Abstract
Tumor-associated macrophages (TAMs) display a spectrum of phenotypes ranging from pro-tumoral/anti-inflammatory “M2-like” to anti-tumoral/pro-inflammatory “M1-like” subtypes and, consequently, high intratumoral M2-to-M1 ratios are typically indicative of poor disease prognosis. Cancer immunotherapies that selectively modulate M2-like TAMs, enabling reversal of the M2-to-M1 ratio, represent a promising anti-cancer intervention but are difficult to implement due to the lack of effective targeting systems. In this study, we report the development of high avidity, M2 macrophage-selective targeted drug delivery platforms based on M2 macrophage-targeting peptides (M2pep) grafted onto poly(N-(2-hydroxypropyl) methacrylamide). Furthermore, these M2pep-grafted polymers also exhibit improved serum stability along with M2 macrophage-selective toxicity.
Keywords: Multivalent polymer, M2 macrophage, Avidity, Serum stability
Graphical Abstract
Over the past decades, our understanding of tumor microenvironment (TME) and its signaling niche in governing cancer progression has been greatly accelerated. Diverse roles of different immune cell populations, either promoting or suppressing tumor development, have been extensively investigated.1 Tumor-associated macrophages (TAMs), in particular, have been recognized for their roles in supporting cancer cell proliferation and migration, suppressing anti-tumor immune responses, as well as stimulating angiogenesis to sustain tumor growth.2 These TAMs preferentially display a gene expression profile characteristic of an anti-inflammatory M2 phenotype although a small population of TAMs is known to exhibit pro-inflammatory, M1-like phenotype and possesses tumoricidal functions.3 Pathologically, a high M2-to-M1 ratio has been implicated in poor disease prognosis in several types of cancers such as ovarian, brain, and gastric cancers.4–6 Significant attention has therefore been drawn towards development of anti-cancer immunomodulating therapies targeting M2-TAMs in order to lower the relative M2-to-M1 abundance and restore the tumoricidal microenvironment.7,8
Active targeting of drugs with appropriate ligands is an effective strategy to promote preferential uptake of the drugs into the cells of interest.9 Utilizing subtractive phage display biopanning against murine M1 and M2 macrophages, our lab has previously identified and optimized M2 macrophage-targeting peptides (M2pep) that can selectively bind to M2 macrophages and M2-TAMs over M1 cells and other leukocyte populations.10–12 To further improve the binding activity of M2pep, we synthesized and evaluated divalent and tetravalent M2pep displayed on lysine-based peptidic scaffolds.13 Only the divalent M2pep was found to have enhanced binding avidity to M2 macrophages with good selectivity over M1 macrophages, whereas tetravalent M2pep, unexpectedly, had less improvement in binding activity and also lost binding selectivity. Since the tetravalent peptidic scaffold used in the previous study positions M2pep closely together (1 glycine spacing between each grafted peptide), we hypothesize that displaying M2pep on a more flexible platform may confer a higher degree of freedom to allow more efficient multivalent interaction and restoration in enhanced binding avidity and selectivity of the higher valency M2pep.
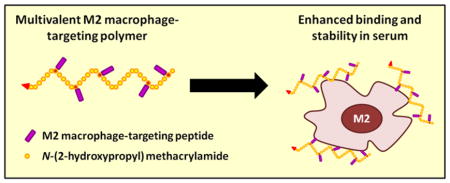
To verify our hypothesis, in this study, we synthesized and evaluated multivalent M2pep-grafted polymers, where the polymer backbone serves as an excellent scaffold that holds M2pep peptides together while allowing each peptide to more flexibly interact with different receptors (Scheme 1). The base polymer was synthesized via reverse addition-fragmentation chain-transfer (RAFT) co-polymerization of N-(2-hydroxypropyl) methacrylamide (HPMA) and N-(3-aminopropyl) methacrylamide (APMA) with Biotin-CTA (chain transfer agent) as a RAFT chain transfer agent and VA-044 as an initiator (Scheme 1 and S1). By using Biotin-CTA in the polymerization reaction, the base polymer, Biotin-poly(HPMA-st-APMA), contained exactly one biotin per polymer chain to enable accurate comparison in binding activity between the peptide-grafted polymers and biotinylated free peptides. HPMA was chosen for its hydrophilicity and biocompatibility, whereas APMA provides free amines for post-conversion to functionalizable groups (maleimide via NHS chemistry with 3-maleimidopropionic acid N-hydroxysuccinimide ester (NHS-Mal) or azide via diazo transfer chemistry with imidazole-1-sulfonyl azide) to facilitate M2pep peptide grafting via thiol-maleimide Michael addition with cysteine-containing peptide or copper(I)-catalyzed alkyne-azide cycloaddition (CuAAC) with alkyne-containing peptide respectively. A series of three M2pep variants with varying affinities (M2pep, AcM2pep(RY), and DFBP-cyclized M2pep(RY)) were grafted to the polymers with comparable efficacy yielding multivalent polymers with valency in the range of 4.5–6.4 (Table 1).
Scheme 1.
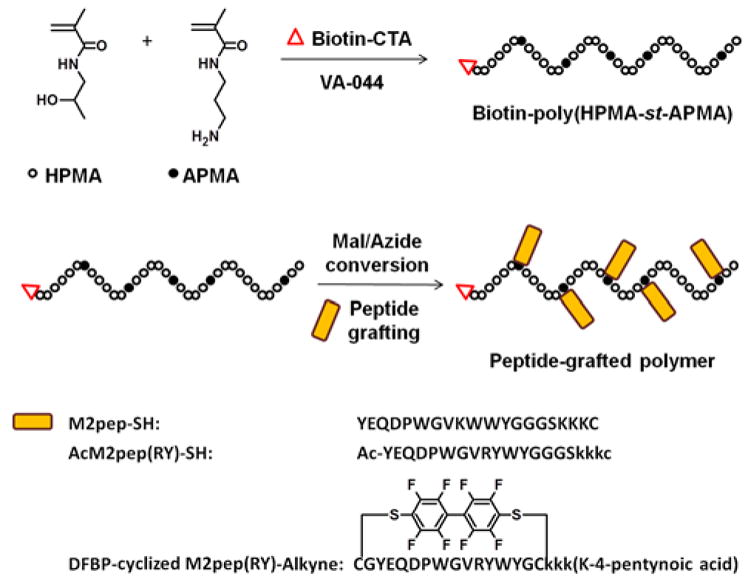
Synthesis of peptide-grafted polymers.
Table 1.
A panel of peptide-grafted polymers, apparent dissociation constant of free peptides, and calculated valency
| Peptide-grafted polymer | Reported apparent dissociation constant of free Peptide (KD,M2 in μM)11 | Valency |
|---|---|---|
| M2pep-polymer | 223 | 4.5 |
| AcM2pep(RY)-polymer | 46.8 | 6.4 |
| DFBP-cyclized M2pep(RY)-polymer | 2.03 | 4.7 |
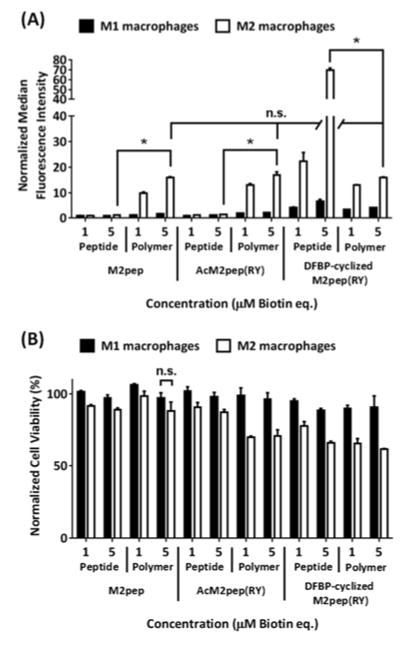
We have previously demonstrated that the M2pep peptides bind preferentially to bone marrow-derived murine macrophages polarized with interleukin 4 (IL-4) to an anti-inflammatory, M2 phenotype compared to the macrophages polarized with interferon γ (IFN-γ) and lipopolysaccharide (LPS) to a pro-inflammatory, M1 phenotype. Therefore, binding studies were performed by incubating biotinylated peptides or peptide-grafted polymers with bone marrow-derived, M1-/M2-polarized, murine macrophages and probing for binding activity with streptavidin-FITC. M1 macrophages were used as a control to evaluate selectivity of the peptide and peptide-grafted polymers. Consistent with their high micromolar KD,M2, binding activities of M2pep and AcM2pep(RY) on M1 and M2 macrophages are weak at 1 and 5 μM (Figure 1A). In regard to both molar equivalence (comparing 1 μM peptide treatment to 1 μM peptide-grafted polymer treatment) and peptide molar equivalence (comparing 5 μM peptide treatment to 1 μM peptide-grafted polymer treatment), multivalent display of these peptides onto the HPMA polymer significantly improves their binding avidity on M2 macrophages while retaining selectivity over M1 macrophages. Hence, the restoration in binding avidity and M2 selectivity supports our hypothesis on the effect of peptide spacing on multivalent binding avidity. Unexpectedly, DFBP-cyclized M2pep(RY), the highest affinity free peptide analog, showed diminished binding activity when presented on the polymer platform (Figure 1A). Although it is unclear why DFBP-cyclized M2pep(RY)-polymer did not show the enhanced binding avidity, one possibility could be that DFBP-cyclized M2pep(RY) peptides, being more hydrophobic from the additional octafluoro-diphenyl moiety in the cyclization region, are more preferentially buried into the polymer coil away from the aqueous environment and, hence, are unable to efficiently engage with target cells.
Figure 1.
(A) Binding study of M2 macrophage-targeting peptides and peptide-grafted polymers in PBS (1% albumin) (PBSA) with M1 and M2 macrophages. * denotes statistical significance (P < 0.05) based on Student’s unpaired t-tests, and n.s. denotes non-statistical significance based on one-way ANOVA with Tukey’s post-hoc tests. For clarity, only statistical analyses of 5 μM treatments are shown. (B) Corresponding cell viabilities. Unless labeled, statistical significance (P < 0.05) was observed on the relative cell viability between M1 and M2 macrophages of the same treatment.
Interestingly, whereas our previously reported peptidic scaffold-based tetravalent M2pep was shown to be very toxic to macrophages, with less than 30% cell viability at 5 μM treatment,13 the peptide-grafted polymers developed here (valency ~ 4.5–6.5) are less toxic with greater than 60% cell viability at the same concentration (Figure 1B). Notably, as observed previously for tetravalent M2pep13 and here for multivalent M2pep-polymers, their binding-associated toxicity is more selective to M2 macrophages, providing a unique feature for future development of M2-TAM-targeted drug-free macromolecular therapeutics analogous to the Kopeček lab’s apoptosis-inducing CD20-crosslinking polymers.14 Since these multivalent M2pep constructs have similar valencies, the difference in toxicity could be due to peptide spacing and/or backbone rigidity, where tetravalent M2pep is more rigid with closely-spaced M2pep, and peptide-grafted polymers are more flexible with higher degree of freedom of the displayed peptides. While the rigid peptidic scaffold-based tetravalent M2pep mediates more potent M2 toxicity, it is poorly water-soluble and hence may not be suitable for in vivo applications. On the other hand, although the M2pep-polymers are less toxic, they retain M2-to-M1 binding selectivity while being more water-soluble (empirical observation) and amenable to further conjugation with more potent cytotoxic cargos, making them an ideal platform for M2-TAM-targeted drug delivery application. In fact, the lower toxicity of M2pep-polymers is more suitable for delivery of therapeutic agents which aim at modulating M2-TAMs without killing them (e.g. M2-to-M1 repolarization).
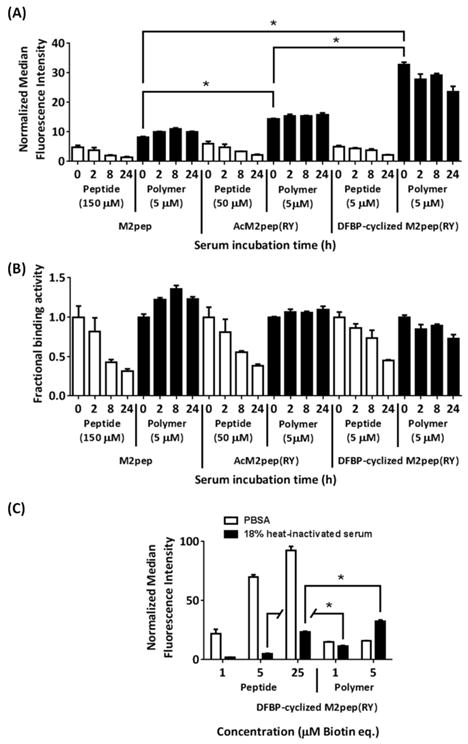
Since stability and binding activity in serum are two important parameters for successful in vivo targeted drug delivery applications, we next evaluated how binding activities of different peptide-grafted polymers are affected by serum incubation over time. Biotinylated free peptides and peptide-grafted polymers were incubated in normal mouse serum, and aliquots were taken at various time points, diluted in PBS, and heat-inactivated for use in binding study on M2 macrophages. Notably, all peptide-grafted polymers retained high binding activities even after incubation in serum for 24 h, unlike their free peptide analogs which lost more than half of the initial binding activities during the same period of serum incubation (Figure 2A and 2B (normalized data)). Linear M2pep and AcM2pep(RY) have been previously shown to be rapidly degraded within 24 h.11 By displaying these peptides onto HPMA polymer, their serum stability is significantly enhanced. Unlike polymeric micelles/polymersomes which may encapsulate peptides away from serum proteases, peptides on the peptide-polymer conjugates are expected to be relatively more exposed to serum environment. Hence, improvement in serum stability of peptide-polymer conjugates, such as the ones demonstrated here, is a relatively less-appreciated advantage of peptide-polymer conjugates as opposed to their well-recognized abilities to mediate multivalent interaction and prolong in vivo circulation time. Interestingly, although the initial binding study of all peptide-grafted polymers, performed in PBS (1% albumin) (PBSA), shows no statistical difference in the extent of binding activity among these peptide-grafted polymer analogs (Figure 1A), their binding activities are significantly altered in the presence of serum with DFBP-cyclized M2pep(RY)-polymer having the highest binding activity (Figure 2A).
Figure 2.
(A) Binding study of M2pep peptides and peptide-grafted polymers after serum incubation, with M2 macrophages. All serum-incubated samples were diluted to 18% serum with PBS and heat-inactivated prior to the binding study. Concentrations are labeled as biotin equivalence. Binding data of the polymer analogs are shaded in black for clarity. * denotes statistical significance (P < 0.05) based on Student’s unpaired t-tests. (B) Fractional binding activities relative to their respective activities at 0 h time point. (C) Binding study of DFBP-cyclized M2pep(RY)-peptide/polymer in PBSA and 18% heat-inactivated serum (in PBS) with M2 macrophages. Statistical analysis comparing treatments of equimolar peptide equivalence was performed by Student’s unpaired t-tests where * denotes statistical significance (P < 0.05).
To further evaluate the effect of serum on binding activity of free peptide versus peptide-grafted polymer, we performed binding studies of DFBP-cyclized M2pep(RY) and its peptide-grafted polymer analog (DFBP-cyclized M2pep(RY)-polymer) at different concentrations both in PBSA and in PBS (18% heat-inactivated mouse serum). In the presence of serum, binding activity of DFBP-cyclized M2pep(RY) on M2 macrophages is significantly reduced whereas the binding activity of its peptide-grafted polymer analog (DFBP-cyclized M2pep(RY)-polymer) is less affected at 1 μM and unexpectedly enhanced at 5 μM (Figure 2C). Although DFBP-cyclized M2pep(RY), as a free peptide, has higher binding activity than its polymer in PBSA, the trend is reversed in the presence of serum. Since binding study in the presence of serum is more relevant to the in vivo environment, we propose DFBP-cyclized M2pep(RY)-polymer as an improved M2 macrophage-targeting platform suitable for further development into anti-cancer M2-TAM-targeted drug-polymer conjugates. As demonstrated in our current study, binding behaviors of targeting peptides or peptide-grafted polymers in a common buffer (PBSA) and in serum-containing buffer could be very different highlighting the importance of in vitro evaluation in physiologically relevant media. Adding on to the well-recognized benefit of prolonged in vivo circulation time, the accompanying improvement in serum stability of peptides on polymers also represents a valuable advantage in development of therapeutic peptide-polymer conjugates.
In summary, we report development of multivalent HPMA-based polymer bearing multiple M2 macrophage-targeting peptides. Enhanced binding avidity and stability in the presence of serum were observed for all peptide-grafted polymers highlighting the advantages of peptide-grafted polymer as a promising targeted drug delivery carrier for nanomedicine applications. These polymers also possess some extent of inherent cytotoxicity to M2 macrophages selectively over M1 macrophages which could complement the activity of their conjugated cytotoxic drugs for M2-TAM depletion application. Our investigation here suggests DFBP-cyclized M2pep(RY)-grafted polymer as a potential platform for future development of TAM-targeted immunomodulating therapeutics.
Supplementary Material
Acknowledgments
This work was supported by NIH 1R01CA177272. Chayanon Ngambenjawong was supported by an Anandamahidol Foundation Fellowship.
Footnotes
Note
The authors have a pending patent on multivalent M2pep-polymers.
The Supporting Information is available free of charge on the ACS Publications website.
Detailed materials and methods. Measured molecular weight of the synthesized peptides and base polymer (PDF)
References
- 1.Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nat Med. 2013;19:1423–1437. doi: 10.1038/nm.3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Noy R, Pollard JW. Tumor-Associated Macrophages: From Mechanisms to Therapy. Immunity. 2014;41:49–61. doi: 10.1016/j.immuni.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schmieder A, Michel J, Schönhaar K, Goerdt S, Schledzewski K. Differentiation and gene expression profile of tumor-associated macrophages. Semin Cancer Biol. 2012;22:289–297. doi: 10.1016/j.semcancer.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 4.He Y, Zhang M, Wu X, Sun X, Xu T, He Q, Di W. High MUC2 Expression in Ovarian Cancer Is Inversely Associated with the M1/M2 Ratio of Tumor-Associated Macrophages and Patient Survival Time. PLoS One. 2013;8:e79769. doi: 10.1371/journal.pone.0079769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ding P, Wang W, Wang J, Yang Z, Xue L. Expression of tumor-associated macrophage in progression of human glioma. Cell Biochem Biophys. 2014;70:1625–1631. doi: 10.1007/s12013-014-0105-3. [DOI] [PubMed] [Google Scholar]
- 6.Pantano F, Berti P, Guida FM, Perrone G, Vincenzi B, Amato MMC, Righi D, Dell’Aquila E, Graziano F, Catalano V, Caricato M, Rizzo S, Muda AO, Russo A, Tonini G, Santini D. The role of macrophages polarization in predicting prognosis of radically resected gastric cancer patients. J Cell Mol Med. 2013;17:1415–1421. doi: 10.1111/jcmm.12109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ngambenjawong C, Gustafson HH, Pun SH. Progress in tumor-associated macrophage (TAM)-targeted therapeutics. Adv Drug Deliv Rev. 2017 doi: 10.1016/j.addr.2017.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mantovani A, Marchesi F, Malesci A, Laghi L, Allavena P. Tumour-associated macrophages as treatment targets in oncology. Nat Rev Clin Oncol. 2017 doi: 10.1038/nrclinonc.2016.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Srinivasarao M, Galliford CV, Low PS. Principles in the design of ligand-targeted cancer therapeutics and imaging agents. Nat Rev Drug Discov. 2015;14:203–219. doi: 10.1038/nrd4519. [DOI] [PubMed] [Google Scholar]
- 10.Cieslewicz M, Tang J, Yu JL, Cao H, Zavaljevski M, Motoyama K, Lieber A, Raines EW, Pun SH. Targeted delivery of proapoptotic peptides to tumor-associated macrophages improves survival. Proc Natl Acad Sci U S A. 2013;110:15919–15924. doi: 10.1073/pnas.1312197110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ngambenjawong C, Gustafson HH, Pineda JM, Kacherovsky NA, Cieslewicz M, Pun SH. Serum Stability and Affinity Optimization of an M2 Macrophage-Targeting Peptide (M2pep) Theranostics. 2016;6:1403–1414. doi: 10.7150/thno.15394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ngambenjawong C, Pineda JMB, Pun SH. Engineering an Affinity-Enhanced Peptide through Optimization of Cyclization Chemistry. Bioconjug Chem. 2016;27:2854–2862. doi: 10.1021/acs.bioconjchem.6b00502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ngambenjawong C, Cieslewicz M, Schellinger JG, Pun SH. Synthesis and evaluation of multivalent M2pep peptides for targeting alternatively activated M2 macrophages. J Control Release. 2016;224:103–111. doi: 10.1016/j.jconrel.2015.12.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chu TW, Kopecek J. Drug-free macromolecular therapeutics - a new paradigm in polymeric nanomedicines. Biomater Sci. 2015;3:908–922. doi: 10.1039/C4BM00442F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sobczynski DJ, Eniola-Adefeso O. Effect of anticoagulants on the protein corona-induced reduced drug carrier adhesion efficiency in human blood flow. Acta Biomater. 2017;48:186–194. doi: 10.1016/j.actbio.2016.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.