Abstract
Cholinesterases are involved in neuronal signal transduction, and perturbation of function has been implicated in diseases, such as Alzheimer’s and Huntington’s disease. For the two major classes of cholinesterases, such as acetylcholinesterase (AChE) and butyrylcholinesterase (BChE), previous studies reported BChE activity is elevated in patients with Alzheimer’s disease, while AChE levels remain the same or decrease. Thus, the development of potent and specific inhibitors of BChE have received much attention as a potential therapeutic in the alleviation of neurodegenerative diseases. In this study, we evaluated amino acid analogs as selective inhibitors of BChE. Amino acid analogs bearing a 9-fluorenylmethyloxycarbonyl (Fmoc) group were tested, as the Fmoc group has structural resemblance to previously described inhibitors. We identified leucine, lysine, and tryptophan analogs bearing the Fmoc group as selective inhibitors of BChE. The Fmoc group contributed to inhibition, as analogs bearing a carboxybenzyl group showed ~tenfold higher values for the inhibition constant (KI value). Inclusion of a t-butoxycarbonyl on the side chain of Fmoc tryptophan led to an eightfold lower KI value compared to Fmoc tryptophan alone suggesting that modifications of the amino acid side chains may be designed to create inhibitors with higher affinity. Our results identify Fmoc-amino acids as a scaffold upon which to design BChE-specific inhibitors and provide the foundation for further experimental and computational studies to dissect the interactions that contribute to inhibitor binding.
Keywords: Butyrylcholinesterase, Cholinesterase, Fmoc-amino acid, Tryptophan, Inhibition
Introduction
Cholinesterases and the inhibition of cholinesterases have been the subject of numerous studies over the past several decades [e.g., reviewed in (Pagano et al. 2015; Hogan 2014; Pinho et al. 2013; Huang et al. 2013; Anand and Singh 2013; Musial et al. 2007)]. The enzymes are divided into two major subfamilies: acetylcholinesterase (AChE) and butyrylcholinesterase (BChE). AChE hydrolyzes acetylcholine resulting in the termination of neurotransmission and the enzyme also been suggested to be involved in activities, such as cell differentiation and development (Nizri and Brenner 2013; Halliday and Greenfield 2012; Abreu-Villaca et al. 2011; Jiang and Zhang 2008; Dori and Soreq 2006; Meshorer et al. 2002; Grisaru et al. 1999). While the biological role of BChE is less well understood, there has been significant interest in the development of molecules that selectively inhibit BChE relative to AChE, as BChE activity was found to increase in Alzheimer’s patients, while AChE activity decreases or remains the same (Mushtaq et al. 2014).
X-ray structures show that the overall AChE and BChE structures are similar, but several structural differences in the active sites and active-site gorges have been identified (Nicolet et al. 2003; Soreq et al. 1992; Dvir et al. 2010; Sussman et al. 1991). While the active site of each enzyme has a serine nucleophile as part of a serine, histidine, glutamate catalytic triad, and two backbone amides from glycine residues are situated to form hydrogen bonds with the substrate carbonyl group in an ‘oxyanion hole’, the size of the active-site cavities is different for the two enzymes. In BChE, aliphatic residues, such as leucine and isoleucine, are found, where two phenylalanine residues are found at the corresponding positions in AChE, and the active-site gorge is larger in BChE relative to AChE. These structural differences are suggested to allow the accommodation of larger substrates in the BChE active site relative to AChE (Macdonald et al. 2012; Radic et al. 1993; Vellom et al. 1993; Saxena et al. 1997). Indeed, over six decades ago, Augustinsson reported that substrates larger than propionylcholine were not efficiently hydrolyzed by AChE, while BChE efficiently catalyzes the hydrolysis of this substrate and the larger butyrylcholine commonly used in biochemical studies (Augustinsson 1948). In addition to the differences in the size of the active-site cavities, the larger gorge leading to the BChE active site has been investigated in studies of inhibitor development [e.g., (Saxena et al. 1997)]. In particular, significant attention has focused on a site at the outer end of the gorge known as the ‘peripheral binding site’, as this site has been suggested to be important for substrate binding, and studies in acetylcholinesterase have focused on developing inhibitors that interact with this distal binding site (Mallender et al. 2000; Szegletes et al. 1999).
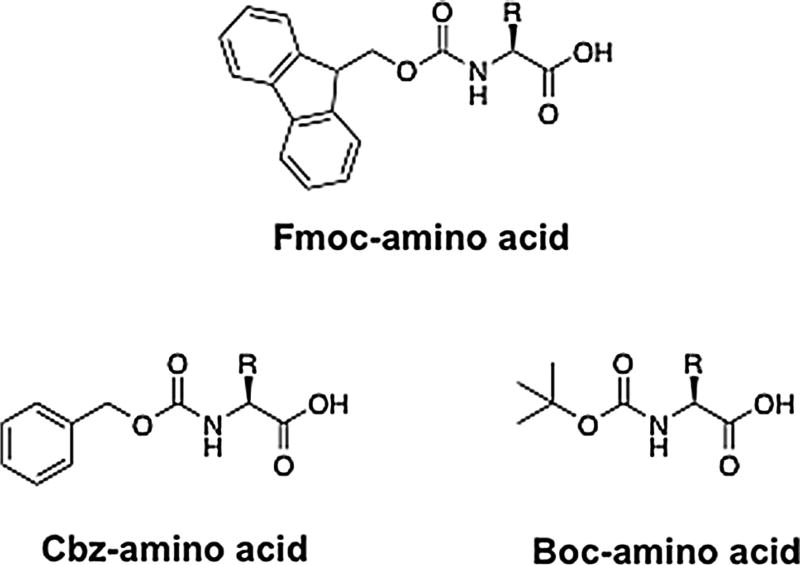
Over the past decades, numerous compounds have been synthesized and tested as cholinesterase inhibitors, including natural products and variants synthesized based on natural products [e.g., (Brus et al. 2014; Huang et al. 2013; Pinho et al. 2013; Anand and Singh 2013; Carolan et al. 2010; Kamal et al. 2008)]. These classes of inhibitors include compounds that have been used as therapeutics, such as donepezil, galantamine, rivastigmine, and tacrine (Fig. 1) (Anand and Singh 2013). Although the structures of cholinesterase inhibitors are widely varied, as shown by the few examples in Fig. 1, many reoccurring features, such as aromatic groups, are found in different inhibitor classes. A model for the importance of aromatic groups in cholinesterase inhibitors is that these groups may lead to favorable binding interactions with aromatic groups within the cholinesterase active site. Based on this observation and previous studies, we postulated that molecules bearing the aromatic 9-fluorenylmethyloxycarbonyl (Fmoc) group might provide a series of compounds that inhibit cholinesterase (Fig. 2). Indeed, a previous study by Zhao et al. evaluated a series of β-carboline and quinolone alkaloids derivatives that bear structural resemblance to the Fmoc-moiety and they found several compounds inhibited BChE and/or AChE (Zhao et al. 2013). We chose to investigate Fmoc-amino acids, as amino acid analogs provide a scaffold upon which to readily vary functional groups and molecular size. The Fmoc group is used extensively in solid-phase peptide synthesis, and numerous Fmoc-protected amino acids and amino acid analogs are commercially available or can be readily synthesized (Fields and Noble 1990; Behrendt et al. 2016). Herein, we report that Fmoc-amino acids inhibit BChE and that the inhibitors are selective for BChE relative to AChE. The compounds show competitive inhibition, but the site or sites of interaction for the analogs, such as the active site, peripheral binding site, or another site, remains to be determined. Nevertheless, the results provide a foundation upon which to evaluate amino acid analogs as BChE-selective inhibitors. Furthermore, the results provide a basis for future computational modeling studies to evaluate the mode or modes of interaction and to make predications to guide the design of more potent inhibitors.
Fig. 1.
Examples of cholinesterase inhibitors
Fig. 2.
Fmoc-, Cbz-, and Boc-amino acids. The ‘R’ group was varied in this study
Materials and methods
Materials
All reagents were of the highest purity commercially available (≥97 %). All buffers were prepared with reagent grade materials or better. Bovine serum albumin (BSA) was from Thermo Fisher Scientific. Butyrylcholinesterase from equine serum and acetylcholinesterase from Electrophorus electricus were from Sigma-Aldrich. S-butyrylthiocholine and S-acetylthiocholine were from Alfa Aesar. Fmoc- and Boc-amino acids were from AAPPTec (Louisville, KY), and the amino acids were from Fisher Scientific and Alfa Aesar. Thin-layer chromatography and NMR spectroscopy were used to analyze amino acid analog purity. Absorbance measurements were made using a PerkinElmer Lamba 25 spectrophotometer fitted with a PCB-1500 water Peltier system (PerkinElmer) for temperature control. Cuvettes were from Starna Cells (Atascadero, CA). Water for all solutions was purified using a Barnstead Nanopure Diamond water system.
Relative enzyme activity measurements
Activity measurements were performed based on the method of Ellman (Ellman et al. 1961). Lyophilized butyrylcholinesterase from equine serum or lyophilized acetylcholinesterase from E. electricus was dissolved in cold 10 mM sodium phosphate, pH 7.5. Reactions were conducted at 25 °C in 100 mM sodium phosphate, pH 7.5, 1 mM MgCl2, 0.2 mM 5,5′-dithiobis-(2-nitrobenzoic acid) (DTNB), 1.2 µg BSA, and 100 µM butyrylthiocholine or 100 µM acetylthiocholine.
To evaluate the effect of amino acids and analogs on cholinesterase activity, stock solutions containing the amino acid or analog were prepared in methanol. An aliquot of the stock solution of the amino acid or analog was added to the reaction mixture (without enzyme) to give a final concentration of 200 µM with 2 % (vol/vol) methanol as a cosolvent. The cosolvent was included to increase Fmoc-amino acid solubility. For the control reaction without an amino acid analog, methanol was added to a final concentration of 2 % (vol/vol). Reactions were initiated by adding enzyme (final concentration 50 nM BChE or 20 nM AChE), and initial rates were determined by monitoring continuously at 412 nm. A molar absorptivity of 14,150 M−1 cm−1 was used to calculate product formation (Riddles et al. 1983). Relative activity was determined by dividing the initial rate for reaction in the presence of each amino acid or analog by the reaction with 2 % methanol and without amino acid or analog. At least three determinations using independently prepared solutions of the amino acids or analogs were measured and averaged. The enzyme and substrate concentrations were varied, typically fourfold, to test if the relative activity was affected by the enzyme and/or substrate concentrations. The relative activities determined at the different enzymes and substrate concentrations tested were experimentally indistinguishable.
Inhibition constant (KI) and half maximal inhibitory concentration (IC50) value determinations
Reactions were conducted at 25 °C in 100 mM sodium phosphate, pH 7.5, 1 mM MgCl2, 0.2 mM DTNB, 1.2 µg BSA, 100 µM butyrylthiocholine, and 40 nM butyrylcholinesterase. Solutions of the Fmoc-amino acid analog at varying concentrations were prepared in methanol and added to the reaction mixture to give a final methanol concentration of 2 % (vol/vol). The reactions were initiated by the addition of enzyme, and the initial rates were determined by monitoring continuously at 412 nm as described above. The KI values were determined by fitting the initial rates as a function of inhibitor concentration (typically 12–14 concentrations) as previously described (Segel 1974). For the calculation of the IC50 dose–response curve, the Fmoc-amino acid concentrations were varied over a minimum of six orders of magnitude. IC50 values were determined by fitting the initial rates as a function of inhibitor concentration using the log(inhibitor) versus the response variable slope equation. At least three determinations using independently prepared inhibitor solutions were averaged to determine KI and IC50 values. All calculations were completed using KaleidaGraph (Synergy Software).
van der Waals volume calculations
Calculations of van der Waals volumes of Fmoc-amino acids were performed using the approach described by Zhao et al. (2003). The number of atoms, bonds, and rings for each compound were determined, and the volume was calculated using the Atomic and Bond Contributions of the van der Waals volume (VABC) method (Zhao et al. 2003). To compare to the previous studies that used the program VOIDOO (version 3.1) to calculate the van der Waals volume (Kleywegt and Jones 1994), the VABC method was used to calculate the van der Waals volume for ethopropazine and compared to the literature value (Saxena et al. 1997).
Results and discussion
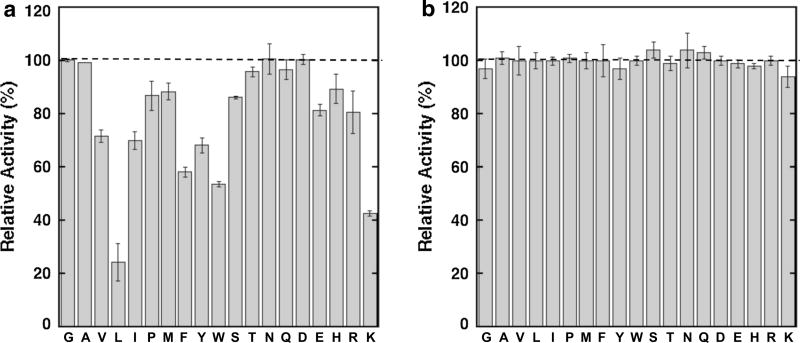
We first tested if Fmoc analogs of 19 of the 20 common amino acids inhibit BChE. The Fmoc analog of cysteine was not tested, because the cysteine sulfhydryl group interferes with the assay used to measure enzyme activity. We used a concentration of 200 µM Fmoc-amino acid in 2 % methanol (vol/vol) to evaluate the effect of each Fmoc-amino acid on BChE activity. Methanol was included in the reaction as a cosolvent to increase the aqueous solubility. We chose a concentration of 200 µM Fmoc-amino acid for the initial survey, as all the analogs were soluble under these conditions. The effect of each Fmoc-amino acid on BChE activity was determined by comparing enzyme activity in the presence of 200 µM Fmoc-amino acid to BChE activity in the presence of 2 % methanol to control for the methanol used as the cosolvent. As shown in Fig. 3a, two Fmoc-amino acids, Fmoc-Leu and Fmoc-Lys, showed the greatest decrease in activity. Several other Fmoc-amino acids decreased BChE activity, including Fmoc-Ile, Fmoc-Phe, Fmoc-Trp, and Fmoc-Tyr. The results show that analogs bearing different side-chain functional groups were found to exhibit the greatest inhibition, including analogs bearing a hydrophobic group (leucine), aromatic group (tryptophan), and cationic group (lysine). Differences in the effect on BChE activity were observed within the series of analogs with hydrophobic side chains. Specifically, Fmoc-Leu showed the greatest reduction of activity, but the constitutional isomer Fmoc-Ile showed an approximately two-fold smaller decrease in activity. A similar smaller effect was observed for Fmoc-Val. Nevertheless, the larger aliphatic side chains appear to facilitate inhibition compared to smaller side chains, as BChE activity in the presence of Fmoc-Ala or Fmoc-Gly was not experimentally distinguishable from activity in the absence of an Fmoc-amino acid. The presence of an aromatic group appeared to contribute to inhibitor binding, as Fmoc-Phe, Fmoc-Tyr, and Fmoc-Trp all showed a reduction in enzyme activity with Fmoc-Trp showing the greatest effect. Other Fmoc-amino acids did not have a significant effect on BChE activity, including analogs bearing carboxylate and amide groups, such as Fmoc-Asn, Fmoc-Gln, Fmoc-Asp, and analogs bearing a hydroxyl group, such as Fmoc-Thr. In addition, relative activity was reduced but within 10 % of reaction without an amino acid added for several analogs, including Fmoc-Pro, Fmoc-Met, and Fmoc-Ser.
Fig. 3.
Effects of Fmoc-amino acids on a BChE and b AChE activities. The Fmoc-amino acids are identified using single-letter amino acid notation. Relative activity is the activity in the presence of 200 µM Fmoc-amino acid relative to the activity in the absence of the analog. Values are from Supplementary Tables 1 and 2
We next individually measured the inhibition constants (KI values) for the analogs that showed the greatest relative decrease in BChE activity, and the results are summarized in Table 1. (IC50 values are reported in Supplementary Table 3). The KI values of 115 and 150 µM for Fmoc-Leu and Fmoc-Lys, respectively, suggest that a hydrophobic or cationic group is important for inhibition. The KI value for Fmoc-Ile was 194 µM suggesting an approximately two-fold difference in the inhibition properties for the two constitutional isomers, Fmoc-Leu and Fmoc-Ile. As a natural substrate for the enzyme, acetylcholine, bears a cationic ammonium group, analogs bearing a positively charged group, such as the cationic Lys side chain, may be favorable for binding and indeed Fmoc-Lys was one of the more potent inhibitors with a KI value of 150 µM. Although Fmoc-Arg is expected to bear a cationic group under the reaction conditions, in the presence of 200 µM Fmoc-Arg, the relative activity was indistinguishable from the reaction without Fmoc-amino acid present (Fig. 3). Minimally, two models may account for the different behaviors shown by Fmoc-Lys and Fmoc-Arg. One model is that the larger guanidinium group of the arginine side chain is not able to access the active site, because it is not accommodated in the active-site gorge compared to the smaller ammonium group of Lys. However, the calculation of van der Waals volumes described below and shown in Supplementary Table 4 suggests that all Fmoc-amino acid analogs tested can be accommodated in the BChE active-site gorge. As the arrangement of hydrogen-bonding groups for the Arg side chain differs compared to Lys, another model is that the differences in these hydrogen-bonding arrangements may prevent Arg from making favorable interactions analogous to Lys, and/or the arrangement could lead to the Arg side chain making unfavorable interactions. Future computational modeling studies will be needed to dissect the physical basis for the difference in inhibition for Fmoc-Lys and Fmoc-Arg.
Table 1.
Inhibition constants for Fmoc-amino acids
| Fmoc-amino acid | KI (µM) |
|---|---|
| Fmoc-Leu-O− | 115 ± 11 |
| Fmoc-Lys-O− | 150 ± 10 |
| Fmoc-Ile-O− | 194 ± 13 |
| Fmoc-Val-O− | 313 ± 14 |
| Fmoc-Phe-O− | 330 ± 15 |
| Fmoc-Tyr-O− | 307 ± 13 |
| Fmoc-Trp-O− | 193 ± 21 |
Do the Fmoc-amino acids selectively inhibit BChE?
To evaluate if the Fmoc-amino acids selectively inhibit BChE relative to AChE, we next tested if the analogs affect AChE activity. An evaluation analogous to that described above for BChE was performed using AChE. In this experiment, we evaluated the effect of the presence of 200 µM Fmoc-amino acid on AChE activity relative to a reaction without an amino acid analog. All reactions contained 2 % methanol (vol/vol) as cosolvent. The results in Fig. 3b show that AChE activity in the presence of 200 µM Fmoc-amino acid was indistinguishable from the activity in the absence of the amino acid analog for all compounds tested. These results indicate that the KI values for inhibitor binding to AChE are >200 µM and suggest that the Fmoc-amino acids that decreased BChE activity are selective for the inhibition of BChE.
As noted above, the active site and active-site gorge of BChE are larger than those in AChE. The simplest model for selective inhibition of BChE is that the Fmoc-amino acids are too large to be accommodated by the smaller active-site gorge in AChE. Saxena et al. previously used X-ray data to calculate the volume of the active-site gorges for AChE from Torpedo californica and a model to calculate the volume of the active-site gorge in human BChE (Saxena et al. 1997). They reported that the volume of the active-site gorge for AChE is 302.31 Å3, while the BChE active-site gorge is 501.91 Å3, suggesting that the BChE active-site gorge is ~200 Å3 larger than that for AChE. In the same study, a small molecule, ethopropazine, was shown to have a 9000-fold difference in the KI value between BChE and AChE. Calculation of the van der Waals volume for ethopropazine showed a volume of 317.6 Å3, and it was suggested that the smaller AChE active-site gorge was unable to accommodate the bulky inhibitor leading to selective binding (Saxena et al. 1997).
We carried out a similar analysis using the available data for the active-site gorges of AChE and BChE from Saxena et al. (Saxena et al. 1997). Although our experiments were performed using AChE from E. electricus, a comparison of X-ray structures show that the two structures are nearly superimposable [an overlay of the structures of AChE from T. californica (PDB ID 1EA5) and E. electricus (PDB ID 1EEA) gave an overall RMSD for of 0.328 Å]. We first calculated the van der Waals volumes for the Fmoc-amino acid analogs using the approach described by Zhao et al. (2003). To compare to the literature, we used the approach of Zhao et al. to calculate the van der Waals volume for ethopropazine and determined a van der Waals volume of 305.6 Å3 similar to 317.6 Å3 reported by Saxena et al. (1997). The results listed in Table 2 show that the van der Waals volumes for all the Fmoc-amino acids that inhibited BChE are larger than the calculated molecular volume for the AChE active-site gorge, but smaller than the calculated molecular volume for the BChE active-site gorge (van der Waals volumes for all Fmoc analogs tested are given in Supplementary Table 4). Together, the results suggest that the Fmoc-amino acids that selectively inhibit BChE are too bulky to be accommodated in the smaller gorge but can be accommodated by BChE.
Table 2.
Calculated volumes of the AChE and BChE active-site gorges from Saxena et al. and calculated van der Waals volumes of Fmoc-amino acids
| Enzyme | Volume of active-site gorge (Å3)a |
|---|---|
| Torpedo AChE | 302.31 |
| Human BChE | 501.91 |
|
| |
| Fmoc-amino acid | van der Waals volume (Å3) |
|
| |
| Fmoc-Leu-O− | 336.65 |
| Fmoc-Lys-O− | 348.97 |
| Fmoc-Ile-O− | 336.65 |
| Fmoc-Val-O− | 319.35 |
| Fmoc-Phe-O− | 357.37 |
| Fmoc-Tyr-O− | 366.16 |
| Fmoc-Trp-O− | 377.07 |
Data from Saxena et al
Is the Fmoc group important for BChE inhibition?
As described in the “Introduction”, Fmoc-amino acids were tested as BChE inhibitors as the aromatic Fmoc group bears structural features found in other cholinesterase inhibitors. An additional structural feature that may contribute to inhibition of BChE is the carbamate group, as carbamates have been used as cholinesterase inhibitors (Verma et al. 2015; Hartsel et al. 2012; Wong et al. 2013; Bar-On et al. 2002). Nevertheless, several Fmoc-amino acids did not show an inhibitory effect under the conditions tested (e.g., Fmoc-Ala). An alternate model for the observed inhibition is the leucine, lysine, and tryptophan side chains that lead to enzyme inhibition independent of the Fmoc group. To test this possibility, we examined if amino acids without the Fmoc group inhibit BChE.
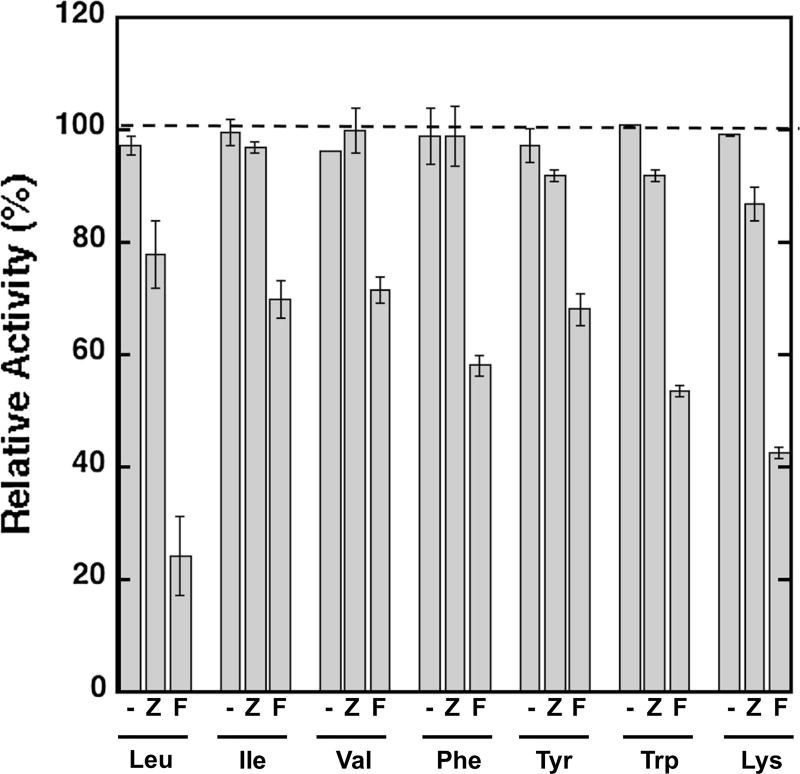
The ability of amino acids to inhibit BChE was tested by comparing BChE activity in the presence of 200 µM amino acid relative to the activity without amino acid as described above. The results summarized in Supplementary Table 5 show that relative to the control activity was unchanged in the presence of all amino acids tested (a similar result shown in Supplementary Table 6 was observed for AChE). These results suggest that the side chains of Lys, Leu, and Trp alone do not lead to BChE inhibition and support the model that the Fmoc group is important for inhibition. An alternate model, however, for the observed differences of the amino acid and Fmoc-amino acid is the cationic amine group in the amino acid that is unfavorable for binding and inhibiting the enzyme. To evaluate further the importance of the Fmoc and carbamate group in inhibition, we investigated the ability of carboxybenzyl (Cbz)-protected amino acids to inhibit BChE.
Amino acids bearing the Cbz group maintain a carbamate group analogous to the Fmoc-amino acids, but a phenyl group replaces the fluorenyl moiety found in the corresponding Fmoc-amino acids (Fig. 2). The ability of each Cbz-containing amino acid to inhibit BChE was evaluated as described for the Fmoc analogs, and the results are shown in Fig. 4. While Cbz-protected Ile, Val, Phe, and Tyr showed ≤5 % reduction in activity compared to reaction in the absence of an amino acid analog, activity was reduced by 22, 8, and 13 % in the presence of Cbz analogs of Leu, Trp, and Lys, respectively. We next determined the KI values for the three analogs that showed a >5 % decrease in activity, and the results are summarized in Table 3. The results show that the Cbz analogs of Leu, Trp, and Lys do inhibit BChE, but the inhibition constants are ~tenfold higher than the corresponding Fmoc analogs. A model to account for this observation is that interactions of the active site or peripheral binding site (or another binding site) with the aromatic moiety contribute to favorable binding, and the larger fluorenyl group relative to the phenyl group leads to more favorable interactions with the Fmoc analogs. Together, these results suggest that the importance of the larger fluorenyl group and additional experimental approaches to vary the size of the aromatic moiety in combination with computational modeling will be aid in identifying the binding site or sites and to dissect the interactions important for binding.
Fig. 4.
Evaluating the effect of varying the amino group modifications on inhibition of BChE. Amino acids lacking a group on the amine (hyphen), Cbz (Z), and Fmoc (F) are compared. Relative activity is the activity in the presence of the amino acid or analog compared to the activity in the absence of an analog. Values are from Supplementary Tables 1, 5, and 7
Table 3.
Inhibition constants (KI values) for the inhibition of BChE by Cbz-amino acids
| Fmoc-amino acid | KI (µM) |
|---|---|
| Cbz-Leu-O− | 1150 ± 130 |
| Cbz-Lys-O− | 1535 ± 502 |
| Cbz-Trp-O− | 4795 ± 630 |
As an additional experiment to survey amino acid analogs, we tested if analogs bearing the tert-butyloxycarbonyl (Boc) protecting group inhibit BChE. The Boc-protected analogs were selected, as the Boc protecting group is commonly used in solid-phase peptide synthesis allowing ready access to the compounds. The results summarized in Supplementary Table 8 show that the Boc-protected amino acids had little effect on BChE activity. A specific feature for differences in the Boc versus Fmoc-amino acids cannot be discerned from these results alone, as multiple features are varied, such as aromaticity and size, but the bulky aliphatic t-butyl moiety of the Boc group may introduce unfavorable steric effects. A future direction will be to explore if analogs bearing smaller aliphatic groups can act as inhibitors.
Evaluating the effect of modifying side-chain substituents on BChE inhibition
An attractive feature of evaluating amino acids analogs as cholinesterase inhibitors is that the analogs offer the ability to explore readily the effect of incorporating a variety of hydrophobic and aromatic substituents on the amino acid side chains. Previously reported analogs often bear hydrophobic and/or aromatic groups and these groups may interact with the hydrophobic and aromatic groups in the BChE active site or at another binding site (Andersson et al. 2013; Berg et al. 2011; Kaboudin et al. 2009; Law et al. 2007). To test if including hydrophobic or aromatic groups on the amino acid side chains of Fmoc-amino acids lead to more potent inhibitors, we evaluated the inhibition properties for a series of commercially available compounds bearing side-chain protecting groups commonly used in peptide synthesis—t-butyl, Boc, and Cbz.
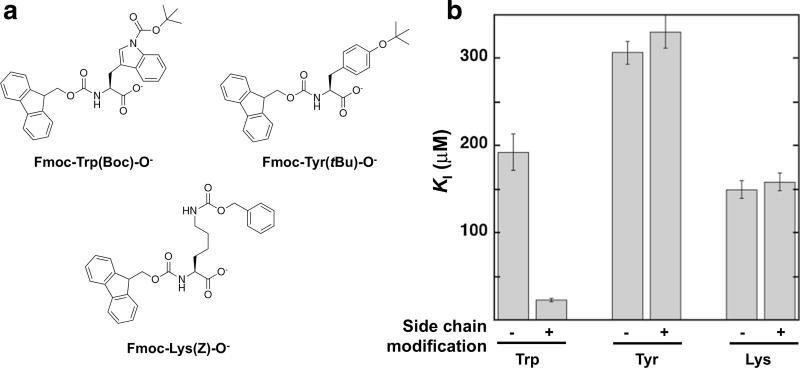
Figure 5 shows the results of evaluating the Fmoc-amino acid analogs relative to analogs without the modified side chains. The greatest decrease in relative activity was observed for Fmoc-Trp(Boc)-OH compared to Fmoc-Trp. Measurement of the KI value shows that the incorporation of a Boc group on the indole nitrogen reduces the KI value approximately eightfold. The Boc group on the Trp indole nitrogen introduces a carbamate group and steric bulk relative to the indole hydrogen. It is possible that the hydrogen on the indole nitrogen makes unfavorable interactions and removal of this hydrogen leads to a better inhibitor. An alternate model is favorable hydrogen bonding, and/or van der Waals interactions are introduced by the carbamate and/or t-butyl group of the Boc modification.
Fig. 5.
Evaluating the effect of introducing side-chain modifications on inhibition of BChE. a Structures of the Fmoc-amino acids evaluated. b KI values determined for inhibition of BChE. Minus symbol indicates that the Fmoc-amino acid side chain is unmodified, and plus symbol indicates the Fmoc-amino acid side chains bear the modifications shown in a. Values are from Supplementary Tables 1 and 9
While the introduction of a Boc group on the Trp indole nitrogen led to a better inhibitor, the KI values determined for Fmoc-Tyr and Fmoc-Lys bearing side-chain protecting groups, t-butyl and carboxybenzyl, respectively, were similar to the corresponding compounds without side-chain protecting groups. Although the carboxybenzyl group introduces a carbamate group, and carbamate groups are parts of numerous cholinesterase inhibitors, the positive charge of the lysine chain may contribute to binding interactions in the BChE active site analogous to the cationic group of the choline substrate and loss of the cationic-side chain in the Fmoc-Lys(Boc)-OH may lead to higher KI value. Together, these results identify that modifications of the amino acid side chain can lead to a better inhibitor than the Fmoc-amino acid alone, and indicate the complexity associated with identifying the types of interactions, e.g., hydrogen bonding, aromatic, van der Waals interactions, that may lead to developing more potent inhibitors.
Conclusions
We identified several Fmoc-amino acids that inhibit BChE revealing the potential of amino acid analogs as a scaffold to develop potent and selective BChE inhibitors. The aromatic fluorenyl group appears to be important for inhibitor binding, as the Fmoc analogs were better inhibitors than the corresponding Cbz analogs, but interactions in addition to the Fmoc group may contribute to inhibitor binding, as Fmoc-Gly and Fmoc-Ala showed little effect on BChE activity. The results with Fmoc-Leu and Fmoc-Trp identify these compounds as good candidates for further optimization, and the Fmoc-Trp analog bearing a Boc group on the side chain was promising to develop even better inhibitors. The cationic-side chain of Fmoc-Lys may introduce a cationic group in the choline-binding site of the enzyme active site and may provide a scaffold to incorporate features of the substrate in the peptide inhibitors. In addition, peptides containing an Fmoc group may provide an attractive scaffold to design inhibitors as the functional groups, and arrangement of functional groups within the inhibitor can be readily varied.
While our result show that Fmoc-amino acids act as BChE-selective inhibitors, the site or sites of inhibitor binding are not revealed by this study. As described above, in addition to the active site, much attention has been focused on the importance of a site distal to the active site, known as the ‘peripheral binding site’, and inhibitors designed to interact with the active site and peripheral binding site in cholinesterases have been reported (Berg et al. 2011; Nicolet et al. 2003; Xie et al. 2013; Zhao et al. 2011). We are currently performing computational modeling studies to evaluate potential binding sites and to make predictions that can be tested experimentally using a larger library of compounds to dissect further the interactions important for binding.
Supplementary Material
Acknowledgments
We thank the reviewers for helpful comments on this manuscript. This work was supported by startup funds provided by California State University, Long Beach. Research reported in this publication was supported by the National Institute of General Medical Sciences of the National Institutes of Health under Award Numbers UL1GM118979 and RL5GM118978. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. NMR instrumentation was provided for by the National Science Foundation (MRI CHE-1337559). Any opinions, findings, and conclusions expressed are those of the author(s) and do not necessarily reflect the views of NSF.
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s00726-016-2310-4) contains supplementary material, which is available to authorized users.
Compliance with ethical standards
Research involving human participants and/or animals This article does not contain any studies with human participants or animals performed by any of the authors.
Conflict of interest The authors declare that they have no conflict of interest.
References
- Abreu-Villaca Y, Filgueiras CC, Manhaes AC. Developmental aspects of the cholinergic system. Behav Brain Res. 2011;221(2):367–378. doi: 10.1016/j.bbr.2009.12.049. [DOI] [PubMed] [Google Scholar]
- Anand P, Singh B. A review on cholinesterase inhibitors for Alzheimer’s disease. Arch Pharmacal Res. 2013;36(4):375–399. doi: 10.1007/s12272-013-0036-3. [DOI] [PubMed] [Google Scholar]
- Andersson CD, Forsgren N, Akfur C, Allgardsson A, Berg L, Engdahl C, Qian W, Ekstrom F, Linusson A. Divergent structure-activity relationships of structurally similar acetylcholinesterase inhibitors. J Med Chem. 2013;56(19):7615–7624. doi: 10.1021/jm400990p. [DOI] [PubMed] [Google Scholar]
- Augustinsson K. On the specificity of cholinesterase. Biol Bull. 1948;95(2):241. [PubMed] [Google Scholar]
- Bar-On P, Millard CB, Harel M, Dvir H, Enz A, Sussman JL, Silman I. Kinetic and structural studies on the interaction of cholinesterases with the anti-Alzheimer drug rivastigmine. Biochemistry. 2002;41(11):3555–3564. doi: 10.1021/bi020016x. [DOI] [PubMed] [Google Scholar]
- Behrendt R, White P, Offer J. Advances in Fmoc solid-phase peptide synthesis. J Pept Sci. 2016;22(1):4–27. doi: 10.1002/psc.2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg L, Andersson CD, Artursson E, Hornberg A, Tunemalm AK, Linusson A, Ekstrom F. Targeting acetylcholinesterase: identification of chemical leads by high throughput screening, structure determination and molecular modeling. PLoS One. 2011;6(11):e26039. doi: 10.1371/journal.pone.0026039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brus B, Kosak U, Turk S, Pislar A, Coquelle N, Kos J, Stojan J, Colletier JP, Gobec S. Discovery, biological evaluation, and crystal structure of a novel nanomolar selective butyrylcholinesterase inhibitor. J Med Chem. 2014;57(19):8167–8179. doi: 10.1021/jm501195e. [DOI] [PubMed] [Google Scholar]
- Carolan CG, Dillon GP, Khan D, Ryder SA, Gaynor JM, Reidy S, Marquez JF, Jones M, Holland V, Gilmer JF. Isosorbide-2-benzyl carbamate-5-salicylate, a peripheral anionic site binding subnanomolar selective butyrylcholinesterase inhibitor. J Med Chem. 2010;53(3):1190–1199. doi: 10.1021/jm9014845. [DOI] [PubMed] [Google Scholar]
- Dori A, Soreq H. ARP, the cleavable C-terminal peptide of “readthrough” acetylcholinesterase, promotes neuronal development and plasticity. J Mol Neurosci. 2006;28(3):247–255. doi: 10.1385/jmn:28:3:247. [DOI] [PubMed] [Google Scholar]
- Dvir H, Silman I, Harel M, Rosenberry TL, Sussman JL. Acetylcholinesterase: from 3D structure to function. Chem-Biol Interact. 2010;187(1–3):10–22. doi: 10.1016/j.cbi.2010.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellman GL, Courtney KD, Andres V, Jr, Feather-Stone RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 1961;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- Fields GB, Noble RL. Solid phase peptide synthesis utilizing 9-fluorenylmethoxycarbonyl amino acids. Int J Pept Protein Res. 1990;35(3):161–214. doi: 10.1111/j.1399-3011.1990.tb00939.x. [DOI] [PubMed] [Google Scholar]
- Grisaru D, Sternfeld M, Eldor A, Glick D, Soreq H. Structural roles of acetylcholinesterase variants in biology and pathology. Eur J Biochem. 1999;264(3):672–686. doi: 10.1046/j.1432-1327.1999.00693.x. [DOI] [PubMed] [Google Scholar]
- Halliday AC, Greenfield SA. From protein to peptides: a spectrum of non-hydrolytic functions of acetylcholinesterase. Protein Pept Lett. 2012;19(2):165–172. doi: 10.2174/092986612799080149. [DOI] [PubMed] [Google Scholar]
- Hartsel JA, Wong DM, Mutunga JM, Ma M, Anderson TD, Wysinski A, Islam R, Wong EA, Paulson SL, Li J, Lam PC, Totrov MM, Bloomquist JR, Carlier PR. Re-engineering aryl methylcarbamates to confer high selectivity for inhibition of Anopheles gambiae versus human acetylcholinesterase. Bioorg Med Chem Lett. 2012;22(14):4593–4598. doi: 10.1016/j.bmcl.2012.05.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan DB. Long-term efficacy and toxicity of cholinesterase inhibitors in the treatment of Alzheimer disease. Can J Psychiatry. 2014;59(12):618–623. doi: 10.1177/070674371405901202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L, Su T, Li X. Natural products as sources of new lead compounds for the treatment of Alzheimer’s disease. Curr Top Med Chem. 2013;13(15):1864–1878. doi: 10.2174/15680266113139990142. [DOI] [PubMed] [Google Scholar]
- Jiang H, Zhang XJ. Acetylcholinesterase and apoptosis. A novel perspective for an old enzyme. FEBS J. 2008;275(4):612–617. doi: 10.1111/j.1742-4658.2007.06236.x. [DOI] [PubMed] [Google Scholar]
- Kaboudin B, Emadi S, Hadizadeh A. Synthesis of novel phosphorothioates and phosphorodithioates and their differential inhibition of cholinesterases. Bioorg Chem. 2009;37(4):101–105. doi: 10.1016/j.bioorg.2009.05.002. [DOI] [PubMed] [Google Scholar]
- Kamal MA, Qu X, Yu QS, Tweedie D, Holloway HW, Li Y, Tan Y, Greig NH. Tetrahydrofurobenzofuran cymserine, a potent butyrylcholinesterase inhibitor and experimental Alzheimer drug candidate, enzyme kinetic analysis. J Neural Transm (Vienna) 2008;115(6):889–898. doi: 10.1007/s00702-008-0022-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleywegt GJ, Jones TA. Detection, delineation, measurement and display of cavities in macromolecular structures. Acta Crystallogr D Biol Crystallogr. 1994;50(Pt 2):178–185. doi: 10.1107/s0907444993011333. [DOI] [PubMed] [Google Scholar]
- Law KS, Acey RA, Smith CR, Benton DA, Soroushian S, Eckenrod B, Stedman R, Kantardjieff KA, Nakayama K. Dialkyl phenyl phosphates as novel selective inhibitors of butyrylcholinesterase. Biochem Biophys Res Commun. 2007;355(2):371–378. doi: 10.1016/j.bbrc.2007.01.186. [DOI] [PubMed] [Google Scholar]
- Macdonald IR, Martin E, Rosenberry TL, Darvesh S. Probing the peripheral site of human butyrylcholinesterase. Biochemistry. 2012;51(36):7046–7053. doi: 10.1021/bi300955k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallender WD, Szegletes T, Rosenberry TL. Acetylthiocholine binds to asp74 at the peripheral site of human acetylcholinesterase as the first step in the catalytic pathway. Biochemistry. 2000;39(26):7753–7763. doi: 10.1021/bi000210o. [DOI] [PubMed] [Google Scholar]
- Meshorer E, Erb C, Gazit R, Pavlovsky L, Kaufer D, Friedman A, Glick D, Ben-Arie N, Soreq H. Alternative splicing and neuritic mRNA translocation under long-term neuronal hypersensitivity. Science. 2002;295(5554):508–512. doi: 10.1126/science.1066752. [DOI] [PubMed] [Google Scholar]
- Mushtaq G, Greig NH, Khan JA, Kamal MA. Status of acetylcholinesterase and butyrylcholinesterase in Alzheimer’s disease and type 2 diabetes mellitus. CNS Neurol Disord Drug Targets. 2014;13(8):1432–1439. doi: 10.2174/1871527313666141023141545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musial A, Bajda M, Malawska B. Recent developments in cholinesterases inhibitors for Alzheimer’s disease treatment. Curr Med Chem. 2007;14(25):2654–2679. doi: 10.2174/092986707782023217. [DOI] [PubMed] [Google Scholar]
- Nicolet Y, Lockridge O, Masson P, Fontecilla-Camps JC, Nachon F. Crystal structure of human butyrylcholinesterase and of its complexes with substrate and products. J Biol Chem. 2003;278(42):41141–41147. doi: 10.1074/jbc.M210241200. [DOI] [PubMed] [Google Scholar]
- Nizri E, Brenner T. Modulation of inflammatory pathways by the immune cholinergic system. Amino Acids. 2013;45(1):73–85. doi: 10.1007/s00726-011-1192-8. [DOI] [PubMed] [Google Scholar]
- Pagano G, Rengo G, Pasqualetti G, Femminella GD, Monzani F, Ferrara N, Tagliati M. Cholinesterase inhibitors for Parkinson’s disease: a systematic review and meta-analysis. J Neurol Neurosurg Psychiatry. 2015;86(7):767–773. doi: 10.1136/jnnp-2014-308764. [DOI] [PubMed] [Google Scholar]
- Pinho BR, Ferreres F, Valentao P, Andrade PB. Nature as a source of metabolites with cholinesterase-inhibitory activity: an approach to Alzheimer’s disease treatment. J Pharm Pharmacol. 2013;65(12):1681–1700. doi: 10.1111/jphp.12081. [DOI] [PubMed] [Google Scholar]
- Radic Z, Pickering NA, Vellom DC, Camp S, Taylor P. Three distinct domains in the cholinesterase molecule confer selectivity for acetyl- and butyrylcholinesterase inhibitors. Biochemistry. 1993;32(45):12074–12084. doi: 10.1021/bi00096a018. [DOI] [PubMed] [Google Scholar]
- Riddles PW, Blakeley RL, Zerner B. Reassessment of Ellman’s reagent. Methods Enzymol. 1983;91:49–60. doi: 10.1016/s0076-6879(83)91010-8. [DOI] [PubMed] [Google Scholar]
- Saxena A, Redman AM, Jiang X, Lockridge O, Doctor BP. Differences in active site gorge dimensions of cholinesterases revealed by binding of inhibitors to human butyrylcholinesterase. Biochemistry. 1997;36(48):14642–14651. doi: 10.1021/bi971425+. [DOI] [PubMed] [Google Scholar]
- Segel IH. Enzyme kinetics behavior and analysis of rapid equilibrium and steady-state enzyme systems. Wiley; New York: 1974. [Google Scholar]
- Soreq H, Gnatt A, Loewenstein Y, Neville LF. Excavations into the active-site gorge of cholinesterases. Trends Biochem Sci. 1992;17(9):353–358. doi: 10.1016/0968-0004(92)90314-y. [DOI] [PubMed] [Google Scholar]
- Sussman JL, Harel M, Frolow F, Oefner C, Goldman A, Toker L, Silman I. Atomic structure of acetylcholinesterase from Torpedo californica: a prototypic acetylcholine-binding protein. Science. 1991;253(5022):872–879. doi: 10.1126/science.1678899. [DOI] [PubMed] [Google Scholar]
- Szegletes T, Mallender WD, Thomas PJ, Rosenberry TL. Substrate binding to the peripheral site of acetylcholinesterase initiates enzymatic catalysis. Substrate inhibition arises as a secondary effect. Biochemistry. 1999;38(1):122–133. doi: 10.1021/bi9813577. [DOI] [PubMed] [Google Scholar]
- Vellom DC, Radic Z, Li Y, Pickering NA, Camp S, Taylor P. Amino acid residues controlling acetylcholinesterase and butyrylcholinesterase specificity. Biochemistry. 1993;32(1):12–17. doi: 10.1021/bi00052a003. [DOI] [PubMed] [Google Scholar]
- Verma A, Wong DM, Islam R, Tong F, Ghavami M, Mutunga JM, Slebodnick C, Li J, Viayna E, Lam PC, Totrov MM, Bloomquist JR, Carlier PR. 3-Oxoisoxazole-2(3H)-carboxamides and isoxazol-3-yl carbamates: resistance-breaking acetylcholinesterase inhibitors targeting the malaria mosquito, Anopheles gambiae. Bioorg Med Chem. 2015;23(6):1321–1340. doi: 10.1016/j.bmc.2015.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong DM, Li J, Lam PC, Hartsel JA, Mutunga JM, Totrov M, Bloomquist JR, Carlier PR. Aryl methylcarbamates: potency and selectivity towards wild-type and carbamate-insensitive (G119S) Anopheles gambiae acetylcholinesterase, and toxicity to G3 strain An. gambiae. Chem Biol Interact. 2013;203(1):314–318. doi: 10.1016/j.cbi.2012.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie R, Zhao Q, Zhang T, Fang J, Mei X, Ning J, Tang Y. Design, synthesis and biological evaluation of organophosphorous-homodimers as dual binding site acetylcholinesterase inhibitors. Bioorg Med Chem. 2013;21(1):278–282. doi: 10.1016/j.bmc.2012.10.030. [DOI] [PubMed] [Google Scholar]
- Zhao YH, Abraham MH, Zissimos AM. Fast calculation of van der Waals volume as a sum of atomic and bond contributions and its application to drug compounds. J Org Chem. 2003;68(19):7368–7373. doi: 10.1021/jo034808o. [DOI] [PubMed] [Google Scholar]
- Zhao Q, Xie R, Zhang T, Fang J, Mei X, Ning J, Tang Y. Homo- and hetero-dimers of inactive organophosphorous group binding at dual sites of AChE. Bioorg Med Chem Lett. 2011;21(21):6404–6408. doi: 10.1016/j.bmcl.2011.08.098. [DOI] [PubMed] [Google Scholar]
- Zhao T, Ding K-M, Zhang Z, Cheng Z-M, Wang C-H, Wang ZT. Acetylcholinesterase and butyrylcholinesterase inhibitory activities of B-carboline and quinoline alkaloids derivatives from the plants of genus Peganum. J Chem. 2013;2013:1–6. doi: 10.1155/2013/717232. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.