Abstract
Genetic and sociodemographic risk factors potentially associated with cannabis use (CU) were investigated in 40 cannabis users and 96 control subjects. DNA methylation analyses were also performed to explore the possibility of epigenetic changes related to CU. We conducted a candidate gene association study that included variants involved in the dopaminergic (ANKK1, NCAM1 genes) and endocannabinoid (CNR1, CNR2 gene) pathways. Sociodemographic data included gender, marital status, level of education, and body mass index. We used MeDIP-qPCR to test whether variations in DNA methylation might be associated with CU. We found a significant association between SNP rs1049353 of CNR1 gene (p = 0.01) and CU. Differences were also observed related to rs2501431 of CNR2 gene (p = 0.058). A higher education level appears to decrease the risk of CU. Interestingly, females were less likely to use cannabis than males. There was a significantly higher level of DNA methylation in cannabis users compared to controls in two of the genes tested: hypermethylation at exon 8 of DRD2 gene (p = 0.034) and at the CpG-rich region in the NCAM1 gene (p = 0.0004). Both genetic variants and educational attainment were also related to CU. The higher rate of DNA methylation, evidenced among cannabis users, may be either a marker of CU or a consequence of long-term exposure to cannabis. The identified genetic variants and the differentially methylated regions may represent biomarkers and/or potential targets for designs of pharmacological therapeutic agents. Our observations also suggest that educational programs may be useful strategies for CU prevention.
Introduction
Marijuana is the most common drug used illicitly throughout the world. Approximately 9% of those exposed to cannabis become addicted (cannabis use disorder, DSM-5). The number increases to 16% when cannabis use (CU) is initiated during adolescence and to 50% when cannabis is used daily1. Because of the gateway hypothesis of early CU2 and because of potential negative effects of the drug3, genetic risk factors for CU have been investigated using a diversity of methods. Twin studies have suggested that genetic markers may explain about 50% of the variance in CU disorders4–7. Association studies have also provided evidence of genetic risks that include differential changes at Taq1A allele (rs1800497, ANKK1) that influences the function of dopamine receptors8. In addition, changes at rs1049353, rs806380, rs2180619, rs806379, rs12720071, rs2023239 and the haplotype rs6454674-rs806380-rs806377-rs1049353 in the cannabinoid receptor 1 gene (CNR1) and at rs2501431 in the cannabinoid receptor 2 gene (CNR2) were found to be associated with working memory dysfunction, enhanced impulsivity, neurocognitive impairments, anxiety disorder, and depression in cannabis users9–13.
Interestingly, genome-wide association studies of cannabis users and dependent individuals have also identified several genetic variants that are known to play a role in neurogenesis and dopaminergic neurotransmission. These include ANKFN1, RP11-206 M11.7, SLC35G1, CSMD1, NCAM1, CADM2, SCOC and KCNT214–16. Whole genome sequencing approaches have also documented variations involved in the regulation of MEF2Band PCCB genes expression17. Moreover, a gene cluster located on chromosome 17q24 [c17orf58, BPTF, PPM1D] was previously reported to be associated with cannabis use disorders (CUD) diagnosed by DSM-5 18.
Several investigators have sought to identify environmental factors and clarify how their interactions with inherited familial risk conditions and related gene variants might impact susceptibility to cannabis abuse. Traumatic and negative life events, including poor parental care, reduced family bonding, childhood maltreatment, physical and sexual abuse, exposure to community violence, low level of school engagement, as well as school drop-outs appear to play important roles in the initiation and persistence of CU19–24. Nevertheless, the mechanisms of interactions between these factors and their relationships to CU remain to be clarified.
The picture is rendered more complicated by epigenetic factors that might also trigger the initiation and/or maintenance of CUD25–30. Specifically, modifications in DNA methylation have been reported in cannabis users, in tetrahydrocannabinol (THC)-dependent subjects, and in a rat model of prenatal THC exposure to CB1 and CB225,29. These included DNA modifications in catechol-O-methyltransferase (COMT) gene26 and in PACSIN1, a kinase involved in neuron morphogenesis and neurodevelopmental processes30. Interestingly, a significant relationship between DNA sequence variants and DNA methylation has also been established26,31,32.
Despite the reported association between genetic risks and CU, more remains to be done to further clarify the role that these genetic changes might play in diverse populations. Therefore, the major aim of our study was to identify risk factors that can trigger or exacerbate the clinical course of CU. Towards that end, we sought to investigate the potential role of gene variants affecting dopamine and cannabinoid receptors function, SNP Taq1A (ANKK1) and the SNP rs1049353 (CNR1) in CU. Second, we also studied interactions of these variants with sociodemographic measures including body mass index (BMI), gender, marital status, and levels of education. Third, we sought to discover if changes in DNA methylation might also play a role in CU.
Materials and methods
Subjects
A total of 136 subjects (Table 1), 40 cannabis users (30 males and 10 females) and 96 control subjects (38 males and 58 females), aged 18–60 years, were selected among samples collected and stored previously at the Intramural Research Program (IRP) of the National Institute of Drug Abuse (NIDA), project #12-DA-N472, NIDA, IRP (Health Outcomes by Neighborhood (HON)—Baltimore). These subjects were recruited among people who live in Baltimore City or one of the surrounding areas. Based on frequency of use, the cannabis participants were moderate to heavy users33, with no other drug use or alcohol abuse (total n = 40; moderate, n = 20 and heavy, n = 20). Ninety-six (96) unrelated healthy individuals who live in the same geographic area and who have never smoked cannabis served as control participants. The study was approved by the NIDA Addiction IRB. Written informed consent was obtained from all participants. The subjects were reimbursed for their time.
Table 1.
Socio-demographic data of samples collected (96 CTRLs subjects and 40 MJ users)
| Controls (n = 96) | MJ users (n = 40) | ||||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Gender | |||||
| Male | 38 | 39.58% | 30 | 75.00% | |
| Female | 58 | 60.42% | 10 | 25.00% | |
| Marital status | |||||
| Not married | 84 | 87.50% | 38 | 95.00% | |
| Married | 12 | 12.50% | 2 | 5.00% | |
| Level of education | |||||
| 1 = some high school/GED | 12 | 12.50% | 12 | 30.00% | |
| 2 = H.S. diploma | 24 | 25.00% | 10 | 25.00% | |
| 3 = some college | 33 | 34.38% | 16 | 40.00% | |
| 4 = college graduate / Masters / Ph.D. | 27 | 28.13% | 2 | 5.00% | |
| BMI | |||||
| <25 | 36 | 39.56% | 18 | 48.65% | |
| ≥25 | 55 | 60.44% | 19 | 51.35% | |
| Ethnicity | |||||
| African American | 63 | 66.32% | 34 | 87.18% | |
| Asian | 2 | 2.11% | 0 | 0.00% | |
| European American | 24 | 25.26% | 3 | 7.69% | |
| More than one race | 5 | 5.26% | 2 | 5.13% | |
| Native Hawaiian or other Pacific | 1 | 1.05% | 0 | 0.00% | |
| Missing | 1 | 1 | |||
Exclusion criteria included inability to sign informed consent and age <18 years old. Participants in the present study were excluded for other illicit drugs or alcohol. In addition to self-report, the use or abuse of other illicit substances was ruled out by obtaining observed urines that were negative for methamphetamine, 3,4-methylenedioxymethamphetamine, benzodiazepines, cocaine metabolites, methadone, oxycodone, phencyclidine, buprenorphine, and morphine. The participants were screened for prevalence of anxiety disorder (measured by Brief Anxiety Scale (BAS)) and PTSD disorder (measured by PTSD Checklist-Civilian Version (PCL-C)). Only four participants were affected by possible co-occurring anxiety and PTSD disorder. The number was too low to allow another statistical analysis.
Design
The study follows the workflow showed in Fig. 1.
Fig. 1.

Workflow diagram
Sociodemographic measures
Sociodemographic data were collected for each subject (see Table 1). A semi-structured questionnaire was utilized to collect socio-demographic data concerning gender, marital status, educational attainment, BMI, and ethnicity.
Sample collection and DNA extraction
Peripheral whole blood was previously collected from participants via venipuncture and 5 mL, maintained at −80 °C, were used in the present study (see Supplementary material for preparation of blood samples). DNA extraction was carried out using the QIAamp DNA Blood Midi/Maxi Kit (Spin Protocol, Qiagen). Aliquots of the genomic DNA extracted were used for both genotyping and DNA methylation analysis.
Genotyping
The polymorphisms related to the genes listed in Table 2A were genotyped in cannabis users and controls. Ten nanograms of genomic DNA were processed with TaqMan Genotyping Assays (Thermo Fisher) for the identification of allelic variants (see Supplementary material for detailed procedure).
Table 2A.
List of candidate genes and analyzed polymorphisms
| Gene | SNP | DNA sequence variation | Position | Functional consequence | Global MAF |
|---|---|---|---|---|---|
| ANKK1 | rs1800497 | C/T (REV) | 11:113400106 | missense: Glu ⇒ Lys | A = 0.3257/1631 |
| CNR1 | rs1049353 | A/G (REV) | 6:88143916 | synonymous codon: Thr ⇒ Thr | T = 0.1294/648 |
| CNR1 | rs2180619 | A/G (FWD) | 6:88168233 | upstream variant 2KB | G = 0.4685/2346 |
| CNR1 | rs806379 | A/T (FWD) | 6:88151548 | intron variant, upstream variant 2KB | T = 0.3952/1979 |
| CNR1 | rs6454674 | G/T (FWD) | 6:88163211 | intron variant | G = 0.3141/1573 |
| CNR1 | rs12720071 | A/G (REV) | 6:88141462 | UTR variant 3’ | C = 0.0899/450 |
| CNR1 | rs2023239 | C/T (FWD) | 6:88150763 | intron variant, upstream variant 2KB | C = 0.1779/891 |
| CNR2 | rs2501431 | A/G (FWD) | 1:23875153 | synonymous codon: Gly ⇒ Gly | G = 0.3466/1736 |
DNA methylation analysis
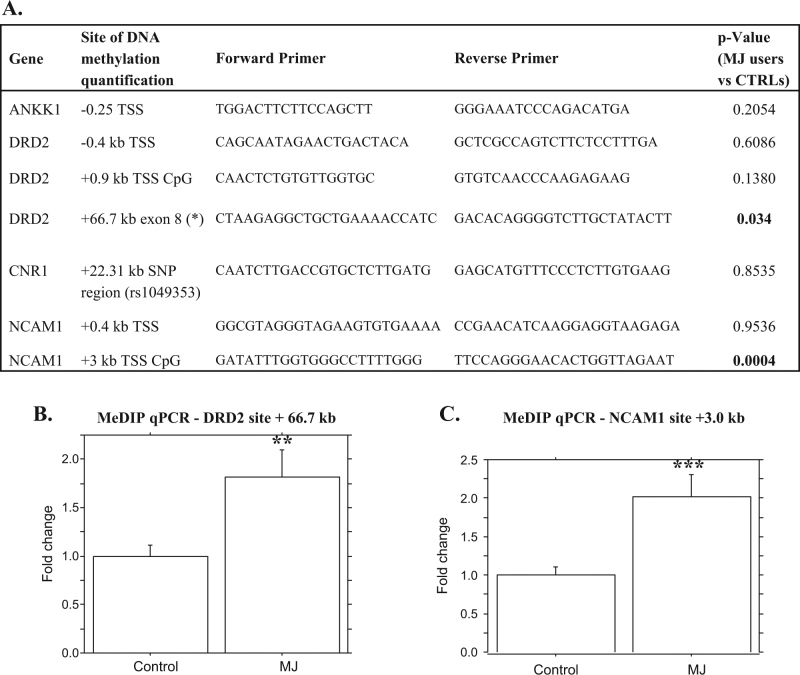
DNA methylation status was analyzed in seven sites, listed in Fig. 2a. These were in regions of ANNK1, CNR1, and in two genes that belong to the same cluster of ANKK1 on chromosome 11, DRD2 and NCAM1 genes. The primer sequences were designed to include sites as close as possible to the polymorphic SNP Taq1A and to the SNP rs1049353 or to the transcription start site (TSS) including CpG regions and intron−exon junctions.
Fig. 2.
a List of the regions where DNA methylation level has been quantified, with related p-value, from the comparison between MJ users and CTRLs. (*) This site was designed as close as possible to the Taq1A SNPs, ANKK1 gene. b c Bar plots related to unpaired t-test, comparing MJ users and control subjects, for b +66.7 kb from TSS, DRD2 gene and c +3 kb from TSS (UCSC Genome Browser on Human Dec. 2013 (GRCh38/hg38) Assembly)
To quantify DNA methylation in specific sites of genes (listed in Fig. 2a) involved in the neurobiology of CUD, MeDIP-qPCR was performed. The procedure includes three steps34: (1) sonication of gDNA (20 µg); (2) immunoprecipitation of the sonicated and denatured gDNA (5 µg); this step was performed overnight at 4 °C using 5 μl of a monoclonal antibody against 5 mC (anti-5-methylcytosine, 5-mC, mouse monoclonal antibody (catalog #33D3, Millipore), 50 µl IP Buffer (10 mm sodium phosphate (pH 7.0), 140 mm NaCl, 0.05% Triton X-100), in a final volume of 500 µl TE; incubated the mixture with 80 µl of Dynabeads (Life Technologies) overnight at 4 °C and washed it three times with 700 µl of IP buffer. The beads were then treated with proteinase K for 3 h at 50 °C and recovered the methylated DNA by phenol-chloroform extraction followed by ethanol precipitation. Sheared “input” DNA samples were collected prior to immunoprecipitation for subsequent comparison with immunoprecipitated DNA (3) quantitative real-time PCR using specific ChIP primers to determine the DNA methylation enrichment. Primers were designed using BLAST Pick up Primer Tool; the CpG regions position was investigated using UCSC genome browser (on Human Dec. 2013 (GRCh38/hg38) Assembly; https://genome.ucsc.edu/cgi-bin/hgGateway). The specific primer sequences used in this study are listed in Fig. 2a and all the details on primer sites annealing are reported in the Supplementary material section.
Statistical analyses
Fisher’s exact test was applied to examine the relationship between both allele frequencies and genotypic distribution with CU.
Haplotype frequencies, haplotype odds ratio and 95% confidence interval, and pairwise linkage disequilibrium were estimated. Haplotype frequencies were determined by using PLINK (1.07, Author: Shaun Purcell, URL: http://pngu.mgh.harvard.edu/purcell/plink/). The SNPs involved in haplotypes analysis (Table 2C) were the six SNPs of CNR1 on chromosome 6 (rs1049353|rs806379|rs6454674|rs2023239|rs12720071|rs2180619).
Table 2C.
Haplotype analysis of the six SNPs of CNR1. Positive associations at the 2–4 SNPs levels are reported
| Sliding window | SNPS forming the haplotype | Haplo-type | Frequency in MJ | Frequency in CTRLs | Test for association CHISQ | DF | p-value |
|---|---|---|---|---|---|---|---|
| 2 SNPs | rs12720071|rs1049353 | TT | 0.009868 | 0.08497 | 5.279 | 1 | 0.02159 |
| rs1049353|rs2023239 | TT | 0.00692 | 0.09218 | 6.402 | 1 | 0.0114 | |
| 3 SNPs | rs12720071|rs104935| rs2023239 | TTT | 0.004799 | 0.0684 | 4.721 | 1 | 0.02979 |
| rs1049353|rs2023239| rs806379 | TTA | 0.008201 | 0.09034 | 6.019 | 1 | 0.01415 | |
| 4 SNPs | rs12720071|rs1049353|rs2023239|rs806379 | TTTA | 0.006228 | 0.07111 | 4.705 | 1 | 0.03007 |
| rs1049353|rs2023239| rs806379|rs6454674 | TTAT | 0.009029 | 0.07984 | 4.907 | 1 | 0.02674 | |
| rs1049353|rs2023239| rs806379|rs6454674 | CCTT | 0.3776 | 0.2373 | 5.339 | 1 | 0.02086 |
Logistic regression models were used as main statistical tool to evaluate the role of potential explanatory factors (gender, marital status (married vs unmarried), educational attainment (1. Some high school/GED, 2. High school diploma, 3. Some college, 4. College Graduate, Masters, Ph.D.)), BMI, on the risk of CU. Two nested models were estimated. The first model is aimed to assess the potential influence of socio-demographic variables on CU, while a second model also included genotyping data. Because of the different gender composition of the two groups, it is considered appropriate to insert gender in the logistic regression models to evaluate its potential confounding effect.
MeDIP-qPCR statistical analysis was performed using STATVIEW 5.0. All the quantitative data are presented as mean + SEM. For data comparing controls (CTRL) and cannabis users (MJ) groups, unpaired Student's t-test was used (StatView version 5.01, St. Louis, Missouri).
In addition, to identify a possible interaction between DNA methylation and respectively the SNP rs1049353 and the SNP rs1800497 (the only two variants for which it was possible to design MeDIP primers). Student's t-test for independent samples was run.
For all the statistical analyses, results were considered statistically significant at p ≤ 0.05.
Results
Socio-demographic findings
In the first logistic regression model (Table 3A), evaluating the influence of socio-demographic factors on CU, gender was found to significantly contribute to CU risk. Male participants had 6.6 times higher risk to be cannabis users compared to females (p = 0.001). In addition, all the levels of educational attainment higher than the reference category seem to decrease the risk of CU. The highest level of education, College Graduate/Masters/Ph.D., reduced significantly the risk by 90% (p = 0.006). Both marital status and BMI did not show any statistical relation with CU.
Table 3A.
Variable(s) entered on step 1: gender, marital status (married (ref cat)-unmarried), BMI, education (4 categories)
| Variables in the equation | ||||||
|---|---|---|---|---|---|---|
| B | S.E. | Wald | df | Sig. | Exp(B) | |
| Gender, female (ref cat), male | 1.889 | 0.505 | 14.006 | 1 | 0.000 | 6.615 |
| Marital status, married (ref cat), unmarried | 1.168 | 0.919 | 1.615 | 1 | 0.204 | 3.216 |
| BMI | 0.024 | 0.031 | .610 | 1 | 0.435 | 1.024 |
| 1-Some high school/2-GED (ref cat) | ||||||
| 3-H.S. diploma (1) | −1.088 | 0.618 | 3.095 | 1 | 0.079 | 0.337 |
| 4-Some college (2) | −0.354 | 0.581 | .370 | 1 | 0.543 | 0.702 |
| 5-College-graduate/6-Masters/7-Ph.D (3) | −2.436 | 0.883 | 7.621 | 1 | 0.006 | 0.087 |
| Constant | −3.001 | 1.488 | 4.068 | 1 | 0.044 | 0.050 |
Significant contributions are indicated as bold values
Genetic findings
G allele and homozygous GG genotype of SNP rs1049353 (G1539A) (CNR1 gene) were significantly higher among cannabis users compared to control subjects (respectively p = 0.002; p = 0.01) (Table 2B). Even excluding homozygous A/A subjects, due to the low number of observations (0% among cannabis users and 4.2% among controls), the differences were confirmed (p = 0.02). Differences were observed (p = 0.058) for the SNP rs2501431of the CNR2 gene. There were no statistical significant differences in the SNP Taq1A (ANKK1) or CNR1 gene (rs806379, rs6454674, rs2023239, rs12720071, rs2180619) (Table 2B).
Table 2B.
Genotype and allele frequencies
| SNP ID (gene) | Genotypes and alleles | Subjects CTRLs | MJ users | Fisher’s exact test |
|---|---|---|---|---|
| rs1800497 (ANKK1) | CC | 50,00% | 51.28% | 0.76 |
| TT | 9.38% | 12.82% | ||
| CT | 40.63% | 35.90% | ||
| C allele | 87.10% | 76.63% | 0.88 | |
| T allele | 12.90% | 23.37% | ||
| rs1049353 (CNR1) | GG | 78.13% | 97.44% | 0.01 |
| AA | 4.17% | 0.00% | ||
| GA | 17.71% | 2.56% | ||
| G | 86.98% | 98.72% | 0.002 | |
| A | 13.02% | 1.28% | ||
| rs2180619 (CNR1) | AA | 21.74% | 23.68% | 0.9 |
| GG | 34.78% | 36.84% | ||
| AG | 43.48% | 39.47% | ||
| A allele | 43.48% | 43.42% | 1 | |
| G allele | 56.52% | 56.58% | ||
| rs806379 (CNR1) | AA | 19.79% | 17.95% | 0.62 |
| TT | 25.00% | 33.33% | ||
| AT | 55.21% | 48.72% | ||
| A allele | 47.40% | 42.31% | 0.5 | |
| T allele | 52.60% | 57.69% | ||
| rs6454674 (CNR1) | GG | 12.77% | 7.50% | 0.21 |
| TT | 36.17% | 52.50% | ||
| GT | 51.06% | 40.00% | ||
| G allele | 38.30% | 27.50% | 0.09 | |
| T allele | 61.70% | 72.50% | ||
| rs12720071 (CNR1) | CC | 21.88% | 20.51% | 1 |
| TT | 0.00% | 0.00% | ||
| TC | 78.13% | 79.49% | ||
| C allele | 60.94% | 60.26% | 1 | |
| T allele | 39.06% | 39.74% | ||
| rs2023239 (CNR1) | CC | 10.42% | 12.50% | 0.47 |
| TT | 48.96% | 37.50% | ||
| TC | 40.63% | 50.00% | ||
| C allele | 30.73% | 37.50% | 0.32 | |
| T allele | 69.27% | 62.50% | ||
| rs2501431 (CNR2) | AA | 47.37% | 58.97% | 0.058 |
| GG | 11.58% | 0.00% | ||
| AG | 41.05% | 41.03% | ||
| A allele | 32.11% | 20.51% | 0.07 | |
| G allele | 67.89% | 79.49% |
The haplotype analysis for the six CNR1 SNPs located on chromosome 6 (rs1049353|rs806379|rs6454674|rs2023239|rs12720071|rs2180619) revealed positive associations for the 2–4 SNPs levels reported in Table 2C.
Gene-sociodemographic interactions
A second logistic regression model (Table 3B) tested simultaneously the influence of genetic and environmental risk factors considering the concurrent influence on CU of the previous significant parameters: the genetic variables rs1049353 (CNR1) and rs2501431 (CNR2), gender and educational attainment. Gender (p < 0.05), and the educational attainment (p = 0.002) were confirmed to have a significant relation with CU. G allele of rs1049353 (CNR1) was confirmed to confer a higher risk to be cannabis users (p = 0.05).
Table 3B.
Variable(s) entered on step 2: gender, education (4 categories) rs1049353, A allele (ref cat)-G allele, rs25GA43A, G allele (ref cat)-A allele
| Variables in the equation | ||||||
|---|---|---|---|---|---|---|
| B | S.E. | Wald | df | Sig. | Exp(B) | |
| Gender, female (ref cat), male | 2.054 | 0.514 | 15.951 | 1 | 0.000 | 7.797 |
| 1-Some high school/2-GED (ref cat) | ||||||
| 3-H.S. diploma (1) | −1.374 | 0.709 | 3.757 | 1 | 0.053 | 0.253 |
| 4-Some college (2) | −.542 | 0.623 | .757 | 1 | 0.384 | 0.582 |
| 5-College graduate/6-Masters/7-Ph.D (3) | −2.851 | 0.926 | 9.491 | 1 | 0.002 | 0.058 |
| rs1049353, A allele (ref cat) G allele | 3.161 | 1.116 | 8.024 | 1 | 0.005 | 23.584 |
| rs25GA43A, G allele (ref cat) A allele | −.183 | 0.503 | .133 | 1 | 0.716 | 0.833 |
| Constant | −3.854 | 1.179 | 10.683 | 1 | 0.001 | 0.021 |
Significant contributions are indicated as bold values
Epigenetic findings
We analyzed DNA methylation status in seven sites of ANNK1, CNR1, DRD2 and NCAM1 genes, listed in Fig. 2a and related to CU. DNA methylation was significantly higher in cannabis users compared to control subjects in two of the regions analyzed (Fig. 2b, c). The first site was in the exon 8 of DRD2 gene at +66.7 kb from the TSS (see Figure 4S) (p = 0.034) and the second was located on a CpG region at +3 kb from the TSS (Figure 6S) in the NCAM1 gene (p = 0.0004). No differences in DNA methylation were found at ANKK1 −0.25 kb, DRD2 −0.4 kb and +0.9 kb, NCAM1 +0.4 kb, CNR1 +22.31 kb, comparing cannabis users and control subjects (Figs. 1S-3S, 5S, 7S).
Discussion
The main findings of our study are: (1) SNP rs1049353 in CNR1 gene is associated with CU; (2) the highest level of education, College graduate/Masters/Ph.D., reduced significantly the risk to be cannabis users; and (3) changes in DNA methylation, comparing cannabis users and control, were observed in two sites related to DRD2 and NCAM1 genes.
Our findings are consistent with the nominal association found between the G allele, SNP rs1049353, and cannabis dependence symptoms10. The SNP rs1049353 polymorphism appears to play a significant role in the function of cannabinoid receptor by influencing mRNA translation as well as secondary structure and stability of the protein35,36. Thus, it is possible that individuals who carry this SNP might experience different marijuana-induced behavioral or physiological effects and may also show different phenotypes that are regulated by the cannabinoid system10,37–40. These suggestions might explain why the G allele may serve as a risk factor for CU41.
To increase the power to detect genetic traits associated with CU, haplotype-based association analysis was also used. Different combinations of SNPs in CNR1 showed significant associations with CU at 2–4 SNPs levels for seven haplotypes in our study. These observations are consistent with another research group that had reported a significant association of CNR1 haplotype rs806368-rs1049353-rs2023239-rs6454674 with the level of cannabis exposure and decreased volume of the right anterior cingulum42.
For the variant rs2501431 in the other cannabinoid receptor CNR2 gene, differences were observed in genotype distribution, with the homozygous A/A genotype being more frequent in the cannabis group. When we excluded GG genotypes, the association did not reach significant values (p = 0.7), leading us to assume a very important role of the G allele. This statement is consistent with the report that the GG genotype is associated with alcoholism in a Japanese population43. Together, these observations suggest the need for further study to elucidate the role of rs2501431 in CNR2 gene as possible risk factor of CU and other addiction diagnoses.
In addition, our data highlight the importance of gender in CU susceptibility. Compared to females, male subjects in the study presented a significantly higher risk to use cannabis. These findings underlie the importance of better exploring gender difference in addiction research. Gender differences in CU are indeed frequently reported, with male proneness to use the drug more frequently or at a higher rate than females44–46. Gender-based differences in cannabinoid effects have been evidenced by preliminary studies investigating the role of gonadal hormones in the regulation of cannabis receptors density and affinity47. Other studies highlighted that sex differences could also be influenced by drug disposition and body fat distribution48. However, the fact that females are underrepresented in our study and others may also lead to incomplete and biased results.
Importantly, the evaluation of sociodemographic risk factors helped to confirm that higher education attainment might serve as a possible protective factor, considering the significant inverse relation between education levels and CU. A previous study had reported that users with higher education do not suffer from cognitive deficits with chronic abuse of cannabis49. Interestingly, strong engagement in school and good school achievements have also been repeatedly reported to be important resilience factors against substance use disorders50–54. Thus, poor school performance and dropping out of schools may not only be risk factors for substance use disorders but may be the consequences of shared genetic/epigenetic risks that predict both school failure and CU initiation55,56. The potential impact of cannabis on school performance is also very important to consider57–60. Logistic regression analysis demonstrated that both genetic (rs1049353, CNR1 gene) and sociodemographic risk factors are coincident, representing endophenotypes associated with CU risks. The role of endophenotypes for the rational development of therapeutic and preventive strategies has previously been assessed and suggests that these are important variables in treatment approaches61.
Our epigenetic findings also appear to suggest that not only inherited gene variants, but also changes in gene expression could be associated to CU. Hypermethylation was reported in cannabis users compared to controls at DRD2 exon 8 and in a CpG-rich region of the NCAM1 gene. The observed NCAM1 differentially methylated region (DMR) was located on the 5′ CpG island of the gene and promoter-associated DMR-CpGs are usually shown to cause transcriptional silencing62. Even if the functional significance of DNA methylation in introns, exons, and intergenic intervals is still less well-understood63,64, the DRD2 DMR, located within the exon 8, suggests the importance of these gene body sites, as already shown in a study on parental THC exposure65.
Both genes, DRD2 and NCAM1 have a central role in the dopaminergic pathway66 and have been recently found significantly associated with lifetime CU16. They are part of the NCAM1–TTC12–ANKK1–DRD2 gene cluster (NTAD), suggested to be associated to nicotine dependence67 and other substance use disorders in general68.
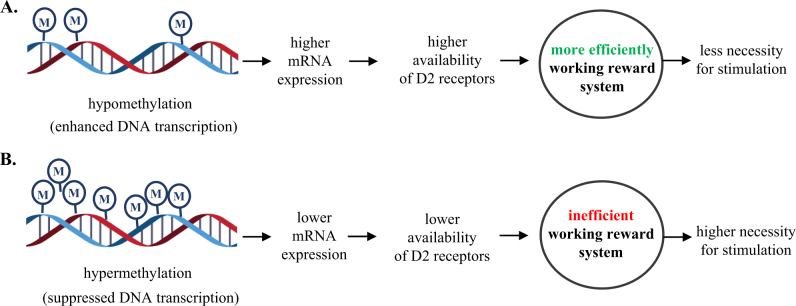
Other behavioral conditions were reported to be associated with DNA methylation in these two genes. DRD2 promoter showed highest methylation levels in patients with gambling behavior, compared to non-gambling participants and, because DNA-hypermethylation is usually associated with transcriptional repression, the subsequently higher availability of D2 receptor was assumed to result in a more active working of the reward system69.
Based on these evidences, we could hypothesize (Fig. 3) that increased DNA methylation observed in cannabis users' blood cells may in some way reflect a lower D2 mRNA expression, lower availability of D2 receptors underlying a reward deficit condition. This difference in the reward system function related to methylation may lead to a reduced need for reinforcing stimuli in non-cannabis users, and a higher necessity of pharmacological stimulation in cannabis-dependent subjects.
Fig. 3.
a Hillemacher’s (2015) hypothesis on altered DNA-methylation in pathological gambling patients. b Based on the current results, hypothesis of DNA hypermethylation in patients with cannabis use disorders
The observed hypermethylation in NCAM1 gene is consistent with the hypermethylated CpG regions of NCAM1 in alcohol-dependent patients compared with controls70,71. This gene that was previously found significantly associated with lifetime CU16 encodes a neural cell adhesion molecule implicated in important functions during development and maintenance of the nervous system. A study in rodents proposed NCAM as a modulator of the dopaminergic pathway and a potential pharmacological target for dopamine-related psychiatric disorders72.
In the interpretation of our findings concerning hypermethylation in cannabis users, it is impossible to establish any causal relationship. Epigenetic changes affecting DRD2 and NCAM1 could be the result of long-term exposure to cannabis and not a pre-existing condition involved in CU neurobiological vulnerability.
The present study has certain limitations. First, because the number of cannabis users was relatively low, statistical power was not as strong as expected. Moreover, since polymorphisms may vary in frequency among different ethnic groups73, it might have been more appropriate to split the samples based on ethnic groups. However, this was not possible due to the relatively small number of subjects per group. The results obtained by the present study could thus be attributed to the ethno-racial background of the participants. Thus, any extension to other ethnic groups should be done with caution and will need to be investigated further.
Another important limitation is that most of the changes in DNA methylation patterns were observed in peripheral cells74 such as WBCs that may not reflect changes in reward brain regions. Moreover, the changes in DNA methylation patterns were investigated at a single time point, thus, precluding the possibility of examining time-dependent effects of marijuana exposure on DNA methylation. Replication studies using specific cell types and brain tissues should help to further clarify the role of DNA methylation in CUD. Another issue that needs to be investigated in future replication studies include assessment of the role of marijuana on other epigenetic markers. This will entail examining changes in histone modifications and DNA methylation during the clinical course of repeated marijuana exposure. Indeed, the use of genome-wide methylation or chromatin immunoprecipitation studies should help to identify other genes that might be involved as either risk factors or consequences of cannabis exposure.
Overall, our results suggest a significant role of genes encoding proteins involved in the endocannabinoid system in the susceptibility for CU. Our study also identified epigenetic modifications affecting dopamine system function as risk factors and/or consequences of marijuana exposure. Confirmation studies that address the shortcomings detailed above are necessary to clarify to what extent changes in peripheral DNA methylation could serve as accessible sources of biological markers and/or targets for pharmacological interventions that might reduce or prevent the effects of marijuana in the brain. Finally, because education attainment was identified as a protective factor for CU, these observations suggest the building and expansion of early childhood educational programs and strategies to prevent CUD in adolescence and later in life.
Electronic supplementary material
Acknowledgements
This work was supported by funds of the Intramural Research Program (IRP) of the National Institute of Drug Abuse (DHHS/NIH/NIDA), project #12-DA-N472, NIDA, IRP [Health Outcomes by Neighbourhood (HON)—Baltimore]. The authors also acknowledge the support of Dr. Antonello Bonci, NIDA IRP Scientific Director, who made it possible for Maria Carla Gerra to work at the NIDA IRP.
Authors' contributions
The study conception and design were by MCG, SJ, JLC, and CD. Samples for the study were kindly provided by KAP. MCG, SJ, and DW worked together in the acquisition of data including genotyping and DNA methylation assays. Data analysis, interpretation, and correlations using environmental data were performed by SJ, MCG, DW, in conjunction with the biostatisticians, MM and JS. All authors contributed to the drafting and revisions of the manuscript.
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary Information accompanies this paper at 10.1038/s41398-017-0087-1.
References
- 1.Anthony, J. C. The epidemiology of cannabis dependence. In Cannabis Dependence: Its Nature, Consequences and Treatment (eds Roffman, R. & Stephens, R. S.) 58–105 (Cambridge University Press, Cambridge, UK, 2006) .
- 2.Anthony JC. Steppingstone and gateway ideas: a discussion of origins, research challenges, and promising lines of research for the future. Drug. Alcohol Depend. 2012;123(Suppl 1):S99–S104. doi: 10.1016/j.drugalcdep.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.WHO (World Health Organization). Management of Substance Abuse: Cannabis (WHO, Geneva, 2016) (http://www.who.int/substance_abuse/facts/cannabis/en/).
- 4.Verweij KJ, et al. Genetic and environmental influences on cannabis use initiation and problematic use: a meta-analysis of twin studies. Addiction. 2010;105:417–430. doi: 10.1111/j.1360-0443.2009.02831.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Agrawal A, Lynskey MT. The genetic epidemiology of cannabis use, abuse and dependence. Addiction. 2006;101:801–812. doi: 10.1111/j.1360-0443.2006.01399.x. [DOI] [PubMed] [Google Scholar]
- 6.Agrawal A, Madden PA, Bucholz KK, Heath AC, Lynskey MT. Initial reactions to tobacco and cannabis smoking: a twin study. Addiction. 2014;109:663–671. doi: 10.1111/add.12449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Agrawal A, Neale MC, Prescott CA, Kendler KS. A twin study of early cannabis use and subsequent use and abuse/dependence of other illicit drugs. Psychol. Med. 2004;34:1227–1237. doi: 10.1017/S0033291704002545. [DOI] [PubMed] [Google Scholar]
- 8.Nacak M, et al. Analysis of dopamine D2 receptor (DRD2) gene polymorphisms in cannabinoid addicts. J. Forensic Sci. 2012;57:1621–1624. doi: 10.1111/j.1556-4029.2012.02169.x. [DOI] [PubMed] [Google Scholar]
- 9.Ruiz-Contreras AE, et al. Performance in working memory and attentional control is associated with the rs2180619 SNP in the CNR1 gene. Genes. Brain Behav. 2014;13:173–178. doi: 10.1111/gbb.12097. [DOI] [PubMed] [Google Scholar]
- 10.Buchmann AF, et al. Role of CNR1 polymorphisms in moderating the effects of psychosocial adversity on impulsivity in adolescents. J. Neural Transm. 2015;122:455–463. doi: 10.1007/s00702-014-1266-3. [DOI] [PubMed] [Google Scholar]
- 11.Ho BC, Wassink TH, Ziebell S, Andreasen NC. Cannabinoid receptor 1 gene polymorphisms and marijuana misuse interactions on white matter and cognitive deficits in schizophrenia. Schizophr. Res. 2011;128:66–75. doi: 10.1016/j.schres.2011.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mitjans M, Gastó C, Catalán R, Fañanás L, Arias B. Genetic variability in the endocannabinoid system and 12-week clinical response to citalopram treatment: the role of the CNR1, CNR2 and FAAH genes. J. Psychopharmacol. 2012;26:1391–1398. doi: 10.1177/0269881112454229. [DOI] [PubMed] [Google Scholar]
- 13.Lester KJ, et al. Genetic variation in the endocannabinoid system and response to Cognitive Behavior Therapy for child anxiety disorders. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2017;174:144–155. doi: 10.1002/ajmg.b.32467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Agrawal A, et al. A genome-wide association study of DSM-IV cannabis dependence. Addict. Biol. 2011;16:514–518. doi: 10.1111/j.1369-1600.2010.00255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sherva R, et al. Genome-wide association study of cannabis dependence severity, novel risk variants, and shared genetic risks. JAMA Psychiatry. 2016;73:472–480. doi: 10.1001/jamapsychiatry.2016.0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stringer S, et al. Genome-wide association study of lifetime cannabis use based on a large meta-analytic sample of 32330 subjects from the International Cannabis Consortium. Transl. Psychiatry. 2016;6:e769. doi: 10.1038/tp.2016.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gizer I. R., Bizon C., Gilder D. A., Ehlers C. L. & Wilhelmsen K. C. Whole genome sequence study of cannabis dependence in two independent cohorts. Addict. Biol. 2017, https://doi.org/10.1111/adb.12489 [Epub ahead of print]. [DOI] [PMC free article] [PubMed]
- 18.Agrawal A, et al. DSM-5 cannabis use disorder: a phenotypic and genomic perspective. Drug. Alcohol Depend. 2014;134:362–369. doi: 10.1016/j.drugalcdep.2013.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Suerken CK, et al. Marijuana use trajectories and academic outcomes among college students. Drug. Alcohol Depend. 2016;162:137–145. doi: 10.1016/j.drugalcdep.2016.02.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Silins E, et al. Cannabis Cohorts Research Consortium. Adolescent substance use and educational attainment: an integrative data analysis comparing cannabis and alcohol from three Australasian cohorts. Drug. Alcohol Depend. 2015;156:90–96. doi: 10.1016/j.drugalcdep.2015.08.034. [DOI] [PubMed] [Google Scholar]
- 21.Stiby AI, et al. Adolescent cannabis and tobacco use and educational outcomes at age 16: birth cohort study. Addiction. 2015;110:658–668. doi: 10.1111/add.12827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Ours JC, Williams J. Why parents worry: initiation into cannabis use by youth and their educational attainment. J. Health Econ. 2009;28:132–142. doi: 10.1016/j.jhealeco.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 23.Harrison PA, Fulkerson JA, Beebe TJ. Multiple substance use among adolescent physical and sexual abuse victims. Child. Abus. Negl. 1997;21:529–539. doi: 10.1016/S0145-2134(97)00013-6. [DOI] [PubMed] [Google Scholar]
- 24.Kilpatrick DG, et al. Risk factors for adolescent substance abuse and dependence: data from a national sample. J. Consult. Clin. Psychol. 2000;68:19–30. doi: 10.1037/0022-006X.68.1.19. [DOI] [PubMed] [Google Scholar]
- 25.Rotter A, et al. CB1 and CB2 receptor expression and promoter methylation in patients with cannabis dependence. Eur. Addict. Res. 2013;19:13–20. doi: 10.1159/000338642. [DOI] [PubMed] [Google Scholar]
- 26.van der Knaap LJ, et al. Catechol-O-methyltransferase gene methylation and substance use in adolescents: the TRAILS study. Genes. Brain Behav. 2014;13:618–625. doi: 10.1111/gbb.12147. [DOI] [PubMed] [Google Scholar]
- 27.DiNieri JA, et al. Maternal cannabis use alters ventral striatal dopamine D2 gene regulation in the offspring. Biol. Psychiatry. 2011;70:763–769. doi: 10.1016/j.biopsych.2011.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tomasiewicz HC, et al. Proenkephalin mediates the enduring effects of adolescent cannabis exposure associated with adult opiate vulnerability. Biol. Psychiatry. 2012;72:803–810. doi: 10.1016/j.biopsych.2012.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Paradisi A, Pasquariello N, Barcaroli D, Maccarrone M. Anandamide regulates keratinocyte differentiation by inducing DNA methylation in a CB1 receptor-dependent manner. J. Biol. Chem. 2008;283:6005–6012. doi: 10.1074/jbc.M707964200. [DOI] [PubMed] [Google Scholar]
- 30.Cecil CA, et al. DNA methylation and substance-use risk: a prospective, genome-wide study spanning gestation to adolescence. Transl. Psychiatry. 2016;6:e976. doi: 10.1038/tp.2016.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gutierrez-Arcelus M, et al. Passive and active DNA methylation and the interplay with genetic variation in gene regulation. Elife. 2013;2:e00523. doi: 10.7554/eLife.00523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jones MJ, Fejes AP, Kobor MS. DNA methylation, genotype and gene expression: who is driving and who is along for the ride? Genome Biol. 2013;14:126. doi: 10.1186/gb-2013-14-7-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vidot DC, Bispo JB, Hlaing WM, Prado G, Messiah SE. Moderate and vigorous physical activity patterns among marijuana users: results from the 2007−2014 National Health and Nutrition Examination Surveys. Drug. Alcohol Depend. 2017;178:43–48. doi: 10.1016/j.drugalcdep.2017.05.004. [DOI] [PubMed] [Google Scholar]
- 34.Weber M, et al. Chromosome-wide and promoter-specific analyses identify sites of differential DNA methylation in normal and transformed human cells. Nat. Genet. 2005;37:853–862. doi: 10.1038/ng1598. [DOI] [PubMed] [Google Scholar]
- 35.Shen LX, Basilion JP, Stanton VP., Jr. Single-nucleotide polymorphisms can cause different structural folds of mRNA. Proc. Natl. Acad. Sci. USA. 1999;96:7871–7876. doi: 10.1073/pnas.96.14.7871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chakrabarti B, Kent L, Suckling J, Bullmore E, Baron-Cohen S. Variations in the human cannabinoid receptor (CNR1) gene modulate striatal responses to happy faces. Eur. J. Neurosci. 2006;23:1944–1948. doi: 10.1111/j.1460-9568.2006.04697.x. [DOI] [PubMed] [Google Scholar]
- 37.Dinu IR, Popa S, Bîcu M, Moţa E, Moţa M. The implication of CNR1 gene’s polymorphisms in the modulation of endocannabinoid system effects. Rom. J. Intern. Med. 2009;47:9–18. [PubMed] [Google Scholar]
- 38.Hill MN, Patel S. Translational evidence for the involvement of the endocannabinoid system in stress-related psychiatric illnesses. Biol. Mood Anxiety Disord. 2013;3:19. doi: 10.1186/2045-5380-3-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Agrawal A, et al. Cannabinoid receptor genotype moderation of the effects of childhood physical abuse on anhedonia and depression. Arch. Gen. Psychiatry. 2012;69:732–740. doi: 10.1001/archgenpsychiatry.2011.2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hopfer CJ, et al. Cannabis receptor haplotype associated with fewer cannabis dependence symptoms in adolescents. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2006;141B:895–901. doi: 10.1002/ajmg.b.30378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hartman CA, et al. The association between cannabinoid receptor 1 gene (CNR1) and cannabis dependence symptoms in adolescents and young adults. Drug. Alcohol Depend. 2009;104:11–16. doi: 10.1016/j.drugalcdep.2009.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hill SY, Sharma V, Jones BL. Lifetime use of cannabis from longitudinal assessments, cannabinoid receptor (CNR1) variation, and reduced volume of the right anterior cingulate. Psychiatry Res. 2016;255:24–34. doi: 10.1016/j.pscychresns.2016.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ishiguro H, et al. Involvement of cannabinoid CB2 receptor in alcohol preference in mice and alcoholism in humans. Pharm. J. 2007;7:380–385. doi: 10.1038/sj.tpj.6500431. [DOI] [PubMed] [Google Scholar]
- 44.Agrawal A, Lynskey MT. Does gender contribute to heterogeneity in criteria for cannabis abuse and dependence? Results from the national epidemiological survey on alcohol and related conditions. Drug. Alcohol Depend. 2007;88:300–307. doi: 10.1016/j.drugalcdep.2006.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Perkonigg A, et al. The natural course of cannabis use, abuse and dependence during the first decades of life. Addiction. 2008;103:439–449. doi: 10.1111/j.1360-0443.2007.02064.x. [DOI] [PubMed] [Google Scholar]
- 46.Farmer RF, et al. Internalizing and externalizing psychopathology as predictors of cannabis use disorder onset during adolescence and early adulthood. Psychol. Addict. Behav. 2015;29:541–551. doi: 10.1037/adb0000059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gorzalka BB, Dang SS. Endocannabinoids and gonadal hormones: bidirectional interactions in physiology and behaviour. Endocrinology. 2012;153:1016–1024. doi: 10.1210/en.2011-1643. [DOI] [PubMed] [Google Scholar]
- 48.Fattore L, Fratta W. How important are sex differences in cannabinoid action? Br. J. Pharmacol. 2010;160:544–548. doi: 10.1111/j.1476-5381.2010.00776.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bolla KI, Brown K, Eldreth D, Tate K, Cadet JL. Dose-related neurocognitive effects of marijuana use. Neurology. 2002;59:1337–1343. doi: 10.1212/01.WNL.0000031422.66442.49. [DOI] [PubMed] [Google Scholar]
- 50.Hodder RK, et al. A school-based resilience intervention to decrease tobacco, alcohol and marijuana use in high school students. BMC Public Health. 2011;11:722. doi: 10.1186/1471-2458-11-722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Council on School Health, American Academy of Pediatrics; Committee on Substance Abuse, American Academy of Pediatrics. Mears CJ, Knight JR. The role of schools in combating illicit substance abuse. Pediatrics. 2007;120:1379–1384. doi: 10.1542/peds.2007-2905. [DOI] [PubMed] [Google Scholar]
- 52.Skinner ML, Haggerty KP, Fleming CB, Catalano RF. Predicting functional resilience among young-adult children of opiate-dependent parents. J. Adolesc. Health. 2009;44:283–290. doi: 10.1016/j.jadohealth.2008.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Napoli M, Marsiglia FF, Kulis S. Sense of belonging in school as a protective factor against drug abuse among native American urban adolescents. J. Soc. Work. Pract. Addict. 2003;3:25–41. doi: 10.1300/J160v03n02_03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lynskey M, Hall W. The effects of adolescent cannabis use on educational attainment: a review. Addiction. 2000;95:1621–1630. doi: 10.1046/j.1360-0443.2000.951116213.x. [DOI] [PubMed] [Google Scholar]
- 55.Kelly AB, et al. A longitudinal study of the association of adolescent polydrug use, alcohol use and high school non-completion. Addiction. 2015;110:627–635. doi: 10.1111/add.12829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Verweij KJ, Huizink AC, Agrawal A, Martin NG, Lynskey MT. Is the relationship between early-onset cannabis use and educational attainment causal or due to common liability? Drug. Alcohol Depend. 2013;133:580–586. doi: 10.1016/j.drugalcdep.2013.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Carey SE, Nestor L, Jones J, Garavan H, Hester R. Impaired learning from errors in cannabis users: dorsal anterior cingulate cortex and hippocampus hypoactivity. Drug. Alcohol Depend. 2015;155:175–182. doi: 10.1016/j.drugalcdep.2015.07.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Crean RD, Crane NA, Mason BJ. An evidence based review of acute and long-term effects of cannabis use on executive cognitive functions. J. Addict. Med. 2011;5:1–8. doi: 10.1097/ADM.0b013e31820c23fa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Meier MH, et al. Persistent cannabis users show neuropsychological decline from childhood to midlife. Proc. Natl. Acad. Sci. USA. 2012;109:E2657–E2664. doi: 10.1073/pnas.1206820109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Volkow ND, Baler RD, Compton WM, Weiss SR. Adverse health effects of marijuana use. N. Engl. J. Med. 2014;370:2219–2227. doi: 10.1056/NEJMra1402309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ersche KD, et al. Cognitive dysfunction and anxious-impulsive personality traits are endophenotypes for drug dependence. Am. J. Psychiatry. 2012;169:926–936. doi: 10.1176/appi.ajp.2012.11091421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Brenet F, et al. DNA methylation of the first exon is tightly linked to transcriptional silencing. PLoS. ONE. 2011;6:e14524. doi: 10.1371/journal.pone.0014524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jones PA. Functions of DNA methylation: islands, start sites, gene bodies and beyond. Nat. Rev. Genet. 2012;13:484–492. doi: 10.1038/nrg3230. [DOI] [PubMed] [Google Scholar]
- 64.Schübeler D. Function and information content of DNA methylation. Nature. 2015;517:321–326. doi: 10.1038/nature14192. [DOI] [PubMed] [Google Scholar]
- 65.Watson CT, et al. Genome-wide DNA methylation profiling reveals epigenetic changes in the rat nucleus accumbens associated with cross-generational effects of adolescent THC exposure. Neuropsychopharmacology. 2015;40:2993–3005. doi: 10.1038/npp.2015.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Xiao MF, et al. Neural cell adhesion molecule modulates dopaminergic signaling and behavior by regulating dopamine D2 receptor internalization. J. Neurosci. 2009;29:14752–14763. doi: 10.1523/JNEUROSCI.4860-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bidwell LC, et al. NCAM1-TTC12-ANKK1-DRD2 variants and smoking motives as intermediate phenotypes for nicotine dependence. Psychopharmacol. (Berl.) 2015;232:1177–1186. doi: 10.1007/s00213-014-3748-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ma Y, Yuan W, Jiang X, Cui WY, Li MD. Updated findings of the association and functional studies of DRD2/ANKK1 variants with addictions. Mol. Neurobiol. 2015;51:281–299. doi: 10.1007/s12035-014-8826-2. [DOI] [PubMed] [Google Scholar]
- 69.Hillemacher T, et al. Alterations in DNA-methylation of the dopamine-receptor 2 gene are associated with abstinence and health care utilization in individuals with a lifetime history of pathologic gambling. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2015;63:30–34. doi: 10.1016/j.pnpbp.2015.05.013. [DOI] [PubMed] [Google Scholar]
- 70.Zhang H, et al. Array-based profiling of DNA methylation changes associated with alcohol dependence. Alcohol Clin. Exp. Res. 2013;37(Suppl 1):E108–E115. doi: 10.1111/j.1530-0277.2012.01928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Barker JM, Zhang Y, Wang F, Taylor JR, Zhang H. Ethanol-induced Htr3a promoter methylation changes in mouse blood and brain. Alcohol Clin. Exp. Res. 2013;37(Suppl 1):E101–E107. doi: 10.1111/j.1530-0277.2012.01906.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mota NR, Araujo-Jnr EV, Paixão-Côrtes VR, Bortolini MC, Bau CH. Linking dopamine neurotransmission and neurogenesis: The evolutionary history of the NTAD (NCAM1-TTC12-ANKK1-DRD2) gene cluster. Genet. Mol. Biol. 2012;35:912–918. doi: 10.1590/S1415-47572012000600004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jorde LB, Wooding SP. Genetic variation, classification and ‘race’. Nat. Genet. 2004;36(11 Suppl):S28–S33. doi: 10.1038/ng1435. [DOI] [PubMed] [Google Scholar]
- 74.Terry MB, Delgado-Cruzata L, Vin-Raviv N, Wu HC, Santella RM. DNA methylation in white blood cells: association with risk factors in epidemiologic studies. Epigenetics. 2011;6:828–837. doi: 10.4161/epi.6.7.16500. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.