Abstract
Progressive supranuclear palsy (PSP), once simply considered a common cause of atypical parkinsonism is now recognized as a spectrum of motor and behavioral syndromes associated with a specific four repeat (4R) tau neuropathology at autopsy. There are currently no effective treatments for PSP, but because PSP is strongly linked biochemically and genetically to tau protein abnormalities, there is a growing interest in pursuing clinical trials of new tau-directed therapies for this disorder. Such new tau therapies are envisioned to have disease-modifying effects by reducing brain levels of toxic forms of tau or compensating for loss of tau function. To be most effective, these treatments will need to be initiated at early stages of disease. New research criteria that recognize early forms of PSP and operationalize diagnosis of the full spectrum of clinical phenotypes have recently been published. These criteria and new clinical trial designs have benefited from rapid advances in MRI, physiological and fluid biomarker development for PSP. As tau pathology is also central to Alzheimer’s disease and chronic traumatic encephalopathy, it is believed that a successful tau therapeutic for PSP would inform the treatment of other neurodegenerative diseases.
Introduction
Progressive supranuclear palsy (PSP) has been traditionally considered to be a cause of atypical Parkinsonism, however over the past decade it has been increasingly recognized to encompass a spectrum of clinical phenotypes involving behavioral, language and a range of movement abnormalities.1 The classic movement disorder clinical phenotype, now referred to as Richardson’s syndrome (PSP-RS), was first described in 1964 by Steele, Richardson and Olszewski. Since then, no effective treatments for this uniformly fatal disorder have been identified. 2 However, because PSP is strongly linked neuropathologically and genetically to tau protein abnormalities, 3 there is an increasing interest in pursuing clinical trials of new tau-directed therapies. PSP-RS is considered a rare disease with a prevalence of approximately 5–7 per 100,000, 4 and a recent UK study 4 found a peak prevalence between the ages of 70–74 of approximately 18 cases per 100,000. However, a study in Japan that included other PSP phenotypes in addition to PSP-RS found a total prevalence of 18 per 100,000 across all ages. 5 This higher prevalence number is consistent with estimates from autopsy series, 6 and suggests that the prevalence of the full spectrum of PSP syndromes is substantially higher than estimates based on PSP-RS alone. A cluster of PSP cases in an industrial city in France and a multicenter case-control study suggest that environmental risk factors influence PSP incidence. 7,8
Our aim is to describe advances in the clinical diagnosis, pathology, genetics, biomarkers and therapeutics of PSP since The Lancet Neurology’s last PSP review.1 Since that time, there has been an increasing focus on therapeutic development for PSP. An important advance has been the development of the new International Parkinson’s and Movement Disorder Society (MDS) Criteria for the Diagnosis of PSP that recognize early, “suggestive” forms and operationalize diagnosis of non-Richardson’s PSP phenotypes. 9 These new research criteria provide a framework for incorporating MRI, physiological and fluid biomarker in diagnostic decision making and novel clinical trial designs. Moreover, they will enable deployment of new therapies for PSP at earlier stages of disease and participation by a wider range of PSP phenotypes in future clinical trials.
The clinical spectrum of PSP
The MDS PSP Diagnostic Criteria were based on a comprehensive literature review by the MDS PSP Working Group, followed by a consensus conference in March, 2016, in collaboration with patient advocacy groups. Here we outline their basic structure and content; the detailed criteria are now published. 9
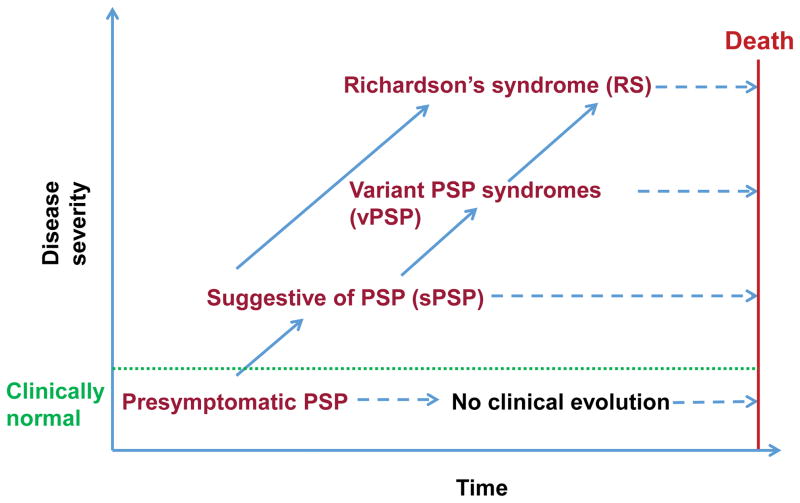
Most neurodegenerative diseases begin with a presymptomatic phase where neuropathology accumulates but has not yet crossed a threshold necessary to produce clinical symptoms. A wealth of evidence supports such a sequence of pathological events in Alzheimer’s disease (AD) and Parkinson’s disease (PD),10,11 and the recent demonstration of mild asymptomatic PSP pathology in some healthy elders suggests that a similar sequence of events might occur in PSP.6,12,13 Therefore, it is likely that PSP starts with a presymptomatic phase in which neuropathological abnormalities begin to accumulate but clinical features are absent, develops to an suggestive of PSP (soPSP) phase in which individuals develop mild or isolated symptoms, but do not meet the full research criteria for PSP, and culminates with a fully symptomatic stage that in many cases would meet the research criteria for PSP-RS or another clinical variant of PSP (vPSP; Figure 1). As the disease progresses, many vPSP syndromes eventually develop features of PSP-RS.1,14 Patients displaying a vPSP phenotype until death are currently difficult to diagnose with confidence as PSP antemortem.15
Figure 1. Model of the clinical trajectory of progressive supranuclear palsy (PSP).
Hypothetical model for the pathological-clinical continuum of PSP. PSP is defined as a continuum of disease from a presymptomatic phase (presymptomatic PSP) through a suggestive phase (soPSP) to a fully symptomatic stage that in many cases would meet the full research criteria for PSP-Richardson’s syndrome (PSP-RS) or another clinical variant of PSP (vPSP). Not all presymptomatic or soPSP will progress to a fully developed PSP phenotype.
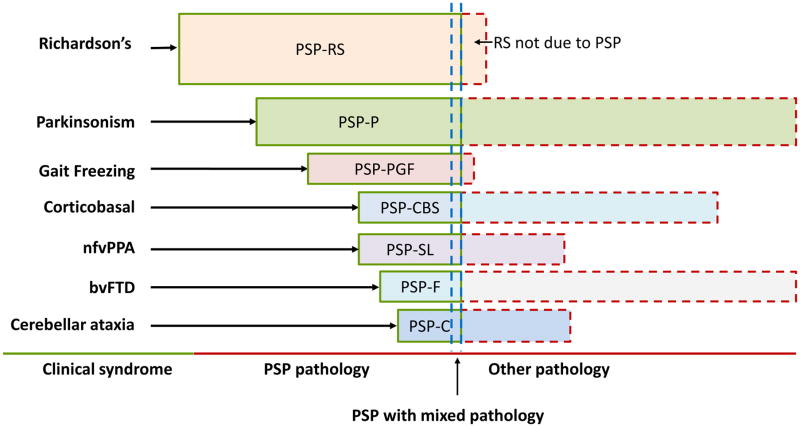
Most PSP syndromes progress to develop some or all of the typical clinical features of PSP-RS; however, approximately two thirds of the neuropathologically-defined cases of PSP in brain banks presented in the first two years with PSP-RS.1,14 Other vPSP syndromes named according to their predominant clinical features may account for about one third of earlier presentations in aggregate and include PSP with predominant parkinsonism (PSP-P), 16 pure akinesia with gait freezing (formerly PAGF, now PSP-PGF), 17 corticobasal syndrome (PSP-CBS), 14,18 primary progressive apraxia of speech or non-fluent variant primary progressive aphasia (nfvPPA; when caused by PSP: PSP with predominant speech/language disorder or PSP-SL), 19–21 behavioral variant frontotemporal dementia (bvFTD, when caused by PSP: PSP with predominant frontal presentation or PSP-F),14,22 and PSP with predominant cerebellar ataxia (PSP-C) (Figure 2). 23
Figure 2. Clinical syndromes of progressive supranuclear palsy (PSP).
PSP, progressive supranuclear palsy; PSP-F, PSP with predominant frontal presentation; PSP-C, PSP with predominant cerebellar ataxia; PSP-CBS, PSP-corticobasal syndrome; nfvPPA, non-fluent variant Primary Progressive Aphasia; bvFTD, behavioral variant Frontotemporal Dementia; PSP-P, PSP with predominant parkinsonism; PSP-SL, PSP with predominant speech/language disorder; PSP-PGF, PSP with progressive gait freezing. Relative proportions of each syndrome are speculative. Nomenclature reflect MDS PSP diagnostic criteria. 9
Presymptomatic PSP
Presymptomatic PSP occurs in individuals who are asymptomatic but possibly destined to develop a full-blown PSP. Currently, the presymptomatic phase can only be identified post mortem by evidence of histological changes typical of PSP pathology in individuals who are considered clinically normal.6,12,13 Because the new MDS PSP criteria focus on clinical diagnosis, they do not include the presymptomatic PSP, but they are consistent with this concept. In one community-derived autopsy series, incipient PSP was present in five out of 233 individuals (2.1%).24 Two similar studies found PSP pathology in 4.6% of normal elderly subjects in a healthy aging cohort 12 and 4.6% of individuals over 60 in a large forensic autopsy series in Japan. 6 These numbers are in striking contrast to the low prevalence of PSP-RS, 4 suggesting that if accurate, most individuals with presymptomatic PSP do not develop overt disease.
Suggestive of PSP
Suggestive of PSP (soPSP) refers to the early symptomatic phase of PSP before the clinical picture is full-blown, in which a few clinical symptoms/signs are clearly present, but do not warrant a diagnosis of PSP. Inherent in the definition of soPSP is some degree of uncertainty as to whether the individual will progress to PSP-RS. In the future, specific biomarkers for PSP may help to mitigate this uncertainty. The soPSP category also includes individuals who have developed one or more major features of PSP-RS, but do not display the complete RS, such as individuals with an isolated saccade slowing or postural instability.14 A diagnosis of soPSP can be made only when individuals are suspected to have PSP pathology, but do not fully meet criteria for PSP, and when other causes have been excluded. Recognition of individuals in the soPSP stage of disease could potentially allow neuroprotective treatment to be initiated early enough to stabilize individuals before the onset of significant disability.
Symptomatic PSP phenotypes
The clinical features of different PSP phenotypes have been described in a number of recent reviews. 1,25 Importantly, language and behavioral presentations of PSP also frequently meet criteria for frontotemporal lobar degeneration (FTLD). We briefly describe each syndrome here with particular attention to new information over the past seven years. We also highlight changes in nomenclature introduced by the new MDS PSP Criteria.
Richardson’s Syndrome
PSP-RS refers to the classically described phenotype of PSP which was originally codified in the 1996 NINDS-SPSP research criteria. The most frequently reported symptoms at onset are unexplained falls, unsteady gait, bradykinesia, subtle personality changes (apathy, disinhibition), cognitive slowing (“bradyphenia”), executive dysfunction (difficulty planning, multitasking), slow, ataxic, spastic and hypophonic speech, dysphagia and impaired ocular movement (i.e., slowing of vertical saccades, difficulty reading, apraxia of eyelid opening [AEO]).1 Vertical supranuclear gaze palsy is the definitive diagnostic feature, but the onset is variable, and may not develop until 3–4 years after disease onset. Decreased velocity (slowing) and gain (amplitude) of vertical greater than horizontal saccadic eye movements and decreased or absent optokinetic nystagmus are early signs on neurological examination.26
PSP-parkinsonism
PSP-P was first defined as a clinical phenotype of PSP based on an autopsy series, in which a subset of cases were found to have a more benign disease course and early features similar to PD. 1 PSP-P patients often present with asymmetric onset of tremor, bradykinesia, rigidity, a moderate initial therapeutic response to levodopa, and a slower rate of progression than PSP-RS. The clinical picture of PSP-P resembles idiopathic PD sufficiently that the two disorders are difficult to distinguish early on. 16 Later in the disease course, most individuals develop features of PSP-RS, and individuals are retrospectively given a diagnosis of PSP-P. It is currently impossible to diagnose patients who don’t evolve into PSP-RS as PSP during life. However, later in the course, drug-induced dyskinesias, autonomic dysfunction, and visual hallucinations are much less common in PSP-P than in PD, which may help to distinguish these diseases. 25
Pure akinesia with gait freezing, now referred to as PSP with progressive gait freezing (PSP-PGF), is a clinical phenotype of PSP initially presenting with an isolated gait disorder years before developing other features of PSP-RS. 17 PSP-PGF is characterized by progressive gait disturbance with start hesitation and subsequent freezing of gait, sometimes also involving speech or writing, without tremor, rigidity, dementia, or eye movement abnormality during the first five years of the disease. PSP-PGF has been reported to be highly predictive of PSP pathology.1
PSP-corticobasal syndrome
CBS is the best recognized presentation of corticobasal degeneration (CBD), a 4R tauopathy closely related to PSP. Clinically and genetically there is considerable overlap between CBD and PSP, and the current CBD research criteria acknowledge a syndrome caused by CBD pathology that resembles PSP-RS, underscoring this frequent overlap.27 In contrast, PSP-CBS refers to a clinical CBS phenotype of pathological PSP, characterized by a variable mixture of progressive asymmetric limb rigidity, apraxia, cortical sensory loss, alien limb, dystonia and bradykinesia that is unresponsive to levodopa. PSP-CBS is a rare presentation of PSP pathology, occurring in only six of the 179 pathologically diagnosed PSP cases in the Queen Square Brain Bank.18 Distinction of PSP-CBS from CBD-CBS on clinical grounds is impossible during life 18 and for this reason, the new MDS PSP Criteria designate PSP-CBS as possible PSP, but probable 4R tauopathy. 9
PSP-speech language
Nonfluent variant primary progressive aphasia (nfvPPA) is a progressive clinical syndrome that is characterized by either agrammatism in language production or effortful, halting speech with inconsistent speech sound errors and distortions (apraxia of speech; AOS).28 The MDS PSP criteria recognize a clinical phenotype of PSP initially presenting with predominant speech and language disorder (PSP-SL) features of nfvPPA before developing other motor features of PSP. 19,20 A recent longitudinal study of 13 subjects with primary progressive AOS (PPAOS), which is similar to nfvPPA, found that five subjects developed a syndrome similar to PSP-RS about five years after onset,19 and 22/25 nfvPPA in a larger series had tau pathology, most commonly 4R tau. 21 Similar to PSP-CBS, the new PSP criteria designate PSP-SL as possible PSP, but probable 4R tauopathy because determining which PSP-SL cases have PSP pathology based on clinical findings is impossible during life. 9
PSP with Frontal Presentation
PSP with a predominant frontal presentation (PSP-F), refers to PSP presenting with the clinical features of behavioral variant frontotemporal dementia (bvFTD) years before motor features of PSP. bvFTD is a clinical syndrome characterized by an early and progressive deterioration of personality, social comportment, behavior and cognition.29 PSP-F is uncommon. Only three out of 66 autopsy-proven PSP cases (4.5%) in the Mayo Clinic series presented with the behavioral and personality changes of bvFTD.22 However, a European series of autopsy proven PSP suggested a higher prevalence of FTD-like symptoms at onset. 14
PSP with predominant cerebellar ataxia
PSP-C is a rare clinical phenotype of PSP presenting with cerebellar ataxia as the initial and principal symptom before developing cardinal features of RS during the disease course. In contrast to the ratio of three out of 22 consecutive patients reported in an earlier Japanese study, a recent Western autopsy study identified only 5 PSP-C in 1,085 pathologically confirmed PSP cases, four of which were clinically misdiagnosed as MSA-C.23 Clinical features of PSP-C were similar to those of MSA-C, but lacking marked dysautonomia sufficient to meet criteria for a clinical diagnosis of MSA. 23 Because PSP-C is difficult to diagnose during life and ataxia is far more often suggestive of other neurodegenerative diseases rather than PSP, this variant is not included in the new MDS PSP criteria.
PSP with mixed pathology
While there are strong links between the hallmark neuropathological features of PSP and clinical features of disease, there is an increasing recognition that a subset of patients may have co-pathologies that influence their clinical phenotype. A variety of co-pathologies have been described in association with PSP, including most commonly, AD, but also PD, TDP-43, argyrophilic grain disease or cerebrovascular disease. In a recent study evaluating 64 cases of pathologically proven PSP, 36% had concomitant AD, 20% PD, 1% DLB, 44% argyrophilic grains, 52% cerebral white matter rarefaction and 25% cerebral amyloid angiography. 30
Pathology
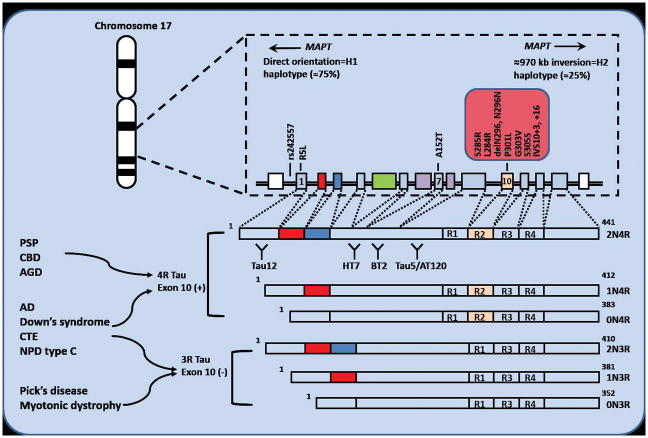
The NINDS neuropathologic criteria for PSP were validated in 1996, requiring the presence of neurofibrillary tangles (NFTs) or neuropil threads (now recognized to be composed of tau protein), or both, in the basal ganglia and brainstem. 31 Microscopic features include neuronal loss, gliosis, NFTs, neuropil threads, tufted astrocytes (TAs) and oligodendroglial coiled bodies (CBs). 31 Six isoforms of the encoded tau protein are expressed in the adult human brain (Figure 3).
Figure 3. The MAPT gene locus, mutations and human brain tau protein isoforms.
The MAPT gene locus located on chr. 17q21 exists as two major haplotypes, termed H1 and H2. Most people are H1/H1 haplotype. Mutations producing PSP-like phenotypes are indicated. Alternative splicing of exons 2, 3 and 10 of the MAPT gene gives rise to six isoforms of the encoded tau protein expressed in the human brain. Increased 4 microtubule binding domain repeat (4R) tau containing exon 10 are associated with PSP and CBD. Equal ratio of 3R and 4R tau in AD. Binding sites for monoclonal antibodies (tau 12, HT7, BT2, and tau5/AT120) from CSF tau assays. 3R, three repeat; 4R, Alzheimer’s disease, AD; Argyrophilic grain disease, AGD; corticobasal degeneration, CBD; chronic traumatic encephalopathy, CTE; MAPT, microtubule-associated protein tau; Niemann–Pick disease type C, NPD type C.
The regional distribution of tau pathology and neuronal loss is a source of pathological and consequentially clinical heterogeneity in PSP. 31 Greater cortical tau pathology has been documented in PSP syndromes with more cortical symptoms during life, such as PSP-CBS, 32 PSP-SL,21 and PSP-F. On the other hand, brainstem predominant PSP syndromes, such as PSP-P and PSP-PGF, have less cortical tau pathology and more severe degeneration in globus pallidus, subthalamic nucleus and substantia nigra when compared with PSP-RS. 1 Studies using brain tissue from human PSP patients have shown that inoculation of tau transgenic mice with this tissue can recapitulate PSP tau inclusions in mouse brains or identify specific strains of transmissible tau in cell culture models.33,34 It is hypothesized that disease phenotypes may emerge from tau spreading preferentially through different brain networks that that are connected functionally and neuroanatomically. 35
Genetics
MAPT polymorphisms and haplotypes associated with sporadic PSP
The first and most strongly linked gene to PSP is the gene for tau itself, microtubule-associated protein tau (MAPT). 3 Genetic studies, including genome-wide association studies, have identified both an inversion polymorphism and haplotype-specific MAPT polymorphisms influencing the risk of PSP (Figure 3). 3 The odds ratio (OR) for PSP in carriers of the MAPT H1/H1 haplotype is 5.5, which is stronger than the OR for the APOE ε3/ε4 genotype as a risk factor for AD. The underlying molecular mechanisms for PSP risk conferred by the H1 haplotype and the related sub-haplotype H1c are unclear. A rare coding variant (A152T) alters microtubule assembly and is a strong risk factor for PSP and FTD. 36
MAPT mutations sometimes produce a PSP phenotype
PSP is generally a sporadic disease, but very rare familial forms of PSP have been recognized,37,38 and 7% of patients with PSP-RS in one series fulfilled criteria for an autosomal dominant mode of transmission,37 however this was not replicated in a different study. 8 Although a clear causative mutation cannot be identified in most familial cases, mutations in the MAPT gene have been identified in several autosomal dominant families, including pathologically-confirmed PSP.38 Interestingly, most of these variants are located in exon 10 and its splicing regulatory region (Figure 3). Given that elevated concentrations of insoluble 4R tau protein are found in PSP, mutations that enhance splicing in MAPT exon 10 may promote disease by enhancing production of 4R tau isoforms. 39
Emerging loci from genome-wide association studies
In 2011, the first large PSP GWAS identified three new genetic risk factors: STX6, EIF2AK3 and MOBP.3 The mechanisms by which these other genes might increase risk for PSP are not known. Recently, the PSP-associated SNP near the MOBP gene was found to correlate more strongly with increased expression of the SLC25A38/Appoptosin gene. 40 Appoptosin overexpression in transgenic mice increases caspase-mediated tau cleavage, tau aggregation, synaptic dysfunction and behavioral deficits.40 Loss of function of EIF2AK3 in humans appears to result in tau pathology.41 These findings suggest that other genetic polymorphisms might also increase risk for PSP via effects on tau.
Biomarkers (Table 1)
Table 1.
PSP Biomarkers
| Class | Measure | Potential uses | Gold | Ref | Key insights | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Bd | An | Pr | Dx | SO | PD | |||||
| Imaging | ||||||||||
| MRI | ||||||||||
| Visual read on conventional MRI | X | X | Yes | 43,83 | Hummingbird and Morning Glory signs specific for PSP, but don’t differentiate from CBD pathology | |||||
| volumetric (VBM, BSI, etc.) | X | Yes | 74,84 | brain atrophy responds to anti-tau therapy in tau transgenic mouse models | ||||||
| X | X | Yes | 19,20 | PSP-like atrophy detectable in nfvPPA and PPAOS | ||||||
| X | Yes | 42,83 | Midbrain atrophy not specific to PSP; also found in CBD pathology | |||||||
| mibrain/pons ratio(s) such as MPRI | X | X | Yes | 45–47 | ratio differentiates path confirmed PSP from PD, MSA; Predicts gaze palsy and other PSP-RS signs (clinically diagnosed cases) | |||||
| X | Yes | 85 | Distinct atrophy rate measureable in PSP | |||||||
| diffusion tensor imaging (DTI) | X | No | 44,86 | DTI difference in PSP phenotypes; DTI sensitive to disease progression | ||||||
| free water imaging | X | No | 87 | Improved differential diagnosis: PSP from PD | ||||||
| resting-state fMRI | X | No | 35 | Reduced connectivity in midbrain-anchored network also present in normals | ||||||
| task fMRI | X | X | No | 88 | Differential diagnosis: PSP from PD | |||||
| PET | ||||||||||
| FDG | X | X | X | Yes |
49 48 |
Medial frontal, caudate, thalamic hypotmetabolism; Network changes in metabolism identify PSP | ||||
| Tau (T807/AV1451, PBB3) | X | X | X | Yes | 51–53,89 | Anecdotal reports of in vivo binding for most tracers; T807/AV1451 low affinity for 4R tau in post mortem brain | ||||
| MEG | X | X | No | 90 | Measurable evoked responses in PSP | |||||
| Fluid | ||||||||||
| CSF | ||||||||||
| Tau | No | 56,91 | Total tau comparable to controls | |||||||
| p181 tau | No | 56,91 | pTau comparable to or lower than controls | |||||||
| Neuro-filament light chain (NfL) | X | X | X | X | X | X | No | 57,58,91,92 | NfL elevated in PSP, increases over time. Changes correlated with clinical and imaging. Elevated in tau transgenic mice | |
| YKL-40 | X | No | 55 | Elevated in PSP | ||||||
| Blood | NfL | X | X | X | X | ? | No | 59,60,92 | Plasma NfL elevated in PSP and predictive of clinical/imaging changes | |
| Physiological | ||||||||||
| eye movements | Infrared oculography | X | X | X | Yes | 26,58 | Decreased saccade velocity and gain in PSP | |||
| eyelid function | Spontaneous and evoked blink rate | X | X | No | 93 | Blink rate parameters associated with disease | ||||
| retinal thickness | Optical coherence tomography | X | X | No | 62 | Decreased retinal thickness in PSP | ||||
| activity | wrist actigraphy | X | X | No | 94 | Disrupted circadian rhythms and sleep | ||||
| finger taps | quantitative | X | No | 95 | Distinct abnormalities: PSP-RS vs PD | |||||
Potential biomarker utility: Bd: bedside, An: animal models, Pr: predictive of PSP pathology, Dx: differential diagnosis, SO: surrogate outcome for clinical trial, PD: pharmacodynamic for clinical trial. Gold = supported by autopsy confirmed cases. BSI: boundary shift integral, VBM: voxel based morphometry
When fully developed, the PSP-RS clinical syndrome is distinctive and usually easily differentiated from other parkinsonian disorders. However, in early or variant cases, increasing evidence suggests that biomarkers may help to improve diagnostic accuracy. Over the past decade, several potential neuroimaging, biological and neurophysiological biomarkers have been described as potentially helpful in differentiating PSP-RS from other parkinsonian syndromes. However, an important lesson learned from early studies is that the diagnostic value of these biomarkers cannot be established without adequately powered studies in autopsy confirmed cases. For example, midbrain atrophy, alone or in combination with other brainstem measures, has been repeatedly identified as a biomarker of PSP, but it was not able to differentiate RS due to PSP from that due to CBD pathology in some studies. 42
There is urgent need for diagnostic biomarkers that can detect PSP pathology in very mildly symptomatic or presymptomatic individuals, allowing for earlier diagnosis and intervention with potentially disease modifying therapies. Such biomarkers could be deployed in vPSP and soPSP to permit expansion of therapeutic studies to earlier disease stages in these patients. Finally, biomarkers that can demonstrate pharmacodynamic effects of new therapies on their intended targets are needed to support clinical trials.
MRI
Atrophy of the midbrain and superior cerebellar peduncles (SCP) has been found to be a useful marker in differentiating PSP-RS from other parkinsonian syndromes and may be tracked longitudinally using diffusion tensor imaging. 43,44 In a cohort including pathologically confirmed PSP, MSA, PD and CBD and controls, conventional structural MRI was more specific but less sensitive than clinical diagnosis in PSP, and the hummingbird and morning glory flower signs each had 100% specificity for PSP but lower sensitivity (68.4% and 50%, respectively).43 Another cohort study including pathologically confirmed cases, found that the MR parkinsonism index (MRPI) yielded sensitivity of 100% and specificity of 99.2%–100% for PSP-RS.45 The pons/midbrain ratio, as calculated from conventional MRI, had high specificity and sensitivity for the diagnosis of pathologically confirmed PSP.46 In studies of the early stages of PSP, 46 MRPI was also able to predict development of PSP-RS in unclassifiable parkinsonism and eye movement abnormalities in PSP-P. 45,47
Resting-state functional MRI (fMRI) is also a potentially promising imaging biomarker for PSP. An intrinsic connectivity network anchored at the dorsal midbrain shows disruptions in PSP that are correlated with physiological and clinical features of disease.35
PET using FDG, 18F-AV1451 and other newer ligands
FDG-PET in autopsy-confirmed 4R-tauopathy patients including PSP variants showed hypometabolism in frontal cortex, caudate, midbrain, and thalamus.48 PSP-related metabolic covariance patterns may also help with differential diagnosis. 49 Despite these observations, the diagnostic utility of FDG-PET in PSP is not well established.
The recent development of tau-specific PET imaging ligands offers the opportunity for in vivo topographical mapping and quantification of tau aggregation and deposition in parallel with clinical assessments of PSP. 50 One such ligand, 18F AV1451, binds avidly to tangles and paired helical filament-tau-containing neurites in AD and produces high quality images in living patients; 51 however, its utility in PSP is still uncertain. Preliminary data have suggested weak AV1451 binding to aggregated 4R tau in autopsy tissue slices from patients with PSP. 51 Preliminary human PET data suggest binding to areas of known tau pathology in PSP-RS as compared to controls in more advanced patients. 52,53 Several other novel selective tau imaging agents, including 11C PBB3, have also been reported to bind to PSP tau, but insufficient clinical data exist at this time to gauge their potential utility.54
CSF and blood
There have been a number of studies attempting to identify CSF biomarkers to achieve accurate diagnosis of PSP.55 None were performed in autopsy confirmed cases. CSF phospho-tau and total tau concentrations are normal or low relative to controls, in contrast to AD, where they are elevated.56 Several CSF studies showed 2–5 fold increased neurofilament light chain (NfL) concentrations in PSP compared with healthy controls, PD, PDD and DLB but not CBS and MSA.55,57 CSF NfL was the only CSF biomarker that changed over time in a recent multicenter PSP clinical trial.58 NfL can now reliably be measured in blood, and PSP-RS patients have elevated levels of plasma NfL compared to age matched controls and patients with PD.59 Baseline plasma NfL concentrations predict disease progression over the course of a year using a variety of clinical and MRI measures.60
Physiological
Slowing of vertical saccades to a greater extent than horizontal saccades is a key distinguishing feature of PSP-RS. 61 Decreases in saccade velocity and gain account for the most characteristic PSP ocular motor deficits, and have been demonstrated to be highly specific findings that allow differentiation of autopsy-confirmed PSP from other disorders.26 Profound changes in saccade velocity and gain over the course of one year have been demonstrated in two autopsy confirmed cases of PSP. 61 Retinal optical coherence tomography (OCT) may also be a promising biomarker for PSP, but it remains in an early stage of investigation. 62
Therapeutics (Table 2)
Table 2. Planned or enrolling clinical trials involving tau therapeutics.
| Drug | Mode of action | Disease | Phase | Ref | ClinicalTrials.gov identifier |
|---|---|---|---|---|---|
| TPI-287 | Microtubule stabilizer | AD, CBD, PSP | 1 | 69 | NCT01966666, NCT02133846 |
| AADvac1 | Tau active vaccination | AD | 1 | n/a | NCT01850238 |
| ACI-35 | Tau active vaccination | AD | 1 | n/a | NCT02738450 |
| LMT-X (TRx0237) | Tau aggregation inhibitor | AD, bvFTD | 3 (both negative) | 73 | NCT01689233, NCT01689246, NCT01626378 |
| Abb-8E12 (C2N-8E12) | Anti-tau monoclonal antibody | PSP, AD | 2 | 84 | NCT02494024, NCT02880956 |
| BMS-986168 | Anti-tau monoclonal antibody | PSP | 2 | 96 |
NCT02460094 NCT03068468 |
| Salsalate | Inhibiting tau acetylation | PSP, AD | 1 | 74 | NCT02422485 |
| Young Plasma | Rejuvenation | PSP, AD | 1 | 82 | NCT02460731 |
| Various | O-GlcNAcase inhibitors | PSP | Pre-clinical | 75 | Not available |
| Tau antisense oligos | Reduce gene expression | Various | Pre-clinical | 39,77 | Not available |
| Various | Anti-tau monoclonal antibody | Various | Pre-clinical, Phase 1 | Not available | Not available |
PSP is a uniformly fatal disease. Individuals with PSP-P often initially experience a symptomatic benefit from levodopa therapy, as do a more limited number of PSP-RS patients, 2 however this benefit is transient in most cases and does not have any known impact on disease duration. Physical therapy is helpful, and leads to measureable improvements on clinical rating scales.63 Botulinum toxin injections (pretarsal) may be effective for AEO. A variety of small randomized placebo controlled clinical trials have been performed in the past in PSP-RS, however none demonstrated any efficacy other than co-enzyme Q10, which showed a modest symptomatic benefit in a small 6 week study that was not replicated in a larger, albeit underpowered study. 58,64 Deep brain stimulation of the pedunculopontine nucleus has been attempted in advanced PSP-RS, however there were no clear benefits observed and unacceptable side effects.65
Lessons learned from recent international clinical trials (riluzole, tideglusib, davunetide)
The rapid growth of knowledge about the potential pathogenic mechanisms of PSP has facilitated the implementation of a series of rational clinical studies with hypothesis-driven therapeutic approaches aimed at disease-modification by targeting tau or mitochondrial dysfunction.58,66–68 Of these, three clinical trials were adequately powered to demonstrate small to moderate disease-modifying effects: the NNIPPS study of riluzole, 67 a phase 2 trial of tideglusib, 68 and a Phase 2/3 trial of davunetide. 58 All three trials failed to show efficacy on clinical endpoints. An important limitation of these studies was the lack of a pharmacodynamic biomarker that could demonstrate that the experimental agent engaged its physiological target and produced its hypothesized biological effect. Thus, it is possible that the studies were negative because the drug’s proposed mechanism of action did not operate in humans in the same manner as in preclinical models.
Tau loss of function therapies
Neurodegeneration in PSP is strongly linked to tau pathology, but the mechanisms by which tau abnormalities lead to cell dysfunction and death are not well understood. Two general types of tau dysfunction are thought to lead to disease: loss of normal tau function or toxic gain of tau function. 69 These mechanisms are not mutually exclusive, and it is possible that toxic gain of function in one cellular compartment might lead to loss of tau function in others.
Microtubule stabilizers
The rationale for treating PSP with microtubule stabilizers is to compensate for the microtubule dysfunction that might result from a disease-associated loss of tau function (Figure 4). 69 A number of microtubule-stabilizing agents have been developed and three have been explored in human neurodegenerative disease clinical trials. Davunetide is a microtubule stabilizing octapeptide that demonstrated benefits in animal models of tauopathy. However, a recent large clinical trial of davunetide for PSP-RS demonstrated a lack of efficacy.58 TPI-287, a new blood brain barrier permeable taxane, has entered phase I clinical trials for AD, PSP and CBD (Table 2).
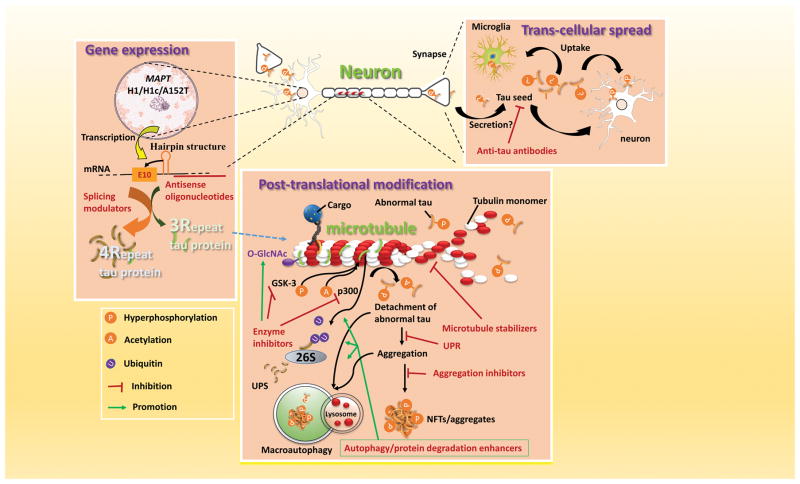
Figure 4. Potential mechanism based therapies for PSP.
Three general categories of intervention are under development. (1) Modulation of MAPT gene expression, via antisense oligonucleotides and splicing modulators. (2) Modulation of tau protein post-translational modifications, including phosphorylation, acetylation and O-GlcNAc modification; degradation by both the ubiquitin proteasome system (UPS) and autophagy; modulation of the unfolded protein response (UPR). (3) Inhibition of tau propagation via trans-synaptic pathways or mediated by microglia. Modulating inflammation may alter tau pathology. 26S/UPS, ubiquitin proteasome system; GSK, glycogen synthetase kinase; p300, acetyltransferase
Tau gain of function therapies
Immunotherapeutic approaches
Evidence that tau may propagate between cells in a prion-like fashion raises the possibility that blocking spread of pathogenic tau by specific anti-tau antibodies might be a viable approach to tauopathy treatment (Figure 4).70 Passive immunization with anti-tau monoclonal antibodies not only suppresses tau pathology but also improves cognitive or motor function in tau transgenic mouse models. Certain three dimensional conformations of tau may be particularly pathogenic and can be targeted by specific antibodies.71 Two N-terminal tau directed monoclonal antibodies, BMS-986168 and Abb-8E12, have progressed to Phase 2 clinical trials for PSP (Table 2).72 Active vaccination against tau epitopes is also under investigation, and two tau vaccines have entered human clinical trials: AADvac1 and ACI-35. 72
Small molecules targeting aggregation or post-translational modification
In PSP, tau aggregates into NFTs and neuropil threads. Therefore, inhibition of tau aggregate assembly and disassembly of existing tau aggregates are potential therapeutic approaches for PSP. Methylene blue derivatives, in particular LMTX, were investigated in phase III clinical trials for AD and bvFTD, 73 however both trials were negative, casting doubt on the potential use of LMTX in PSP.
Hyperphosphorylation of tau is a well-studied post-translational modification and has historically been a focus for drug development (Figure 4). Inhibition of glycogen synthetase kinase 3 (GSK-3) reduces tau phosphorylation in vitro and GSK-3 may constitute a therapeutic target for PSP.68 However, the GSK-3 inhibitor tideglusib did not demonstrate efficacy in a phase 2 clinical trial for PSP,68 and a clinical trial of lithium, thought to work by a similar mechanism, was abandoned due to poor tolerability (clinicaltrials.gov: NCT00703677). Several studies suggest that acetylation of soluble tau species could be a precursor to hyperphosphorylation of tau, and that inhibition of this process could be a potential therapeutic strategy.74 Recently, salsalate, a tau acetylation inhibitor, has entered phase I clinical trial for PSP (Table 2). Finally, inhibitors of the enzyme that cleaves the posttranslational modification O-GlcNAc (O-GlcNAcase), protected against neurodegeneration in tau transgenic mice. 75 Clinical trials of O-GlcNAcase inhibitors are planned for PSP.
Antisense oligonucleotides (ASO’s) and splicing modulators
Alteration or down-regulation of tau gene expression could be beneficial in tauopathies by preventing buildup of toxic forms of tau. 76 A recent study demonstrated the feasibility of reducing human tau protein levels using MAPT ASOs both in vitro and in vivo. 77 Exon 10 splicing is regulated by a hairpin RNA structure that is destabilized by many MAPT point mutations that often lead to a PSP phenotype. Normalization of the 3R/4R tau ratio using ASOs or splicing modulators may also be a viable therapeutic approach to PSP. Both ASOs and small molecules have been developed to stabilize the hairpin and inhibit 4R-tau expression. 39 Intrathecally administered ASOs have been used in clinical trials for SOD1 related amyotrophic lateral sclerosis, and a similar delivery approach might be used in PSP. 77
Microglial targeted approaches
A growing body of evidence suggests that microglial activation drives tau pathology and contributes to the spreading of pathological tau in the brain(Figure 4). 78 Preclinical data suggest that therapeutic strategies targeting microglia-specific fractalkine receptor (CX3CR1) and IL1 β signaling may also help decrease spread of tau. 79
Other strategies
Other strategies under development for targeting tau pathology include enhanced degradation of misfolded tau or NFTs by various proteolytic systems including autophagy, 80 the unfolded protein response, 41 agents targeting mitochondrial dysfunction, 64 cell replacement 81 and young plasma transfusions designed to reverse brain aging.82 A small phase I study with young plasma transfusions is underway for PSP (Table 2).
Conclusions and future directions
There are no effective therapies for PSP, however, insights into the etiology and ontogeny of PSP over the past decade are bearing fruit in the form of new experimental therapeutic agents that have entered PSP clinical trials. Both neuropathological and genetic data point to a central role of the tau protein in PSP pathogenesis. The new MDS PSP diagnostic criteria expand the clinical spectrum of PSP by incorporating the concept of conditions suggestive of PSP and a variety of symptomatic PSP phenotypes. A variety of promising biomarkers for PSP have been described to aid in differential diagnosis and evaluation of novel therapeutics. Increasing numbers of clinical trials are planned for PSP, offering hope for patients and their families for the possibility of effective PSP therapies. Since tau pathology is also central to AD and chronic traumatic encephalopathy, it is believed that a successful tau therapeutic for PSP would also inform the treatment of other neurodegenerative diseases.
There is still a great deal of work to be done. While many clues have emerged from genetics, cell biology and neuropathology of PSP and other tauopathies, the cellular mechanisms of PSP pathogenesis remain elusive. Transcellular spread of tau pathology is an attractive hypothesis with clear therapeutic implications, but its existence in humans has yet to be convincingly demonstrated. Moreover it is unclear how well findings in preclinical tauopathy models will translate to human patients. There are exciting possibilities for human therapies, but the number of available clinical stage agents remains modest.
Important challenges remain for clinicians. Many patients are diagnosed too late to optimally benefit from a disease-modifying therapy. While the new diagnostic criteria may help to improve this problem, international efforts will be necessary to better educate physicians and establish reliable biomarkers for early and accurate differential diagnosis to enable clinical trials at early stages of disease when new therapies are most likely to be efficacious. Such biomarkers will need to be validated in autopsy confirmed cases. Tau-sensitive PET imaging is an attractive possibility, however the low affinity of current ligands for tau in PSP post mortem specimens raises concerns that these agents may have limited utility in PSP, and are not ready for widespread use.
Large, multicenter clinical trials have been successfully executed in PSP-RS, but PSP remains a rare disease. If multiple tau therapeutic programs reach late stage clinical development, there may be insufficient numbers of eligible patients to enroll in large, competing clinical trials. Careful planning and international partnerships involving patients, families, academic and industry researchers, and advocacy groups will be necessary to ensure the success of such studies. Moreover, assuring that clinical trial results, data and biospecimens are rapidly returned to the PSP research community at large will also be critical for advancing therapeutic development.
Acknowledgments
ALB is supported by National Institutes of Health grants U54NS092089, R01AG038791 and P01AG19724 and the Tau Research Consortium. GUH is funded by the Deutsche Forschungsgemeinschaft (DFG, HO2402/6-2 & Munich Cluster for Systems Neurology SyNergy). IL receives support from the National Institutes of Health grants P50AG005131, T35HL007491, U01NS086659 and U54NS092089.
Footnotes
Contributors
ALB generated the outline, wrote portions of and revised the manuscript. He reviewed the published articles, discussed the content, and helped produce the figures. JTY searched the published works, wrote the first draft, produced the figures, and edited the manuscript. GUH, LIG, IL, and AEL contributed to the discussion, editing and revision of the manuscript. AEL also contributed to producing the figures.
Declaration of interest
ALB has receives research support from the NIH (R01AG038791, U54NS092089), the University of California, the Tau Consortium, CBD Solutions, the Bluefield Project to Cure FTD, the Alzheimer’s Association and the following companies: Avid, Biogen, Bristol Myers Squibb, C2N Diagnostics, Cortice Biosciences, Eli Lilly, Forum Pharmaceuticals, Genentech, Roche and TauRx; has served as a consultant for Abbvie, Asceneuron, Celgene, Ionis Pharmaceuticals, Janssen, Merck and Novartis; serves on a Data and Safety Monitoring Board for Neurogenetics Pharmaceuticals; has stock and/or options in Alector and Delos. GUH received research support from CurePSP, the German Academic Exchange Service (DAAD), German Center for Neurodegenerative Diseases (DZNE), German Research Foundation (DFG), German Ministry of Education and Research (BMBF), the Sellas Life Sciences Group, Neuropore; served as a consultant for Abbvie, Asceneuron, Bristol-Myers Squibb, Novartis, Roche, UCB; has received honoraria for scientific presentations from Abbvie, Roche, Teva, UCB. LIG is supported by research funding from Bristol-Myers Squibb, AbbVie and the American Parkinson’s Disease Association and consults for BMS, AbbVie, SJO Research and the University of California. IL is also supported by the Parkinson Study Group, Michael J Fox Foundation, AVID Pharmaceuticals, C2N Diagnostics/Abbvie and Bristol-Myers Squibb; was member of the advisory board for Cynapsus, Lundbeck, Biogen and Bristol-Myers Squibb Advisory Boards; is a member of the Biotie/Parkinson Study Group Medical Advisory Board; and receives her salary from the University of California San Diego. AEL has served as an advisor for Abbvie, Acorda, Avanir Pharmaceuticals, Bristol-Myers Squibb, Ceregene, Lilly, Merck, and UCB; received honoraria from Medtronic, Teva, UCB, AbbVie; received grants from Brain Canada, Canadian Institutes of Health Research, Edmond J Safra Philanthropic Foundation, Michael J. Fox Foundation, the Ontario Brain Institute, National Parkinson Foundation, Parkinson Society Canada, Physicians Services Incorporated (PSI), W. Garfield Weston Foundation; received publishing royalties from Saunders, Wiley-Blackwell, Johns Hopkins Press, and Cambridge University Press; and has served as an expert witness in cases related to the welding industry.
References
- 1.Williams DR, Lees AJ. Progressive supranuclear palsy: clinicopathological concepts and diagnostic challenges. Lancet Neurol. 2009;8(3):270–9. doi: 10.1016/S1474-4422(09)70042-0. [DOI] [PubMed] [Google Scholar]
- 2.Lamb R, Rohrer JD, Lees AJ, Morris HR. Progressive Supranuclear Palsy and Corticobasal Degeneration: Pathophysiology and Treatment Options. Curr Treat Options Neurol. 2016;18(9):42. doi: 10.1007/s11940-016-0422-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hoglinger GU, Melhem NM, Dickson DW, et al. Identification of common variants influencing risk of the tauopathy progressive supranuclear palsy. Nature genetics. 2011;43(7):699–705. doi: 10.1038/ng.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coyle-Gilchrist IT, Dick KM, Patterson K, et al. Prevalence, characteristics, and survival of frontotemporal lobar degeneration syndromes. Neurology. 2016;86(18):1736–43. doi: 10.1212/WNL.0000000000002638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Takigawa H, Kitayama M, Wada-Isoe K, Kowa H, Nakashima K. Prevalence of progressive supranuclear palsy in Yonago: change throughout a decade. Brain Behav. 2016;6(12):e00557. doi: 10.1002/brb3.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yoshida K, Hata Y, Kinoshita K, Takashima S, Tanaka K, Nishida N. Incipient progressive supranuclear palsy is more common than expected and may comprise clinicopathological subtypes: a forensic autopsy series. Acta Neuropathol. 2017 doi: 10.1007/s00401-016-1665-7. [DOI] [PubMed] [Google Scholar]
- 7.Caparros-Lefebvre D, Golbe LI, Deramecourt V, et al. A geographical cluster of progressive supranuclear palsy in northern France. Neurology. 2015;85(15):1293–300. doi: 10.1212/WNL.0000000000001997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Litvan I, Lees PS, Cunningham CR, et al. Environmental and occupational risk factors for progressive supranuclear palsy: Case-control study. Mov Disord. 2016;31(5):644–52. doi: 10.1002/mds.26512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoglinger G, Respondek G, Stamelou M, et al. Clinical Diagnosis of Progressive Supranuclear Palsy - The Movement Disorder Society Criteria. Movement Disorders. 2017 doi: 10.1002/mds.26987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jack CR, Jr, Holtzman DM. Biomarker modeling of Alzheimer’s disease. Neuron. 2013;80(6):1347–58. doi: 10.1016/j.neuron.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berg D, Postuma RB, Adler CH, et al. MDS research criteria for prodromal Parkinson’s disease. Mov Disord. 2015;30(12):1600–11. doi: 10.1002/mds.26431. [DOI] [PubMed] [Google Scholar]
- 12.Dugger BN, Hentz JG, Adler CH, et al. Clinicopathological outcomes of prospectively followed normal elderly brain bank volunteers. J Neuropathol Exp Neurol. 2014;73(3):244–52. doi: 10.1097/NEN.0000000000000046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nogami A, Yamazaki M, Saito Y, et al. Early Stage of Progressive Supranuclear Palsy: A Neuropathological Study of 324 Consecutive Autopsy Cases. J Nippon Med Sch. 2015;82(6):266–73. doi: 10.1272/jnms.82.266. [DOI] [PubMed] [Google Scholar]
- 14.Respondek G, Stamelou M, Kurz C, et al. The phenotypic spectrum of progressive supranuclear palsy: a retrospective multicenter study of 100 definite cases. Movement disorders : official journal of the Movement Disorder Society. 2014;29(14):1758–66. doi: 10.1002/mds.26054. [DOI] [PubMed] [Google Scholar]
- 15.Respondek G, Roeber S, Kretzschmar H, et al. Accuracy of the National Institute for Neurological Disorders and Stroke/Society for Progressive Supranuclear Palsy and neuroprotection and natural history in Parkinson plus syndromes criteria for the diagnosis of progressive supranuclear palsy. Mov Disord. 2013;28(4):504–9. doi: 10.1002/mds.25327. [DOI] [PubMed] [Google Scholar]
- 16.Williams DR, Lees AJ. What features improve the accuracy of the clinical diagnosis of progressive supranuclear palsy-parkinsonism (PSP-P)? Mov Disord. 2010;25(3):357–62. doi: 10.1002/mds.22977. [DOI] [PubMed] [Google Scholar]
- 17.Owens E, Josephs KA, Savica R, et al. The clinical spectrum and natural history of pure akinesia with gait freezing. Journal of neurology. 2016;263(12):2419–23. doi: 10.1007/s00415-016-8278-x. [DOI] [PubMed] [Google Scholar]
- 18.Ling H, O’Sullivan SS, Holton JL, et al. Does corticobasal degeneration exist? A clinicopathological re-evaluation. Brain. 2010;133(Pt 7):2045–57. doi: 10.1093/brain/awq123. [DOI] [PubMed] [Google Scholar]
- 19.Josephs KA, Duffy JR, Strand EA, et al. The evolution of primary progressive apraxia of speech. Brain. 2014;137(Pt 10):2783–95. doi: 10.1093/brain/awu223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Santos-Santos MA, Mandelli ML, Binney RJ, et al. Features of Patients With Nonfluent/Agrammatic Primary Progressive Aphasia With Underlying Progressive Supranuclear Palsy Pathology or Corticobasal Degeneration. JAMA Neurol. 2016;73(6):733–42. doi: 10.1001/jamaneurol.2016.0412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Spinelli EG, Mandelli ML, Miller ZA, et al. Typical and atypical pathology in primary progressive aphasia variants. Ann Neurol. 2017 doi: 10.1002/ana.24885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hassan A, Parisi JE, Josephs KA. Autopsy-proven progressive supranuclear palsy presenting as behavioral variant frontotemporal dementia. Neurocase. 2012;18(6):478–88. doi: 10.1080/13554794.2011.627345. [DOI] [PubMed] [Google Scholar]
- 23.Koga S, Josephs KA, Ogaki K, et al. Cerebellar ataxia in progressive supranuclear palsy: An autopsy study of PSP-C. Mov Disord. 2016;31(5):653–62. doi: 10.1002/mds.26499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kovacs GG, Milenkovic I, Wohrer A, et al. Non-Alzheimer neurodegenerative pathologies and their combinations are more frequent than commonly believed in the elderly brain: a community-based autopsy series. Acta neuropathologica. 2013;126(3):365–84. doi: 10.1007/s00401-013-1157-y. [DOI] [PubMed] [Google Scholar]
- 25.Ling H. Clinical Approach to Progressive Supranuclear Palsy. J Mov Disord. 2016;9(1):3–13. doi: 10.14802/jmd.15060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boxer AL, Garbutt S, Seeley WW, et al. Saccade abnormalities in autopsy-confirmed frontotemporal lobar degeneration and Alzheimer disease. Archives of neurology. 2012;69(4):509–17. doi: 10.1001/archneurol.2011.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Armstrong MJ, Litvan I, Lang AE, et al. Criteria for the diagnosis of corticobasal degeneration. Neurology. 2013;80(5):496–503. doi: 10.1212/WNL.0b013e31827f0fd1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gorno-Tempini ML, Hillis AE, Weintraub S, et al. Classification of primary progressive aphasia and its variants. Neurology. 2011;76(11):1006–14. doi: 10.1212/WNL.0b013e31821103e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rascovsky K, Hodges JR, Knopman D, et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain : a journal of neurology. 2011;134(Pt 9):2456–77. doi: 10.1093/brain/awr179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dugger BN, Adler CH, Shill HA, et al. Concomitant pathologies among a spectrum of parkinsonian disorders. Parkinsonism & related disorders. 2014;20(5):525–9. doi: 10.1016/j.parkreldis.2014.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dickson DW, Ahmed Z, Algom AA, Tsuboi Y, Josephs KA. Neuropathology of variants of progressive supranuclear palsy. Curr Opin Neurol. 2010;23(4):394–400. doi: 10.1097/WCO.0b013e32833be924. [DOI] [PubMed] [Google Scholar]
- 32.Ling H, de Silva R, Massey LA, et al. Characteristics of progressive supranuclear palsy presenting with corticobasal syndrome: a cortical variant. Neuropathology and applied neurobiology. 2014;40(2):149–63. doi: 10.1111/nan.12037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Clavaguera F, Akatsu H, Fraser G, et al. Brain homogenates from human tauopathies induce tau inclusions in mouse brain. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(23):9535–40. doi: 10.1073/pnas.1301175110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sanders DW, Kaufman SK, DeVos SL, et al. Distinct Tau Prion Strains Propagate in Cells and Mice and Define Different Tauopathies. Neuron. 2014;92(4):796–812. doi: 10.1016/j.neuron.2014.04.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gardner RC, Boxer AL, Trujillo A, et al. Intrinsic Connectivity Network Disruption in Progressive Supranuclear Palsy. Annals of Neurology. 2013;73:603–16. doi: 10.1002/ana.23844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Coppola G, Chinnathambi S, Lee JJ, et al. Evidence for a role of the rare p. A152T variant in MAPT in increasing the risk for FTD-spectrum and Alzheimer’s diseases. Hum Mol Genet. 2012;21(15):3500–12. doi: 10.1093/hmg/dds161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Donker Kaat L, Boon AJ, Azmani A, et al. Familial aggregation of parkinsonism in progressive supranuclear palsy. Neurology. 2009;73(2):98–105. doi: 10.1212/WNL.0b013e3181a92bcc. [DOI] [PubMed] [Google Scholar]
- 38.Fujioka S, Sanchez Contreras MY, Strongosky AJ, et al. Three sib-pairs of autopsy-confirmed progressive supranuclear palsy. Parkinsonism Relat Disord. 2015;21(2):101–5. doi: 10.1016/j.parkreldis.2014.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schoch KM, DeVos SL, Miller RL, et al. Increased 4R-Tau Induces Pathological Changes in a Human-Tau Mouse Model. Neuron. 2016;90(5):941–7. doi: 10.1016/j.neuron.2016.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhao Y, Tseng IC, Heyser CJ, et al. Appoptosin-Mediated Caspase Cleavage of Tau Contributes to Progressive Supranuclear Palsy Pathogenesis. Neuron. 2015;87(5):963–75. doi: 10.1016/j.neuron.2015.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bruch J, Kurz C, Vasiljevic A, Nicolino M, Arzberger T, Hoglinger GU. Early Neurodegeneration in the Brain of a Child Without Functional PKR-like Endoplasmic Reticulum Kinase. Journal of neuropathology and experimental neurology. 2015;74(8):850–7. doi: 10.1097/NEN.0000000000000224. [DOI] [PubMed] [Google Scholar]
- 42.Whitwell JL, Jack CR, Jr, Parisi JE, et al. Midbrain atrophy is not a biomarker of progressive supranuclear palsy pathology. European journal of neurology : the official journal of the European Federation of Neurological Societies. 2013;20(10):1417–22. doi: 10.1111/ene.12212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Massey LA, Micallef C, Paviour DC, et al. Conventional magnetic resonance imaging in confirmed progressive supranuclear palsy and multiple system atrophy. Mov Disord. 2012;27(14):1754–62. doi: 10.1002/mds.24968. [DOI] [PubMed] [Google Scholar]
- 44.Zhang Y, Walter R, Ng P, et al. Progression of Microstructural Degeneration in Progressive Supranuclear Palsy and Corticobasal Syndrome: A Longitudinal Diffusion Tensor Imaging Study. PLoS ONE. 2016;11(6):e0157218. doi: 10.1371/journal.pone.0157218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Morelli M, Arabia G, Novellino F, et al. MRI measurements predict PSP in unclassifiable parkinsonisms: a cohort study. Neurology. 2011;77(11):1042–7. doi: 10.1212/WNL.0b013e31822e55d0. [DOI] [PubMed] [Google Scholar]
- 46.Massey LA, Jager HR, Paviour DC, et al. The midbrain to pons ratio: a simple and specific MRI sign of progressive supranuclear palsy. Neurology. 2013;80(20):1856–61. doi: 10.1212/WNL.0b013e318292a2d2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Quattrone A, Morelli M, Williams DR, et al. MR parkinsonism index predicts vertical supranuclear gaze palsy in patients with PSP-parkinsonism. Neurology. 2016;87(12):1266–73. doi: 10.1212/WNL.0000000000003125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zalewski N, Botha H, Whitwell JL, Lowe V, Dickson DW, Josephs KA. FDG-PET in pathologically confirmed spontaneous 4R-tauopathy variants. Journal of neurology. 2014;261(4):710–6. doi: 10.1007/s00415-014-7256-4. [DOI] [PubMed] [Google Scholar]
- 49.Tang CC, Poston KL, Eckert T, et al. Differential diagnosis of parkinsonism: a metabolic imaging study using pattern analysis. Lancet Neurol. 2010;9(2):149–58. doi: 10.1016/S1474-4422(10)70002-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dani M, Brooks DJ, Edison P. Tau imaging in neurodegenerative diseases. Eur J Nucl Med Mol Imaging. 2016;43(6):1139–50. doi: 10.1007/s00259-015-3231-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Marquie M, Normandin MD, Vanderburg CR, et al. Validating novel tau positron emission tomography tracer [F-18]-AV-1451 (T807) on postmortem brain tissue. Ann Neurol. 2015;78(5):787–800. doi: 10.1002/ana.24517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cho H, Choi JY, Hwang MS, et al. Subcortical 18 F-AV-1451 binding patterns in progressive supranuclear palsy. Movement disorders : official journal of the Movement Disorder Society. 2017;32(1):134–40. doi: 10.1002/mds.26844. [DOI] [PubMed] [Google Scholar]
- 53.Whitwell JL, Lowe VJ, Tosakulwong N, et al. [18 F]AV-1451 tau positron emission tomography in progressive supranuclear palsy. Movement disorders : official journal of the Movement Disorder Society. 2017;32(1):124–33. doi: 10.1002/mds.26834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ono M, Sahara N, Kumata K, et al. Distinct binding of PET ligands PBB3 and AV-1451 to tau fibril strains in neurodegenerative tauopathies. Brain. 2017 doi: 10.1093/brain/aww339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Magdalinou NK, Paterson RW, Schott JM, et al. A panel of nine cerebrospinal fluid biomarkers may identify patients with atypical parkinsonian syndromes. J Neurol Neurosurg Psychiatry. 2015;86(11):1240–7. doi: 10.1136/jnnp-2014-309562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wagshal D, Sankaranarayanan S, Guss V, et al. Divergent CSF tau alterations in two common tauopathies: Alzheimer’s disease and progressive supranuclear palsy. J Neurol Neurosurg Psychiatry. 2015;86(3):244–50. doi: 10.1136/jnnp-2014-308004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Scherling CS, Hall T, Berisha F, et al. CSF neurofilament concentration reflects disease severity in frontotemporal degeneration. Annals of Neurology. 2014;75(1):116–26. doi: 10.1002/ana.24052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Boxer AL, Lang AE, Grossman M, et al. Davunetide in patients with progressive supranuclear palsy: a randomised, double-blind, placebo-controlled phase 2/3 trial. Lancet Neurol. 2014 doi: 10.1016/S1474-4422(14)70088-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hansson O, Janelidze S, Hall S, et al. Blood-based NfL: A biomarker for differential diagnosis of parkinsonian disorder. Neurology. 2017;88(10):930–7. doi: 10.1212/WNL.0000000000003680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rojas JC, Karydas A, Bang J, et al. Plasma neurofilament light chain predicts progression in progressive supranuclear palsy. Annals of Clinical and Translational Neurology. 2016;3(3):16–25. doi: 10.1002/acn3.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen AL, Riley DE, King SA, et al. The disturbance of gaze in progressive supranuclear palsy: implications for pathogenesis. Front Neurol. 2010;1:147. doi: 10.3389/fneur.2010.00147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schneider M, Muller HP, Lauda F, et al. Retinal single-layer analysis in Parkinsonian syndromes: an optical coherence tomography study. J Neural Transm. 2014;121(1):41–7. doi: 10.1007/s00702-013-1072-3. [DOI] [PubMed] [Google Scholar]
- 63.Clerici I, Ferrazzoli D, Maestri R, et al. Rehabilitation in progressive supranuclear palsy: Effectiveness of two multidisciplinary treatments. PLoS One. 2017;12(2):e0170927. doi: 10.1371/journal.pone.0170927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Apetauerova D, Scala SA, Hamill RW, et al. CoQ10 in progressive supranuclear palsy: A randomized, placebo-controlled, double-blind trial. Neurol Neuroimmunol Neuroinflamm. 2016;3(5):e266. doi: 10.1212/NXI.0000000000000266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Scelzo E, Lozano AM, Hamani C, et al. Peduncolopontine nucleus stimulation in progressive supranuclear palsy: a randomised trial. J Neurol Neurosurg Psychiatry. 2017 doi: 10.1136/jnnp-2016-315192. [DOI] [PubMed] [Google Scholar]
- 66.Tsai RM, Boxer AL. Clinical trials: past, current, and future for atypical Parkinsonian syndromes. Semin Neurol. 2014;34(2):225–34. doi: 10.1055/s-0034-1381739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bensimon G, Ludolph A, Agid Y, Vidailhet M, Payan C, Leigh PN. Riluzole treatment, survival and diagnostic criteria in Parkinson plus disorders: the NNIPPS study. Brain. 2009;132(Pt 1):156–71. doi: 10.1093/brain/awn291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tolosa E, Litvan I, Hoglinger GU, et al. A phase 2 trial of the GSK-3 inhibitor tideglusib in progressive supranuclear palsy. Mov Disord. 2014 doi: 10.1002/mds.25824. [DOI] [PubMed] [Google Scholar]
- 69.Khanna MR, Kovalevich J, Lee VM, Trojanowski JQ, Brunden KR. Therapeutic strategies for the treatment of tauopathies: Hopes and challenges. Alzheimer’s & dementia : the journal of the Alzheimer’s Association. 2016;12(10):1051–65. doi: 10.1016/j.jalz.2016.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yanamandra K, Kfoury N, Jiang H, et al. Anti-Tau Antibodies that Block Tau Aggregate Seeding In Vitro Markedly Decrease Pathology and Improve Cognition In Vivo. Neuron. 2013;80(2):402–14. doi: 10.1016/j.neuron.2013.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kondo A, Shahpasand K, Mannix R, et al. Antibody against early driver of neurodegeneration cis P-tau blocks brain injury and tauopathy. Nature. 2015;523(7561):431–6. doi: 10.1038/nature14658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pedersen JT, Sigurdsson EM. Tau immunotherapy for Alzheimer’s disease. Trends in molecular medicine. 2015;21(6):394–402. doi: 10.1016/j.molmed.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 73.Gauthier S, Feldman HH, Schneider LS, et al. Efficacy and safety of tau-aggregation inhibitor therapy in patients with mild or moderate Alzheimer’s disease: a randomised, controlled, double-blind, parallel-arm, phase 3 trial. Lancet. 2016;388(10062):2873–84. doi: 10.1016/S0140-6736(16)31275-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Min SW, Chen X, Tracy TE, et al. Critical role of acetylation in tau-mediated neurodegeneration and cognitive deficits. Nature medicine. 2015;21(10):1154–62. doi: 10.1038/nm.3951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yuzwa SA, Shan X, Macauley MS, et al. Increasing O-GlcNAc slows neurodegeneration and stabilizes tau against aggregation. Nature chemical biology. 2012;8(4):393–9. doi: 10.1038/nchembio.797. [DOI] [PubMed] [Google Scholar]
- 76.Vossel KA, Zhang K, Brodbeck J, et al. Tau reduction prevents Abeta-induced defects in axonal transport. Science. 2010;330(6001):198. doi: 10.1126/science.1194653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.DeVos SL, Miller RL, Schoch KM, et al. Tau reduction prevents neuronal loss and reverses pathological tau deposition and seeding in mice with tauopathy. Sci Transl Med. 2017;9(374) doi: 10.1126/scitranslmed.aag0481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Asai H, Ikezu S, Tsunoda S, et al. Depletion of microglia and inhibition of exosome synthesis halt tau propagation. Nat Neurosci. 2015;18(11):1584–93. doi: 10.1038/nn.4132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bhaskar K, Konerth M, Kokiko-Cochran ON, Cardona A, Ransohoff RM, Lamb BT. Regulation of tau pathology by the microglial fractalkine receptor. Neuron. 2010;68(1):19–31. doi: 10.1016/j.neuron.2010.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gentry EG, Henderson BW, Arrant AE, et al. Rho Kinase Inhibition as a Therapeutic for Progressive Supranuclear Palsy and Corticobasal Degeneration. J Neurosci. 2016;36(4):1316–23. doi: 10.1523/JNEUROSCI.2336-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hampton DW, Webber DJ, Bilican B, Goedert M, Spillantini MG, Chandran S. Cell-mediated neuroprotection in a mouse model of human tauopathy. J Neurosci. 2010;30(30):9973–83. doi: 10.1523/JNEUROSCI.0834-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Villeda SA, Plambeck KE, Middeldorp J, et al. Young blood reverses age-related impairments in cognitive function and synaptic plasticity in mice. Nature medicine. 2014;20(6):659–63. doi: 10.1038/nm.3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Josephs KA, Whitwell JL, Dickson DW, et al. Voxel-based morphometry in autopsy proven PSP and CBD. Neurobiol Aging. 2008;29(2):280–9. doi: 10.1016/j.neurobiolaging.2006.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yanamandra K, Jiang H, Mahan TE, et al. Anti-tau antibody reduces insoluble tau and decreases brain atrophy. Ann Clin Transl Neurol. 2015;2(3):278–88. doi: 10.1002/acn3.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dutt S, Binney RJ, Heuer HW, et al. Progression of brain atrophy in PSP and CBS over 6 months and 1 year. Neurology. 2016 doi: 10.1212/WNL.0000000000003305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Agosta F, Pievani M, Svetel M, et al. Diffusion tensor MRI contributes to differentiate Richardson’s syndrome from PSP-parkinsonism. Neurobiol Aging. 2012;33(12):2817–26. doi: 10.1016/j.neurobiolaging.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 87.Planetta PJ, Ofori E, Pasternak O, et al. Free-water imaging in Parkinson’s disease and atypical parkinsonism. Brain. 2016;139(Pt 2):495–508. doi: 10.1093/brain/awv361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Burciu RG, Chung JW, Shukla P, et al. Functional MRI of disease progression in Parkinson disease and atypical parkinsonian syndromes. Neurology. 2016;87(7):709–17. doi: 10.1212/WNL.0000000000002985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Smith R, Schain M, Nilsson C, et al. Increased basal ganglia binding of 18 F-AV-1451 in patients with progressive supranuclear palsy. Movement disorders : official journal of the Movement Disorder Society. 2017;32(1):108–14. doi: 10.1002/mds.26813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hughes LE, Rowe JB, Ghosh BC, Carlyon RP, Plack CJ, Gockel HE. The binaural masking level difference: cortical correlates persist despite severe brain stem atrophy in progressive supranuclear palsy. J Neurophysiol. 2014;112(12):3086–94. doi: 10.1152/jn.00062.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hall S, Ohrfelt A, Constantinescu R, et al. Accuracy of a panel of 5 cerebrospinal fluid biomarkers in the differential diagnosis of patients with dementia and/or parkinsonian disorders. Archives of neurology. 2012;69(11):1445–52. doi: 10.1001/archneurol.2012.1654. [DOI] [PubMed] [Google Scholar]
- 92.Bacioglu M, Maia LF, Preische O, et al. Neurofilament Light Chain in Blood and CSF as Marker of Disease Progression in Mouse Models and in Neurodegenerative Diseases. Neuron. 2016;91(1):56–66. doi: 10.1016/j.neuron.2016.05.018. [DOI] [PubMed] [Google Scholar]
- 93.Bologna M, Agostino R, Gregori B, et al. Voluntary, spontaneous and reflex blinking in patients with clinically probable progressive supranuclear palsy. Brain : a journal of neurology. 2009;132(Pt 2):502–10. doi: 10.1093/brain/awn317. [DOI] [PubMed] [Google Scholar]
- 94.Walsh CM, Ruoff L, Varbel J, et al. Rest-activity rhythm disruption in progressive supranuclear palsy. Sleep medicine. 2016;22:50–6. doi: 10.1016/j.sleep.2016.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ling H, Massey LA, Lees AJ, Brown P, Day BL. Hypokinesia without decrement distinguishes progressive supranuclear palsy from Parkinson’s disease. Brain : a journal of neurology. 2012;135(Pt 4):1141–53. doi: 10.1093/brain/aws038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bright J, Hussain S, Dang V, et al. Human secreted tau increases amyloid-beta production. Neurobiology of aging. 2015;36(2):693–709. doi: 10.1016/j.neurobiolaging.2014.09.007. [DOI] [PubMed] [Google Scholar]