Abstract
Background
While androgen-deprivation-therapy with the recently developed anti-androgen enzalutamide (Enz) shows promising therapeutic benefits in men with metastatic castration-resistant prostate cancer (PCa), many patients develop resistance to Enz, which may involve the induction of the androgen receptor (AR) splicing variant 7 (AR-v7).
Objective
Our aim is to identify the mechanisms responsible for AR-v7 production and to develop novel preclinical approaches to suppress the Enz-resistant (EnzR) PCa.
Design, setting, and participants
We established EnzR-PCa cell lines and examined the long noncoding RNA Malat1 (Malat1) function in conferring Enz resistance. We also examined the in vivo effects of Malat1 short interfering RNA and the AR-v7 degradation enhancer, ASC-J9®.
Outcome measurements and statistical analysis
Enz resistance and expression of Malat1 and AR-v7. All statistical comparisons were analyzed with a t-test or one way analysis of variance followed by t-test.
Results and limitations
We demonstrated that Malat1 is indispensable for Enz-induced AR-v7 production in VCaP and EnzR-C4-2 cells. We observed increased AR-v7 and Malat1 expression in our established EnzR-PCa cell lines and in some PCa patients who received Enz treatment. Targeting the Malat1/AR-v7 axis resulted in altering the PCa resistance to androgen deprivation therapy with Enz. The limitation of this study includes the small sample size from the same human patients before and after receiving Enz treatment.
Conclusions
Targeting the Malat1/AR-v7 axis via Malat1-short interfering RNA or AR-v7 degradation enhancer ASC-J9® in EnzR-PCa cell lines and mouse models suppressed EnzR-PCa progression.
Patient summary
Androgen deprivation therapy-enzalutamide treatment may not be the best choice for prostate cancer patients who have higher expression of the Malat1/ androgen receptor splicing variant 7 axis, and new therapies using Malat1-short interfering RNA or ASC-J9® may be developed in the future to better suppress enzalutamide-resistant prostate cancer.
Keywords: PCa AR-v7, Malat1, Enzalutamide
1. Introduction
Castration-resistant prostate cancer (CRPC) is characterized by its continuous androgen receptor (AR) function and resistance to androgen-deprivation therapy (ADT) [1,2]. The recently developed antiandrogen, enzalutamide (Enz, also called MDV3100), was approved recently by the Food and Drug Administration after a demonstration that it could prolong survival in men with metastatic CRPC [3]. However, Enz resistance eventually occurs in most patients due to a variety of AR-dependent and AR-independent mechanisms. Relevant in this setting is the recent demonstration that Enz resistance is strongly associated with the expression of the most common AR splicing variant 7 (AR-v7, also called AR3) in circulating tumor cells (CTCs) from CRPC patients. Importantly, AR-v7 was also expressed higher in the tumors of Enz-treated patients compared with tumors of Enz-naïve patients [4]. The detailed mechanism(s) of how AR-v7 is induced and its linkage to Enz resistance, however, remain unclear. Here, we show a long noncoding RNA (lncRNA), Malat1, is required for Enz-induced AR-v7 production, and targeting the Malat1/AR-v7 axis could overcome Enz resistance and may become a future therapeutic choice to better suppress the Enz-resistant (EnzR) CRPC progression.
2. Materials and methods
2.1. Generation of Enz-resistant (R1 and R2) cell lines
R1 cells were generated by culturing C4-2 cells under increasing Enz concentrations from 10 μM to 40 μM (every 20 d) for 3 mo (Supplementary Fig. 1). For R2, cells were cultured at 10 μM Enz for 3 mo before experiments. After generation, both R1 and R2 were maintained in media with 10 μM Enz.
2.2. In vivo xenograft mouse model
EnzR cells (R1), 1 × 106, were injected subcutaneously into 6-wk-old nude mice 1:1 with Matrigel. After 4 wk, tumor bearing mice were randomly grouped and injected with scramble RNA (10 mg/kg), Malat1-short interfering RNA (siRNA; 10 mg/kg), or ASC-J9® (75 mg/kg) for 2 wk. Invivofectamine 2.0 kit (#1377501; Invitrogen, Carslbad, CA, USA) was used to deliver the Malat1-siRNA. Briefly, 50-μl 3-mg/ml Malat1-siRNA was diluted with 50-μl complexation buffer, then mixed 1:1 with invivofectamine 2.0 for several seconds. The mixture was injected into the peritumor region. Enz (30 mg/kg) was diluted with corn oil and intraperitoneal injected every other day during the therapies. Tumors were measured by caliper every week, then mice sacrificed and tumors removed for final measurement and immunohistochemistry studies.
2.3. Analysis of CTCs
Blood collection, processing, and CTC isolation procedures were described previously [4]. CTC samples used for study included 113 frozen samples, each representing a unique blood draw from men with metastatic CRPC who signed consent forms for blood draw before, during, and after standard-of-care treatment with abiraterone, Enz, or taxane chemotherapies. Samples were processed and data generated while blinded to treatment status. From this dataset, 10 pairs of pre- and post-Enz treatment Malat1 expression data were avaialble following unblinding. Expression data was normalized to a control gene (RPL13A), and normalized data presented for each pretreatment and post-treatment pairs. Expression levels of full-length AR (AR-FL), AR-v7, and Malat1 were quantified by quantitative polymerase chain reaction (qPCR) using specific primers listed in Supplementary Table 2. Other materials and methods used are described in Supplementary data.
3. Results
3.1. Enhanced expression of AR-v7 and Malat1 in EnzR-PCa cells
Recent clinical data suggested that CRPC patients with higher AR-v7 expression in their CTCs responded poorly to Enz therapy [4]. However, the mechanism(s) how AR-v7 was generated and its linkage to the development of Enz resistance remain largely unclear. Here we established two EnzR-PCa C4-2 cell lines (R1 and R2) using different Enz treatment strategies (Supplementary Fig. 1A), and confirmed their resistance phenotype (Supplementary Fig. 1B–D).
Since lncRNAs have been reported to play essential roles in cancer development and drug resistance, we speculated that some selective lncRNAs might be altered after development of Enz resistance. We focused on 32 lncRNAs whose expression was either relatively specific in the prostate or have higher expression in PCa, and were intrigued by two of them, PCGEM1 and Malat1 (Supplementary Table 1). PCGEM1 has been identified as an AR/AR-v7 signaling regulatory lncRNA [5], and Malat1 has been reported to be upregulated in CRPC [6], and may contribute to RNA splicing via binding to the serine/arginine rich splicing factor 1 (SF2) complex [7].
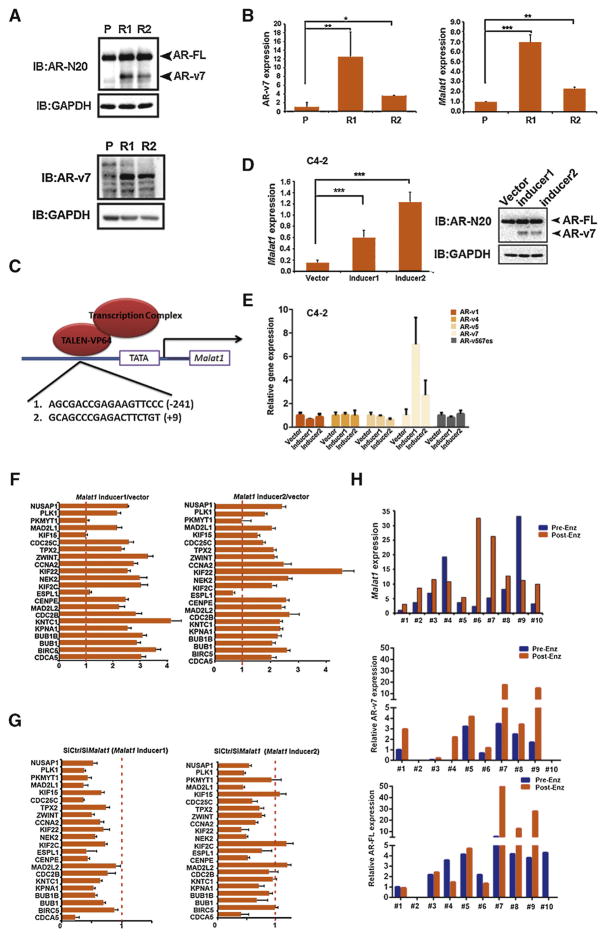
Since AR-v7 is the predominant splicing variant of AR and could confer Enz resistance to PCa cells (Supplementary Fig. 1E), we were interested to see the potential linkage of the increased Malat1 expression in EnzR cell lines to the AR-v7 expression. The results revealed that AR-v7 and Malat1 expression at both protein (Fig. 1A) and messenger RNA levels (Fig. 1B) was increased in those EnzR-PCa cells compared with their parental Enz-sensitive cells. Also, AR-FL was also elevated in the EnzR-PCa cells (Fig. 1A). Since the R1 cells have a relatively higher expression of AR-v7, we focused on this EnzR cell line for subsequent experiments.
Fig. 1.
Enzalutamide (Enz) treatment results in higher expression of the androgen receptor splicing variant 7 (AR-v7) and Malat1. (A) AR-v7 expression is higher in Enz resistant cell lines (R1 and R2) compared with parental (P) cells, using Western blotting to measure full length androgen receptor (AR-FL) and AR-v7 with AR-N20 and AR-v7 specific antibody, respectively, while glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as a loading control. (B) The quantitative polymerase chain reaction (qPCR) analysis suggests Malat1 (right) and AR-v7 messenger RNA (left) were dramatically increased after cells acquired Enz resistance. (C) Cartoon showing transcription activator-like effector (TALE) targeting sites on the Malat1 promoter. (D) TALE-based transactivation induces Malat1. Left, Malat1 expression after TALE-based transcription factors were transduced into C4-2 cells. Right, AR-FL and AR-v7 were detected by western blotting in two cell populations infected with Malat1 inducers. (E) AR variants were detected by qPCR in two Malat1-inducers-infected cells. (F) Malat1-expressing cells showed AR-v7 gene signature. Cell cycle genes regulated by AR-v7 were tested with qPCR in Malat1-expressing cells that were induced by TALE expression. (G) The short interfering Malat1 (siMalat1) reversed the induced expression of AR-v7 target genes in cell lines. (H) Malat1, AR-v7, and AR-FL expression in circulating tumor cells of metastatic castration-resistant prostate cancer patients before (Pre-Enz) and after (Post-Enz) Enz treatment (n = 10).
* p < 0.05.
** p < 0.01.
*** p < 0.001.
To prove higher Malat1 expression may lead to increased AR-v7 expression, we manipulated Malat1 expression by transducing two individual transcription activator-like effector (TALE)-based transcription factors into the upstream promoter region of Malat1 (Fig. 1C), and found this induction of Malat1 expression (Fig. 1D) increased the AR-v7 expression at the mRNA/protein levels in C4-2 cells (Fig. 1D and E) with little impact on other AR variants (Fig. 1E), suggesting the specific role of Malat1 on the induction of AR-v7 production.
Importantly, the forced expression of Malat1 by two TALE-based Malat1-inducers also altered the expressions of 22 AR-v7-regulated downstream genes [8] (Fig. 1F), and adding the Malat1-siRNA into these two inducer-harboring cells led to blocking/reversing the Malat1-induced AR-v7 target gene expressions (Fig. 1G).
Importantly, in a cohort of men with metastatic CRPC undergoing ADT treatment with Enz (ADT-Enz) a longitudinal analysis of CTCs from the same 10 patients before and after ADT-Enz demonstrated an increase of Malat1 and AR-v7 expression in eight of 10 patients evaluated (Fig. 1H, Supplementary Table 3), consistent with our in vitro study. Furthermore, analysis of the The Cancer Genome Atlas database revealed PCa patients harboring higher expression of Malat1 have poor overall survival (Supplementary Fig. 1F).
Together, results from multiple EnzR-PCa cells (Fig. 1A–G) and human clinical data (Fig. 1H), all suggest that Malat1 may play essential roles in the development of EnzR-CRPC.
3.2. Enz-induced Malat1 expression via suppressing AR function
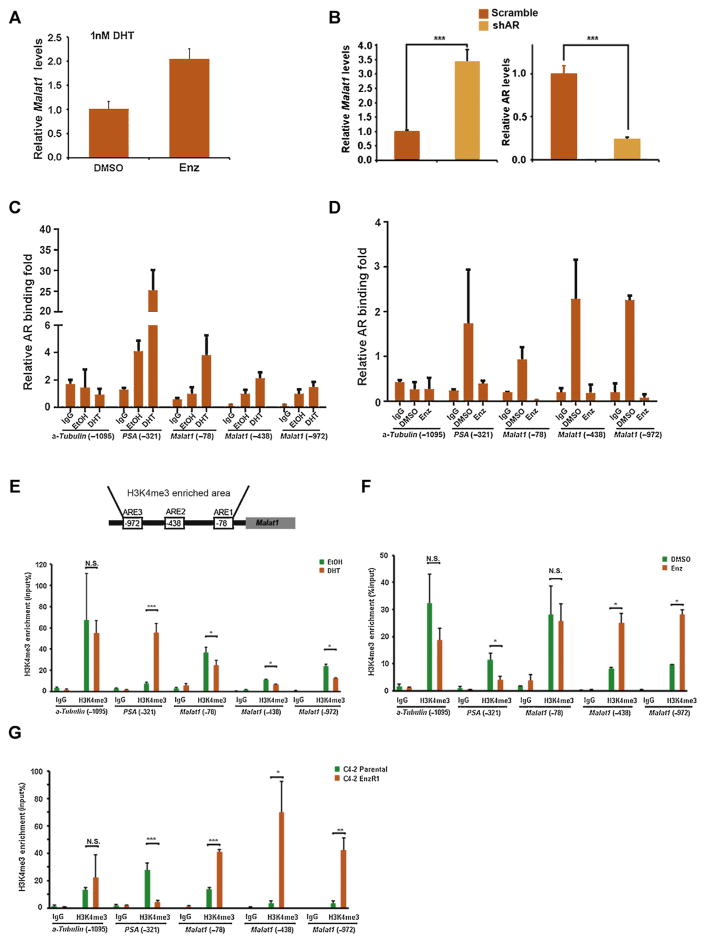
We further dissected mechanism(s) by which Malat1 expression was enhanced during or after development of Enz resistance. We found that transient treatment with Enz increased Malat1 expression in the presence of 1 nM dihydrotestosterone (DHT), the androgen concentration found in both CRPC C4-2 and and VCaP cells (Fig. 2A, supplementary Fig. 2B, respectively), and knocking down AR with short hairpin RNA (shRNA; Fig. 2B), led to increased Malat1 expression at 1 nM DHT in C4-2 cells (Fig. 2B). In contrast, adding functional AR-complementary DNA or treating with DHT (both 1 nM DHT and 10 nM DHT) resulted in suppressing the Malat1 expression in the EnzR cells (Supplementary Figs. 2C and 2D).
Fig. 2.
Androgen receptor (AR) regulates Malat1 expression by directly binding to its promoter. (A) C4-2 cells were cultured in media containing 1 nM dihydrotestosterone (DHT), the treatment with 10 μM enzalutamide (Enz) could result in increased expression of Malat1. (B) Suppression of AR expression in C4-2 cells through short hairpin RNA (right) increases Malat1 expression (left). (C) AR binding to regulatory elements of Malat1 promoter was enhanced in response to 10 nM DHT, and (D) was reduced in response to 10 μM Enz in C4-2 cells. Cells were treated with either 10 nM DHT or 10 μM Enz for 24 h. Chromatin immunoprecipitation assay was performed using anti-AR antibody followed by qPCR with specific primers for predicted AR binding sequence, ARE at the PSA promoter region served as positive controls and non-ARE at alpha-Tubulin promoter region was used as negative controls. (E) Top, schematic map of AR binding to Malat1 promoter and the H3K4me3 enrichment in Malat1 promoter. Bottom, DHT reduces H3K4me3 levels of Malat1 promoter after C4-2 cells were cultured with 10 nM DHT for 24 h. (F) Enz increases H3K4me3 levels of Malat1 promoter after C4-2 cells were treated with 10 μM Enz for 24 h. (G) H3K4me3 levels in Malat1 promoter are higher in C4-2 Enz-resistant cells compared to parental C4-2 cells. H3K4me3 enrichment in the promoter region of PSA or alpha-Tubulin as experimental positive or negative controls, respectively.
DMSO = dimethyl sulfoxide; IgG = immunoglobulin-G; N.S. = not significant.
* p < 0.05.
** p < 0.01.
*** p < 0.001.
3.3. Enz induced Malat1 expression via altering AR binding to Malat1 promoter
Further mechanism dissection found AR could bind directly to the predicted AR response elements (AREs) located on the proximal promoter of the Malat1 gene, and adding DHT increased AR binding to these AREs (Fig. 2C) which might then lead to suppress Malat1 expression (Supplementary Fig. 2C). In contrast, adding Enz might suppress the AR binding to these AREs (Fig. 2D), which might then lead to increase the Malat1 expression (Fig. 2A).
Sequence analysis found a H3K4me3 enrichment region (see the University of California Santa Cruz database; Fig. 2E, indicating the promoter activity) in the 2 kb upstream of Malat1 gene locus. The results from manipulating AR activity with DHT or Enz revealed that adding DHT could suppress H3K4me3 levels in Malat1 promoter in the C4-2 cells (Fig. 2E lower panel), and transient or long-term Enz (R1 cells) treatment might increase the Malat1 promoter activity (Fig. 2F and G, respectively).
3.4. Malat1 is indispensable for AR-v7 production and may function via interacting with SF2 to splice the AR transcript
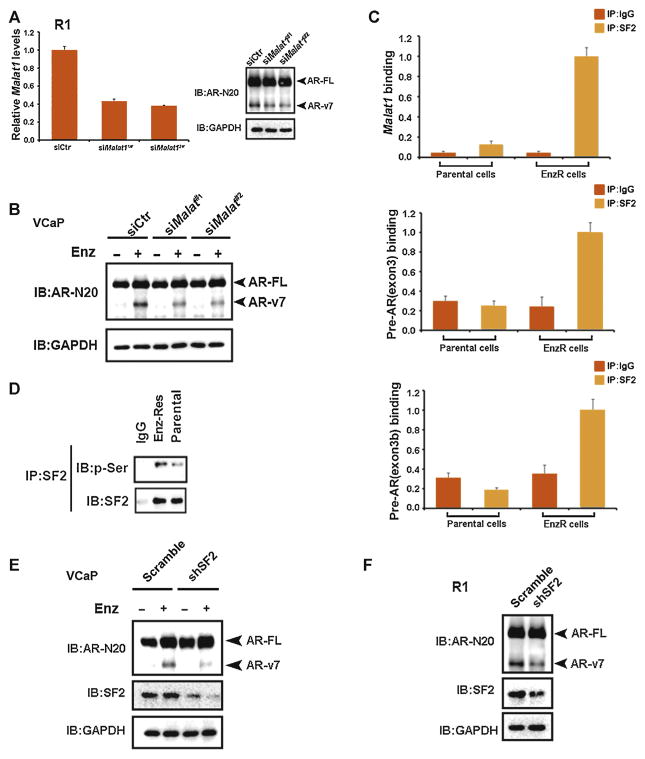
Next, to examine if Enz-enhanced Malat1 expression is required for AR-v7 expression, we knocked-down Malat1 with two different siRNAs (Fig. 3A) and results revealed that suppressing Malat1 by Malat1-siRNAs led to decrease AR-v7 expression in the EnzR C4-2 cells (Fig. 3A) and VCaP cells (Fig. 3B, Supplementary Fig. 3A), suggesting that Malat1 is indispensable for Enz-induced AR-v7 expression.
Fig. 3.
Malat1 is indispensable for androgen receptor splicing variant 7 (AR-v7) production. (A) Left, the efficiency of knocking down Malat1. Right, AR-v7 expression in enzalutamide-resistant (EnzR) C4-2 cells was reduced in response to small interfering Malat1 of two different sequences. (B) Knockdown of Malat1 attenuates Enz-induced AR-v7 production in VCaP cells. Cells were transfected with siMalat1 and si-control (siCtr) for 24 h, maintained in 1 nM dihydrotestosterone (DHT) with/without 10 μM Enz for 24 h, then collected for full length AR (AR-FL) and AR-v7 detection by anti-AR N-20 antibody. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) served as a loading control. (C) Malat1 and AR transcripts bind to serine/arginine rich splicing factor 1 (SF2). RNA immunoprecipitation was performed using anti-SF2 antibody, the RNA in immunoprecipitate was extracted with Trizol, followed by reverse transcription and detection with quantitative polymerase chain reaction for Malat1 and AR transcript, immunoglobulin-G (IgG) was used as a negative control. (D) EnzR cells have a higher SF2 activity. SF2 was immunoprecipitated from parental (P) and EnzR C4-2 cells followed by immunoblotting with anti-SF2 antibody and anti-phosphoserine antibody. (E) Knockdown of SF2 blocks Enz-induced AR-v7 production in VCaP cells. VCaP cells were infected with short hairpin (sh)RNA-SF2 lentivirus for 24 h then maintained in 1 nM DHT with/without 10 μM Enz for 24 h followed by AR-FL and AR-v7 protein detection. GAPDH served as loading control. (F) Knocking down SF2 can decrease AR-v7 level in EnzR cells. GAPDH served as a loading control.
Ser = serine.
Early studies suggested Malat1 might function through binding to (SF2, also called ARSF1) to increase the RNA splicing capacity for the targets [7,9]. We examined the possible complex formation of Malat1-SF2 on the AR transcript by immunoprecipitating SF2 complex followed by analysis of associated RNAs including Malat1 and AR transcript. The results revealed that at the 1 nM DHT castration condition, there is a significant increase in binding of Malat1 and AR transcript to SF2 in EnzR cells compared with parental Enz-sensitive cells, supporting the hypothesis that Malat1 can enhance splicing of AR-v7 through direct modulation of SF2 binding and activity (Fig. 3C). Furthermore, we found that in EnzR cells there is a higher level of SF2 activity determined by elevated phosphorylation level, indicating enhanced splicing activity of SF2 likely as a result of increased Malat1 expression (Fig. 3D). Importantly, knocking down of SF2 in VCaP cells (Fig. 3E) and EnzR1 cells (Fig. 3F) also led to attenuate AR-v7 production, suggesting that SF2 is indeed involved in the splicing of AR-v7 as reported earlier [10].
3.5. Malat1-enhanced AR-v7 expression contributes to the development of Enz resistance
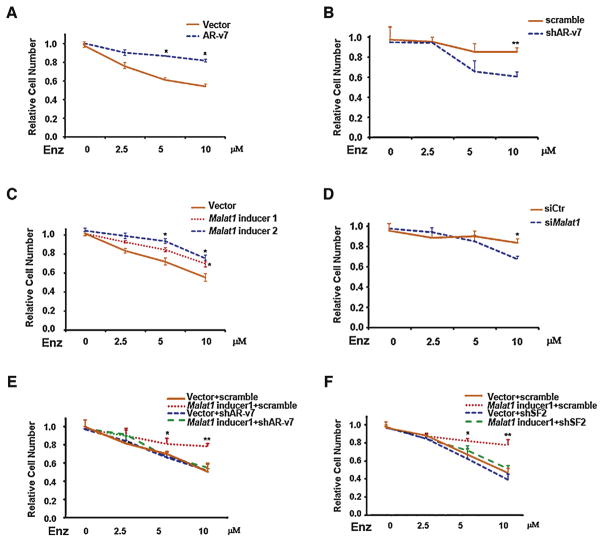
To confirm our hypothesis that Enz could function through induction of the Malat1/AR-v7 axis to accelerate development of Enz-resistance, we examined the effects of Enz on the growth of C4-2 cells stably transfected with AR-v7, and MTT proliferation assay results revealed that Enz had a reduced effect on cell growth suppression (Fig. 4A), and transducing AR-v7-shRNA could resensitize EnzR1 cells to respond to Enz treatment (Fig. 4B).
Fig. 4.
Androgen receptor splicing variant 7 (AR-v7) and Malat1 confer enzalutamide (Enz)-resistance to prostate cancer cells. (A) AR-v7 stably transfected C4-2 cell line displays Enz insensitivity compared with the control. Equal numbers of cells were seeded and subjected to various concentrations of Enz. After 4 d, cell growth/viability was determined by MTT assay. (B) Knockdown of AR-v7 in EnzR cell line (R1) makes cells more sensitive to Enz treatment. (C) Malat1-expressing cells show Enz insensitivity compared with control cells. Equal numbers of cells were seeded and treated as indicated for MTT. (D) The deficiency of Malat1 in EnzR cell line makes cells sensitive to Enz treatment. (E) Malat1-mediated Enz-resistance can be reversed by short hairpin (sh)AR-v7. After two rounds of virus infection, equal numbers of cells were seeded and treated as indicated for MTT. (F) Malat1-mediated Enz-resistance can be reversed by shRNA-serine/arginine rich splicing factor 1 (SF2) in cells seeded and treated as indicated for MTT. For A-D the statistical analysis was made between Malat1-expressing cells and controls cells. For E-F ANOVA t test was performed among groups.
siCtr = si-control.
* p < 0.05; ** p < 0.05.
We then applied another approach using the TALE-based Malat1 induction to prove that increased Malat1 (therefore increased AR-v7) in C4-2 cells also resulted in a reduced effect of Enz to suppress the cell growth (Fig. 4C), and adding Malat1-siRNA in EnzR cells can overcome Enz resistance (Fig. 4D). Importantly, we found the contribution of Malat1 to Enz resistance can be blocked by AR-v7-shRNA or SF2-shRNA (Fig. 4E and F). Furthermore, reciprocal expression of AR-v7 could reverse Malat1-siRNA-mediated effects on both Enz sensitivity and cell growth (Supplementary Figs. 4A and 4B).
Together, results from Fig. 4A–F and Supplementary Fig. 4A-B strengthen the notion that the Malat1/SF2/AR-v7 axis is critically involved in Enz-resistance development.
3.6. New therapies to suppress EnzR-PCa in in vitro cell lines and in vivo mouse models
To prove the key roles of our newly identified Enz/Malat1/ AR-v7 axis in the development of Enz-resistance in the in-vivo mouse model and seek new therapeutic approaches to better suppress EnzR-PCa, we used our newly developed EnzR cell lines to test the efficacy of targeting Malat1 and AR-v7 with Malat1-siRNA or AR-v7 degradation enhancer ASC-J9® [11–15]. The results revealed that silencing Malat1 expression by Malat1-siRNA or degrading AR-v7 by ASC-J9® suppressed the growth of EnzR cells (Fig. 5A and B). Of note, Malat1 induction (to induce AR-v7) is insufficient to render ASC-J9® resistance to PCa cells (Supplementary Fig. 5), suggesting targeting AR-v7 protein by ASC-J9® play a key role to suppress AR-v7-mediated EnzR-PCa progression.
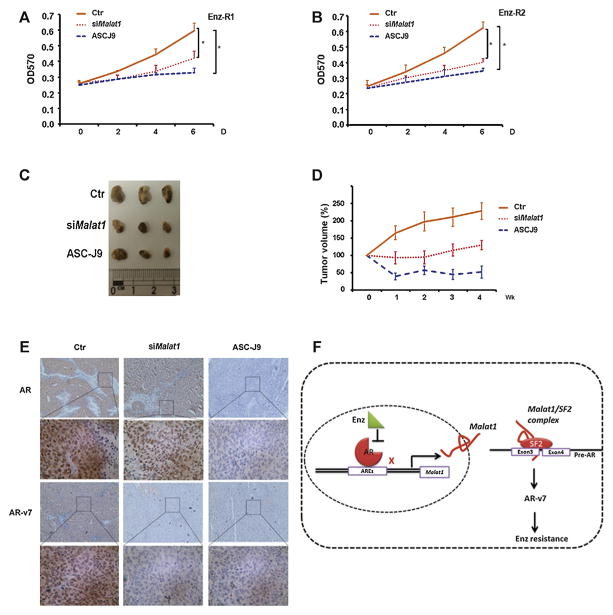
Fig. 5.
The Malat1-small interfering RNA or anti-androgen receptor splicing variant 7 (AR-v7) with ASC-J9 suppresses enzalutamide-resistant (EnzR) cell lines in vitro and in vivo growth compared with a control (Ctr). (A) The Malat1-small interfering RNA and ASC-J9 (5 μM) suppresses cell growth of EnzR1 and (B) EnzR2 cell lines. (C, D) Mouse tumor volumes (C) were measured (1/2[short axis2 × long axis]) and plotted against the control volume of various treatments and days (D). Tumor dissection of EnzR injected mice with indicated therapies. Control small interfering RNA (n = 4), Malat1-small interfering RNA (n = 6), and ASC-J9 (n = 6) were injected in the periphery of tumors of mice. After 4 wk of weight monitoring, mice were sacrificed. Representative images (Rows 1 and 3 are 100× magnification and rows 2 and 4 are 400× magnification of the boxes in rows 1 and 3) of tumors after mice were sacrificed. (E) Immunohistochemistry staining to monitor AR-v7 and full length AR (AR) expression as indicated with above treatments. (F) A schematic depiction of molecular mechanism underlying Enz resistance development.
* p < 0.01.
We then established in vivo mouse models via subcutaneously xenografting EnzR (R1) cells (1 × 106) into nude mice. After 3 wk, 16 of 30 injected mice developed tumors. We divided these 16 mice into three groups and injected Group 1 with Malat1-siRNA (n = 6; 10 mg/kg body weight every 2 wk), Group 2 with ASC-J9® (75 mg/kg body weight every 24 h; n = 6), and Group 3 with anti-GFP oligonucleotides (10 mg/kg body weight every 2 wk) as a control (n = 4). During the entire experimental duration, Enz (30 mg/kg body weight every 24 h) was administered to all groups of mice. Mouse tumor growth was measured weekly and mice sacrificed after 4 wk. The results revealed that both Malat1-siRNA and ASC-J9® significantly suppressed EnzR tumors (Fig. 5C and D).
We also examined the AR-v7 and AR expression in vivo through immunohistochemistry staining and results indicated a significant reduction of AR-v7 expression in mice treated with Malat1-siRNA and ASC-J9® while Malat1-siRNA had little effect on AR (Fig. 5E).
4. Discussion
Among several mechanisms involved in the development of Enz resistance in CRPC, including induction of AR-v7 [4], ARF876L mutation [16–18], and altered glucocorticoid receptor signals [19], the induction of AR-v7 [4] has the strongest clinical data support derived from a clinical study showing CRPC patients with detectable AR-v7 in CTCs had poor responses to ADT-Enz [4]. Furthermore, AR-v7 might be linked to bone metastases in CRPC [20]. These clinical data point to the possible reason why ADT-Enz may always fail after an initial clinical response. Therefore, it is important to develop new approaches to suppress AR function beyond antiandrogens since induced AR-v7 lacks the ligand-binding domain and can be activated in the castration condition [21–24]. In this study, we found that Malat1 expression and SF2 activity are upregulated in EnzR C4-2 cells, which contributed to AR-v7 production and led to Enz resistance (Fig. 5F). Our finding is consistent with previous work showing that SF2 could recognize and bind to the intron between exon 3 and exon 4 of the AR transcript to facilitate AR-v7 production [25].
The linkage of Enz resistance to the induction of the Malat1/SF2/AR-v7 axis strongly suggests a new and better therapy to further suppress CRPC during or after development of Enz resistance, via targeting the Malat1, SF2, and AR-v7. Targeting Malat1 with siRNA has been effective for many cancers including PCa, lung cancer, and osteosarcoma [6,25,26]. However, the efficiency and toxicity of siRNA delivery remains a major concern for this approach. Interestingly, bioactive small molecules have been recently designed to better target RNA based on folding of the RNA [27]. As a lncRNA, Malat1 may contain functionally significant RNA motifs that can be targeted by small bioactive molecules, and since Malat1 is highly induced in EnzR-PCa, Malat1 expression can be used as a marker in drug screening to identify molecules that can decrease the Malat1 expression to provide therapeutic efficacy.
Early studies indicated that treating with Enz could induce AR-v7 expression in VCaP, but not in LNCaP or C4-2 cells [8], suggesting that a higher expression of Malat1 in VCaP may be required to induce AR-v7 (Supplementary Fig. 3B), which might be important clinically. It is possible that the endogenous Malat1 existing in CRPC before ADT-Enz may not reach a critical threshold to induce AR-v7 expression and only after induction (via ADT-Enz) may reach that threshold to induce AR-v7, leading to Enz-resistance development.
Additionally, we found that AR activity failed to regulate Malat1 expression in CWR22Rv1 and LNCaP cells (Supplementary Figs. 2A and 2B), suggesting that LNCaP cells may represent the early stage of PCa that may lack certain cofactors for Malat1 induction by AR. However, Malat1 in CWR22Rv1 cells may be regulated primarily by AR-v7, thus are insensitive to treatment with androgen or antiandrogens. In a reciprocal manner, AR-v7 expression in CWR22Rv1 cells is also not sensitive to alterations of Malat1 expression (Supplementary Fig. 3C), as AR-v7 expression can occur without the need for additional factors in cellular splicing machinery like Malat1, due to genomic duplication in the AR locus [28].
5. Conclusions
In summary, these results suggest that targeting the Malat1/AR-v7 axis via Malat1-siRNA or ASC-J9 can be developed as a new therapy to better suppress the EnzR-PCa progression.
Supplementary Material
Acknowledgments
Funding/Support and role of the sponsor: This work was supported by NIH Grants (CA127300 and CA156700), Taiwan Department of Health Clinical Trial and Research Center of Excellence Grant DOH99-TD-B-111-004 (China Medical University, Taichung, Taiwan).
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.eururo.2016.04.035.
Footnotes
Author contributions: Chawnshang Chang had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Chang, Sun.
Acquisition of data: Wang, Lin, Lin, Antonarakis, Luo.
Analysis and interpretation of data: Sun, Li, Antonarakis, Luo.
Drafting of the manuscript: Wang, Sun, Chang.
Critical revision of the manuscript for important intellectual content: Chang.
Statistical analysis: Luo, Antonarakis.
Obtaining funding: Chang.
Administrative, technical, or material support: Niu, Yeh.
Supervision: None.
Other: None.
Financial disclosures: Chawnshang Chang certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: ASC-J9 was patented by the University of Rochester, University of North Carolina, and AndroScience, and then licensed to AndroScience. All authors declare there are no potential conflicts of interest related to this manuscript.
References
- 1.Sun S, Sprenger CC, Vessella RL, et al. Castration resistance in human prostate cancer is conferred by a frequently occurring androgen receptor splice variant. J Clin Invest. 2010;120:2715–30. doi: 10.1172/JCI41824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heinlein CA, Chang C. Androgen receptor in prostate cancer. Endocr Rev. 2004;25:276–308. doi: 10.1210/er.2002-0032. [DOI] [PubMed] [Google Scholar]
- 3.Vogelzang NJ. Enzalutamide—a major advance in the treatment of metastatic prostate cancer. N Engl J Med. 2012;367:1256–7. doi: 10.1056/NEJMe1209041. [DOI] [PubMed] [Google Scholar]
- 4.Antonarakis ES, Lu C, Wang H, et al. AR-V7 and resistance to enzalutamide and abiraterone in prostate cancer. N Engl J Med. 2014;371:1028–38. doi: 10.1056/NEJMoa1315815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang L, Lin C, Jin C, et al. lncRNA-dependent mechanisms of androgen-receptor-regulated gene activation programs. Nature. 2013;500:598–602. doi: 10.1038/nature12451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ren S, Liu Y, Xu W, et al. Long noncoding RNA MALAT-1 is a new potential therapeutic target for castration resistant prostate cancer. J Urol. 2013;190:2278–87. doi: 10.1016/j.juro.2013.07.001. [DOI] [PubMed] [Google Scholar]
- 7.Tripathi V, Ellis JD, Shen Z, et al. The nuclear-retained noncoding RNA MALAT1 regulates alternative splicing by modulating SR splicing factor phosphorylation. Mol Cell. 2010;39:925–38. doi: 10.1016/j.molcel.2010.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hu R, Lu C, Mostaghel EA, et al. Distinct transcriptional programs mediated by the ligand-dependent full-length androgen receptor and its splice variants in castration-resistant prostate cancer. Cancer Res. 2012;72:3457–62. doi: 10.1158/0008-5472.CAN-11-3892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang J, Su L, Chen X, et al. MALAT1 promotes cell proliferation in gastric cancer by recruiting SF2/ASF. Biomed Pharmacother. 2014;68:557–64. doi: 10.1016/j.biopha.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 10.Liu LL, Xie N, Sun S, et al. Mechanisms of the androgen receptor splicing in prostate cancer cells. Oncogene. 2014;33:3140–50. doi: 10.1038/onc.2013.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Soh SF, Huang CK, Lee SO, et al. Determination of androgen receptor degradation enhancer ASC-J9((R)) in mouse sera and organs with liquid chromatography tandem mass spectrometry. J Pharm Biomed Anal. 2014;88:117–22. doi: 10.1016/j.jpba.2013.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lu T, Lin WJ, Izumi K, et al. Targeting androgen receptor to suppress macrophage-induced EMT and benign prostatic hyperplasia (BPH) development. Mol Endocrinol. 2012;26:1707–15. doi: 10.1210/me.2012-1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yamashita S, Lai KP, Chuang KL, et al. ASC-J9 suppresses castration-resistant prostate cancer growth through degradation of full-length and splice variant androgen receptors. Neoplasia. 2012;14:74–83. doi: 10.1593/neo.111436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu MH, Ma WL, Hsu CL, et al. Androgen receptor promotes hepatitis B virus-induced hepatocarcinogenesis through modulation of hepatitis B virus RNA transcription. Sci Transl Med. 2010;2:32ra35. doi: 10.1126/scitranslmed.3001143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lai KP, Huang CK, Chang YJ, et al. New therapeutic approach to suppress castration-resistant prostate cancer using ASC-J9 via targeting androgen receptor in selective prostate cells. Am J Pathol. 2013;182:460–73. doi: 10.1016/j.ajpath.2012.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Korpal M, Korn JM, Gao X, et al. An F876L mutation in androgen receptor confers genetic and phenotypic resistance to MDV3100 (enzalutamide) Cancer Discov. 2013;3:1030–43. doi: 10.1158/2159-8290.CD-13-0142. [DOI] [PubMed] [Google Scholar]
- 17.Joseph JD, Lu N, Qian J, et al. A clinically relevant androgen receptor mutation confers resistance to second-generation antiandrogens enzalutamide and ARN-509. Cancer Discov. 2013;3:1020–9. doi: 10.1158/2159-8290.CD-13-0226. [DOI] [PubMed] [Google Scholar]
- 18.Wang R, Lin W, Lin C, et al. ASC-J9((R)) suppresses castration resistant prostate cancer progression via degrading the enzalutamide-induced androgen receptor mutant AR-F876L. Cancer Lett. 2016;379:154–60. doi: 10.1016/j.canlet.2016.05.018. [DOI] [PubMed] [Google Scholar]
- 19.Arora VK, Schenkein E, Murali R, et al. Glucocorticoid receptor confers resistance to antiandrogens by bypassing androgen receptor blockade. Cell. 2013;155:1309–22. doi: 10.1016/j.cell.2013.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hornberg E, Ylitalo EB, Crnalic S, et al. Expression of androgen receptor splice variants in prostate cancer bone metastases is associated with castration-resistance and short survival. PLoS One. 2011;6:e19059. doi: 10.1371/journal.pone.0019059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li L, Xie H, Liang L, et al. Increased PrLZ-mediated androgen receptor transactivation promotes prostate cancer growth at castration-resistant stage. Carcinogenesis. 2013;34:257–67. doi: 10.1093/carcin/bgs337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhu ML, Kyprianou N. Androgen receptor and growth factor signaling cross-talk in prostate cancer cells. Endocr Relat Cancer. 2008;15:841–9. doi: 10.1677/ERC-08-0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dai B, Chen H, Guo S, et al. Compensatory upregulation of tyrosine kinase Etk/BMX in response to androgen deprivation promotes castration-resistant growth of prostate cancer cells. Cancer Res. 2010;70:5587–96. doi: 10.1158/0008-5472.CAN-09-4610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Culig Z, Bartsch G, Hobisch A. Interleukin-6 regulates androgen receptor activity and prostate cancer cell growth. Mol Cell Endocrinol. 2002;197:231–8. doi: 10.1016/s0303-7207(02)00263-0. [DOI] [PubMed] [Google Scholar]
- 25.Gutschner T, Hammerle M, Eissmann M, et al. The noncoding RNA MALAT1 is a critical regulator of the metastasis phenotype of lung cancer cells. Cancer Res. 2013;73:1180–9. doi: 10.1158/0008-5472.CAN-12-2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cai X, Liu Y, Yang W, et al. Long noncoding RNA MALAT1 as a potential therapeutic target in osteosarcoma. J Orthop Res. 2016;34:932–41. doi: 10.1002/jor.23105. [DOI] [PubMed] [Google Scholar]
- 27.Yang WY, Gao R, Southern M, et al. Design of a bioactive small molecule that targets r(AUUCU) repeats in spinocerebellar ataxia 10. Nat Commun. 2016;7:11647. doi: 10.1038/ncomms11647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li Y, Hwang TH, Oseth LA, et al. AR intragenic deletions linked to androgen receptor splice variant expression and activity in models of prostate cancer progression. Oncogene. 2012;31:4759–67. doi: 10.1038/onc.2011.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.