Abstract
Parkinson’s disease (PD) is a progressive movement disorder characterized by neuroinflammation and dopaminergic neurodegeneration in the brain. 1-methyl-4-phenylpyridinium (MPP+), a metabolite of the parkinsonian neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) induces the release of inflammatory mediators from glial cells and neurons. Glia maturation factor (GMF), a brain proinflammatory protein, MPP+, and mast cell-derived inflammatory mediators induce neurodegeneration which eventually leads to PD. However, the precise mechanisms underlying interaction between glial cells, neurons and mast cells in PD still remain elusive. In the present study, mouse bone marrow-derived mast cells (BMMCs) and mouse fetal brain-derived mixed glia/neurons, astrocytes and neurons were incubated with MPP+, GMF and mast cell-derived inflammatory mediators mouse mast cell protease-6 (MMCP-6), MMCP-7 or tryptase/brain-specific serine protease-4 (tryptase/BSSP-4). Inflammatory mediators released from these cells in the culture medium were quantitated by enzyme-linked immunosorbent assay. Neurodegeneration was quantified by measuring total neurite outgrowth following microtubule-associated protein-2 immunocytochemistry. MPP+− induced significant neurodegeneration with reduced total neurite outgrowth. MPP+− induced the release of tryptase/BSSP-4 from the mouse mast cells, and tryptase/BSSP-4 induced chemokine (C-C motif) ligand 2 (CCL2) release from astrocytes and glia/neurons. Overall our results suggest that MPP+, GMF, MMCP-6 or MMCP-7 stimulate glia/neurons, astrocytes or neurons to release CCL2 and matrix metalloproteinase-3. Additionally, CD40L expression is increased in BMMCs after incubation with MPP+ in a co-culture system consisting of BMMCs and glia/neurons. We propose that mast cell interaction with glial cells and neurons during neuroinflammation can be explored as a new therapeutic target for PD.
Keywords: CCL2, Cytokines, Glia maturation factor, Mast cells, 1-methyl-4-phenylpyridinium, Microtubule-associated protein-2, Neuroinflammation, Parkinson’s disease
Introduction
Parkinson’s disease (PD) is a multifactorial chronic progressive neurodegenerative disease characterized by the presence of neuroinflammation and accumulation of α-synuclein in the dopaminergic neurons which is associated with the formation of Lewy bodies and neurodegeneration (Phani et al., 2012). Mast cells are implicated in neuroinflammation and in the pathogenesis of PD, Multiple sclerosis (MS) and its animal model, experimental autoimmune encephalomyelitis (EAE) (Secor et al., 2000; Sayed et al., 2011; Kempuraj et al., 2015). Mast cells are generally co-localized adjacent to glial cells in the brain during neuroinflammatory responses (Kim et al., 2010). Resident brain mast cells are present adjacent to blood vessels, glial cells and nerves in the central nervous system (CNS), and communicate with these cells in pathophysiological conditions (Chikahisa et al., 2013). Activated mast cells release several multifunctional proinflammatory mediators including interleukin-1beta (IL-1β), IL-6, IL-8, IL-17, IL-18, IL-33, tumor necrosis factor-alpha (TNF-α), granulocyte macrophage-colony stimulating factor (GM-CSF), chemokine (C-C motif) ligand 2 (CCL2), CCL5, matrix metalloproteinase-3 (MMP-3), substance P, histamine, tryptase, prostaglandins, reactive oxygen species (ROS), reactive nitrogen species (RNS) and nitric oxide (NO) during an inflammatory response (Mekori and Metcalfe, 2000; Kalesnikoff and Galli, 2008; Sismanopoulos et al., 2012; Theoharides et al., 2012; Kempuraj et al., 2013). Persistent microglial activation and chronic neuroinflammation are implicated in the PD pathogenesis (Grimmig et al., 2016; Li et al., 2016).
Protease-activated receptors (PARs) expressed on the neurons are cleaved by the mast cell proteases and mediate neuroinflammation (Saito and Bunnett, 2005). Mast cells express proteases such as tryptase, chymase and carboxypeptidase in the granules. Human mast cells express α tryptase and β tryptase and several chymases. Mouse mast cell protease-6 (MMCP-6) and MMCP-7 are considered to be similar to human tryptase β and α, respectively. Mast cell tryptase activates rodent microglia to release TNF-α, IL-6, and ROS (Zhang et al., 2012). PAR2 is expressed on neurons as well as mast cells and plays an important role in promoting neuroinflammation when activated by mast cell proteases (Cottrell et al., 2003). Cross-talk between astrocytes and mast cells through CD40 and CD40L during neuroinflammation causes the release of many inflammatory mediators from these cells (Kim et al., 2010; Kim et al., 2011; Skaper et al., 2012; Walker et al., 2012). The cross-talk between glial cells, neurons, and mast cells contributes to the release of proinflammatory mediators that induce neurodegeneration in the brain (Skaper et al., 2012; Dong et al., 2016). However, the nature of the molecular and cellular interactions between glial cells, neurons and mast cells is not very clear. In this study, we have investigated if PD-relevant stimuli MPP+ and glia maturation factor (GMF) could activate mast cells, and that the mast cell-derived proteases activate glial and neuronal cells to further release inflammatory mediators relevant to PD pathogenesis. Our current data support the paradigm that the cross-talk between neurons, glial cells and mast cells, which is regulated by neuroinflammatory cytokines and chemokines, is involved in the neurodegeneration in PD. We suggest that the mast cell interactions with neurons and glia represents a novel target against PD.
Methods
Reagents
Iscove’s Modified Dulbecco’s Medium (IMDM), Dulbecco’s phosphate buffered saline (DPBS), Dulbecco’s Modified Eagle Medium Nutrient Mixture F-12 (DMEM F-12), Neurobasal medium, Fetal bovine serum (FBS), 2-Mercaptoethanol, GlutaMAX-1, Penicillin-Streptomycin, 0.25% Trypsin-EDTA were purchased from Life Technologies (Grand Island, NY). Rabbit IgG control antibody was obtained from Proteintech (Chicago, IL). Mouse IgG1 isotype control antibody was purchased from Thermo Scientific (Rockford, IL). Cell culture flasks and cell culture plates were obtained from Costar (Corning Incorporated, and Corning, NY). Recombinant mouse mast cell protease-6/Mcpt6, Recombinant mouse tryptase beta-1/Mcpt7, tryptase/BSSP-4, Enzyme-linked immunosorbent assay (ELISA) kits for mouse CCL2, MMP-3, tryptase/BSSP-4, monoclonal anti-mouse CD40L/TNFSF5 Phycoerythrin conjugated Rat IgG2A antibodies and flow cytometry reagents were purchased from R&D Systems (Minneapolis, MN). ImmPACT 3,3′-diaminobenzidine (DAB) peroxidase and avidin-biotin complex (ABC) kits were purchased from Vector Laboratories (Burlingame, CA). C57BL/6 wild type (Wt) mice and C57BL/6 Wt pregnant mice were purchased from Charles River Laboratories (Wilmington, MA). 1-methyl-4-phenylpyridinium (MPP+) and Poly-D-lysine were purchased from Sigma-Aldrich (St. Louis, MO). Paraformaldehyde was purchased from USB Corporation (Cleveland, OH). Microtubule-associated protein-2 (MAP-2) monoclonal antibody was obtained from Chemicon International (Temecula, CA).
Mouse Primary Mast Cell Culture
GMF-knockout (GMF-KO) mice was previously developed in our laboratory and a colony of these mice was always maintained for our studies (Lim et al., 2004). Bone marrow-derived mast cells (BMMCs) were grown by culturing bone marrow cells from the femur of Wt mice (C57BL/6) as well as from GMF-KO mice (C57BL/6) as reported previously (Tagen et al., 2009; Kim et al., 2011; Sayed et al., 2011). Briefly, bone marrow cells were aspirated and cultured in DMEM supplemented with IL-3 (10 ng/ml), 10% heat-inactivated FBS, 1% penicillin-streptomycin, 20 μM 2-mercaptoethanol, 1% L-glutamine for 4–7 weeks at 37°C in a 5% CO2 incubator. During this culture period, non-adherent cells were removed twice every week with the addition of fresh culture medium. After 4–7 weeks of culture, >99% of the cells in the culture were mast cells as determined by 0.1% toluidine blue staining as we have previously reported (Tagen et al., 2009). BMMCs cultured over 5 weeks were used for the experiments. Bone marrow cells from 5–10 mice were pooled together in order to get more number of BMMCs in the culture. This study was carried out in accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health (NIH). The Committee on the Ethics of Animal Experiments of the University of Iowa (Iowa City, IA) and the University of Missouri (Columbia, MO) approved the protocol.
Stimulation of Neuronal Cells and MAP-2 Immunohistochemistry to Study Neuronal Morphology
In order to analyze the morphology of neurons in the culture, MAP-2 immunocytochemistry (ICC) was performed as reported previously (Marx et al., 2001; Kempuraj et al., 2013). Briefly, mouse fetal brain cells from Wt mice and GMF-KO mice were isolated and grown in the neurobasal medium in 24 well cell culture plates coated with poly-D-lysine. These neuronal cells were incubated with MPP+, MMCP-6 and MMCP-7 at the indicated doses for different times and the culture media was removed from the wells. The cells were washed twice with DPBS and were immunostained for MAP-2 to analyze the neuronal morphology. First, the cells were fixed with 4% paraformaldehyde in DPBS for 10 min at room temperature and blocked with 5% normal goat serum. MAP-2 (1:500 dilution) monoclonal antibody was added to the wells and incubated overnight at 4°C. After washing, the cells were further incubated with goat anti-mouse biotinylated IgG secondary antibody for 1 h and ABC reagent for 30 min and subsequently with ImmPACT DAB peroxidase substrate solution for 5 min at room temperature. Development of brown color in the wells indicated positive reaction for MAP-2 in the neurons. The cells were washed with DPBS in between the incubations. Finally, the neurons were observed under the microscope (Nikon DIAPHOT microscope, Garden City, NY) and photographed. The total neurite length was measured in the whole area of photomicrographs using MetaMorph Software (Meta Imaging Series 7.8, Molecular Devices, Sunnyvale, CA) to quantitate the neuronal outgrowth (Perez-Martinez and Jaworski, 2005; Ould-yahoui et al., 2009). Mouse astrocytes and glia/neurons were incubated with MPP+, GMF, MMCP-6, MMCP-7 or tryptase/BSSP-4 for various time intervals as indicated in the results and legends sections. After the incubation period, the culture media was collected and assayed for inflammatory mediators by ELISA.
Mouse Primary Glia/neurons, Astrocytes, and Neuronal Cells Culture
Wt (C57BL/6) and GMF-KO pregnant mice were euthanized on the 16–17th day of gestation. Brains were harvested from the fetuses and the cells were used to grow glia/neurons, astrocytes and neuronal cells as reported previously (Zaheer et al., 2001; Zaheer et al., 2002). Glia/neurons were grown in DMEM nutrient mixture F-12 (Ham) (DMEM F-12) with 5–10% FBS and 1% penicillin -streptomycin at 37°C in a 5% CO2 and 95% air atmosphere in 25 cm2 or 75 cm2 tissue culture flasks. We obtained pure astrocytes by removing the microglia from the culture by shaking the culture flasks at 200 rpm on a shaker as reported previously (Zaheer et al., 2007). Cells from astrocyte culture were positive for the astrocyte marker glial fibrillary acidic protein (GFAP) by ICC staining. Mouse neuronal cultures were prepared from Wt mice as well as GMF-KO mice fetal brains as described previously (Kempuraj et al., 2013). Briefly, mouse fetal brain cells were cultured in neurobasal medium supplemented with B27, 2 mM L-glutamine and 1% penicillin/streptomycin at 37°C in humidified 5% CO2 and 95% air. Neurons were cultured on poly-D-lysine coated 24 well tissue culture plates for 2 weeks. These cultures represent a nearly pure neuronal population based upon immunostaining for MAP-2.
Mouse Mast Cell Stimulation with MPP+ or GMF, and Inflammatory Mediators Assay
BMMCs grown from Wt mice and GMF-KO mice bone marrow were counted and plated in separate 24 well culture plates at 0.5 to 1×106 cells/ml in BMMCs culture medium containing 1% FBS and cultured overnight at 37°C. The cells were then incubated with MPP+ or GMF at the indicated concentrations for different times as mentioned in the results section and figure legends. The culture supernatants were collected, centrifuged and either stored at −80°C until inflammatory mediator assays or used immediately. CCL2, MMP-3 or tryptase/BSSP-4 were assayed in these culture supernatants by ELISA. The working concentrations of stimulants were prepared in sterile 0.1% bovine serum albumin (BSA) in DPBS. The untreated control cells were incubated with equal volume of culture medium containing 0.1% BSA in DPBS.
Mouse Mast Cells and Mouse Glia/neurons Co-culture and Stimulation
Primary mouse glia/neurons were first cultured in 24 well cell culture plates and the BMMCs were seeded in the same wells containing the glia/neurons at a ratio of 1:3; similar to inflammatory conditions. Glia/neurons or BMMCs were also cultured separately in different wells. Glial culture medium and BMMCs culture medium were used in equal (50:50) ratios in the co-culture conditions as reported previously (Kempuraj et al., 2015). These cells were incubated with MPP+ or GMF or mast cell proteases as indicated in the results and figure legends. The supernatants were collected, centrifuged and stored at -80°C for inflammatory mediators assay by ELISA kits. In another set of experiments, BMMCs were co-cultured with neurons in six well tissue culture plate, incubated with MPP+ and the culture supernatants were used to measure tryptase/BSSP-4 release by ELISA Kit.
Analysis of CD40L (CD154) Expression in Mouse Mast Cells Co-cultured with Glia/neurons by Flow Cytometry
BMMCs and glia/neurons can activate each other through direct cell-to-cell contact and through their inflammatory cytokines and chemokines. Here, we analyzed if the inhibition of direct cell-to-cell contact reduces the expression of CD40L on mast cells. First, glia/neurons were plated and grown to confluency in 24 well tissue culture plate. BMMCs were added into the wells and co-cultured for 72 h at 37°C. These co-cultured cells were incubated with MPP+ (15 μM) for 72 h. In additional wells, after the confluence of glia/neurons; transwell inserts were placed and then BMMCs were added to the transwell inserts. This is to avoid direct contact of glia/neurons and BMMCs. The cells were incubated with MPP+ for 72 h as mentioned above. The BMMCs were collected by centrifugation and processed for the analysis of CD40L expression (n=3) by flow cytometry (BD LSR II with violet laser, BD Biosciences, San Jose, CA) using monoclonal anti-mouse CD40L/TNFSF5 Phycoerythrin conjugated Rat IgG2A antibodies as we have reported previously (Kempuraj et al., 2015). Rat IgG2A isotype control Allophycocyanin conjugated or Rat IgG2A Isotype control Phycoerythrin conjugated were used as isotype matched controls.
Statistical Analysis
The results were analyzed using GraphPad InStat 3 statistical software. Mean ± SEM was calculated and analyzed using One-way Analysis of Variance (ANOVA) followed by Tukey-Kramer post hoc analysis to determine statistical significance for differences between the groups/conditions. Only one-way ANOVA and Tukey-Kramer post hoc analysis were used for the analysis unless otherwise mentioned. An unpaired t-test was used when comparing only two groups. A p-value of <0.05 was considered statistically significant.
Results
MPP+ Induces Neuronal Degeneration as Determined by Immunocytochemistry for MAP-2
To investigate whether MPP+ and mouse mast cell proteases induce neurodegeneration, mouse primary neurons obtained from Wt and GMF-KO mice fetal brains were incubated with MPP+ (10 μM) or mast cell proteases MMCP-6 and 100 ng/ml MMCP-7 at 100 ng/ml) for 24 h. Then the neurons were immunostained for MAP-2 to analyze the neuronal morphology. MPP+, MMCP-6 and MMCP-7-induced significantly more neuronal degeneration in the neurons obtained from Wt mice fetal brains as compared to untreated control neuronal cells (Fig. 1A, arrows). Further, neurons obtained from GMF-KO mice showed reduced neuronal degeneration (Fig. 1B) as compared to neurons obtained from Wt mice. Untreated neuronal cells did not show degeneration. Neurite outgrowth was measured by MetaMorph software was significantly (*p<0.05) reduced in the neurons incubated with MPP+, MMCP-6 and MMCP-7 (Fig. 1C, D; *p<0.05, n=3). Our results show that the total neurite length is reduced more in Wt neurons as compared to GMF-KO neurons after the treatments, indicating that GMF deficiency provides neuroprotection in the brain.
Fig. 1.
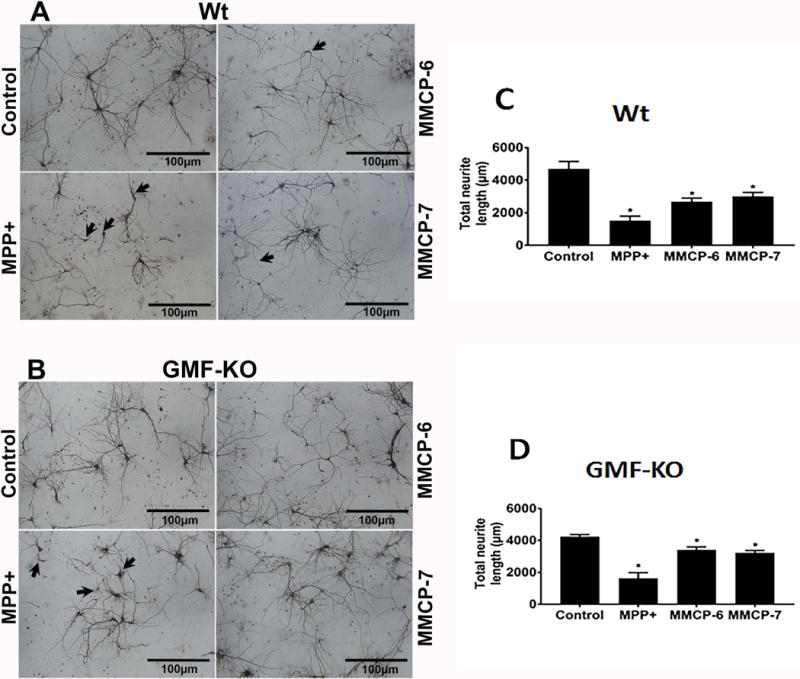
MPP+ and mast cell proteases-induce neuronal degeneration. Primary mouse neurons from Wt mice and GMF-KO mice fetal brains were cultured for 2 weeks. These cells were incubated with MPP+ (10 μM) or mast cell proteases (MMCP-6 or MMCP-7 at 100 ng/ml) for 24 h. MAP-2 ICC was performed to analyze the neuronal morphology. (A) MPP+, MMCP-6 and MMCP-7-induced significant neuronal degeneration (arrows) in the neurons obtained from Wt mice fetal brains as compared to non-treated control neuronal cells. (B) GMF-KO neurons showed less neuronal degeneration when compared to the extent of neurodegeneration observed in the neurons grown from Wt mice fetal brains. Control neuronal cells did not show any degeneration. Scale bar = 100 μm. (C, D) The total neuronal outgrowth length was measured in the whole photomicrographs using MetaMorph software to determine the neuronal degeneration. MPP+, MMCP-6 and MMCP-7 significantly reduced the total neurite length as compared with control cells as shown in the bar graphs (One-way ANOVA and Tukey-Kramer post hoc, n=3, *p<0.05). Neurodegeneration is relatively higher in Wt neurons than in GMF-KO neurons.
MPP+ Induces the Release of Tryptase/BSSP-4 from Wt BMMCs, Which Induces Release of CCL2 from Astrocytes and Glia/neurons
We examined whether MPP+ induces mast cell protease tryptase/BSSP-4 release from Wt BMMCs. BMMCs incubated with MPP+ (10 μM) for 24 h significantly induced the release of tryptase as compared to the release from untreated BMMCs (Fig. 2A, n=4, p<0.05). In another experiment, co-cultured neurons and BMMCs obtained from Wt mice were incubated with MPP+ (10 μM) for 24 h and assayed for tryptase release in the culture media. Our results showed that MPP+ released significantly more tryptase in this co-culture system as compared to BMMCs alone (Fig. 2B, n=4). Further, co-culture also increased the overall amount of tryptase release from BMMCs. Next, we investigated if tryptase/BSSP-4 induces CCL2 release from astrocytes, and glia/neurons. Our results show that tryptase/BSSP-4 induces significant (*p<0.05) release of CCL2 from astrocytes (Fig. 2C, n=6) as well as from glia/neurons (Fig. 2D, n=5) when compared to control untreated cells. Our results suggest that tryptase-mediated CCL2 release from Wt BMMCs could potentially induce neurodegeneration along with other inflammatory mediators.
Fig. 2.
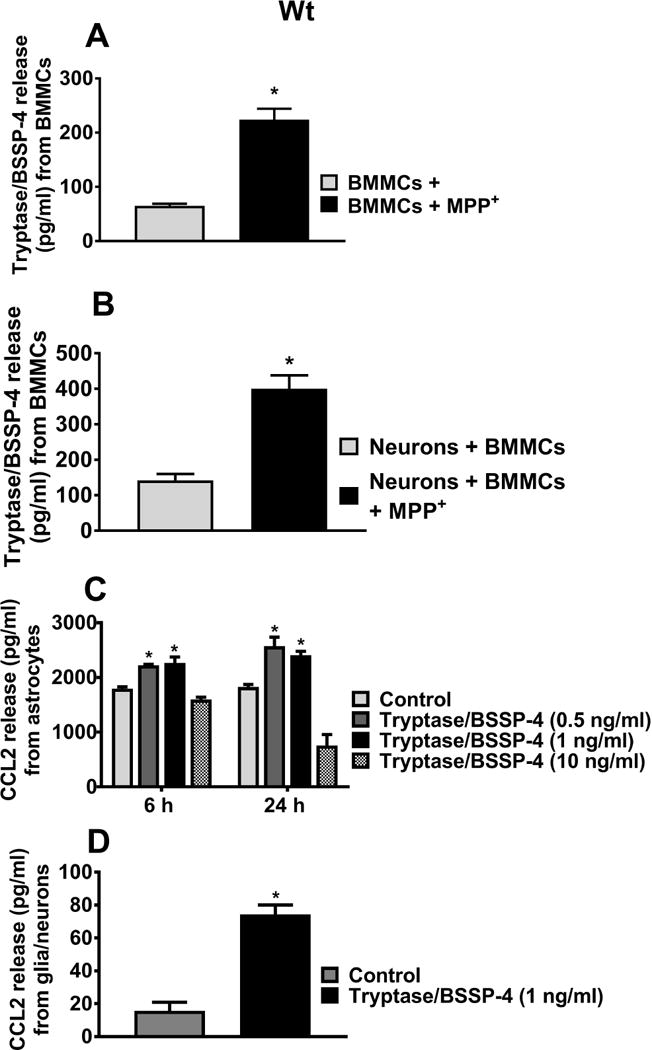
MPP+-induces tryptase/BSSP-4 release from Wt BMMCs, and tryptase release CCL2 from astrocytes and glia/neurons. (A) Wt BMMCs were incubated with MPP+ (10 μM) and the culture media were used for tryptase assay by ELISA (n=4). MPP+ induced significant tryptase/BSSP-4 release from BMMCs as compared to untreated control cells. (B) Wt mouse neurons and Wt BMMCs co-cultured cells were treated with MPP+ (10 μM) for 24 h (n=4) and the tryptase released in the medium was assayed. MPP+ released significantly more tryptase in this co-culture system as compared to co-cultured control cells (*p<0.05; t test, control vs treated). (C, D) Wt mouse astrocytes (n=6) or glia/neurons (n=4) were incubated with tryptase/BSSP-4 (0.5–10 ng/ml) and the release of CCL2 was assayed in the culture media by ELISA. Tryptase/BSSP-4 induced CCL2 release from the astrocytes and glia/neurons. Results were shown as mean ± SEM (*p<0.05; control vs treated, C= One-way ANOVA and Tukey-Kramer post hoc, D = t test).
MMCP-6 and MMCP-7 Induce Release of CCL2 from Wt Mouse Glia/neurons
Here we tested whether mouse mast cell protease MMCP-6 induces Wt mice glia/neurons to release chemokine CCL2. In dose-response (5, 25, 50, 100 and 200 ng/ml) and time-course (6, 24 and 48 h) studies, glia/neurons were incubated with MMCP-6 and assayed for CCL2 levels in the culture media by ELISA. MMCP-6 significantly induced CCL2 release from glia/neurons obtained from Wt mice (Fig. 3A, n=3). This release starts at 6 h at 25 ng/ml as compared to untreated control cells and is maximum at 24 h (*p<0.05 control vs MMCP-6 treated cells, ANOVA and Tukey-Kramer). We also found that Wt glia/neurons incubated with MMCP-7 (100 ng/ml) for 24 h also induced the release of CCL2 (Fig. 3B, n=5, *p<0.05, t-test). Additionally, we tested the effect of MMCP-6 on GMF-KO glia/neurons at 100 ng/ml concentration. Our results show that glia/neurons obtained from GMF-KO mice did not show any significant increase of CCL2 release except at 48 h of incubation (Fig. 4).
Fig. 3.
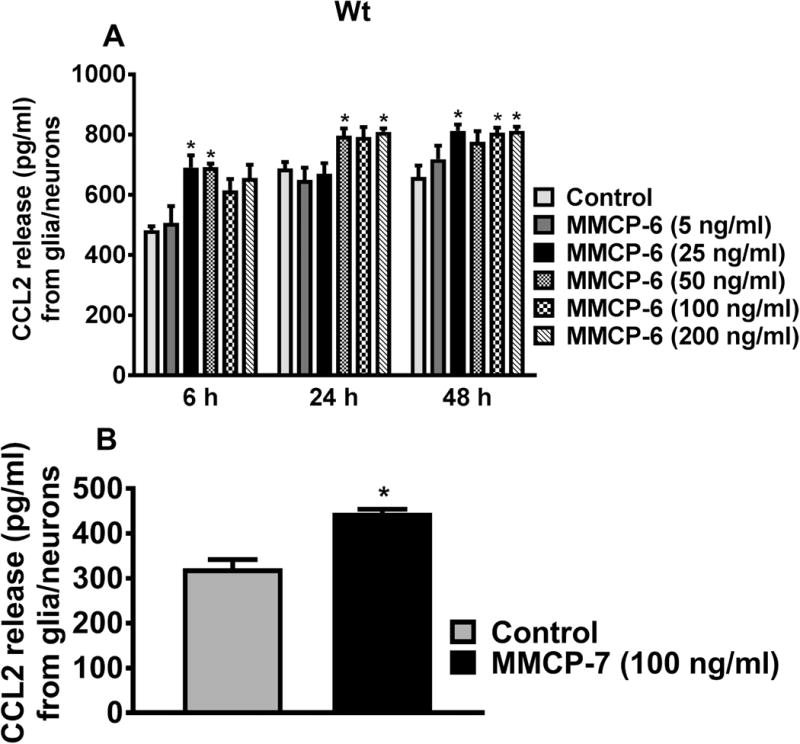
MMCP-6 -induces CCL2 release from Wt mouse glia/neurons. (A) Wt mouse glia/neurons were incubated with MMCP-6 for dose-response (5 to 200 ng/ml) and time-course studies (6 to 48 h), and CCL2 levels in the culture media were determined by ELISA (n=3). MMCP-6 induced significant CCL2 release from 6 h (at 25 ng/ml concentration) onwards from glia/neurons. Results were presented as mean ± SEM (*p<0.05 control vs MMCP-6 treated cells, One-way ANOVA and Tukey-Kramer post hoc). (B) Wt glia/neurons were incubated with MMCP-7 (100 ng/ml) for 24 h and CCL2 release was measured by ELISA (n=5). MMCP-7 induced significant CCL2 release from glia/neurons as compared to untreated control cells (*p<0.05 control vs cells incubated with MMCP-7, t-test).
Fig. 4. Effect of.
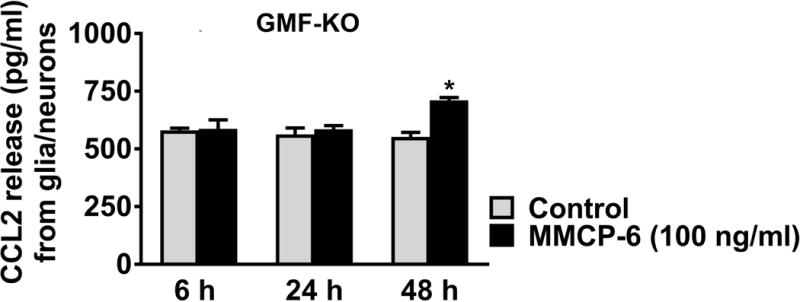
MMCP-6 on CCL2 release from GMF-KO mouse glia/neurons. Glia/neurons obtained from GMF-KO mice were incubated with MMCP-6 (100 ng/ml) for 6, 24 and 48 h. CCL2 release was measured in the culture media by ELISA (n=4). MMCP-6 significantly released CCL2 only at 48 h when compared to untreated control cells. Results were presented as mean ± SEM (*p<0.05 control vs MMCP-6 treated cells, t-test).
MMCP-6 and MMCP-7 Induce CCL2 Release from Wt Mouse Astrocytes
Wt mice astrocytes were similarly incubated with MMCP-6 and MMCP-7 at 25, 50, 100 and 250 ng/ml for 6 h and 24 h and the release of CCL2 was measured in the culture media (n=3–8). Both MMCP-6 (Fig. 5A) and MMCP-7 (Fig. 5B) induced significant release of CCL2 from astrocytes (*p<0.05 control vs MMCP-6 treated cells, one-way ANOVA and Tukey-Kramer post hoc). Astrocytes incubated with MMCP-6 for 6 h at 100 and 250 ng/ml (Fig. 5A) and 24 h at 50, 100 and 250 ng/ml induced significant release of CCL2 as compared to untreated control cells. Incubation of mouse astrocytes for 6 h or for 24 h with MMCP-7 at various concentrations induced significant release of CCL2 (Fig. 5B). These results suggest that mouse mast cell proteases can activate astrocytes to release inflammatory mediators.
Fig. 5.
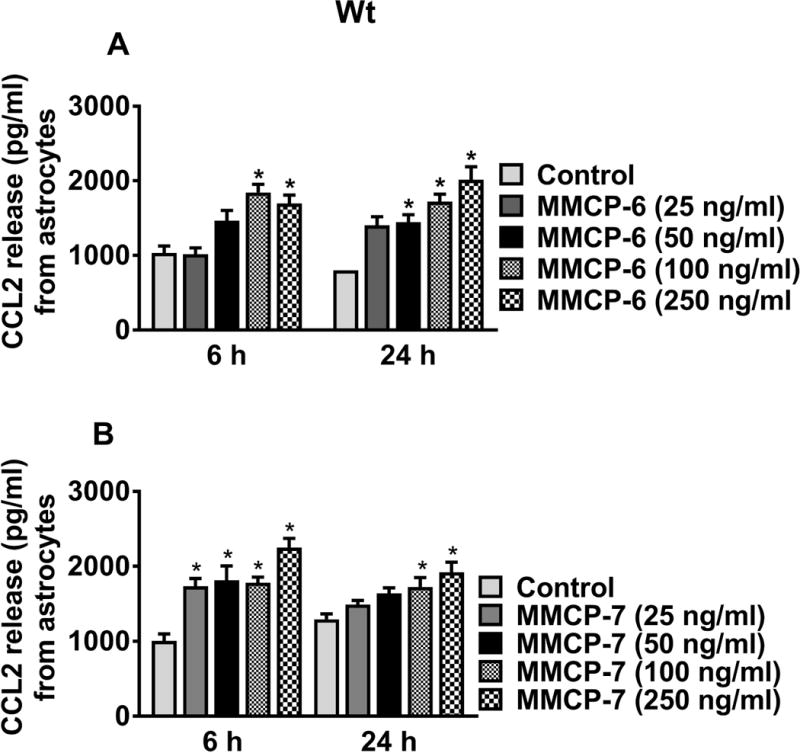
MMCP-6 and MMCP-7-induce CCL2 release from Wt mouse astrocytes. Wt mouse astrocytes were grown and stimulated with MMCP-6 and MMCP-7 for 6 h and 24 h, and CCL2 release was measured in the culture media by ELISA (n=3–8). Both (A) MMCP-6 and (B) MMCP-7 induced CCL2 release in a dose-dependent manner from mouse astrocytes when compared with non-treated control cells. Results were presented as mean ± SEM (*p<0.05 control vs MMCP-6 or MMCP-7 treated cells, One-way ANOVA and Tukey-Kramer post hoc).
Release of CCL2 from Wt and GMF-KO Mice Neurons by MMCP-6, MMCP-7 and MPP+
We next examined if MMCP-6, MMCP-7, and MPP+ activate mouse neurons to release CCL2 (n=3). Neuronal cells obtained from Wt mice as well as from GMF-KO mice were incubated with MMCP-6 (200 ng/ml), MMCP-7 (200 ng/ml) and MPP+ (20 μM) for 24 h and the CCL2 release was assayed in the culture media. Neuronal cells obtained from Wt mice released significantly higher amounts of CCL2 after incubation with MMCP-6, MMCP-7, and MPP+ when compared to untreated control cells (Fig. 6). However, only MPP+ significantly released CCL2 from neuronal cells obtained from GMF-KO mice (Fig. 6, *p<0.05 control vs MMCP-6, MMCP-7 and MPP+ treated cells, one-way ANOVA and Tukey-Kramer post hoc). Our results show that MMCP-6, MMCP-7 and MPP+ can induce neurons to release chemokine CCL2 from Wt astrocytes and it appears that this release of CCL2 by MMCP-6 and MMCP-7 is dependent on the presence of GMF.
Fig. 6.
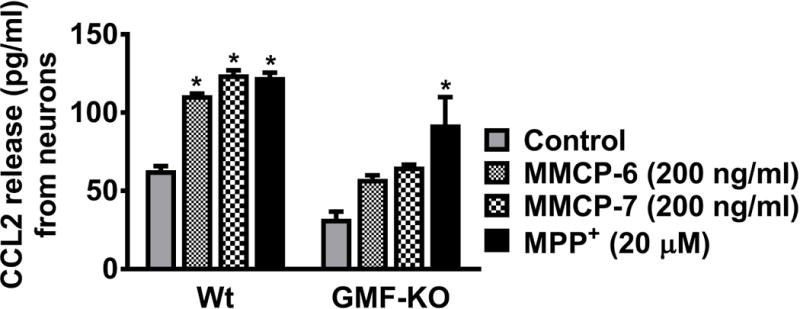
MMCP-6, MMCP-7, and MPP+ -induce CCL2 release from mouse neurons. We examined whether MMCP-6, MMCP-7 and MPP+ activate mouse neurons to release CCL2. Mouse neuronal cells grown from Wt mice as well as from GMF-KO mice were incubated with MMCP-6 (200 ng/ml), MMCP-7 (200 ng/ml) or MPP+ (20 μM) for 24 h and the CCL2 release was assayed in the culture media by ELISA (n=3). Neurons obtained from Wt mice significantly released CCL2 after incubation with MMCP-6, MMCP-7, and MPP+ when compared to control non-treated cells. However, only MPP+ significantly released CCL2 from neuronal cultures obtained from GMF-KO mice. Results were presented as mean ± SEM (*p<0.05 control vs treated cells, One-way ANOVA and Tukey-Kramer post hoc).
GMF and MPP+ Induce the Release of MMP-3 from Mast Cells, Astrocytes and Glia/neurons, Obtained from Wt Mouse
MMP-3 is implicated in the pathogenesis of neurodegenerative diseases. In this study, we investigated whether MPP+, GMF and mouse mast cell proteases activate brain cells or mast cells to release MMP-3. First, we incubated Wt BMMCs with GMF (100 ng/ml) or MPP+ (20 μM) for 24 h and measured MMP-3 release in the cell culture media. GMF (Fig. 7A, n=3) and MPP+ (Fig. 7B, n=4) treatment significantly increased (*p<0.05; t test, control vs treated) the release of MMP-3 as compared to untreated control BMMCs. Next, Wt BMMCs and glia/neurons separately or in combination were incubated with MPP+ (10 μM) for 48 h and assayed for the release of MMP-3 (Fig. 7C, n=3). Incubation of co-cultures of BMMCs and glia/neurons with MPP+ induced significant release of MMP-3 as compared to control cells (Fig. 7C, *p<0.05; t-test, control vs treated). In another set of experiments, Wt mice glia/neurons were incubated with MPP+ (15 μM) or GMF (100 ng/ml) and the release of MMP-3 was measured in the culture media. Both MPP+ and GMF induced significant release of MMP-3 from glia/neurons as compared with untreated control cells (Fig. 7D, n=3). We also incubated glia/neurons with GMF in the presence or absence of BMMCs. GMF augments the release of MMP-3 in the presence of BMMCs (glia/neuron + BMMCs) compared to glia/neurons cultures alone (Fig. 7D, *p<0.05; control vs treated). Our results show that co-culture of mast cells and glia/neurons increases MMP-3 release.
Fig. 7.
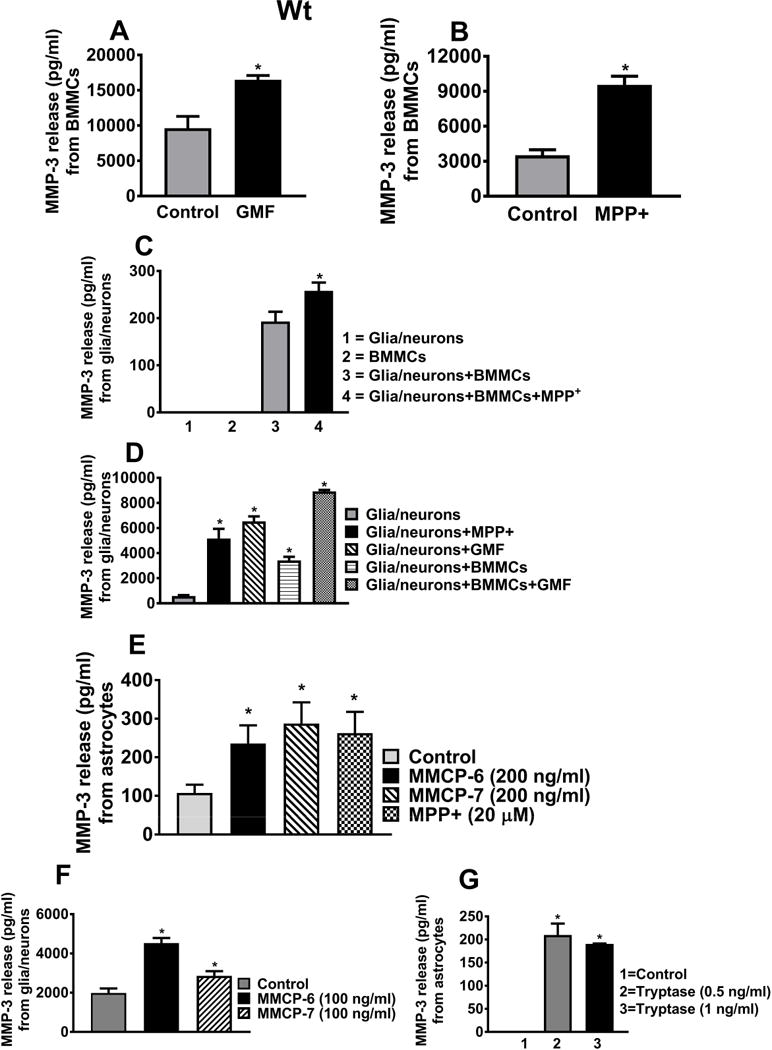
GMF, MPP+, MMCP-6, MMCP-7 and tryptase release MMP-3 from Wt brain cells or mast cells. (A, B) BMMCs grown from Wt mice were incubated with GMF (100 ng/ml) or MPP+ (20 μM) for 24 h and MMP-3 release in the cell culture media was measured by ELISA. (A) GMF (n=3) and (B) MPP+ (n=4) significantly increased the release of MMP-3 when compared with non-treated control BMMCs (*p<0.05; t test, control vs treated). (C) Wt mouse BMMCs plus glia/neurons incubated with MPP+ significantly induced MMP-3 release as compared to non-treated control cells (n=3, *p<0.05; t test, control vs treated). (D) Wt mouse glia/neurons were then incubated with MPP+ (15 μM) or GMF (100 ng/ml) for 24 h and the release of MMP-3 was measured in the culture media (n=3). MPP+ and GMF significantly induced the release of MMP-3 from glia/neurons. Wt glia/neurons and BMMCs were co-cultured and then stimulated with GMF. GMF augments the release of MMP-3 in this co-culture system as compared to single glia/neurons culture condition (*p<0.05; t test, control vs treated). (E, F, G) Wt mouse astrocytes or glia/neurons were incubated with MMCP-6, MMCP-7, MPP+ or tryptase/BSSP-4 and the release of MMCP-3 in the culture media was measured by ELISA. MMCP-7 (n=6), MMP-6 (n=6), MPP+ (n=6) and tryptase/BSSP-4 (n=3)-induced significantly increased MMP-3 release as compared to control cells. Results were presented as mean ± SEM (*p<0.05 control vs treated cells, One-way ANOVA and Tukey-Kramer post hoc).
Further, Wt mouse astrocytes incubated with MMCP-6 (200 ng/ml), MMCP-7 (200 ng/ml) and MPP+ (20 μM) for 24 h showed the significant release of MMP-3 as compared to control cells (Fig. 7E, *p<0.05; t test, control vs treated, n=6). Both MMCP-6 (100 ng/ml) and MMCP-7 (100 ng/ml) also induced MMP-3 release from glia/neurons (Fig. 7F, n=6). In another set of experiments, Wt astrocytes incubated with tryptase/BSSP-4 for 24 h showed increased MMP-3 release as compared with untreated control cells (Fig. 7G, n=3, *p<0.05). These results show that mast cell proteases activate brain cells to release MMP-3. Our overall results show that MPP+, GMF, MMCP-6, MMCP-7 and tryptase/BSSP-4 activate brain cells or mast cells to release the neurotoxic mediator MMP-3 to induce neurodegeneration.
Increased Expression of CD40L in Wt BMMCs Co-cultured with Mouse Wt Glia/neurons as Determined by Flow Cytometry
Wt glia/neurons co-cultured with Wt BMMCs were incubated with MPP+ (15 μM) for 72 h (n=3). In additional wells, glia/neurons and BMMCs were separated using transwell inserts to avoid direct contacts between the cell populations. The expression of CD40L on BMMCs was analyzed by flow cytometry to determine the effect of direct cell-to-cell contact. MPP+ increased the expression of CD40L on BMMCs co-cultured with glia/neurons in direct cell-to-cell contact as compared to BMMCs that were contact inhibited by using transwell inserts. CD40L expression was low in untreated control BMMCs (Fig. 8). These results indicate that prevention of direct cell-to-cell contact between BMMCs and glia/neurons downregulates CD40L expression.
Fig. 8.
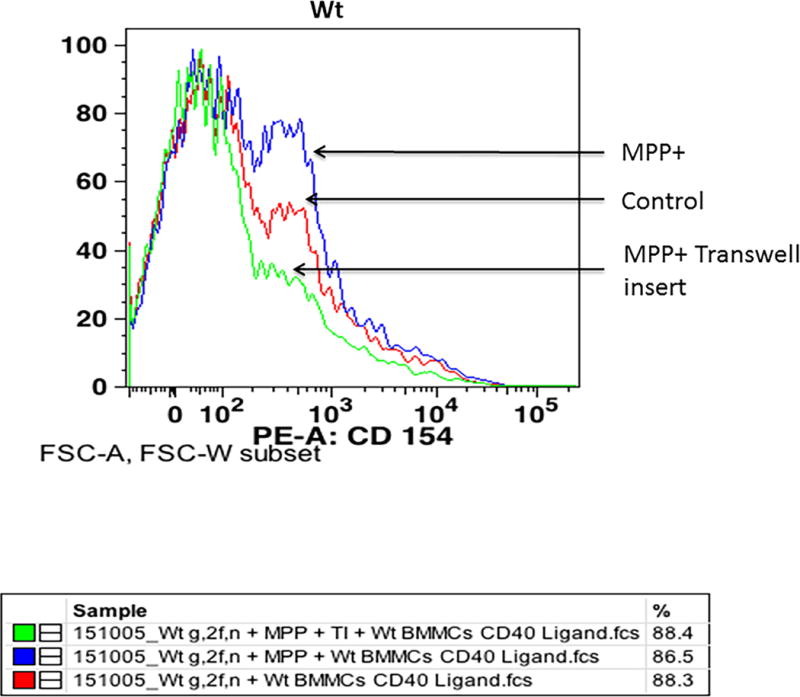
Expression of CD40L in Wt BMMCs co-cultured with glia/neurons as analyzed by flow cytometry. BMMCs and glia/neurons were co-cultured in 24 well culture plates for 72 h and subsequently incubated with MPP+ (15 μM) for 72 h at 37°C. In additional wells, transwell inserts were placed to avoid direct contact between glia/neurons and BMMCs. BMMCs were added on top of transwell inserts and incubated with MPP+. The expression of CD40L on BMMCs was analyzed by flow cytometry using anti-mCD40L/TNFSF5 antibody (n=3). MPP+ increased the expression of CD40L on BMMCs as compared to control untreated cells where there was no direct cell-to-cell contact inhibition. However, CD40L expression was reduced in the wells where transwell inserts were used to avoid direct contact of glia/neurons and BMMCs.
Discussion
The present study shows that the PD-relevant toxin MPP+-induced neurodegeneration with reduced total neurite outgrowth. MPP+ also induced tryptase/BSSP-4 release from Wt mouse BMMCs. Mouse mast cell proteases release chemokine CCL2 from glia/neurons, astrocytes, and neurons in vitro. Further, MPP+, GMF, MMCP-6, MMCP-7 and tryptase/BSSP-4 stimulate glia/neurons, astrocytes or BMMCs to release neurotoxic MMP-3. Tryptase, CCL2, and MMP-3 are implicated in the mechanism of neurodegeneration. In this study, we demonstrate that an absence of GMF reduces the activation and release of CCL2 from mouse glia/neurons. We further report that MPP+ up-regulates the expression of CD40L in Wt BMMCs co-cultured with glia/neurons in an in vitro co-culture system. Mast cells play an important role in the mechanism of BBB dysfunction, neuroinflammation and frequently co-localized next to glial cells in neuroinflammatory conditions in the brain (Seeldrayers et al., 1992; Kim et al., 2010; McKittrick et al., 2015). Previous studies have shown that intravenously administered BMMCs as well as peripheral mast cells infiltrate the brain in pathological conditions and thus exacerbate neuroinflammatory response (Silverman et al., 2000; Tanzola et al., 2003; Bennett et al., 2009; Skaper et al., 2012; Skaper et al., 2013a). Resident mast cells in the brain can recruit and activate other types of inflammatory cells and cause vasodilation during neuroinflammatory conditions (Nelissen et al., 2013). Additionally, peripheral mast cells have also been shown to influence the CNS inflammatory responses. Mast cells are implicated in demyelinating and neuroinflammatory diseases such as MS/EAE and PD (Skaper et al., 2014). Mast cells are both a target and a source of various inflammatory mediators that are involved in the neuroinflammatory processes. Mast cells can selectively release several neuroactive mediators, including cytokines, chemokines, ROS, RNS and NO depending upon the tissue microenvironment and the type of stimuli (Mekori and Metcalfe, 2000; Kalesnikoff and Galli, 2008; Sismanopoulos et al., 2012; Theoharides et al., 2012; Kempuraj et al., 2013; Nelissen et al., 2013). Proinflammatory mediators released from the activated mast cells could influence neuroinflammation leading to neurodegeneration in the CNS. Though mast cells are known to be involved in neuroinflammation, the exact mechanism how mast cells interact with glial cells and neurons in neuroinflammation is not yet clearly known.
Our present study shows the release of CCL2, tryptase/BSSP-4 and MMP-3 from glia, neurons or BMMCs or under co-culture conditions incubated with the PD-relevant toxin MPP+. CCL2 is expressed in glia, neurons, and mast cells and plays an important role in the pathogenesis of neurodegenerative diseases as a chemoattractant (Madrigal and Caso, 2014; Kempuraj et al., 2016). CCL2 released from brain cells and mast cells in response to the PD-relevant stimulant could increase the infiltration of other types of inflammatory cells into the substantia nigra in the brain and then further exacerbate neuroinflammation. Mast cells interact/cross-talk with astrocytes, neurons, microglia and oligodendrocytes in the pathogenesis of neurodegenerative diseases (Skaper and Facci, 2012; Skaper et al., 2012; Skaper et al., 2013a; Frieri et al., 2015). It has been reported that mast cells but not the microglia were the first responders in the brain injury (Jin et al., 2009) and also release TNF-α before the other cells, indicating its immediate response in the brain (Zhang et al., 2016). Mast cell protease tryptase is an important serine-protease and plays an important role in inflammation. Tryptase is stored in mast cell granules and released once activated. A recent report suggests that new and specific inhibitors targeting tryptase could represent a specific and potent therapeutic option to treat various inflammatory disorders including neuroinflammatory conditions (Ni et al., 2017). Mast cell protease is known to activate microglia and neurons through PAR-2 and release TNF-α and IL-6 (Zhang et al., 2012). PAR-2 are G protein-coupled receptors for proteases from inflammatory cells such as mast cells and play an important role in neuronal functions and in neuroinflammation (Cottrell et al., 2003; Saito and Bunnett, 2005), and it also induce mast cell accumulation (Liu et al., 2016). PAR-2 expressed on astrocytes, microglia, neurons, and mast cells are involved in the process of neurodegeneration (Cottrell et al., 2003; Rothmeier and Ruf, 2012; Zhang et al., 2012). MMCP-6 and MMCP-7 are proteases of connective tissue-type mast cells in the mouse and are similar to human β and α tryptases, respectively (Caughey, 2007). These proinflammatory molecules activate PAR-2 expression in glial cells, neurons as well as mast cells (Cui et al., 2014). Mast cells are the source of proteases that can activate PAR-2 and induce local neuroinflammation in the CNS (Rothmeier and Ruf, 2012). In this study, we have analyzed whether mouse mast cell proteases MMCP-6 and MMCP-7 activate glial cells and neuronal cells to release neuroinflammatory mediators relevant to PD pathogenesis. We observed that mouse mast cell proteases MMCP-6, MMCP-7 or tryptase/BSSP-4 activate glial cells, neurons, and release CCL2 and MMP-3 that are implicated in neurodegeneration. We speculate that this release may involve PAR-2, mitogen-activated protein kinases (MAPKs) and nuclear factor-kappa B (NF-κB) pathways as reported previously for microglial activation by proteases (Zhang et al., 2012). Our current results and previous other reports have shown that mast cell proteases act as neuroinflammatory mediators in the neuroinflammatory disorders including PD (Zhang et al., 2012; Shaik-Dasthagirisaheb and Conti, 2016). We did not measure MMCP-6 and MMCP-7 in this study, as the exact ELISA kits for their measurements are not available. Further, the exact functional differences between the mouse proteases are not yet clearly known.
Mast cells, endothelial cells, and leukocytes in the brain secrete MMPs. MMPs digest the tight junctions and basement membrane proteins and thus further increase the BBB permeability in neuroinflammation (Rempe et al., 2016). MMP-3 is normally present in low concentration but increases in various inflammatory conditions. MMP-3 is an inflammatory mediator released from activated microglia (Lee et al., 2010a) as well as mast cells (Johnson et al., 1998). MMPs are highly expressed in several neuropathological disorders and induces neuroinflammatory responses causing the breakdown of the BBB, infiltration of the peripheral immunocytes, demyelination, and neuronal death (Woo et al., 2008; Rosenberg, 2009; Rempe et al., 2016). The expression of MMP-3 is also upregulated in α-synuclein-stimulated microglia and mediates neuroinflammatory reactions relevant to PD. MMPs cleave the N-terminal extracellular domain of PAR-1 and activate intracellular inflammatory signaling pathways in α-synuclein-activated microglia (Lee et al., 2015). MMPs induce dopaminergic neuronal degeneration in PD. MMP-3 released from dopaminergic neurons activates microglia and releases TNF-α which in turn mediate neurodegeneration (Ronnberg et al., 2012). MMP-3 is also involved in caspase-3 activation in apoptotic pathways, α-synuclein cleavage, and generation of toxic aggregates. MMP-3 deficient mice show attenuated BBB permeability, decreased number of immune cells infiltration in the substantia nigra in 1-methyl-4-phenyl-1, 2,3,6-tetrahydropyridine (MPTP)-treated mouse model of PD, indicating that MMPs play an important role in neuroinflammatory mechanisms (Rempe et al., 2016). BBB breakdown allows the entry of immune/inflammatory cells to the brain from the peripheral system in neuroinflammatory conditions. Immune cells from the blood can cross the BBB through MMPs-independent transcellular and MMPs-dependent para cellular routes. In para cellular pathway, immune cells such as T cells and mast cells release MMPs which opens the brain endothelial tight junctions and cross the BBB (Rempe et al., 2016). In this study, we have measured the release of MMP-3 from activated mast cells and brain cells since it is implicated in neuroinflammation and neurodegenerative mechanisms. MMP-3 is an inflammatory neurotoxic protein that induces neuronal death, BBB dysfunction in demyelination/neurodegenerative diseases such as PD, AD and MS (Woo et al., 2008). Mast cell activation was associated with proinflammatory cytokine and metalloproteinase expression by neighboring cells, suggesting an important contributory role for the mast cells in neurodegeneration (Tetlow and Woolley, 1995). Our study shows that MPP+, MMCP-6, MMCP-7 and tryptase/BSSP-4 induce the release of MMP-3 from mouse glia, neurons and mast cells suggesting that these cells can release neuroinflammatory molecules including MMP-3 that are involved in neurodegeneration such as in PD.
GMF, an inflammatory brain protein was previously isolated, sequenced and cloned in our laboratory (Lim et al., 1989; Kaplan et al., 1991; Lim and Zaheer, 1991; Zaheer et al., 1993). GMF is expressed in astrocytes, microglia and many neurons in the brains and the expression is increased in neurodegenerative diseases (Wang et al., 1992; Zaheer et al., 2011a; Thangavel et al., 2013). We have previously reported that GMF-induces neurodegeneration by acting on glial cells and neurons (Lim et al., 2004; Zaheer et al., 2008a; Zaheer et al., 2008b; Kempuraj et al., 2013). Additionally, we have also previously shown that GMF activates mast cells to release various inflammatory mediators (Kempuraj et al., 2015; Kempuraj et al., 2016). GMF-induced neuroinflammation leads to the death of neurons in the CNS (Zaheer et al., 2007). GMF expression level is increased in the CNS of neurodegenerative diseases including EAE (Zaheer et al., 2011b; Thangavel et al., 2012), while GMF knockdown suppresses dopaminergic neuronal loss, glial activation and the expression levels of proinflammatory mediators in the substantia nigra of MPTP-treated animal model of PD (Khan et al., 2015). We have previously reported the expression of GMF in human and mouse mast cells, as GMF was also reported in several extra CNS cells in the body (Kempuraj et al., 2015). GMF could act in an autocrine and paracrine manner in the activation of mast cells in the CNS. It is known that GMF (Zaheer et al., 2007; Kempuraj et al., 2013) and MPP+ (Brahmachari et al., 2009) activate glial cells and induce neuroinflammation (Hirsch and Hunot, 2009; Lee et al., 2010b; Kalia et al., 2013). We have previously reported reduced expression of inflammatory mediators in astrocytes and microglia obtained from GMF-KO mice and increased levels of inflammatory mediators in GMF-KO brain cells reconstituted to overexpress GMF (Zaheer et al., 2008b). Therefore, we have used GMF-KO cells in only some experiments to study the effect of the mast cell proteases on brain cells. Our previous studies have demonstrated that GMF activates astrocytes through p38 MAPK and NF-κB signaling pathways (Zaheer et al., 2001; Zaheer et al., 2007) and inhibition of these pathways in GMF-KO glial cells also reduced the inflammatory mediator release in vitro (Khan et al., 2014). The reduced release of CCL2 from GMF-KO cells observed in the present study could be due to the inhibition of MAPKs and NF-κB activation as we reported previously (Zaheer et al., 2007). Further, we have also demonstrated that GMF-deficiency in astrocytes upregulates the antioxidant status and limits the extent of lipid peroxidation and production of ROS along with diminished NF-κB-mediated inflammations in MPP+-mediated toxicity (Khan et al., 2014). Our present study shows decreased neurodegeneration with reduced total neurite outgrowth in MPP+ treated neuronal cultures obtained from GMF-KO mice brains than from Wt mice brains.
Glial cells, neurons and mast cells communicate with each other through several signaling pathways involving CD40L, CD40, toll-like receptor 2 (TLR2), TLR4, PAR-2, chemokine (C-X-C motif) receptor 4 (CXCR4)/CXCL12 and C5a receptor to promote glial cell migration and activation associated with inflammatory mediator release in neuroinflammatory conditions (Kim et al., 2011; Skaper et al., 2012; Zhang et al., 2012; Skaper et al., 2013b). It has been suggested that mast cells are the major linking cells between neurons and neuroinflammatory responses (Tore and Tuncel, 2009). CD40 and CD40L interaction activate inflammatory cells to release proinflammatory mediators. In the present study, our observation that MPP+ upregulates the expression of CD40L in BMMCs in the co-culture indicates an important role of mast cells in neuroinflammatory pathways. Glial expression of GMF at their cell surface could activate the adjacent mast cells in the brain to express/release neuroinflammatory proteins/mediators (Lim et al., 1990). Glial cells, as well as brain mast cells, mediate both neurotoxic as well as neurotrophic effects. Inflammatory mediators released from glia in the substantia nigra can recruit and activate mast cells in the substantia nigra. Inflammatory mediators released from activated mast cells can further activate and release inflammatory mediators from glial cells in the substantia nigra leading to neurodegeneration in PD. Further studies are required to understand the mechanism of mast cell activation in the human PD brains especially in the substantia nigra region in vivo, and using dopaminergic neurons in vitro. In conclusion, our present results indicate that mast cells cross-talk with glial cells and neuronal cells in the pathogenesis of neurodegeneration, and this interaction can be explored as a new therapeutic target for neurodegenerative diseases including PD.
Acknowledgments
The Flow cytometry data presented herein were obtained at the Flow Cytometry Facility, which is a Carver College of Medicine/Holden Comprehensive Cancer Center core research facility at the University of Iowa, Iowa City, IA. The Facility is funded through user fees and the generous financial support of the Carver College of Medicine, Holden Comprehensive Cancer Center, and Iowa City Veteran’s Administration Medical Center. We thank Mr. Justin Fishbaugh, Technical Director, Flow Cytometry Facility for his help in Flow Cytometry data acquisition and analysis in this study.
Funding
This work was supported by Veteran Affairs Merit Award I01BX002477 and National Institutes of Health Grants AG048205 & NS073670 to AZ.
Footnotes
Conflict of interest disclosure
Authors declare that they have no competing interests.
Compliance with Ethics Standards
The Committee on the Ethics of Animal Experiments of the University of Iowa (Iowa City, IA) and the University of Missouri (Columbia, MO) approved the protocol.
References
- Bennett JL, Blanchet MR, Zhao L, Zbytnuik L, Antignano F, Gold M, Kubes P, McNagny KM. Bone marrow-derived mast cells accumulate in the central nervous system during inflammation but are dispensable for experimental autoimmune encephalomyelitis pathogenesis. J Immunol. 2009;182:5507–5514. doi: 10.4049/jimmunol.0801485. [DOI] [PubMed] [Google Scholar]
- Brahmachari S, Jana A, Pahan K. Sodium benzoate, a metabolite of cinnamon and a food additive, reduces microglial and astroglial inflammatory responses. J Immunol. 2009;183:5917–5927. doi: 10.4049/jimmunol.0803336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caughey GH. Mast cell tryptases and chymases in inflammation and host defense. Immunol Rev. 2007;217:141–154. doi: 10.1111/j.1600-065X.2007.00509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chikahisa S, Kodama T, Soya A, Sagawa Y, Ishimaru Y, Sei H, Nishino S. Histamine from brain resident MAST cells promotes wakefulness and modulates behavioral states. PLoS One. 2013;8:e78434. doi: 10.1371/journal.pone.0078434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottrell GS, Amadesi S, Schmidlin F, Bunnett N. Protease-activated receptor 2: activation, signalling and function. Biochem Soc Trans. 2003;31:1191–1197. doi: 10.1042/bst0311191. [DOI] [PubMed] [Google Scholar]
- Cui Y, Dahlin JS, Feinstein R, Bankova LG, Xing W, Shin K, Gurish MF, Hallgren J. Mouse mast cell protease-6 and MHC are involved in the development of experimental asthma. J Immunol. 2014;193:4783–4789. doi: 10.4049/jimmunol.1302947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong H, Zhang X, Wang Y, Zhou X, Qian Y, Zhang S. Suppression of Brain Mast Cells Degranulation Inhibits Microglial Activation and Central Nervous System Inflammation. Mol Neurobiol. 2016;54:997–1007. doi: 10.1007/s12035-016-9720-x. [DOI] [PubMed] [Google Scholar]
- Frieri M, Kumar K, Boutin A. Role of mast cells in trauma and neuroinflammation in allergy immunology. Ann Allergy Asthma Immunol. 2015;115:172–177. doi: 10.1016/j.anai.2015.06.025. [DOI] [PubMed] [Google Scholar]
- Grimmig B, Morganti J, Nash K, Bickford PC. Immunomodulators as Therapeutic Agents in Mitigating the Progression of Parkinson’s Disease. Brain Sci. 2016;6 doi: 10.3390/brainsci6040041. pii: E41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch EC, Hunot S. Neuroinflammation in Parkinson’s disease: a target for neuroprotection? Lancet Neurol. 2009;8:382–397. doi: 10.1016/S1474-4422(09)70062-6. [DOI] [PubMed] [Google Scholar]
- Jin Y, Silverman AJ, Vannucci SJ. Mast cells are early responders after hypoxia-ischemia in immature rat brain. Stroke. 2009;40:3107–3112. doi: 10.1161/STROKEAHA.109.549691. [DOI] [PubMed] [Google Scholar]
- Johnson JL, Jackson CL, Angelini GD, George SJ. Activation of matrix-degrading metalloproteinases by mast cell proteases in atherosclerotic plaques. Arterioscler Thromb Vasc Biol. 1998;18:1707–1715. doi: 10.1161/01.atv.18.11.1707. [DOI] [PubMed] [Google Scholar]
- Kalesnikoff J, Galli SJ. New developments in mast cell biology. Nat Immunol. 2008;9:1215–1223. doi: 10.1038/ni.f.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalia LV, Kalia SK, McLean PJ, Lozano AM, Lang AE. alpha-Synuclein oligomers and clinical implications for Parkinson disease. Ann Neurol. 2013;73:155–169. doi: 10.1002/ana.23746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan R, Zaheer A, Jaye M, Lim R. Molecular cloning and expression of biologically active human glia maturation factor-beta. J Neurochem. 1991;57:483–490. doi: 10.1111/j.1471-4159.1991.tb03777.x. [DOI] [PubMed] [Google Scholar]
- Kempuraj D, Khan MM, Thangavel R, Xiong Z, Yang E, Zaheer A. Glia maturation factor induces interleukin-33 release from astrocytes: implications for neurodegenerative diseases. J Neuroimmune Pharmacol. 2013;8:643–650. doi: 10.1007/s11481-013-9439-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempuraj D, Thangavel R, Yang E, Pattani S, Zaheer S, Santillan DA, Santillan MK, Zaheer A. Dopaminergic Toxin 1-Methyl-4-Phenylpyridinium, Proteins alpha-Synuclein and Glia Maturation Factor Activate Mast Cells and Release Inflammatory Mediators. PLoS One. 2015;10:e0135776. doi: 10.1371/journal.pone.0135776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempuraj D, Thangavel R, Fattal R, Pattani S, Yang E, Zaheer S, Santillan DA, Santillan MK, Zaheer A. Mast Cells Release Chemokine CCL2 in Response to Parkinsonian Toxin 1-Methyl-4-Phenyl-Pyridinium (MPP(+)) Neurochem Res. 2016;41:1042–1049. doi: 10.1007/s11064-015-1790-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan MM, Kempuraj D, Zaheer S, Zaheer A. Glia Maturation Factor Deficiency Suppresses 1-Methyl-4-Phenylpyridinium-Induced Oxidative Stress in Astrocytes. J Mol Neurosci. 2014 doi: 10.1007/s12031-013-0225-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan MM, Zaheer S, Thangavel R, Patel M, Kempuraj D, Zaheer A. Absence of Glia Maturation Factor Protects Dopaminergic Neurons and Improves Motor Behavior in Mouse Model of Parkinsonism. Neurochem Res. 2015;53:590–599. doi: 10.1007/s11064-015-1553-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DY, Jeoung D, Ro JY. Signaling pathways in the activation of mast cells cocultured with astrocytes and colocalization of both cells in experimental allergic encephalomyelitis. J Immunol. 2010;185:273–283. doi: 10.4049/jimmunol.1000991. [DOI] [PubMed] [Google Scholar]
- Kim DY, Hong GU, Ro JY. Signal pathways in astrocytes activated by cross-talk between of astrocytes and mast cells through CD40-CD40L. J Neuroinflammation. 2011;8:25. doi: 10.1186/1742-2094-8-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee EJ, Ko HM, Jeong YH, Park EM, Kim HS. beta-Lapachone suppresses neuroinflammation by modulating the expression of cytokines and matrix metalloproteinases in activated microglia. J Neuroinflammation. 2015;12:133. doi: 10.1186/s12974-015-0355-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee EJ, Woo MS, Moon PG, Baek MC, Choi IY, Kim WK, Junn E, Kim HS. Alpha-synuclein activates microglia by inducing the expressions of matrix metalloproteinases and the subsequent activation of protease-activated receptor-1. J Immunol. 2010a;185:615–623. doi: 10.4049/jimmunol.0903480. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Kim C, Lee SJ. Alpha-synuclein stimulation of astrocytes: Potential role for neuroinflammation and neuroprotection. Oxid Med Cell Longev. 2010b;3:283–287. doi: 10.4161/oxim.3.4.12809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Zheng Z, Ruan J, Li Z, Tzeng CM. Chronic Inflammation Links Cancer and Parkinson’s Disease. Front Aging Neurosci. 2016;8:126. doi: 10.3389/fnagi.2016.00126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim R, Zaheer A. Structure and function of glia maturation factor beta. Adv Exp Med Biol. 1991;296:161–164. doi: 10.1007/978-1-4684-8047-4_16. [DOI] [PubMed] [Google Scholar]
- Lim R, Miller JF, Zaheer A. Purification and characterization of glia maturation factor beta: a growth regulator for neurons and glia. Proc Natl Acad Sci U S A. 1989;86:3901–3905. doi: 10.1073/pnas.86.10.3901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim R, Liu YX, Zaheer A. Cell-surface expression of glia maturation factor beta in astrocytes. FASEB J. 1990;4:3360–3363. doi: 10.1096/fasebj.4.15.2253851. [DOI] [PubMed] [Google Scholar]
- Lim R, Zaheer A, Khosravi H, Freeman JH, Jr, Halverson HE, Wemmie JA, Yang B. Impaired motor performance and learning in glia maturation factor-knockout mice. Brain Res. 2004;1024:225–232. doi: 10.1016/j.brainres.2004.08.003. [DOI] [PubMed] [Google Scholar]
- Liu X, Wang J, Zhang H, Zhan M, Chen H, Fang Z, Xu C, Chen H, He S. Induction of Mast Cell Accumulation by Tryptase via a Protease Activated Receptor-2 and ICAM-1 Dependent Mechanism. Mediators Inflamm. 2016;2016:6431574. doi: 10.1155/2016/6431574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madrigal JL, Caso JR. The chemokine (C-C motif) ligand 2 in neuroinflammation and neurodegeneration. Adv Exp Med Biol. 2014;824:209–219. doi: 10.1007/978-3-319-07320-0_15. [DOI] [PubMed] [Google Scholar]
- Marx CE, Jarskog LF, Lauder JM, Lieberman JA, Gilmore JH. Cytokine effects on cortical neuron MAP-2 immunoreactivity: implications for schizophrenia. Biol Psychiatry. 2001;50:743–749. doi: 10.1016/s0006-3223(01)01209-4. [DOI] [PubMed] [Google Scholar]
- McKittrick CM, Lawrence CE, Carswell HV. Mast cells promote blood brain barrier breakdown and neutrophil infiltration in a mouse model of focal cerebral ischemia. J Cereb Blood Flow Metab. 2015;35:638–647. doi: 10.1038/jcbfm.2014.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mekori YA, Metcalfe DD. Mast cells in innate immunity. Immunol Rev. 2000;173:131–140. doi: 10.1034/j.1600-065x.2000.917305.x. [DOI] [PubMed] [Google Scholar]
- Nelissen S, Lemmens E, Geurts N, Kramer P, Maurer M, Hendriks J, Hendrix S. The role of mast cells in neuroinflammation. Acta Neuropathol. 2013;125:637–650. doi: 10.1007/s00401-013-1092-y. [DOI] [PubMed] [Google Scholar]
- Ni WW, Cao MD, Huang W, Meng L, Wei JF. Tryptase Inhibitors: A Patent Review. Expert Opin Ther Pat. 2017;27:919–928. doi: 10.1080/13543776.2017.1322064. [DOI] [PubMed] [Google Scholar]
- Ould-yahoui A, Tremblay E, Sbai O, Ferhat L, Bernard A, Charrat E, Gueye Y, Lim NH, Brew K, Risso JJ, Dive V, Khrestchatisky M, Rivera S. A new role for TIMP-1 in modulating neurite outgrowth and morphology of cortical neurons. PLoS One. 2009;4:e8289. doi: 10.1371/journal.pone.0008289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Martinez L, Jaworski DM. Tissue inhibitor of metalloproteinase-2 promotes neuronal differentiation by acting as an anti-mitogenic signal. J Neurosci. 2005;25:4917–4929. doi: 10.1523/JNEUROSCI.5066-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phani S, Loike JD, Przedborski S. Neurodegeneration and inflammation in Parkinson’s disease. Parkinsonism Relat Disord. 2012;18(Suppl 1):S207–209. doi: 10.1016/S1353-8020(11)70064-5. [DOI] [PubMed] [Google Scholar]
- Rempe RG, Hartz AM, Bauer B. Matrix metalloproteinases in the brain and blood-brain barrier: versatile breakers and makers. J Cereb Blood Flow Metab. 2016;36:1481–1507. doi: 10.1177/0271678X16655551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronnberg E, Calounova G, Pejler G. Mast cells express tyrosine hydroxylase and store dopamine in a serglycin-dependent manner. Biol Chem. 2012;393:107–112. doi: 10.1515/BC-2011-220. [DOI] [PubMed] [Google Scholar]
- Rosenberg GA. Matrix metalloproteinases and their multiple roles in neurodegenerative diseases. Lancet Neurol. 2009;8:205–216. doi: 10.1016/S1474-4422(09)70016-X. [DOI] [PubMed] [Google Scholar]
- Rothmeier AS, Ruf W. Protease-activated receptor 2 signaling in inflammation. Semin Immunopathol. 2012;34:133–149. doi: 10.1007/s00281-011-0289-1. [DOI] [PubMed] [Google Scholar]
- Saito T, Bunnett NW. Protease-activated receptors: regulation of neuronal function. Neuromolecular Med. 2005;7:79–99. doi: 10.1385/NMM:7:1-2:079. [DOI] [PubMed] [Google Scholar]
- Sayed BA, Walker ME, Brown MA. Cutting edge: mast cells regulate disease severity in a relapsing-remitting model of multiple sclerosis. J Immunol. 2011;186:3294–3298. doi: 10.4049/jimmunol.1003574. [DOI] [PubMed] [Google Scholar]
- Secor VH, Secor WE, Gutekunst CA, Brown MA. Mast cells are essential for early onset and severe disease in a murine model of multiple sclerosis. J Exp Med. 2000;191:813–822. doi: 10.1084/jem.191.5.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeldrayers PA, Levin LA, Johnson D. Astrocytes support mast cell viability in vitro. J Neuroimmunol. 1992;36:239–243. doi: 10.1016/0165-5728(92)90056-q. [DOI] [PubMed] [Google Scholar]
- Shaik-Dasthagirisaheb YB, Conti P. The Role of Mast Cells in Alzheimer’s Disease. Adv Clin Exp Med. 2016;25:781–787. doi: 10.17219/acem/61914. [DOI] [PubMed] [Google Scholar]
- Silverman AJ, Sutherland AK, Wilhelm M, Silver R. Mast cells migrate from blood to brain. J Neurosci. 2000;20:401–408. doi: 10.1523/JNEUROSCI.20-01-00401.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sismanopoulos N, Delivanis DA, Alysandratos KD, Angelidou A, Therianou A, Kalogeromitros D, Theoharides TC. Mast cells in allergic and inflammatory diseases. Curr Pharm Des. 2012;18:2261–2277. doi: 10.2174/138161212800165997. [DOI] [PubMed] [Google Scholar]
- Skaper SD, Facci L. Mast cell-glia axis in neuroinflammation and therapeutic potential of the anandamide congener palmitoylethanolamide. Philos Trans R Soc Lond B Biol Sci. 2012;367:3312–3325. doi: 10.1098/rstb.2011.0391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skaper SD, Giusti P, Facci L. Microglia and mast cells: two tracks on the road to neuroinflammation. FASEB J. 2012;26:3103–3117. doi: 10.1096/fj.11-197194. [DOI] [PubMed] [Google Scholar]
- Skaper SD, Facci L, Giusti P. Mast cells, glia and neuroinflammation: partners in crime? Immunology. 2013a;141:314–327. doi: 10.1111/imm.12170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skaper SD, Facci L, Giusti P. Glia and Mast Cells as Targets for Palmitoylethanolamide, an Anti-inflammatory and Neuroprotective Lipid Mediator. Mol Neurobiol. 2013b;48:340–352. doi: 10.1007/s12035-013-8487-6. [DOI] [PubMed] [Google Scholar]
- Skaper SD, Facci L, Giusti P. Neuroinflammation, microglia and mast cells in the pathophysiology of neurocognitive disorders: a review. CNS Neurol Disord Drug Targets. 2014;13:1654–1666. doi: 10.2174/1871527313666141130224206. [DOI] [PubMed] [Google Scholar]
- Tagen M, Elorza A, Kempuraj D, Boucher W, Kepley CL, Shirihai OS, Theoharides TC. Mitochondrial uncoupling protein 2 inhibits mast cell activation and reduces histamine content. J Immunol. 2009;183:6313–6319. doi: 10.4049/jimmunol.0803422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanzola MB, Robbie-Ryan M, Gutekunst CA, Brown MA. Mast cells exert effects outside the central nervous system to influence experimental allergic encephalomyelitis disease course. J Immunol. 2003;171:4385–4391. doi: 10.4049/jimmunol.171.8.4385. [DOI] [PubMed] [Google Scholar]
- Tetlow LC, Woolley DE. Mast cells, cytokines, and metalloproteinases at the rheumatoid lesion: dual immunolocalisation studies. Ann Rheum Dis. 1995;54:896–903. doi: 10.1136/ard.54.11.896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thangavel R, Stolmeier D, Yang X, Anantharam P, Zaheer A. Expression of glia maturation factor in neuropathological lesions of Alzheimer’s disease. Neuropathol Appl Neurobiol. 2012;38:572–581. doi: 10.1111/j.1365-2990.2011.01232.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thangavel R, Kempuraj D, Stolmeier D, Anantharam P, Khan M, Zaheer A. Glia maturation factor expression in entorhinal cortex of Alzheimer’s disease brain. Neurochem Res. 2013;38:1777–1784. doi: 10.1007/s11064-013-1080-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theoharides TC, Alysandratos KD, Angelidou A, Delivanis DA, Sismanopoulos N, Zhang B, Asadi S, Vasiadi M, Weng Z, Miniati A, Kalogeromitros D. Mast cells and inflammation. Biochim Biophys Acta. 2012;1822:21–33. doi: 10.1016/j.bbadis.2010.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tore F, Tuncel N. Mast cells: target and source of neuropeptides. Curr Pharm Des. 2009;15:3433–3445. doi: 10.2174/138161209789105036. [DOI] [PubMed] [Google Scholar]
- Walker ME, Hatfield JK, Brown MA. New insights into the role of mast cells in autoimmunity: evidence for a common mechanism of action? Biochim Biophys Acta. 2012;1822:57–65. doi: 10.1016/j.bbadis.2011.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang BR, Zaheer A, Lim R. Polyclonal antibody localizes glia maturation factor beta-like immunoreactivity in neurons and glia. Brain Res. 1992;591:1–7. doi: 10.1016/0006-8993(92)90971-b. [DOI] [PubMed] [Google Scholar]
- Woo MS, Park JS, Choi IY, Kim WK, Kim HS. Inhibition of MMP-3 or -9 suppresses lipopolysaccharide-induced expression of proinflammatory cytokines and iNOS in microglia. J Neurochem. 2008;106:770–780. doi: 10.1111/j.1471-4159.2008.05430.x. [DOI] [PubMed] [Google Scholar]
- Zaheer A, Fink BD, Lim R. Expression of glia maturation factor beta mRNA and protein in rat organs and cells. J Neurochem. 1993;60:914–920. doi: 10.1111/j.1471-4159.1993.tb03237.x. [DOI] [PubMed] [Google Scholar]
- Zaheer A, Yorek MA, Lim R. Effects of glia maturation factor overexpression in primary astrocytes on MAP kinase activation, transcription factor activation, and neurotrophin secretion. Neurochem Res. 2001;26:1293–1299. doi: 10.1023/a:1014241300179. [DOI] [PubMed] [Google Scholar]
- Zaheer A, Mathur SN, Lim R. Overexpression of glia maturation factor in astrocytes leads to immune activation of microglia through secretion of granulocyte-macrophage-colony stimulating factor. Biochem Biophys Res Commun. 2002;294:238–244. doi: 10.1016/S0006-291X(02)00467-9. [DOI] [PubMed] [Google Scholar]
- Zaheer A, Knight S, Zaheer A, Ahrens M, Sahu SK, Yang B. Glia maturation factor overexpression in neuroblastoma cells activates glycogen synthase kinase-3beta and caspase-3. Brain Res. 2008a;1190:206–214. doi: 10.1016/j.brainres.2007.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaheer A, Zaheer S, Thangavel R, Wu Y, Sahu SK, Yang B. Glia maturation factor modulates beta-amyloid-induced glial activation, inflammatory cytokine/chemokine production and neuronal damage. Brain Res. 2008b;1208:192–203. doi: 10.1016/j.brainres.2008.02.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaheer A, Zaheer S, Sahu SK, Knight S, Khosravi H, Mathur SN, Lim R. A novel role of glia maturation factor: induction of granulocyte-macrophage colony-stimulating factor and pro-inflammatory cytokines. J Neurochem. 2007;101:364–376. doi: 10.1111/j.1471-4159.2006.04385.x. [DOI] [PubMed] [Google Scholar]
- Zaheer S, Wu Y, Sahu SK, Zaheer A. Suppression of neuro inflammation in experimental autoimmune encephalomyelitis by glia maturation factor antibody. Brain Res. 2011a;1373:230–239. doi: 10.1016/j.brainres.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaheer S, Thangavel R, Sahu SK, Zaheer A. Augmented expression of glia maturation factor in Alzheimer’s disease. Neuroscience. 2011b;194:227–233. doi: 10.1016/j.neuroscience.2011.07.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Zeng X, Yang H, Hu G, He S. Mast cell tryptase induces microglia activation via protease-activated receptor 2 signaling. Cell Physiol Biochem. 2012;29:931–940. doi: 10.1159/000171029. [DOI] [PubMed] [Google Scholar]
- Zhang X, Wang Y, Dong H, Xu Y, Zhang S. Induction of Microglial Activation by Mediators Released from Mast Cells. Cell Physiol Biochem. 2016;38:1520–1531. doi: 10.1159/000443093. [DOI] [PubMed] [Google Scholar]


