/arpa.2010-0740-OA. [DOI] [PubMed] [Google Scholar]
- 7.Chang SS, Reuter VE, Heston WD, et al. Five different anti–prostate-specific membrane antigen (PSMA) antibodies confirm PSMA expression in tumor-associated neovasculature. Cancer Res. 1999;59:3192–3198. [PubMed] [Google Scholar]
- 8.Mhawech-Fauceglia P, Zhang S, Terracciano L, et al. Prostate-specific membrane antigen (PSMA) protein expression in normal and neoplastic tissues and its sensitivity and specificity in prostate adenocarcinoma: an immunohistochemical study using multiple tumour tissue microarray technique. Histopathology. 2007;50:472–483. doi: 10.1111/j.1365-2559.2007.02635.x. [DOI] [PubMed] [Google Scholar]


