Abstract
Atrial fibrillation is the most common sustained arrhythmia and its prevalence is rapidly rising with the aging of the population. Cardiac alternans, defined as cyclic beat-to-beat alternations in contraction force, action potential (AP) duration and intracellular Ca2+ release at constant stimulation rate, has been associated with the development of ventricular arrhythmias. Recent clinical data also provide strong evidence that alternans plays a central role in arrhythmogenesis in atria. The aim of this article is to review the mechanisms that are responsible for repolarization alternans and contribute to the transition from spatially concordant alternans to the more arrhythmogenic spatially discordant alternans in atria.
Keywords: Alternans, Atria, Arrhythmias, Action potential, Calcium signaling
1. Introduction
Atrial fibrillation (AF), the most common cardiac arrhythmia, currently affects 1–2% of the population, and over the next several decades the prevalence of AF is expected to reach unprecedented levels as the population of developed countries ages. AF is associated with increased risk of stroke, cardiomyopathies as well as heart failure, and accounts for significant morbidity and mortality [1,2].
Several mechanisms of AF have been described. Now it is well recognized that both arrhythmogenic triggers and an appropriate substrate are required for the initiation and perpetuation of AF [3–5]. It has been suggested that action potential (AP) repolarization alternans that are observed to precede AF episodes, plays a major role in generation of proarrhythmic substrate and facilitates re-entry phenomena that ultimately lead to sustained AF [3,6–14]. At the cellular level cardiac alternans is defined as cyclic, beat-to-beat alternations in contraction force, AP duration and intracellular Ca2+ release at constant stimulation rate.
Most of our understanding of the mechanisms and the role of alternans stems from studies of ventricular tissue. While sharing many similarities, ventricular and atrial tissues bare distinct characteristics of excitation-contraction coupling (ECC) and intracellular Ca2+ regulation which also suggests differences in alternans generation and regulation. This review focuses on the mechanisms of atrial alternans, its clinical relevance for the initiation of AF, and highlights differences between atrial and ventricular alternans.
2. Putative mechanisms of alternans
Initially cardiac alternans was described as mechanical [15] and electrical alternans [16] at the whole heart level. Later alternans was also observed in isolated cardiomyocytes suggesting that the origin of this phenomenon resides at the cellular level. The strong spatial and temporal correlation between AP and Ca2+ alternans at both whole heart and single cell levels is well established (Fig. 1), [17,18] and it is generally agreed that the bi-directional relationship between cytosolic Ca2+ concentration ([Ca2+]i) and membrane potential (Vm) plays a key role in the generation of alternans. Bi-directional coupling of Vm and [Ca2+]i (Vm↔[Ca2+]i) is defined by the facts that (1) Vm directly determines the activity of Ca2+ handling mechanisms that are voltage-dependent (Vm→[Ca2+]i coupling), whereas (2) [Ca2+]i dynamics affect Vm regulation through Ca2+-dependent ion currents and transporters ([Ca2+]i→Vm). Whether disturbances of Vm or [Ca2+]i regulation is the predominant mechanism of alternans remains an ongoing matter of debate. Theoretical and computational studies have supported both hypotheses [19–24]. However, due to the complex nature of the bi-directional coupling between Vm dynamics and intracellular Ca2+ handling and the many feedback pathways between the two parameters, the experimental distinction between effects of Ca2+ and Vm is difficult and therefore the mechanisms of alternans still remains incompletely understood, especially in atrial tissue.
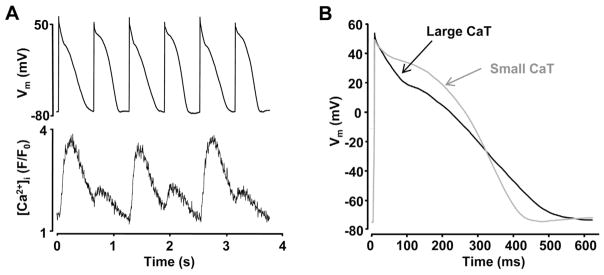
Fig. 1.
AP and Ca2+ alternans occur simultaneously.
(A) Simultaneously recorded APs and Ca2+ transients in current-clamped atrial myocytes. (B) Superimposed AP traces recorded during large (black) and small (gray) amplitude Ca2+ transients.
Figure modified with permission from [17].
2.1. Vm as key mechanism for the development of alternans
A possible contribution of Vm→[Ca2+]i coupling to alternans is well supported by computational studies [19,21,25–29]. Nolasco and Dahlen [25] suggested that at high stimulation rates beat-to-beat Vm alternation is determined by AP duration (APD) restitution and is an underlying cause for the development of alternans. APD restitution refers to the APD dependence on the preceding diastolic interval. The slope of the APD restitution curve is determined by the recovery of ion channels from inactivation and their dependence on Vm. Computational [21,25,26,28,29] as well as several experimental studies [30,31] have suggested that self-sustaining oscillations of APD can occur if this relationship is steep enough, and that the role of Vm as a causative factor of alternans becomes more prominent with increasing pacing rates [28,32]. However, simulation results still differ and remain dependent on the computational models applied, even if they are designed to recreate processes of the same tissue [33]. Contrary to computational results, numerous experimental studies could not confirm these theoretical findings and actually show a poor relationship between experimentally determined APD restitution kinetics and inducibility of alternans [34–38]. While it was shown that in ventricular myocytes AP kinetics affect sarcoplasmic reticulum (SR) Ca2+ release [39,40], activity of the electrogenic Na+/Ca2+ exchanger (NCX) [41–43] and L-type Ca2+ channels (LCC) [42,44–46], experimental evidence for the intricate details of how Vm→ [Ca2+]i coupling modulates the occurrence of alternans is still lacking. Such discrepancy in theoretical and experimental findings can be explained, at least to some extent, by the fact that because of the slow recovery of ion channels and gradual change in intracellular ionic concentrations, cardiac myocytes exhibit a “memory” of the preceding stimulation conditions and thus APD is not determined solely by the preceding diastolic interval, and therefore constitute more complex APD dynamics than are simulated by most computational models (discussed in detail in [47,48]). Due to “cell memory” real cardiac tissue APD restitution curves depend greatly on the conditions under which they have been recorded such as basal pacing frequency and rate of APD adaptation to the change in pacing rate [34,48]. Also, electrical restitution is determined by inactivation and recovery thereof of different ion channels (Na+, LCCs, slow rectifier K+ channels and others), each of which has individual properties and kinetics and consequently experimentally measured restitution curves rarely follow a simple mathematical function [48]. Furthermore, APD restitution curves may also be poor predictors of alternans occurrence because AP morphology is strongly influenced by intracellular Ca2+ dynamics [18,49,50], and Ca2+ alternans can be initiated independently of Vm alternans [17,51] (see discussion below). While the question whether Vm→ [Ca2+]i or [Ca2+]i→Vm coupling plays a leading role in the initiation of alternans is still debatable, there is little doubts that Vm→ [Ca2+]i coupling is critical for development and sustainability of atrial alternans. A recent study by Kanaporis and Blatter [52] in voltage-clamped rabbit atrial myocytes using AP waveforms observed during Ca2+ alternans (APCaT_Large observed during large systolic Ca2+ transient (CaT) and APCaT_Small observed with small CaT) demonstrated that both threshold for induction of Ca2+ alternans and degree of Ca2+ alternans are strongly modulated by the morphology of the AP (Fig. 2A) [52]. This study also demonstrated that AP morphology affected Ca alternans by two mechanisms: (1) by determining SR Ca2+ content (Fig. 2B) and (2) by regulating kinetics of L-type Ca2+ current (Fig. 2C) [52].
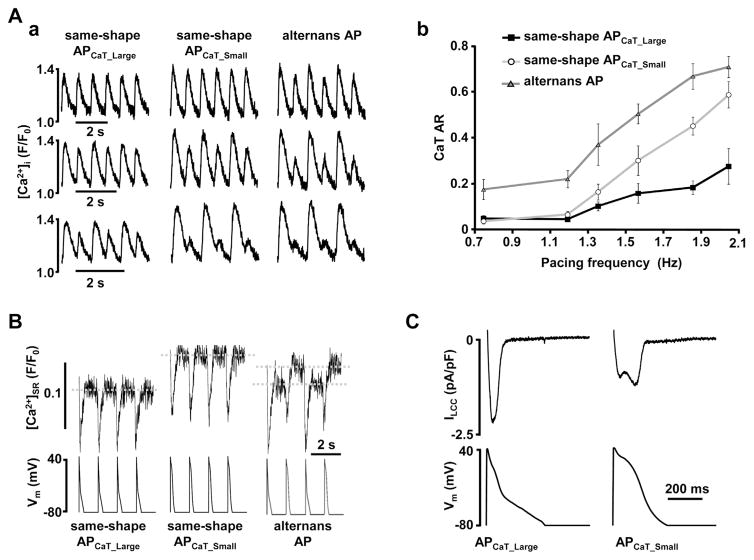
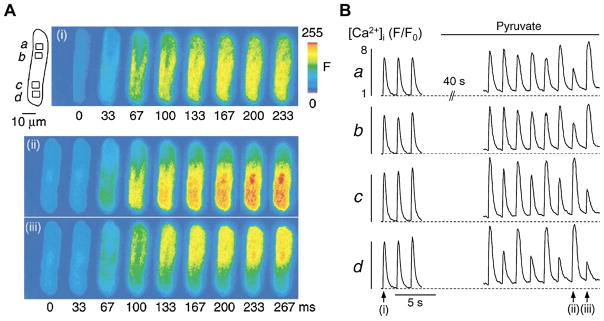
Fig. 2.
Membrane potential determines calcium alternans through modulation of sarcoplasmic reticulum Ca2+ load and L-type Ca2 + current.
Two distinct AP-like voltage commands were generated from prerecorded atrial APs observed during Ca2+ alternans: APCaT_Large (observed during large systolic Ca2+ release) and APCaT_Small (observed during small CaTs). Morphology of these voltage commands is shown on the bottom of panel C. Sequences with only APCaT_Large, only APCaT_Small or alternating AP waveforms (as shown in B bottom) were applied to rabbit atrial myocytes. (A) (a) CaTs elicited in the same voltage-clamped atrial myocyte stimulated with different sequences of AP waveforms at various pacing frequencies. (b) Pacing with same- shape APCaT_Small waveforms (open circles) enhances degree of CaT alternans compared to APCaT_Large stimuli (black squares). CaT alternans ratio (AR, where 0 indicates conditions without alternans and 1 indicates a full skipping of Ca2+ release on every other beat) is further increased during alternans AP voltage clamp protocol (grey triangles). (B) Sarcoplasmic reticulum Ca2+ load ([Ca2+]SR) measurements with Fluo-5N from the same voltage-clamped atrial myocyte exposed to three different AP clamp protocols (bottom). End-diastolic [Ca2+]SR was higher during the same-shape APCaT_Small protocol compared to APCaT_Large and revealed [Ca2+]SR alternans during the alternans AP clamp protocol. (C) Representative traces of L-type Ca2+ currents elicited with APCaT_Large and APCaT_Small voltage commands from the same atrial myocyte.
Figure modified with permission from [52].
2.2. Intracellular Ca2+ cycling as an underlying mechanism of alternans
The inconsistent correlation between APD restitution properties and occurrence of alternans suggests that mechanisms other than Vm play a major role for the development of cardiac alternans. Indeed, there is growing evidence that alternans is initiated and sustained by disturbances of intracellular Ca2+ handling [17,50,51,53,54]. Major support for this hypothesis stems from the demonstration that Ca2+ alternans can be elicited in voltage-clamped ventricular myocytes where beat-to-beat Vm is kept constant. Recently we demonstrated that the same principle also holds for atrial myocytes (Fig. 3A) [17]. In addition, beat-to-beat alternation in AP morphology was abolished when intracellular Ca2+ release was blocked (Fig. 3B). These studies have provided strong evidence that AP alternations are not required for Ca2+ alternans to occur and thus instabilities of inherent Ca2+ handling properties of cardiac myocytes underlie cardiac alternans.
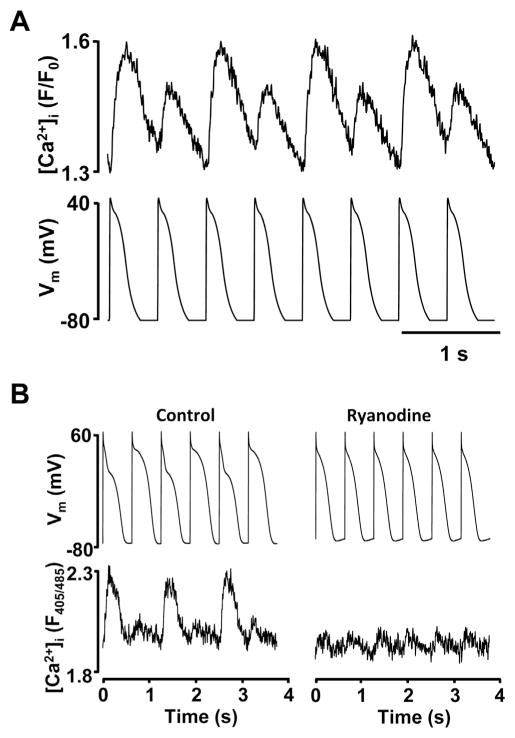
Fig. 3.
Disturbances in intracellular Ca2+ cycling as a key mechanism for the development of alternans.
(A) Ca2+ transient alternans recorded in voltage-clamped atrial myocytes under AP-clamp conditions when beat-to-beat Vm is kept constant. (B) Inhibition of cytosolic Ca2+ release by ryanodine abolishes AP alternans. APs and [Ca2+]i recorded simultaneously from a current-clamped atrial myocyte.
Panel B is from [17].
Several hypotheses on the mechanisms of Ca2+ alternans have been proposed. SR Ca2+ load hypothesis. Under steady-state conditions influx of Ca2+ through LCCs, SR Ca2+ release, Ca2+ uptake by sarco/endoplasmic reticulum Ca2+ ATP-ase (SERCA) and extrusion from the cell by NCX are well balanced and therefore there is little beat-to-beat variation in diastolic [Ca2+]SR. If the balance between Ca2+ uptake and release is disturbed beat-to-beat alternation in diastolic [Ca2+]SR can occur. Consequently, due to the steep SR load – Ca2+ release relationship [55], a higher SR load would lead to a larger Ca2+ release and vice versa. In support of this hypothesis beat-to-beat alternations in diastolic SR Ca2+ load in conjunction with Ca2+ alternans have been reported by several studies [53,56]. However, contrary to these observations, cytosolic Ca2+ alternans occurring without significant beat-to-beat oscillations in diastolic SR load in single myocytes [57–59] and intact heart [60] was also demonstrated. These findings suggest that alternation in diastolic [Ca2+]SR is not an obligatory condition for Ca2+ alternans to occur. Such observation was particularly common in atrial myocytes [57,59,61] and might be related to the higher activity of SERCA in the atrium [62–64] and therefore the higher capacity to refill the SR. L-type Ca2+ channel hypothesis. LCCs are activated by AP-dependent membrane depolarization and serve as the trigger for SR Ca2+ release in a process known as Ca2+-induced Ca2+ release (CICR). Importantly, the activity and inactivation of ILCC is controlled by both voltage and [Ca2+]i and these channels play a central role in the bi-directional coupling between Vm and intracellular Ca2+ dynamics. Therefore, incomplete recovery from inactivation of LCCs on a beat-to-beat basis has been proposed as a causative factor of Ca2+ alternans [56,65,66]. Partial inhibition of LCCs was indeed shown to increase susceptibility to Ca2+ alternans [53]. However numerous other studies have demonstrated that L-type Ca2+ currents can remain unchanged from beat to beat during Ca2+ alternans in both ventricular and atrial myocytes [17,51,53,57,59,67]. Refractoriness of ryanodine receptors (RyR) Ca2+ release hypothesis. Finally, refractoriness of the SR Ca2+ release was suggested as a possible mechanism responsible for Ca2+ alternans [59,60]. In this case, it is hypothesized that Ca2+ alternans can arise due to varying beat-to-beat recovery from inactivation of RyR Ca2+ release channels of the SR. Since the magnitude of an intracellular Ca2+ release, referred as Ca2+ transient (CaT), is dictated by the number of activated RyRs, it is suggested that during a large CaT a larger number of RyRs is activated and therefore at high pacing rates these channels become unavailable for subsequent release resulting in a smaller CaT. The study of Shkryl et al. [54] demonstrated that in rabbit atrial myocytes a large amplitude CaT indeed prolongs RyR refractoriness and that the kinetics of RyR recovery from inactivation is a key factor in the generation of Ca2+ alternans. This hypothesis also found support from in silico simulations [68].
3. Differences between atrial and ventricular alternans
To date, the mechanisms of alternans have been investigated primarily in ventricular tissue and to a much lesser extent in atria. While it can be anticipated that mechanisms of alternans in atrial and ventricular cells share similarities, recent experimental data point towards important differences. For example, we reported subtle differences in APD alternans in atrial and ventricular rabbit myocytes where atrial myocytes exhibited a higher degree of beat-to-beat alternation in APD and a higher pacing frequency threshold to induce alternans [17].
The atria have unique structural properties that affect development of atrial alternans. The atrium has a complex geometry and regional structural features, such as the atrial appendages, the pectinate muscle network and specialized tissues like the sinus node, the Bachmann’s bundle and crista terminalis, as well as multiple orifices for veins, arteries, and valves, which play an important role in AF initiation and maintenance [69]. An important difference between ventricular and atrial myocytes is also encountered at the single cell level. Atrial cells lack or have only a poorly or irregularly developed transversal tubule (t-tubule) system (Fig. 4A) [24,70,71], resulting in unique Ca2+ cycling features during ECC. T-tubules, deep invaginations of the sarcolemmal membrane, allows action potential penetration to the interior of the cell and ensures fast and uniform SR Ca2+ release in ventricular myocytes. In contrast, in atrial myocytes lacking t-tubules LCCs are restricted to the periphery of the cell and thus, membrane depolarization induced Ca2+ release first occurs in subsarcolemmal regions and subsequently propagates via CICR to the center of the cell (Fig. 4B) [72]. Computer simulations using cell models with and without t-tubules have predicted significant differences in possible alternans mechanisms [23,68,73]. The cardiac cell models lacking t-tubules exhibited higher likelihood to develop Ca2+ alternans and pointed towards the role of Ca2 + diffusion, inhomogeneity in [Ca2+]i [73] and RyR refractoriness [68] in the process. This is consistent with experimental observations of intracellular gradients of the degree of Ca2+ alternans and that Ca2+ alternans can be spatially and temporally inhomogeneous even at the level of a single atrial myocyte and, in the extreme cases, subcellular regions can even alternate out-of-phase (Fig. 5) [57,61,74]. Another difference in Ca2+ handling between atrial and ventricular cells is the lower expression of phospholamban that leads to higher SERCA activity in the atria [62–64]. Since beat-to-beat fluctuation in SR Ca2+ load was proposed as a possible cause of Ca2+ alternans [53,75], it is conceivable that the lower SERCA activity in ventricle may contribute to Ca2+ alternans at increased pacing frequencies due to incomplete filling of the SR, whereas a more rapid filling is consistent with the observation that in atrial cells end-diastolic [Ca2+]SR typically did not alternate during Ca2+ alternans [59,61]. This notion is also consistent with findings that upregulation of SERCA suppresses alternans in murine [76] and guinea pig [77] ventricular myocytes.
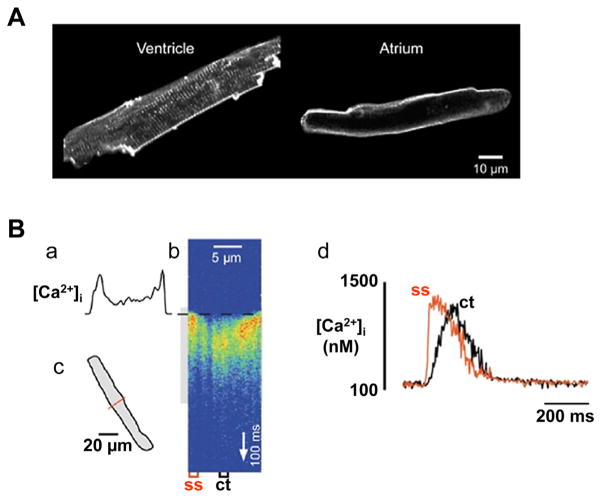
Fig. 4.
Ca2+ signaling during excitation-contraction coupling in atrial myocytes.
(A) Confocal images of a ventricular and an atrial myocyte from the same cat heart stained with the membrane-bound fluorescent dye Di-8-ANEPPS. The regular structures spaced in a sarcomeric pattern in the ventricular cell represent t-tubules. In contrast, the atrial myocyte is devoid of any t-tubular staining. (B) Ca2+ transient recorded in the confocal linescan mode. The scanned line was positioned perpendicular to the longitudinal axis of the cell (c). Electrical stimulation of the cell during acquisition of the linescan image triggered a ‘U’-shaped Ca2+ transient (b), indicating that [Ca2+]i increased first at the periphery of the cell (a) before propagating towards the center of the myocyte. Panel d shows local Ca2+ transients measured in the subsarcolemmal space (ss) and the center of the cell (ct).
The Figure is modified with permission from [72].
Fig. 5.
Neighboring regions within an atrial myocyte can alternate out-of-phase.
(A) Series of fluo-4 fluorescence images recorded under control conditions and during Ca2+ alternans. The images illustrate the rising phase of the Ca2+ transients marked by the arrows in (B). (B) Subcellular Ca2+ transients recorded from the regions marked by the boxes a–d (A). [Ca2+]i images and subcellular Ca2+ transients reveal that the time of onset, the magnitude, and the phase of Ca2+ alternans exhibit large subcellular variations and that the upper and the lower half of the cell alternate out-of-phase.
The Figure is modified with permission from [74].
Furthermore, atrial and ventricle cells contain unique sets of ion channels [78,79] leading to distinctive AP morphologies and Ca2+-dependent modulation of AP properties in these two cell types. For example, ventricle and atrium differ in activity of small-conductance Ca2+-activated K+ (SK) channels [80,81], Ca2+-activated Cl− channels [82], while acetylcholine-activated and ultrarapid rectifier K+ channels are expressed exclusively in the atria [83–85]. Recently we demonstrated that Ca2+-activated Cl− channels play a major role in sustaining APD alternans in rabbit atrial cells [86]. Our data indicated that while these channels might be also important in ventricular alternans [87], atrial myocytes exhibit a higher density of this current that could explain in part the significantly higher degree of beat-to-beat alternation in APD in atrial myocytes [17]. In addition, atrial tissue displays substantial regional heterogeneity in conduction velocity (CV) and AP morphology, ranging from a triangular shape with no sustained plateau to ventricular like APs [88]. This phenomenon results in significant regional differences in electrical restitution and local intrinsic differences in rate-adaptation [88].
Finally, under pathological conditions such as heart failure, ischemia or during the progression of AF, the electrophysiological and structural properties of the atrium become significantly remodeled. Remodeling includes changes in CV [89,90], AP morphology and heterogeneity, intracellular Ca2+ signaling [91], ion channel expression [92,93] and fibrosis [94]. All these changes are considered to be key factors that contribute to the development of sustained atrial arrhythmias.
4. Clinical relevance of cardiac alternans
To this date the majority of clinical data that relates cardiac alternans and arrhythmias was obtained in ventricle. The beat-to-beat alternations in the time course of ventricular AP repolarization are reflected in the ECG as T-wave alternans (TWA). Even subtle TWA at microvolt levels (referred to as microvolt TWA) was demonstrated as a valuable prognostic tool for ventricular arrhythmia risk stratification [95]. The clinical use of atrial AP repolarization alternans as a diagnostic tool, however, is hindered by the fact that the atrial repolarization signal is masked in the conventional ECG recordings by the ventricular QRS complex and therefore the clinical exploitation of the relationship between atrial alter-nans and the development of atrial arrhythmias for risk assessment has been limited. However, recently progress has been made in several experimental [12,90,96] and clinical studies [7,8,11,97,98] using monophasic AP electrodes to monitor atrial repolarization alternans in vivo. These and other studies involving computer simulations [99], animal models [12,90,96,100] and studies in humans [7,8,11,97,98] have provided convincing evidence that AP alternans in atria may lead directly to AF (Fig. 6) or its transition from atrial flutter. In addition, newer clinical studies have demonstrated that atrial repolarization alternans, similarly to ventricular TWA, can be used to predict vulnerability to AF [11,98]. For example, Narayan et al. [11] have demonstrated that APD alternans preceded AF episodes, while APD alternans was absent in subjects with no AF. Furthermore, in patients with persistent AF APD alternans was typically induced at relatively low pacing rates (100–120 bpm). In contrast, in control subjects alternans developed only at rapid pacing rates (>230 bpm) indicating that atrial repolarization alternans has potential as a prognostic tool to identify susceptibility to atrial arrhythmias. Similarly, Lalani et al. [98] established that AP alternans was larger and more prevalent in patients with persistent AF than in subjects with paroxysmal AF, while alternans was not observed in the control group. Taken together, evidence is accumulating that AP alternans in both ventricle and atrium precedes development of fibrillation, and thus, has prognostic value for arrhythmia prediction.
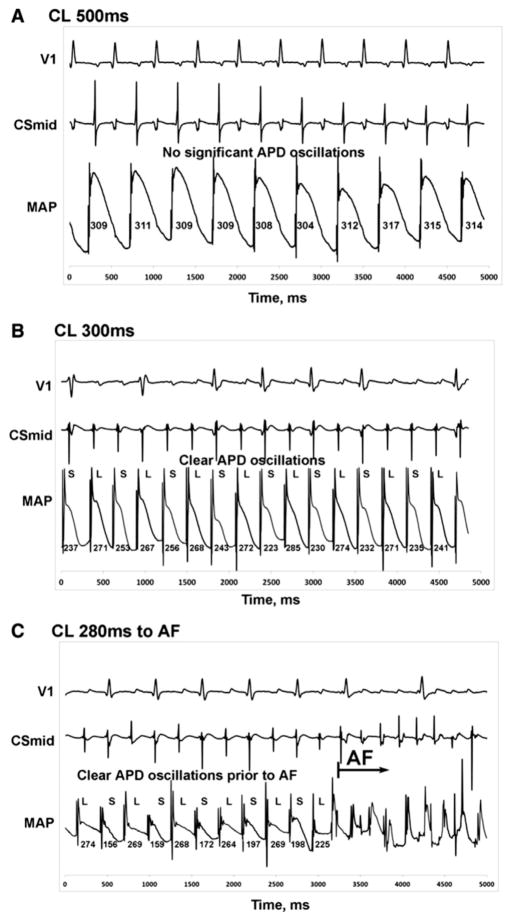
Fig. 6.
Atrial alternans precedes initiation of atrial fibrillation.
Rate dependence of AP alternans in a 61-year-old male patient with paroxysmal atrial fibrillation. (A) No significant atrial AP alternans is observed at baseline pacing with cycle length (CL) of 500 ms. (B) At CL 300 ms, APD alternans is observed. (C) APD alternans were detectable and preceded AF initiation while pacing at CL 280 ms. V1, first ECG precordial lead; CSmid, middle coronary sinus; MAP, monophasic action potential.
Figure is modified with permission from [98].
The mechanisms linking AP alternans to ventricular arrhythmias involve the development of spatially discordant alternans [101–104]. Usually AP alternans starts as concordant alternans, i.e. APD either prolongs or shortens simultaneously in all cells of a particular region of the ventricular myocardium. Concordant AP alternans itself may not exacerbate into fibrillation, i.e. its arrhythmogenic potential is relatively low. However, as development of alternans progresses, AP alternation can turn discordant where different regions of the heart alternate out-of-phase, i.e. in some regions APD is prolonged while during the same beat APD is shortened in other regions of the heart. Development of discordant alternans is believed to have major effects on the spatial organization of repolarization across the cardiac tissue and significantly contributes to arrhythmogenicity [101–104]. This notion is supported experimentally by the demonstration that ventricular fibrillation is always preceded by discordant AP alternans [102]. Similarly to findings in ventricle, discordant alternans was also observed in atria and has been directly linked to the development of atrial fibrillation [7,12,96]. The underlying mechanisms for the formation of spatially discordant alternans remain uncertain and most likely are determined by the combination of factors that are discussed below.
5. Mechanisms of spatially discordant alternans
Several mechanisms for discordant alternans have been suggested that revolve around Ca2+ cycling, cell-to-cell communication and electrical tissue properties.
5.1. Calcium cycling heterogeneity
As discussed above disturbances in intracellular Ca2+ handling have been proposed as an underlying mechanism of alternans at the cellular level [17,32,50,51,53,54,75]. Spatial heterogeneities in electrical as well as Ca2+ cycling properties of cardiac tissue between the endocardium and epicardium or between the base and apex of the heart are essential for maintaining normal function of the heart [105,106], including excitation spread and coordinated contractility. However, under pathological conditions, such heterogeneity in Ca2+ cyclingcan lead to the development of spatially discordant Ca2+ alternans. Furthermore, since [Ca2+]i dynamics feedback on ion conductances of myocytes and thus in turn affect APD, the heterogeneity in Ca2+ handling contributes to spatial differences in AP morphology as well. Additional support for Ca2+-dependent development of discordant alternans comes from computational models, demonstrating that discordant APD alternans can be induced if [Ca2+]i→Vm coupling results in APD shortening [107] or, alternatively, by SR Ca2+ accumulation during rapid pacing [108]. Furthermore, suppression of intracellular Ca2+ release from SR by ryanodine significantly reduces development of spatially discordant alternans during tachypacing induced cardiomyopathy in transgenic rabbits with Long QT type-1 syndrome [49]. The aforementioned insights come from ventricular tissue, however experimental evidence that heterogeneity in Ca2+ cycling underlies generation of discordant alternans also in atria is still lacking.
5.2. Insufficient cell-to-cell coupling
Cardiac myocytes are electrically coupled via gap junctions that allow the flow of ionic currents between cells. In the heart, different isoforms of gap junction forming proteins connexins exhibit regional expression. While in the ventricle electrical coupling between myocytes is achieved almost exclusively by connexin 43 (Cx43), in the atria three types of connexins are expressed – Cx40, Cx43 and Cx45 (with levels of Cx45 being very low). A strong coupling between myocytes results in well-coordinated and relatively homogeneous repolarization of the cardiac tissue. However, in various pathological situations decreased gap junction expression and/or increased inhomogeneity of gap junction distribution in both ventricle and atrium occurs [109]. In humans with AF and in AF animal models altered expression and distribution of atrial Cx40 is well documented which can lead to dispersed conduction and thus formation of a substrate for atrial arrhythmias [93,109]. Furthermore, pharmacological enhancement of intercellular coupling effectively suppressed development of discordant alternans, and decreased susceptibility to ventricular arrhythmias [101] and atrial fibrillation [110]. In addition to the changes in connexin expression, cell-to-cell coupling can be disrupted also by increased fibrosis of the tissue. While insufficient coupling between cells is likely to contribute to increased susceptibility to arrhythmias in remodeled myocardium, it can hardly explain development of discordant alternans in relatively healthy hearts with normal intercellular coupling. Also, it is noteworthy that cardiomyocytes have a large safety margin with respect to cell-to-cell coupling, i.e. the myocardium maintains near normal conduction velocities even when electrical coupling is significantly reduced [111]. Therefore, it is unlikely that moderate reduction in gap junction protein levels alone is sufficient for induction of cardiac arrhythmias. However, decreased intercellular coupling may amplify cell-to-cell differences in AP morphology, conduction velocity and Ca2+ cycling which in turn facilitates the development of spatially discordant alternans and arrhythmic events.
5.3. Conduction velocity and APD restitution
Computer modeling studies predict that impairment of CV or APD restitution can result in discordant alternans [22,47]. CV restitution refers to the relationship between CV and the preceding diastolic interval. In cardiac tissue CV is determined by intercellular electric coupling and the activity and kinetics of Na+ channels which drive the upstroke of cardiac APs. Under normal conditions recovery of Na+ channels from inactivation is fast and thus slowing of CV is observed only at very fast beating rates, however slowing of CV was demonstrated in several pathological conditions including AF [89,90]. Data addressing the relationship between CV and APD restitution heterogeneities and development of alternans in atria are scarce. Heterogeneous and slower CV together with prolongated and spatially dispersed APDs were demonstrated in atria of diabetic rats and these changes were associated with the increased inducibility of APD alternans and susceptibility to atrial tachyarrhythmias [112]. Also, the spatial dispersion of APD restitution was significantly increased in a canine vagally mediated AF model and in rapid pacing-induced AF [100]. Similarly, a greater spatial dispersion of APD restitution kinetics was reported in patients with chronic AF compared to patients with paroxysmal AF and control subjects [10].
6. Conclusions
While numerous clinical and animal research data obtained in ventricular tissue have greatly contributed to the understanding of putative mechanisms of alternans and their relation to ventricular arrhythmias, alternans in atrial tissue remains much less investigated. The availability of clinical data on atrial alternans is hindered by the fact that measurements of atrial repolarization require invasive methods as atrial repolarization is masked by electrical activity of ventricles in the conventional ECGs. The available clinical data, however, suggest that alternans plays a vital role in AF. In this review we summarized the current knowledge about mechanisms of alternans and their relevance to arrhythmogenesis in atria.
Acknowledgments
This work was supported by National Institutes of Health grants (HL057832, HL080101, HL101235, HL132871) and the Fondation Leducq (to L.A.B.). G.K. was supported by American Heart Association grant 16GRNT30130011.
Footnotes
Conflict of interest
The authors state no conflict of interest.
References
- 1.Chugh SS, Havmoeller R, Narayanan K, Singh D, Rienstra M, Benjamin EJ, et al. Worldwide epidemiology of atrial fibrillation: a Global Burden of Disease 2010 Study. Circulation. 2014;129:837–47. doi: 10.1161/CIRCULATIONAHA.113.005119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chinitz JS, Vaishnava P, Narayan RL, Fuster V. Atrial fibrillation through the years: contemporary evaluation and management. Circulation. 2013;127:408–16. doi: 10.1161/CIRCULATIONAHA.112.120758. [DOI] [PubMed] [Google Scholar]
- 3.Comtois P, Nattel S. Atrial repolarization alternans as a path to atrial fibrillation. J Cardiovasc Electrophysiol. 2012;23:1013–5. doi: 10.1111/j.1540-8167.2012.02391.x. [DOI] [PubMed] [Google Scholar]
- 4.Berenfeld O, Jalife J. Mechanisms of atrial fibrillation: rotors, ionic determinants, and excitation frequency. Cardiol Clin. 2014;32:495–506. doi: 10.1016/j.ccl.2014.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kottkamp H. Human atrial fibrillation substrate: towards a specific fibrotic atrial cardiomyopathy. Eur Heart J. 2013;34:2731–8. doi: 10.1093/eurheartj/eht194. [DOI] [PubMed] [Google Scholar]
- 6.Franz MR, Jamal SM, Narayan SM. The role of action potential alternans in the initiation of atrial fibrillation in humans: a review and future directions. Europace. 2012;14(Suppl 5):v58–64. doi: 10.1093/europace/eus273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hiromoto K, Shimizu H, Furukawa Y, Kanemori T, Mine T, Masuyama T, et al. Discordant repolarization alternans-induced atrial fibrillation is suppressed by verapamil. Circ J. 2005;69:1368–73. doi: 10.1253/circj.69.1368. [DOI] [PubMed] [Google Scholar]
- 8.Narayan SM, Bode F, Karasik PL, Franz MR. Alternans of atrial action potentials during atrial flutter as a precursor to atrial fibrillation. Circulation. 2002;106:1968–73. doi: 10.1161/01.cir.0000037062.35762.b4. [DOI] [PubMed] [Google Scholar]
- 9.Coyne KS, Paramore C, Grandy S, Mercader M, Reynolds M, Zimetbaum P. Assessing the direct costs of treating nonvalvular atrial fibrillation in the United States. Value Health. 2006;9:348–56. doi: 10.1111/j.1524-4733.2006.00124.x. [DOI] [PubMed] [Google Scholar]
- 10.Kim BS, Kim YH, Hwang GS, Pak HN, Lee SC, Shim WJ, et al. Action potential duration restitution kinetics in human atrial fibrillation. J Am Coll Cardiol. 2002;39:1329–36. doi: 10.1016/s0735-1097(02)01760-6. [DOI] [PubMed] [Google Scholar]
- 11.Narayan SM, Franz MR, Clopton P, Pruvot EJ, Krummen DE. Repolarization alternans reveals vulnerability to human atrial fibrillation. Circulation. 2011;123:2922–30. doi: 10.1161/CIRCULATIONAHA.110.977827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jousset F, Tenkorang J, Vesin JM, Pascale P, Ruchat P, Rollin AG, et al. Kinetics of atrial repolarization alternans in a free-behaving ovine model. J Cardiovasc Electrophysiol. 2012;23:1003–12. doi: 10.1111/j.1540-8167.2012.02336.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gaeta SA, Christini DJ. Non-linear dynamics of cardiac alternans: subcellular to tissue-level mechanisms of arrhythmia. Front Physiol. 2012;3:157. doi: 10.3389/fphys.2012.00157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Myles RC, Burton FL, Cobbe SM, Smith GL. The link between repolarisation alternans and ventricular arrhythmia: does the cellular phenomenon extend to the clinical problem? J Mol Cell Cardiol. 2008;45:1–10. doi: 10.1016/j.yjmcc.2008.03.024. [DOI] [PubMed] [Google Scholar]
- 15.Traube L. Ein fall von pulsus bigeminus nebst bemerkungen tiber die lebershwellungen bei klappenfehlern und uber acute leberatrophic. Ber Klin Wschr. 1872:185. [Google Scholar]
- 16.Lewis T. Notes upon alternation of the heart. Quart J Med. 1911:141–4. [Google Scholar]
- 17.Kanaporis G, Blatter LA. The mechanisms of calcium cycling and action potential dynamics in cardiac alternans. Circ Res. 2015;116:846–56. doi: 10.1161/CIRCRESAHA.116.305404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pruvot EJ, Katra RP, Rosenbaum DS, Laurita KR. Role of calcium cycling versus restitution in the mechanism of repolarization alternans. Circ Res. 2004;94:1083–90. doi: 10.1161/01.RES.0000125629.72053.95. [DOI] [PubMed] [Google Scholar]
- 19.Watanabe MA, Fenton FH, Evans SJ, Hastings HM, Karma A. Mechanisms for discordant alternans. J Cardiovasc Electrophysiol. 2001;12:196–206. doi: 10.1046/j.1540-8167.2001.00196.x. [DOI] [PubMed] [Google Scholar]
- 20.Shiferaw Y, Sato D, Karma A. Coupled dynamics of voltage and calcium in paced cardiac cells. Phys Rev E Stat Nonlin Soft Matter Phys. 2005;71:021903. doi: 10.1103/PhysRevE.71.021903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Watanabe MA, Koller ML. Mathematical analysis of dynamics of cardiac memory and accommodation: theory and experiment. Am J Physiol Heart Circ Physiol. 2002;282:H1534–47. doi: 10.1152/ajpheart.00351.2001. [DOI] [PubMed] [Google Scholar]
- 22.Qu Z, Garfinkel A, Chen PS, Weiss JN. Mechanisms of discordant alternans and induction of reentry in simulated cardiac tissue. Circulation. 2000;102:1664–70. doi: 10.1161/01.cir.102.14.1664. [DOI] [PubMed] [Google Scholar]
- 23.Tao T, O’Neill SC, Diaz ME, Li YT, Eisner DA, Zhang H. Alternans of cardiac calcium cycling in a cluster of ryanodine receptors: a simulation study. Am J Physiol Heart Circ Physiol. 2008;295:H598–609. doi: 10.1152/ajpheart.01086.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pawlak Cieslik A, Szturmowicz M, Fijalkowska A, Gatarek J, Gralec R, Blasinska-Przerwa K, et al. Diagnosis of malignant pericarditis: a single centre experience. Kardiol Pol. 2012;70:1147–53. [PubMed] [Google Scholar]
- 25.Nolasco JB, Dahlen RW. A graphic method for the study of alternation in cardiac action potentials. J Appl Physiol. 1968;25:191–6. doi: 10.1152/jappl.1968.25.2.191. [DOI] [PubMed] [Google Scholar]
- 26.Tolkacheva EG, Romeo MM, Guerraty M, Gauthier DJ. Condition for alternans and its control in a two-dimensional mapping model of paced cardiac dynamics. Phys Rev E Stat Nonlin Soft Matter Phys. 2004;69:031904. doi: 10.1103/PhysRevE.69.031904. [DOI] [PubMed] [Google Scholar]
- 27.Shiferaw Y, Karma A. Turing instability mediated by voltage and calcium diffusion in paced cardiac cells. Proc Natl Acad Sci U S A. 2006;103:5670–5. doi: 10.1073/pnas.0511061103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jordan PN, Christini DJ. Characterizing the contribution of voltage- and calcium-dependent coupling to action potential stability: implications for repolarization alternans. Am J Physiol Heart Circ Physiol. 2007;293:H2109–18. doi: 10.1152/ajpheart.00609.2007. [DOI] [PubMed] [Google Scholar]
- 29.Jordan PN, Christini DJ. Action potential morphology influences intracellular calcium handling stability and the occurrence of alternans. Biophys J. 2006;90:672–80. doi: 10.1529/biophysj.105.071340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koller ML, Maier SK, Gelzer AR, Bauer WR, Meesmann M, Gilmour RF., Jr Altered dynamics of action potential restitution and alternans in humans with structural heart disease. Circulation. 2005;112:1542–8. doi: 10.1161/CIRCULATIONAHA.104.502831. [DOI] [PubMed] [Google Scholar]
- 31.Tolkacheva EG, Anumonwo JM, Jalife J. Action potential duration restitution portraits of mammalian ventricular myocytes: role of calcium current. Biophys J. 2006;91:2735–45. doi: 10.1529/biophysj.106.083865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bayer JD, Narayan SM, Lalani GG, Trayanova NA. Rate-dependent action potential alternans in human heart failure implicates abnormal intracellular calcium handling. Heart Rhythm. 2010;7:1093–101. doi: 10.1016/j.hrthm.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cherry EM, Hastings HM, Evans SJ. Dynamics of human atrial cell models: restitution, memory, and intracellular calcium dynamics in single cells. Prog Biophys Mol Biol. 2008;98:24–37. doi: 10.1016/j.pbiomolbio.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kalb SS, Dobrovolny HM, Tolkacheva EG, Idriss SF, Krassowska W, Gauthier DJ. The restitution portrait: a new method for investigating rate-dependent restitution. J Cardiovasc Electrophysiol. 2004;15:698–709. doi: 10.1046/j.1540-8167.2004.03550.x. [DOI] [PubMed] [Google Scholar]
- 35.Pruvot EJ, Katra RP, Rosenbaum DS, Laurita KR. Role of calcium cycling versus restitution in the mechanism of repolarization alternans. Circ Res. 2004;94:1083–90. doi: 10.1161/01.RES.0000125629.72053.95. [DOI] [PubMed] [Google Scholar]
- 36.Saitoh H, Bailey JC, Surawicz B. Alternans of action potential duration after abrupt shortening of cycle length: differences between dog Purkinje and ventricular muscle fibers. Circ Res. 1988;62:1027–40. doi: 10.1161/01.res.62.5.1027. [DOI] [PubMed] [Google Scholar]
- 37.Banville I, Gray RA. Effect of action potential duration and conduction velocity restitution and their spatial dispersion on alternans and the stability of arrhythmias. J Cardiovasc Electrophysiol. 2002;13:1141–9. doi: 10.1046/j.1540-8167.2002.01141.x. [DOI] [PubMed] [Google Scholar]
- 38.Wu R, Patwardhan A. Mechanism of repolarization alternans has restitution of action potential duration dependent and independent components. J Cardiovasc Electrophysiol. 2006;17:87–93. doi: 10.1111/j.1540-8167.2005.00319.x. [DOI] [PubMed] [Google Scholar]
- 39.Sah R, Ramirez RJ, Kaprielian R, Backx PH. Alterations in action potential profile enhance excitation-contraction coupling in rat cardiac myocytes. J Physiol. 2001;533:201–14. doi: 10.1111/j.1469-7793.2001.0201b.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bassani RA, Altamirano J, Puglisi JL, Bers DM. Action potential duration determines sarcoplasmic reticulum Ca2+ reloading in mammalian ventricular myocytes. J Physiol. 2004;559:593–609. doi: 10.1113/jphysiol.2004.067959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Livshitz LM, Rudy Y. Regulation of Ca2+ and electrical alternans in cardiac myocytes: role of CAMKII and repolarizing currents. Am J Physiol Heart Circ Physiol. 2007;292:H2854–66. doi: 10.1152/ajpheart.01347.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wan X, Cutler M, Song Z, Karma A, Matsuda T, Baba A, et al. New experimental evidence for mechanism of arrhythmogenic membrane potential alternans based on balance of electrogenic I(NCX)/I(Ca) currents. Heart Rhythm. 2012;9:1698–705. doi: 10.1016/j.hrthm.2012.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weber CR, Piacentino V, 3rd, Ginsburg KS, Houser SR, Bers DM. Na+-Ca2+ exchange current and submembrane [Ca2+] during the cardiac action potential. Circ Res. 2002;90:182–9. doi: 10.1161/hh0202.103940. [DOI] [PubMed] [Google Scholar]
- 44.Guo D, Zhao X, Wu Y, Liu T, Kowey PR, Yan GX. L-type calcium current reactivation contributes to arrhythmogenesis associated with action potential triangulation. J Cardiovasc Electrophysiol. 2007;18:196–203. doi: 10.1111/j.1540-8167.2006.00698.x. [DOI] [PubMed] [Google Scholar]
- 45.Clark RB, Bouchard RA, Giles WR. Action potential duration modulates calcium influx, Na+-Ca2+ exchange, and intracellular calcium release in rat ventricular myocytes. Ann N Y Acad Sci. 1996;779:417–29. doi: 10.1111/j.1749-6632.1996.tb44817.x. [DOI] [PubMed] [Google Scholar]
- 46.Linz KW, Meyer R. Profile and kinetics of L-type calcium current during the cardiac ventricular action potential compared in guinea-pigs, rats and rabbits. Pflugers Arch. 2000;439:588–99. doi: 10.1007/s004249900212. [DOI] [PubMed] [Google Scholar]
- 47.Qu Z, Xie Y, Garfinkel A, Weiss JN. T-wave alternans and arrhythmogenesis in cardiac diseases. Front Physiol. 2010;1:154. doi: 10.3389/fphys.2010.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Franz MR. The electrical restitution curve revisited: steep or flat slope – which is better? J Cardiovasc Electrophysiol. 2003;14:S140–7. doi: 10.1046/j.1540.8167.90303.x. [DOI] [PubMed] [Google Scholar]
- 49.Lau E, Kossidas K, Kim TY, Kunitomo Y, Ziv O, Song Z, et al. spatially discordant alternans and arrhythmias in tachypacing-induced cardiac myopathy in transgenic LQT1 rabbits: the importance of IKs and Ca2+ cycling. PLoS One. 2015;10:e0122754. doi: 10.1371/journal.pone.0122754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Goldhaber JI, Xie LH, Duong T, Motter C, Khuu K, Weiss JN. Action potential duration restitution and alternans in rabbit ventricular myocytes: the key role of intracellular calcium cycling. Circ Res. 2005;96:459–66. doi: 10.1161/01.RES.0000156891.66893.83. [DOI] [PubMed] [Google Scholar]
- 51.Chudin E, Goldhaber J, Garfinkel A, Weiss J, Kogan B. Intracellular Ca2+ dynamics and the stability of ventricular tachycardia. Biophys J. 1999;77:2930–41. doi: 10.1016/S0006-3495(99)77126-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kanaporis G, Blatter LA. Membrane potential determines calcium alternans through modulation of SR Ca2+ load and L-type Ca2+ current. J Mol Cell Cardiol. 2017;105:49–58. doi: 10.1016/j.yjmcc.2017.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Diaz ME, O’Neill SC, Eisner DA. Sarcoplasmic reticulum calcium content fluctuation is the key to cardiac alternans. Circ Res. 2004;94:650–6. doi: 10.1161/01.RES.0000119923.64774.72. [DOI] [PubMed] [Google Scholar]
- 54.Shkryl VM, Maxwell JT, Domeier TL, Blatter LA. Refractoriness of sarcoplasmic reticulum Ca2+ release determines Ca2+ alternans in atrial myocytes. Am J Physiol Heart Circ Physiol. 2012;302:H2310–2. doi: 10.1152/ajpheart.00079.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shannon TR, Ginsburg KS, Bers DM. Potentiation of fractional sarcoplasmic reticulum calcium release by total and free intra-sarcoplasmic reticulum calcium concentration. Biophys J. 2000;78:334–43. doi: 10.1016/S0006-3495(00)76596-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li Y, Diaz ME, Eisner DA, O’Neill S. The effects of membrane potential, SR Ca2+ content and RyR responsiveness on systolic Ca2+ alternans in rat ventricular myocytes. J Physiol. 2009;587:1283–92. doi: 10.1113/jphysiol.2008.164368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Huser J, Wang YG, Sheehan KA, Cifuentes F, Lipsius SL, Blatter LA. Functional coupling between glycolysis and excitation-contraction coupling underlies alternans in cat heart cells. J Physiol. 2000;524(Pt 3):795–806. doi: 10.1111/j.1469-7793.2000.00795.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Picht E, DeSantiago J, Blatter LA, Bers DM. Cardiac alternans do not rely on diastolic sarcoplasmic reticulum calcium content fluctuations. Circ Res. 2006;99:740–8. doi: 10.1161/01.RES.0000244002.88813.91. [DOI] [PubMed] [Google Scholar]
- 59.Shkryl VM, Maxwell JT, Domeier TL, Blatter LA. Refractoriness of sarcoplasmic reticulum Ca release determines Ca alternans in atrial myocytes. Am J Physiol Heart Circ Physiol. 2012;302:H2310–2. doi: 10.1152/ajpheart.00079.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang L, Myles RC, De Jesus NM, Ohlendorf AK, Bers DM, Ripplinger CM. Optical mapping of sarcoplasmic reticulum Ca2+ in the intact heart: ryanodine receptor refractoriness during alternans and fibrillation. Circ Res. 2014;114:1410–21. doi: 10.1161/CIRCRESAHA.114.302505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Edwards JN, Blatter LA. Cardiac alternans and intracellular calcium cycling. Clin Exp Pharmacol Physiol. 2014;41:524–32. doi: 10.1111/1440-1681.12231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Boknik P, Unkel C, Kirchhefer U, Kleideiter U, Klein-Wiele O, Knapp J, et al. Regional expression of phospholamban in the human heart. Cardiovasc Res. 1999;43:67–76. doi: 10.1016/s0008-6363(99)00053-x. [DOI] [PubMed] [Google Scholar]
- 63.Vangheluwe P, Schuermans M, Zador E, Waelkens E, Raeymaekers L, Wuytack F. Sarcolipin and phospholamban mRNA and protein expression in cardiac and skeletal muscle of different species. Biochem J. 2005;389:151–9. doi: 10.1042/BJ20050068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Luss I, Boknik P, Jones LR, Kirchhefer U, Knapp J, Linck B, et al. Expression of cardiac calcium regulatory proteins in atrium v ventricle in different species. J Mol Cell Cardiol. 1999;31:1299–314. doi: 10.1006/jmcc.1999.0962. [DOI] [PubMed] [Google Scholar]
- 65.Fox JJ, McHarg JL, Gilmour RF., Jr Ionic mechanism of electrical alternans. Am J Physiol Heart Circ Physiol. 2002;282:H516–30. doi: 10.1152/ajpheart.00612.2001. [DOI] [PubMed] [Google Scholar]
- 66.Shiferaw Y, Watanabe MA, Garfinkel A, Weiss JN, Karma A. Model of intracellular calcium cycling in ventricular myocytes. Biophys J. 2003;85:3666–86. doi: 10.1016/S0006-3495(03)74784-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wan X, Laurita KR, Pruvot EJ, Rosenbaum DS. Molecular correlates of repolarization alternans in cardiac myocytes. J Mol Cell Cardiol. 2005;39:419–28. doi: 10.1016/j.yjmcc.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 68.Lugo CA, Cantalapiedra IR, Penaranda A, Hove-Madsen L, Echebarria B. Are SR Ca content fluctuations or SR refractoriness the key to atrial cardiac alternans?: insights from a human atrial model. Am J Physiol Heart Circ Physiol. 2014;306:H1540–52. doi: 10.1152/ajpheart.00515.2013. [DOI] [PubMed] [Google Scholar]
- 69.Ellis WS, SippensGroenewegen A, Auslander DM, Lesh MD. The role of the crista terminalis in atrial flutter and fibrillation: a computer modeling study. Ann Biomed Eng. 2000;28:742–54. doi: 10.1114/1.1289456. [DOI] [PubMed] [Google Scholar]
- 70.Bootman MD, Smyrnias I, Thul R, Coombes S, Roderick HL. Atrial cardiomyocyte calcium signalling. Biochim Biophys Acta. 2011;1813:922–34. doi: 10.1016/j.bbamcr.2011.01.030. [DOI] [PubMed] [Google Scholar]
- 71.Sheehan KA, Zima AV, Blatter LA. Regional differences in spontaneous Ca2+ spark activity and regulation in cat atrial myocytes. J Physiol. 2006;572:799–809. doi: 10.1113/jphysiol.2005.103267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Blatter LA, Kockskamper J, Sheehan KA, Zima AV, Huser J, Lipsius SL. Local calcium gradients during excitation–contraction coupling and alternans in atrial myocytes. J Physiol. 2003;546:19–31. doi: 10.1113/jphysiol.2002.025239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Li Q, O’Neill SC, Tao T, Li Y, Eisner D, Zhang H. Mechanisms by which cytoplasmic calcium wave propagation and alternans are generated in cardiac atrial myocytes lacking T-tubules-insights from a simulation study. Biophys J. 2012;102:1471–82. doi: 10.1016/j.bpj.2012.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kockskamper J, Blatter LA. Subcellular Ca2+ alternans represents a novel mechanism for the generation of arrhythmogenic Ca2+ waves in cat atrial myocytes. J Physiol. 2002;545:65–79. doi: 10.1113/jphysiol.2002.025502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Eisner DA, Li Y, O’Neill SC. Alternans of intracellular calcium: mechanism and significance. Heart Rhythm. 2006;3:743–5. doi: 10.1016/j.hrthm.2005.12.020. [DOI] [PubMed] [Google Scholar]
- 76.Stary V, Puppala D, Scherrer-Crosbie M, Dillmann WH, Armoundas AA. SERCA2a upregulation ameliorates cellular alternans induced by metabolic inhibition. J Appl Physiol (1985) 2016;120:865–75. doi: 10.1152/japplphysiol.00588.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cutler MJ, Wan X, Laurita KR, Hajjar RJ, Rosenbaum DS. Targeted SERCA2a gene expression identifies molecular mechanism and therapeutic target for arrhythmogenic cardiac alternans. Circ Arrhythm Electrophysiol. 2009;2:686–94. doi: 10.1161/CIRCEP.109.863118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ordog B, Brutyo E, Puskas LG, Papp JG, Varro A, Szabad J, et al. Gene expression profiling of human cardiac potassium and sodium channels. Int J Cardiol. 2006;111:386–93. doi: 10.1016/j.ijcard.2005.07.063. [DOI] [PubMed] [Google Scholar]
- 79.Wang Z, Yue L, White M, Pelletier G, Nattel S. Differential distribution of inward rectifier potassium channel transcripts in human atrium versus ventricle. Circulation. 1998;98:2422–8. doi: 10.1161/01.cir.98.22.2422. [DOI] [PubMed] [Google Scholar]
- 80.Tuteja D, Xu D, Timofeyev V, Lu L, Sharma D, Zhang Z, et al. Differential expression of small-conductance Ca2+-activated K+ channels SK1, SK2, and SK3 in mouse atrial and ventricular myocytes. Am J Physiol Heart Circ Physiol. 2005;289:H2714–23. doi: 10.1152/ajpheart.00534.2005. [DOI] [PubMed] [Google Scholar]
- 81.Hsueh CH, Chang PC, Hsieh YC, Reher T, Chen PS, Lin SF. Proarrhythmic effect of blocking the small conductance calcium activated potassium channel in isolated canine left atrium. Heart Rhythm. 2013;10:891–8. doi: 10.1016/j.hrthm.2013.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Szigeti G, Rusznak Z, Kovacs L, Papp Z. Calcium-activated transient membrane currents are carried mainly by chloride ions in isolated atrial, ventricular and Purkinje cells of rabbit heart. Exp Physiol. 1998;83:137–53. doi: 10.1113/expphysiol.1998.sp004097. [DOI] [PubMed] [Google Scholar]
- 83.Dobrzynski H, Marples DD, Musa H, Yamanushi TT, Henderson Z, Takagishi Y, et al. Distribution of the muscarinic K+ channel proteins Kir3.1 and Kir3. 4 in the ventricle, atrium, and sinoatrial node of heart. J Histochem Cytochem. 2001;49:1221–34. doi: 10.1177/002215540104901004. [DOI] [PubMed] [Google Scholar]
- 84.Bingen BO, Neshati Z, Askar SF, Kazbanov IV, Ypey DL, Panfilov AV, et al. Atrium-specific Kir3.x determines inducibility, dynamics, and termination of fibrillation by regulating restitution-driven alternans. Circulation. 2013;128:2732–44. doi: 10.1161/CIRCULATIONAHA.113.005019. [DOI] [PubMed] [Google Scholar]
- 85.Ravens U, Wettwer E. Ultra-rapid delayed rectifier channels: molecular basis and therapeutic implications. Cardiovasc Res. 2011;89:776–85. doi: 10.1093/cvr/cvq398. [DOI] [PubMed] [Google Scholar]
- 86.Kanaporis G, Blatter LA. Calcium-activated chloride current determines action potential morphology during calcium alternans in atrial myocytes. J Physiol. 2016;594:699–714. doi: 10.1113/JP271887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kanaporis G, Blatter LA. Ca2+-activated chloride channel activity during Ca2+ alternans in ventricular myocytes. Channels (Austin) 2016;10:507–17. doi: 10.1080/19336950.2016.1207020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Al Abed A, Lovell NH, Dokos S. Local heterogeneous electrical restitution properties of rabbit atria. J Cardiovasc Electrophysiol. 2016;27:743–53. doi: 10.1111/jce.12968. [DOI] [PubMed] [Google Scholar]
- 89.Hori Y, Nakahara S, Anjo N, Nakagawa A, Nishiyama N, Yamada K, et al. Investigation of the atrial conduction time measured by tissue Doppler imaging at the left atrial appendage and the actual electrical conduction time: consideration of left atrial remodeling in atrial fibrillation patients. J Interv Card Electrophysiol. 2016 doi: 10.1007/s10840-016-0185-7. [DOI] [PubMed] [Google Scholar]
- 90.Monigatti-Tenkorang J, Jousset F, Pascale P, Vesin JM, Ruchat P, Fromer M, et al. Intermittent atrial tachycardia promotes repolarization alternans and conduction slowing during rapid rates, and increases susceptibility to atrial fibrillation in a free-behaving sheep model. J Cardiovasc Electrophysiol. 2014;25:418–27. doi: 10.1111/jce.12353. [DOI] [PubMed] [Google Scholar]
- 91.Greiser M, Schotten U. Dynamic remodeling of intracellular Ca2+ signaling during atrial fibrillation. J Mol Cell Cardiol. 2013;58:134–42. doi: 10.1016/j.yjmcc.2012.12.020. [DOI] [PubMed] [Google Scholar]
- 92.Gaborit N, Steenman M, Lamirault G, Le Meur N, Le Bouter S, Lande G, et al. Human atrial ion channel and transporter subunit gene-expression remodeling associated with valvular heart disease and atrial fibrillation. Circulation. 2005;112:471–81. doi: 10.1161/CIRCULATIONAHA.104.506857. [DOI] [PubMed] [Google Scholar]
- 93.Gemel J, Levy AE, Simon AR, Bennett KB, Ai X, Akhter S, et al. Connexin40 abnormalities and atrial fibrillation in the human heart. J Mol Cell Cardiol. 2014;76:159–68. doi: 10.1016/j.yjmcc.2014.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Boldt A, Wetzel U, Lauschke J, Weigl J, Gummert J, Hindricks G, et al. Fibrosis in left atrial tissue of patients with atrial fibrillation with and without underlying mitral valve disease. Heart. 2004;90:400–5. doi: 10.1136/hrt.2003.015347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Merchant FM, Sayadi O, Moazzami K, Puppala D, Armoundas AA. T-wave alternans as an arrhythmic risk stratifier: state of the art. Curr Cardiol Rep. 2013;15:398. doi: 10.1007/s11886-013-0398-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Verrier RL, Fuller H, Justo F, Nearing BD, Rajamani S, Belardinelli L. Unmasking atrial repolarization to assess alternans, spatiotemporal heterogeneity, and susceptibility to atrial fibrillation. Heart Rhythm. 2016;13:953–61. doi: 10.1016/j.hrthm.2015.11.019. [DOI] [PubMed] [Google Scholar]
- 97.Narayan SM, Wright M, Derval N, Jadidi A, Forclaz A, Nault I, et al. Classifying fractionated electrograms in human atrial fibrillation using monophasic action potentials and activation mapping: evidence for localized drivers, rate acceleration, and nonlocal signal etiologies. Heart Rhythm. 2011;8:244–53. doi: 10.1016/j.hrthm.2010.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lalani GG, Schricker AA, Clopton P, Krummen DE, Narayan SM. Frequency analysis of atrial action potential alternans: a sensitive clinical index of individual propensity to atrial fibrillation. Circ Arrhythm Electrophysiol. 2013;6:859–67. doi: 10.1161/CIRCEP.113.000204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gong Y, Xie F, Stein KM, Garfinkel A, Culianu CA, Lerman BB, et al. Mechanism underlying initiation of paroxysmal atrial flutter/atrial fibrillation by ectopic foci: a simulation study. Circulation. 2007;115:2094–102. doi: 10.1161/CIRCULATIONAHA.106.656504. [DOI] [PubMed] [Google Scholar]
- 100.Lu Z, Cui B, He B, Hu X, Wu W, Wu L, et al. Distinct restitution properties in vagally mediated atrial fibrillation and six-hour rapid pacing-induced atrial fibrillation. Cardiovasc Res. 2011;89:834–42. doi: 10.1093/cvr/cvq334. [DOI] [PubMed] [Google Scholar]
- 101.Hsieh YC, Lin JC, Hung CY, Li CH, Lin SF, Yeh HI, et al. Gap junction modifier rotigaptide decreases the susceptibility to ventricular arrhythmia by enhancing conduction velocity and suppressing discordant alternans during therapeutic hypothermia in isolated rabbit hearts. Heart Rhythm. 2016;13:251–61. doi: 10.1016/j.hrthm.2015.07.023. [DOI] [PubMed] [Google Scholar]
- 102.Wilson LD, Jeyaraj D, Wan X, Hoeker GS, Said TH, Gittinger M, et al. Heart failure enhances susceptibility to arrhythmogenic cardiac alternans. Heart Rhythm. 2009;6:251–9. doi: 10.1016/j.hrthm.2008.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gelzer AR, Koller ML, Otani NF, Fox JJ, Enyeart MW, Hooker GJ, et al. Dynamic mechanism for initiation of ventricular fibrillation in vivo. Circulation. 2008;118:1123–9. doi: 10.1161/CIRCULATIONAHA.107.738013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wilson LD, Rosenbaum DS. Mechanisms of arrythmogenic cardiac alternans. Europace. 2007;9(Suppl 6):vi77–82. doi: 10.1093/europace/eum210. [DOI] [PubMed] [Google Scholar]
- 105.Katra RP, Pruvot E, Laurita KR. Intracellular calcium handling heterogeneities in intact guinea pig hearts. Am J Physiol Heart Circ Physiol. 2004;286:H648–56. doi: 10.1152/ajpheart.00374.2003. [DOI] [PubMed] [Google Scholar]
- 106.Laurita KR, Katra R, Wible B, Wan X, Koo MH. Transmural heterogeneity of calcium handling in canine. Circ Res. 2003;92:668–75. doi: 10.1161/01.RES.0000062468.25308.27. [DOI] [PubMed] [Google Scholar]
- 107.Sato D, Shiferaw Y, Garfinkel A, Weiss JN, Qu Z, Karma A. Spatially discordant alternans in cardiac tissue: role of calcium cycling. Circ Res. 2006;99:520–7. doi: 10.1161/01.RES.0000240542.03986.e7. [DOI] [PubMed] [Google Scholar]
- 108.Sato D, Bers DM, Shiferaw Y. Formation of spatially discordant alternans due to fluctuations and diffusion of calcium. PLoS One. 2013;8:e85365. doi: 10.1371/journal.pone.0085365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Severs NJ, Bruce AF, Dupont E, Rothery S. Remodelling of gap junctions and connexin expression in diseased myocardium. Cardiovasc Res. 2008;80:9–19. doi: 10.1093/cvr/cvn133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Guerra JM, Everett THt, Lee KW, Wilson E, Olgin JE. Effects of the gap junction modifier rotigaptide (ZP123) on atrial conduction and vulnerability to atrial fibrillation. Circulation. 2006;114:110–8. doi: 10.1161/CIRCULATIONAHA.105.606251. [DOI] [PubMed] [Google Scholar]
- 111.van Rijen HV, Eckardt D, Degen J, Theis M, Ott T, Willecke K, et al. Slow conduction and enhanced anisotropy increase the propensity for ventricular tachyarrhythmias in adult mice with induced deletion of connexin43. Circulation. 2004;109:1048–55. doi: 10.1161/01.CIR.0000117402.70689.75. [DOI] [PubMed] [Google Scholar]
- 112.Watanabe M, Yokoshiki H, Mitsuyama H, Mizukami K, Ono T, Tsutsui H. Conduction and refractory disorders in the diabetic atrium. Am J Physiol Heart Circ Physiol. 2012;303:H86–95. doi: 10.1152/ajpheart.00010.2012. [DOI] [PubMed] [Google Scholar]