Practical Implications
Clinicians encountering catatonic presentations in the context of late-onset psychiatric symptoms should consider familial frontotemporal dementia with C9ORF72 genetic mutation in the differential diagnosis.
Behavioral variant frontotemporal dementia (bvFTD) may present with atypical features of disinhibition, cognitive decline, and other neuropsychiatric symptoms. We present a case of bvFTD presenting with malignant catatonia and later revealing repeat expansion of chromosome 9 open reading frame 72 (C9ORF72) alleles. We describe the clinical course, physical and mental examination findings, and diagnostic features; a brief and cursory literature review is also offered.
Case report
A 61-year-old Caucasian woman presented with cognitive dysfunction of 20 months. Her past medical history included postpartum depression. Her mother had dementia with psychosis in her 70s without further available details. The patient's initial symptoms included soliloquy, increased anxiety, and a delusion of possessing ghosts inside herself. A year later she developed uncontrollable crying and difficulty with activities of daily living. She was diagnosed with an atypical late-onset form of schizophrenia and treated with olanzapine 10 mg daily. Three months later, she developed disorganized thinking and abnormal behavior, the latter manifesting as difficulty driving, running into a garage, parking sideways, and arriving at unscheduled appointments, possible manifestations of hypomania. Mini-Mental State Examination score was 20 out of 30. She demonstrated diminished purposeful communication, palilalia, jerking limb movement, and a stooped posture, leading to hospitalization in October 2014. Discontinuation of olanzapine did not lead to clinical improvement.
On examination, the patient was observed to be a well-nourished agitated woman lying in bed in a tense position with bilateral arms flexed toward her chest and bilateral knees bent. There was no fever. She was moaning loudly, incessantly, and rhythmically. She had forced eye closure and systemic lead-pipe rigidity with intermittent waxy flexibility. She rarely made spontaneous limb movements, and passive movement of her rigid limbs elicited louder and more persistent vocalization. Most of her produced sounds were nonspecific and nonmeaningful screaming, mixed occasionally with isolated out-of-context words. She could not repeat and was mostly unresponsive to verbal commands. She was diffusely hyperreflexic with bilateral extensor plantar response but lacked notable fasciculations and muscle wasting. She was promptly diagnosed with malignant catatonia.
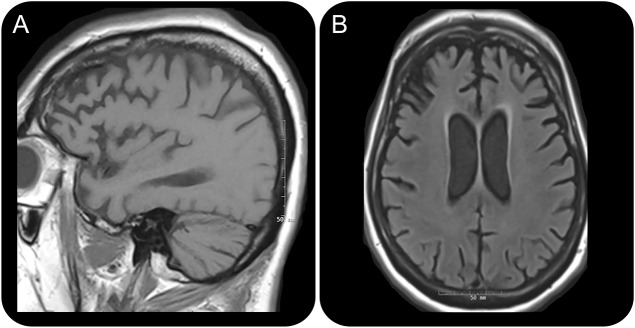
Extensive investigations including laboratory and toxicologic screening were negative. There was no evidence of infectious, inflammatory, or paraneoplastic etiology. EEG monitoring revealed only diffuse slowing activity of delta frequency, maximally in the bilateral frontal region. CSF was only remarkable for mildly elevated tau level at 615 pg/mL. Brain MRI was notable for bilateral frontal and temporal atrophy (figure), and brain fluorodeoxyglucose PET demonstrated global hypometabolism. EMG revealed scattered abnormal spontaneous activity suggestive of sparse active denervation in the cervical and lumbosacral segments. Motor unit potential morphology could not be adequately evaluated due to poor patient cooperation and a diagnosis of amyotrophic lateral sclerosis (ALS) could not be made.
Brain MRI
Figure. Brain MRI fluid-attenuated inversion recovery (FLAIR) sequence demonstrated considerable atrophy in the frontal and temporal lobes while parenchymal volumes are relatively preserved in the parietal and occipital lobes. Predominantly frontotemporal atrophy is seen on T1-weighted sagittal (A) and FLAIR axial (B) images.
Extensive treatments included high-dose lorazepam, quetiapine, levodopa, amantadine, memantine, dextromethorphan and quinidine combination, and 8 cycles of electroconvulsive therapy (ECT), leading to no improvement. Eventually palliative care was provided and the patient died nearly 2 years after her symptomatic onset. Postmortem genetic testing revealed significant GGGGCC repeat expansion in C9ORF72 alleles, confirming the clinical diagnosis of frontotemporal dementia (FTD).
DISCUSSION
FTD accounts for 5%–15% of all dementia cases and is the third most common type after Alzheimer disease and dementia with Lewy bodies.1 FTD has 2 major syndromes: the behavioral variant (bvFTD) with an initial presentation of behavioral changes and the aphasic variant presenting with language impairment including semantic dementia and progressive nonfluent aphasia.2 The clinical differentiation between bvFTD and primary psychiatric illnesses such as major depression, bipolar disorder, or schizophrenia can be difficult at times.
Most FTD cases are sporadic, with familial etiology accounting for slightly more than 20% of cases. The abnormal hexanucleotide repeat expansion in C9ORF72 is probably the most common genetic cause of bvFTD and FTD-ALS spectrum disorder.3 Approximately 6% of the sporadic bvFTD cases are also due to this mutation.4 Delusions or hallucinations have been viewed as markers for C9ORF72-associated bvFTD cases.5 Genetic testing was pursued in this patient given evidence of frontotemporal atrophy on MRI and active denervation on EMG, although the latter was limited because of the lack of cooperation from the patient for motor unit potential configuration. Her symptoms and signs of early behavioral disinhibition, stereotypies, and executive dysfunction with a gradual decline suggest bvFTD, which was further confirmed via imaging and genetic studies.
Literature review indicates that there are few reported familial FTD cases due to C9ORF72 mutation presenting with catatonia. A recent report described a 67-year-old man with familial FTD due to C9ORF72 mutation who presented with catatonia and responded favorably to standard psychiatric therapies, including ECT.6 The pathophysiology of catatonia has been described as a dysfunction of frontal circuits, including the prefrontal cortices.7 Overlapping symptoms of catatonia and FTD may be attributed to the sharing of frontal network dysfunction given that corticostriatothalamocortical (i.e., frontal-subcortical) circuits8 link basal ganglia that are implicated in catatonia9 with prefrontal areas that are disrupted in FTD. This relationship between catatonia and FTD warrants further investigation.
Approximately 1 in 7 patients with bvFTD has motor neuron disease.1 The coexistence of ALS and bvFTD indicates poor survival, with an estimated lifespan of 6 years from symptom onset and 1 year from diagnosis.1 Our patient had diffuse hyperreflexia, plantar extensor response, and evidence of denervation in the cervical and lumbosacral segments on EMG. Despite the lack of a clear ALS diagnosis, a FTD-ALS spectrum disorder was nonetheless suspected. This case highlights familial FTD as an important consideration in the differential diagnosis for a patient with new-onset psychosis, progressive cognitive decline, and rigidity.
AUTHOR CONTRIBUTIONS
L. Sheikhi: clinical care, case vignette, images, literature review. Y. Li: clinical care, case vignette, editing. X.F. Jimenez: clinical care, case vignette, editing, submission, corresponding author.
STUDY FUNDING
No targeted funding reported.
DISCLOSURES
The authors report no disclosures. Full disclosure form information provided by the authors is available with the full text of this article at http://cp.neurology.org/lookup/doi/10.1212/CPJ.0000000000000173.
Correspondence to: jimenex2@ccf.org
Funding information and disclosures are provided at the end of the article. Full disclosure form information provided by the authors is available with the full text of this article at http://cp.neurology.org/lookup/doi/10.1212/CPJ.0000000000000173.
Footnotes
Correspondence to: jimenex2@ccf.org
Funding information and disclosures are provided at the end of the article. Full disclosure form information provided by the authors is available with the full text of this article at http://cp.neurology.org/lookup/doi/10.1212/CPJ.0000000000000173.
REFERENCES
- 1.Karageorgiou E, Miller BL. Frontotemporal lobar degeneration: a clinical approach. Semin Neurol. 2014;34:189–201. doi: 10.1055/s-0034-1381735. [DOI] [PubMed] [Google Scholar]
- 2.Rademakers R, Neumann M, Mackenzie IR. Advances in understanding the molecular basis of frontotemporal dementia. Nat Rev Neurol. 2012;8:423–434. doi: 10.1038/nrneurol.2012.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rohrer JD, Isaacs AM, Mizielinska S. C9orf72 expansions in frontotemporal dementia and amyotrophic lateral sclerosis. Lancet Neurol. 2015;14:291–301. doi: 10.1016/S1474-4422(14)70233-9. [DOI] [PubMed] [Google Scholar]
- 4.Majounie E, Renton AE, Mok K. Frequency of the C9orf72 hexanucleotide repeat expansion in patients with amyotrophic lateral sclerosis and frontotemporal dementia: a cross-sectional study. Lancet Neurol. 2012;11:323–330. doi: 10.1016/S1474-4422(12)70043-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Takada LT, Sha SJ. Neuropsychiatric features of C9orf72-associated behavioral variant frontotemporal dementia and frontotemporal dementia with motor neuron disease. Alzheimers Res Ther. 2012;4:38. doi: 10.1186/alzrt141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holm AC. Neurodegenerative and psychiatric overlap in frontotemporal lobar degeneration: a case of familial frontotemporal dementia presenting with catatonia. Int Psychogeriatr. 2014;26:345–347. doi: 10.1017/S1041610213001403. [DOI] [PubMed] [Google Scholar]
- 7.Fink M, Taylor TA. Catatonia: A Clinician's Guide to Diagnosis and Treatment. New York: Cambridge University Press; 2003.
- 8.Tekin S, Cummings JL. Frontal-subcortical neuronal circuits and clinical neuropsychiatry: an update. J Psychosom Res. 2002;53:647–654. doi: 10.1016/s0022-3999(02)00428-2. [DOI] [PubMed] [Google Scholar]
- 9.Dhossche DM, Stoppelbein L, Rout UK. Etiopathogenesis of catatonia: generalizations and working hypotheses. J ECT. 2010;26:253–258. doi: 10.1097/YCT.0b013e3181fbf96d. [DOI] [PubMed] [Google Scholar]