The American Thoracic Society CORE Curriculum updates clinicians annually in adult and pediatric pulmonary diseases, medical critical care, and sleep medicine in a 3-year recurring cycle of topics. The 2015 course was presented in May during the annual International Conference and is published monthly in four parts beginning with the September issue of the journal. Part I covers advances in adult pulmonary medicine. An ABIM Maintenance of Certification module (MOC) and a Continuing Medical Education (CME) exercise covering the contents of the CORE Curriculum can be accessed online at thoracic.org until November 2018.
Cellular and Molecular Biology and Genetics
Chad R. Marion and Robert M. Tighe
Pulmonary and critical care physicians treat a diverse set of acute and chronic diseases. Understanding the molecular and genetic basis of these diseases allows for treatments with less drug toxicity and improved clinical response through personalized disease management.
Cellular and Molecular Biology
Cellular and molecular biology is a scientific approach to understanding cell development and complex cellular processes including modification and synthesis of both RNA and protein that are required for normal cellular homeostasis and response to injury and/or stress. Dysregulation of normal cellular functions frequently leads to the development of pathological states. Although much of this work is laboratory-based, these concepts are increasingly becoming mainstays in the diagnosis and treatment of both common and rare pulmonary diseases, such as asthma and pulmonary alveolar proteinosis. Asthma is a type-2 helper T-cell predominant disease characterized by reversible bronchoconstriction. This is especially true of atopic asthma, which is classically associated with type-2 helper T-cell cytokine production. Investigations have revealed that type-2 helper T-cell cytokines, such as IL-4 and IL-13, play an important role in asthma pathogenesis and are promising therapeutic targets (1).
Pulmonary alveolar proteinosis is characterized by filling of alveoli with periodic acid–Schiff-positive lipoproteinaceous material, much of which is surfactant and its associated proteins (2). This accumulation of surfactant is thought to occur because of a defect in normal alveolar macrophage–derived homeostasis, which is regulated by granulocyte-macrophage colony-stimulating factor (GM-CSF) (3). A deficiency of GM-CSF, due to either GM-CSF antibodies or a defect in GM-CSF production, leads to subsequent accumulation of alveolar surfactant. As a consequence of this understanding, GM-CSF antibody detection as a diagnostic modality and therapeutic target is under development.
Genetics
Genetics includes the study of gene sequences, variation, and inheritance. With robotic technology and high-throughput analysis platforms, the genetic basis of human disease is becoming more accessible for clinicians, leading to targeted therapies. Pulmonologists use genetic analysis to determine lung cancer treatment regimens based on the unique disease-causing genetic mutations, including epithelial growth factor receptor and anaplastic lymphoma kinase inhibitors (4, 5). Similar approaches are likely to play a role in the treatment of noncancerous lung diseases.
Genetic approaches are also employed to understand the pathogenesis of rare diseases, such as pulmonary lymphangioleiomyomatosis (LAM) and common diseases, such as sepsis. Pulmonary LAM, characterized by dyspnea and spontaneous pneumothorax in young females, is due to multiple small, thin-walled pulmonary cysts. Mutations in tuberous sclerosis complex 1 and 2 lead to uncontrolled cell proliferation in LAM and are believed to play a role in cyst formation (6). Sepsis is responsible for up to 250,000 deaths per year in the United States and is frequently caused by gram-negative infections (7). The most well-studied pattern recognition receptor in sepsis is Toll-like receptor-4 (TLR4), which recognizes and binds LPS derived from gram-negative bacteria. LPS-mediated activation of TLR4 induces signal transduction that results in the secretion of proinflammatory cytokines. Polymorphisms in TLR4 can lead to decreased recognition of LPS and increased susceptibility to sepsis (8).
Advancements in cellular and molecular biology and genetics impact the diagnosis and treatment of pulmonary and critical care diseases. The key points described previously are listed in Table 1. Although these advancements are paving the way for real-time personalized medicine, they are rapidly changing and evolving. Pulmonologists must stay abreast of these advancements to provide the most up-to-date care for their patients.
Table 1.
Key points: Cellular and molecular biology
| • Lung cancer treatment regimens are frequently based on the unique disease-causing genetic mutations including EGFR and ALK |
| • TLR4, the most well-studied pattern recognition receptor in sepsis, recognizes and binds LPS derived from gram-negative bacteria |
| • In pulmonary LAM, mutations in TSC1 and TSC2 lead to uncontrolled cell proliferation and are believed to play a role in cyst formation |
Definition of abbreviations: ALK = anaplastic lymphoma kinase; EGFR = epidermal growth factor receptor; LAM = lymphangioleiomyomatosis; LPS = lipopolysaccharide; TLR = Toll-like receptor; TSC = tuberous sclerosis complex.
References
- 1.Oh CK, Geba GP, Molfino N. Investigational therapeutics targeting the IL-4/IL-13/STAT-6 pathway for the treatment of asthma. Eur Respir Rev. 2010;19:46–54. doi: 10.1183/09059180.00007609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Trapnell BC, Whitsett JA, Nakata K. Pulmonary alveolar proteinosis. N Engl J Med. 2003;349:2527–2539. doi: 10.1056/NEJMra023226. [DOI] [PubMed] [Google Scholar]
- 3.Wang T, Lazar CA, Fishbein MC, Lynch JP., III Pulmonary alveolar proteinosis. Semin Respir Crit Care Med. 2012;33:498–508. doi: 10.1055/s-0032-1325160. [DOI] [PubMed] [Google Scholar]
- 4.Sharma SV, Bell DW, Settleman J, Haber DA. Epidermal growth factor receptor mutations in lung cancer. Nat Rev Cancer. 2007;7:169–181. doi: 10.1038/nrc2088. [DOI] [PubMed] [Google Scholar]
- 5.Lindeman NI, Cagle PT, Beasley MB, Chitale DA, Dacic S, Giaccone G, Jenkins RB, Kwiatkowski DJ, Saldivar JS, Squire J, et al. Molecular testing guideline for selection of lung cancer patients for EGFR and ALK tyrosine kinase inhibitors: guideline from the College of American Pathologists, International Association for the Study of Lung Cancer, and Association for Molecular Pathology. J Thorac Oncol. 2013;8:823–859. doi: 10.1097/JTO.0b013e318290868f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Henske EP, McCormack FX. Lymphangioleiomyomatosis—a wolf in sheep’s clothing. J Clin Invest. 2012;122:3807–3816. doi: 10.1172/JCI58709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 8.O’Neill LA, Golenbock D, Bowie AG. The history of Toll-like receptors—redefining innate immunity. Nat Rev Immunol. 2013;13:453–460. doi: 10.1038/nri3446. [DOI] [PubMed] [Google Scholar]
Exercise and Pulmonary Function Testing
James A. Town and Andrew M. Luks
Physiological testing is routinely used to narrow the differential diagnosis, grade the severity of impairment, and monitor for disease progression in patients with lung disease.
Cardiopulmonary Exercise Testing
Cardiopulmonary exercise testing can quantify maximal exercise capacity and identify the major problem limiting exercise (1). Performance is judged relative to predicted values based on population norms but, when possible, an individual’s performance should be interpreted relative to their baseline. Careful observation of the exercising patient is useful to exclude poor effort as a cause of low observed exercise capacity.
Table 2 summarizes the exercise responses in different patterns of exercise limitation including cardiac limitation, ventilatory limitation, and limitation due to pulmonary vascular or interstitial lung disease (1). Normal individuals manifest a cardiac pattern of limitation for which the presence of a ventilatory threshold is a key component. This is best identified by finding a change in the slope of the relationship between Vco2 and Vo2 (volumes of exhaled carbon dioxide and oxygen, respectively) or the point at which the ventilatory equivalents for O2 and CO2 start increasing. Patients limited by heart disease manifest a similar pattern but have reduced maximal exercise capacity. The hallmark of ventilatory limitation is rising PetCO2 (end-tidal CO2) or PaCO2 (arterial oxygen tension) at end-exercise, with other key factors being the absence of a ventilatory threshold and dyspnea as the primary symptom at end-exercise. Patients limited by pulmonary vascular or interstitial lung diseases manifest similar responses as cardiac-limited patients, but are distinguished by hypoxemia at end-exercise and, in some cases, an unchanged or increasing dead-space fraction.
Table 2.
Basic patterns of exercise limitation
| Variable | Pattern of Limitation |
||
|---|---|---|---|
| Cardiac | Ventilatory | Pulmonary Vascular/Interstitial Lung Disease | |
| Ventilatory threshold | Present | Absent | Present |
| PaCO2 or PetCO2 at end-exercise | Decreased | Unchanged or increased | Decreased |
| PaO2 at end-exercise | Stable | Decreased | Decreased |
| Dead-space fraction | Decreased | Unchanged or decreased | Unchanged or increased |
| Metabolic acidosis at end-exercise | Present | Absent | Present |
| Reason for stopping test | Leg fatigue | Dyspnea | Dyspnea and/or leg fatigue |
Definition of abbreviation: PetCO2 = end-tidal (partial) carbon dioxide pressure.
Note: This table summarizes the most common patterns seen in cardiac, ventilatory, and pulmonary vascular limitations to exercise.
Six-Minute-Walk Test
The six-minute-walk test (6MWT) is a submaximal exercise test whose primary outcome is distance walked. It is used to assess baseline exercise capacity, disease progression, and response to therapy in patients with chronic respiratory diseases and has no role in evaluating fit individuals (2). When comparing an individual’s performance over time, results must be interpreted in light of the minimal clinically important difference, which for the 6MWT is 30 m (3). The Incremental Shuttle Walk Test and Endurance Shuttle Walk Test are alternative submaximal exercise tests whose primary outcomes are distance and time walked, respectively (4, 5).
Pulmonary Function Testing
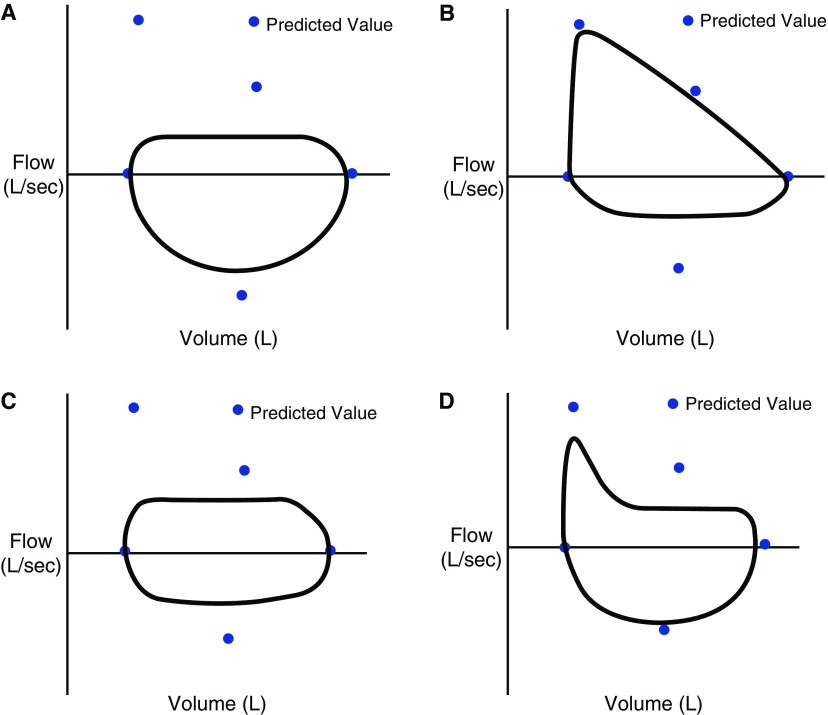
Interpretation of spirometry should always include analysis of the graphical data, as the flow–volume loop may help differentiate among the various causes of obstruction (Figure 1) (6). Methacholine challenge testing is a sensitive test of airway hyperresponsiveness useful for excluding asthma when other diagnostic testing is equivocal. At high concentrations of methacholine there is increased likelihood of inducing airflow obstruction in most individuals and, as a result, test specificity decreases at higher concentrations. The tests should be interpreted in light of the pretest probability of asthma (7, 8).
Figure 1.
Classic flow–volume patterns. Flow–volume loop patterns suggestive of upper airway obstruction as the source of airflow obstruction on spirometry. (A) Variable intrathoracic obstruction, marked by flattening of the expiratory limb of the flow–volume loop. (B) Variable extrathoracic obstruction marked by flattening of the inspiratory limb of the flow–volume loop. (C) Fixed upper airway obstruction with flattening of the expiratory and inspiratory limbs of the flow–volume loop. (D) Unilateral mainstem obstruction marked by a rapid decline in airflow in the initial portion of exhalation and a flattening of the curve over the remainder of that phase.
The diffusing capacity for carbon monoxide (DlCO) assesses the surface area for gas exchange across the alveolar–capillary barrier (9). Abnormal results have different diagnostic implications depending on other findings on pulmonary function testing (Table 3). The DlCO can be adjusted for hemoglobin and alveolar volume; however, caution is advised with the latter adjustment as the effects of alveolar volume on diffusing capacity have not been validated in processes such as emphysema and diffuse parenchymal lung disease.
Table 3.
Interpreting abnormal, unadjusted values for diffusing capacity of the lung for carbon monoxide
| Decreased DlCO Plus Airflow Obstruction | Decreased DlCO Plus Restrictive Pattern | Decreased DlCO with Normal Spirometry and Lung Volumes | Increased DlCO |
|---|---|---|---|
| Emphysema | Idiopathic pulmonary fibrosis | Pulmonary vascular diseases | Asthma |
| Lymphangioleiomyomatosis | Asbestosis | Early diffuse parenchymal lung disease | Obesity |
| Severe cystic fibrosis | Other diffuse parenchymal lung diseases | Anemia | Left-to-right shunt |
| Pulmonary edema | Increased carboxyhemoglobin | Pulmonary hemorrhage | |
| Polycythemia |
Definition of abbreviation: DlCO = diffusing capacity of the lung for carbon monoxide.
References
- 1.American Thoracic Society; American College of Chest Physicians. ATS/ACCP statement on cardiopulmonary exercise testing. Am J Respir Crit Care Med. 2003;167:211–277. doi: 10.1164/rccm.167.2.211. [DOI] [PubMed] [Google Scholar]
- 2.ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: guidelines for the Six-Minute Walk Test. Am J Respir Crit Care Med. 2002;166:111–117. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 3.Mathai SC, Puhan MA, Lam D, Wise RA. The minimal important difference in the 6-Minute Walk Test for patients with pulmonary arterial hypertension. Am J Respir Crit Care Med. 2012;186:428–433. doi: 10.1164/rccm.201203-0480OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parreira VF, Janaudis-Ferreira T, Evans RA, Mathur S, Goldstein RS, Brooks D. Measurement properties of the Incremental Shuttle Walk Test: a systematic review. Chest. 2014;145:1357–1369. doi: 10.1378/chest.13-2071. [DOI] [PubMed] [Google Scholar]
- 5.Singh SJ, Puhan MA, Andrianopoulos V, Hernandes NA, Mitchell KE, Hill CJ, Lee AL, Camillo CA, Troosters T, Spruit MA, et al. An official systematic review of the European Respiratory Society/American Thoracic Society: measurement properties of field walking tests in chronic respiratory disease. Eur Respir J. 2014;44:1447–1478. doi: 10.1183/09031936.00150414. [DOI] [PubMed] [Google Scholar]
- 6.Pellegrino R, Viegi G, Brusasco V, Crapo RO, Burgos F, Casaburi R, Coates A, van der Grinten CP, Gustafsson P, Hankinson J, et al. Interpretative strategies for lung function tests. Eur Respir J. 2005;26:948–968. doi: 10.1183/09031936.05.00035205. [DOI] [PubMed] [Google Scholar]
- 7.Crapo RO, Casaburi R, Coates AL, Enright PL, Hankinson JL, Irvin CG, MacIntyre NR, McKay RT, Wanger JS, Anderson SD, et al. Guidelines for methacholine and exercise challenge testing—1999. Am J Respir Crit Care Med. 2000;161:309–329. doi: 10.1164/ajrccm.161.1.ats11-99. [DOI] [PubMed] [Google Scholar]
- 8.Cockcroft DW. Direct challenge tests: airway hyperresponsiveness in asthma: its measurement and clinical significance. Chest. 2010;138(2) Suppl:18S–24S. doi: 10.1378/chest.10-0088. [DOI] [PubMed] [Google Scholar]
- 9.Macintyre N, Crapo RO, Viegi G, Johnson DC, van der Grinten CP, Brusasco V, Burgos F, Casaburi R, Coates A, Enright P, et al. Standardisation of the single-breath determination of carbon monoxide uptake in the lung. Eur Respir J. 2005;26:720–735. doi: 10.1183/09031936.05.00034905. [DOI] [PubMed] [Google Scholar]
Asthma
Jeremy B. Richards
Asthma is characterized by intermittent airway inflammation resulting in epithelial injury, mucous gland proliferation and hypertrophy, airway smooth muscle hypertrophy, and basement membrane thickening. These pathophysiological changes typically occur in response to an environmental allergen or an acute infection (typically viral) in a patient with a predisposition for reactive airway responses. Clinically, asthma is characterized by intermittent and typically completely reversible obstructive expiratory airflow. Consequences are stratified on the basis of the severity of attributable symptoms.
Severe asthma is defined as asthma necessitating treatment with high-dose inhaled corticosteroids (ICS) and a second controller medication (long-acting β-agonist [LABA] or long-acting muscarinic antagonist [LAMA]) and/or systemic corticosteroids. Severe asthma is not a single disease process, but rather the consequence of a heterogeneous array of genetic and phenotypic features.
Diagnosis
The initial evaluation of patients presenting with severe or difficult asthma should include diagnostic testing to confirm the diagnosis of asthma. Up to 30% of patients presenting to specialists for management of “asthma” may be found, after rigorous assessment, not to have asthma (1). If severe asthma is confirmed, assessment of control is indicated to guide subsequent therapeutic interventions. Clinical criteria for uncontrolled asthma are detailed in Table 4.
Table 4.
Clinical criteria for uncontrolled asthma
| Major criteria (at least one required) | 1. Oral corticosteroids for >50% of the past year |
| 2. Continuous high-dose inhaled corticosteroids (≥1,000 μg of fluticasone per day) | |
| Minor criteria (at least two required) | 1. Concurrent use of at least one other controller medication |
| 2. Daily symptoms requiring a short-acting β-agonist | |
| 3. FEV1 < 80% predicted | |
| 4. One or more urgent care visits in the past year | |
| 5. Three or more oral corticosteroid bursts in the past year | |
| 6. Deterioration with a decrease in corticosteroid dose of 25% | |
| 7. History of a near-fatal asthma-related event |
Although there have been marked advances in genetic, phenotypic, and biomarker characterizations and assessments for severe asthma, research advances have not yet translated to changes in clinical practice. Promising preliminary findings for biomarkers such as the fraction of exhaled nitric oxide have not been corroborated by multiple studies, and the current clinical standard for diagnosis and longitudinal monitoring of severe asthma remains the combination of clinical assessment and patients’ spirometric performance (2). Advanced radiographic imaging, molecular markers, and airway biopsy remain experimental interventions and are not components of routine clinical practice. One potential exception is sputum eosinophil counts, which may have some clinical usefulness in guiding treatment at centers with experience in performing these measurements, although supporting data are limited (3).
Epidemiological studies have demonstrated that Hispanic ethnicity, family history of atopic dermatitis, pet(s) at home, and dust sensitivity are protective regarding both persistent airflow obstruction and increased rate of decline in FEV1 over time.
Treatment
Patients with severe asthma demonstrate persistent symptoms despite high-dose ICS (Table 5) (4). Up to 30% of these patients require oral corticosteroids. Additional options for patients with severe asthma include LABAs, which have been shown to improve symptoms when added to ICS. However, there is significant variability in patient responses to LABAs, and there are concerns regarding increased asthma mortality from these agents, particularly at high doses (5).
Table 5.
Stepwise approach for managing asthma
| Intermittent Asthma (Step 1) | Persistent Asthma (Step 2) | Persistent Asthma (Step 3) | Persistent Asthma (Step 4) | Persistent Asthma (Step 5) | Persistent Asthma (Step 6) | |
|---|---|---|---|---|---|---|
| Preferred treatment | SABA as needed | Low-dose ICS | Low-dose ICS + LABA (or medium-dose ICS) | Medium-dose ICS + LABA | High-dose ICS + LABA (and consider omalizumab for select patients) | High-dose ICS + LABA + oral corticosteroid (and consider omalizumab for select patients) |
| Alternative treatment | Cromolyn, LTRA, or theophylline (and consider allergen immunotherapy as needed) | Low-dose ICS + LTRA, theophylline (and consider allergen immunotherapy as needed) | Medium-dose ICS + LTRA, theophylline (and consider allergen immunotherapy as needed) |
Definition of abbreviations: ICS = inhaled corticosteroid; LABA = long-acting β-agonist; LTRA = leukotriene receptor antagonist; SABA = short-acting β-agonist.
LAMAs (specifically tiotropium), when added to ICS and LABA, have been shown to increase FEV1, decrease symptoms and rescue inhaler use, and increase the interval between severe exacerbations (6). Leukotriene receptor antagonists may benefit patients with aspirin-sensitive severe asthma, but are not as effective as adding an LABA to ICS. The monoclonal anti-IgE antibody, omalizumab, decreases adverse events including hospitalization and severe exacerbations and improves symptoms when added to ICS and LABA treatment (7). The current National Institutes of Health guideline recommendations for treatment of asthma and severe asthma for adults (≥12 yr old) are summarized in Table 5 (8).
Future Directions
Experimental treatments for severe asthma include alternative monoclonal antibodies, such as mepolizumab. This anti–IL-5 antibody demonstrated a decline in severe exacerbations compared with placebo in a randomized, double-blind, multicenter study (9). Of note, this study included only patients with elevated airway and sputum eosinophil counts. The role of other therapies including immunomodulatory medications (e.g., methotrexate), bronchial thermoplasty, and macrolide antibiotics remains to be determined.
References
- 1.Aaron SD, Vandemheen KL, Boulet LP, McIvor RA, Fitzgerald JM, Hernandez P, Lemiere C, Sharma S, Field SK, Alvarez GG, et al. Canadian Respiratory Clinical Research Consortium. Overdiagnosis of asthma in obese and nonobese adults. CMAJ. 2008;179:1121–1131. doi: 10.1503/cmaj.081332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Calhoun WJ, Ameredes BT, King TS, Icitovic N, Bleecker ER, Castro M, Cherniack RM, Chinchilli VM, Craig T, Denlinger L, et al. Asthma Clinical Research Network of the National Heart, Lung, and Blood Institute. Comparison of physician-, biomarker-, and symptom-based strategies for adjustment of inhaled corticosteroid therapy in adults with asthma: the BASALT randomized controlled trial. JAMA. 2012;308:987–997. doi: 10.1001/2012.jama.10893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Veen IH, Ten Brinke A, Gauw SA, Sterk PJ, Rabe KF, Bel EH. Consistency of sputum eosinophilia in difficult-to-treat asthma: a 5-year follow-up study. J Allergy Clin Immunol. 2009;124:615–617, 617.e1–617.e2. doi: 10.1016/j.jaci.2009.06.029. [DOI] [PubMed] [Google Scholar]
- 4.Lemanske RF, Jr, Mauger DT, Sorkness CA, Jackson DJ, Boehmer SJ, Martinez FD, Strunk RC, Szefler SJ, Zeiger RS, Bacharier LB, et al. Childhood Asthma Research and Education (CARE) Network of the National Heart, Lung, and Blood Institute. Step-up therapy for children with uncontrolled asthma receiving inhaled corticosteroids. N Engl J Med. 2010;362:975–985. doi: 10.1056/NEJMoa1001278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Suissa S, Ernst P, Boivin JF, Horwitz RI, Habbick B, Cockroft D, Blais L, McNutt M, Buist AS, Spitzer WO. A cohort analysis of excess mortality in asthma and the use of inhaled β-agonists. Am J Respir Crit Care Med. 1994;149:604–610. doi: 10.1164/ajrccm.149.3.8118625. [DOI] [PubMed] [Google Scholar]
- 6.Kerstjens HA, Engel M, Dahl R, Paggiaro P, Beck E, Vandewalker M, Sigmund R, Seibold W, Moroni-Zentgraf P, Bateman ED. Tiotropium in asthma poorly controlled with standard combination therapy. N Engl J Med. 2012;367:1198–1207. doi: 10.1056/NEJMoa1208606. [DOI] [PubMed] [Google Scholar]
- 7.Rodrigo GJ, Neffen H, Castro-Rodriguez JA. Efficacy and safety of subcutaneous omalizumab vs placebo as add-on therapy to corticosteroids for children and adults with asthma: a systematic review. Chest. 2011;139:28–35. doi: 10.1378/chest.10-1194. [DOI] [PubMed] [Google Scholar]
- 8.National Institute of Health, National Heart, Lung and Blood InstituteExpert Panel Report 3: guidelines for the diagnosis and management of asthma. Washington, DC: Government Printing Office; 2007. NIH Publication No. 07-4051 [Google Scholar]
- 9.Ortega HG, Liu MC, Pavord ID, Brusselle GG, FitzGerald JM, Chetta A, Humbert M, Katz LE, Keene ON, Yancey SW, et al. MENSA Investigators. Mepolizumab treatment in patients with severe eosinophilic asthma. N Engl J Med. 2014;371:1198–1207. doi: 10.1056/NEJMoa1403290. [DOI] [PubMed] [Google Scholar]
Obstructive Airway Disorders
Sucharita Kher and Prerna Mota
Obliterative Bronchiolitis
Obliterative bronchiolitis is characterized by cough, dyspnea, and progressive worsening of airway obstruction. Inflammation of small airways results in excessive fibroproliferation. Obliterative bronchiolitis can occur with autoimmune conditions (rheumatoid arthritis), inhalational toxin exposures, infections (viral, Mycoplasma), and posttransplantation (1–3).
Spirometry typically shows an obstructive defect with poor response to bronchodilators. Whereas chest radiographs are often unremarkable, expiratory films on high-resolution computed tomography of the chest frequently reveal a “mosaic pattern” with areas of decreased attenuation suggesting hyperinflation. Bronchoscopy with bronchoalveolar lavage and transbronchial biopsy is often performed to rule out infections and acute rejection in transplant recipients (1).
Bronchiolitis Obliterans Syndrome
Symptoms and spirometric findings of bronchiolitis obliterans in patients who have undergone lung transplantation or hematopoietic stem cell transplantation are known as bronchiolitis obliterans syndrome (BOS). BOS represents a chronic allograft rejection in lung transplant recipients and graft-versus-host disease after stem cell transplantation. It is the leading cause of death beyond 1 year after lung transplantation.
BOS is defined clinically by a decline in FEV1 to less than or equal to 80% of baseline posttransplantation FEV1 occurring after 3 months posttransplantation, for a minimal period of 3 weeks. It is also important to rule out all reversible causes. BOS treatment guidelines are still evolving but the current recommendations include switching immunosuppression to tacrolimus (if currently taking cyclosporine), administering azithromycin (with bronchoalveolar lavage neutrophilia), avoiding long-term, high-dose steroids, referral for fundoplication for proven reflux disease, and consideration for retransplantation for end-stage BOS (4).
Paradoxical Vocal Fold Motion Disorder
Paradoxical vocal fold motion disorder is defined as inappropriate adduction of true vocal cords on inspiration. It is a mimicker of asthma and presentation is variable including dyspnea, upper airway wheezing, and/or stridor. Triggers include reflux, strong smells, respiratory infections, and emotional stressors (5). The gold standard for diagnosis is direct observation of the inspiratory adduction of vocal cords with a posterior diamond-shaped chink on laryngoscopy. The flow–volume loop may show a truncated inspiratory limb suggesting variable extrathoracic obstruction. Structural glottic disorders including vocal cord palsy must be ruled out. Treatment includes patient reassurance, education, treating reflux (if present), speech therapy, and psychotherapy by a multidisciplinary team.
Bronchiectasis
Bronchiectasis is defined as abnormal dilation of bronchi and conducting airways. Causes include infections (atypical mycobacteria), congenital disorders (primary ciliary dyskinesia), inflammatory diseases (rheumatoid arthritis, ulcerative colitis), immunodeficiencies, and reflux; or it may be idiopathic. Cough with copious sputum (especially in absence of smoking), chronic nonproductive cough, hemoptysis, difficult-to-control asthma, and recurrent lung infections should trigger evaluation for possibility of bronchiectasis.
High-resolution computed tomography of the chest establishes the diagnosis and helps to identify a cause (e.g., infection, structural lung disease). Spirometry reveals an obstructive ventilatory defect (Figure 1D) and should be performed annually in all adults with bronchiectasis and more frequently in the presence of immunodeficiency or a ciliary defect. Testing for immunoglobulin deficiency, allergic bronchopulmonary aspergillosis, α1-antitrypsin deficiency, and rheumatological disease can be done on the basis of history.
Sputum culture should be obtained in all patients. Bronchoscopy with bronchoalveolar lavage is performed when high-resolution computed tomography demonstrates evidence of atypical mycobacteria with nondiagnostic sputum, when the patient does not respond to medical treatment, and to exclude proximal obstruction for localized disease (6).
Table 6 lists a heterogeneous group of obstructive airway disorders with potentially similar clinical features.
Table 6.
Key points: Obstructive airway disorders
| • Obliterative bronchiolitis can occur with autoimmune conditions, inhalational toxin exposures, infections, and posttransplantation and may present with irreversible airflow obstruction and mosaic pattern on high-resolution computed tomography |
| • Bronchiolitis obliterans syndrome represents a chronic allograft rejection in lung transplant recipients and is the leading cause of death beyond 1 yr after lung transplantation |
| • Paradoxical vocal fold motion disorder can mimic asthma with a presentation that includes dyspnea, upper airway wheezing, and/or stridor |
| • Bronchiectasis may be idiopathic or caused by infections, congenital disorders, inflammatory diseases, immunodeficiencies, and reflux |
References
- 1.Barker AF, Bergeron A, Rom WN, Hertz MI. Obliterative bronchiolitis. N Engl J Med. 2014;370:1820–1828. doi: 10.1056/NEJMra1204664. [DOI] [PubMed] [Google Scholar]
- 2.King MS, Eisenberg R, Newman JH, Tolle JJ, Harrell FE, Jr, Nian H, Ninan M, Lambright ES, Sheller JR, Johnson JE, et al. Constrictive bronchiolitis in soldiers returning from Iraq and Afghanistan. N Engl J Med. 2011;365:222–230. doi: 10.1056/NEJMoa1101388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kreiss K, Gomaa A, Kullman G, Fedan K, Simoes EJ, Enright PL. Clinical bronchiolitis obliterans in workers at a microwave-popcorn plant. N Engl J Med. 2002;347:330–338. doi: 10.1056/NEJMoa020300. [DOI] [PubMed] [Google Scholar]
- 4.Meyer KC, Raghu G, Verleden GM, Corris PA, Aurora P, Wilson KC, Brozek J, Glanville AR ISHLT/ATS/ERS BOS Task Force Committee; ISHLT/ATS/ERS BOS Task Force Committee. An international ISHLT/ATS/ERS clinical practice guideline: diagnosis and management of bronchiolitis obliterans syndrome. Eur Respir J. 2014;44:1479–1503. doi: 10.1183/09031936.00107514. [DOI] [PubMed] [Google Scholar]
- 5.Hoyte FC. Vocal cord dysfunction. Immunol Allergy Clin North Am. 2013;33:1–22. doi: 10.1016/j.iac.2012.10.010. [DOI] [PubMed] [Google Scholar]
- 6.Pasteur MC, Bilton D, Hill AT British Thoracic Society Non-CF Bronchiectasis Guideline Group. British Thoracic Society guideline for non-CF bronchiectasis. Thorax. 2010;65:577. doi: 10.1136/thx.2010.142778. [DOI] [PubMed] [Google Scholar]
Rare and Congenital Lung Diseases
Gina Hong and Natalie E. West
By definition in the United States, rare diseases affect fewer than 200,000 Americans. Many rare pulmonary diseases affect far fewer patients.
Cystic Fibrosis
Cystic fibrosis (CF) is the most common autosomal recessive disorder in the white population, with a frequency of 1 in 2,500 births. CF can be caused by approximately 2,000 genetic mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) gene, resulting in abnormal chloride transport (1).
The leading cause of mortality is respiratory failure from recurrent pulmonary infections. Pseudomonas aeruginosa, Staphylococcus aureus (both methicillin sensitive and resistant), and Stenotrophomonas maltophilia contribute to chronic infections resulting in progressive loss of lung function, with the first two pathogens associated with a more rapid decline in lung function. Other manifestations of CF include chronic sinusitis, pancreatic insufficiency, diabetes, hepatobiliary disease, intestinal obstruction, and male infertility (2).
Improvement in management of CF has increased survival from 5 to 41 years of age in the past six decades. Airway clearance, inhaled dornase alfa, hypertonic saline, and antimicrobial therapy remain the cornerstones of therapy. A new era of therapy for CF includes the use of ivacaftor, a CFTR potentiator, which is an oral agent that improves CFTR function in approximately 10% of individuals with CF, resulting in improved lung function and decreased pulmonary exacerbations (3).
Primary Ciliary Dyskinesia
Primary ciliary dyskinesia (PCD) is an autosomal recessive disease caused by structural abnormalities of motile cilia. Diagnosis is classically made by biopsy of nasal epithelium. The latest diagnostic advances include measurement of nasal nitric oxide and genetic testing of 21 known mutations. The triad of chronic sinusitis, bronchiectasis, and situs inversus is known as Kartagener syndrome, occurring in 50% of PCD, and is readily diagnosed by clinical assessment alone.
Impairment of mucociliary clearance results in chronic cough, purulent sputum, and chronic infection, similar to CF (4). Therefore, pulmonary management of PCD mirrors that of CF with airway clearance, antibiotics, and nebulized hypertonic saline. However, inhaled dornase alfa is associated with increased pulmonary exacerbations and lung function decline and is not recommended (5).
Lung Disease Related to Impaired Immunity
Common variable immunodeficiency is caused by defective immunoglobulin production. The diagnosis is most commonly made in the second or third decade of life by discovery of low IgG and low IgM and/or IgA levels and poor vaccination response.
Because of inadequate immunoglobulin levels, recurrent sinopulmonary infections secondary to Streptococcus pneumoniae, Haemophilus influenzae, and Mycoplasma pneumoniae result in chronic sinusitis, recurrent pneumonias, and bronchiectasis. More rarely, lymphoid interstitial pneumonitis, granulomatous lung disease, and lymphoma related to common variable immunodeficiency are seen (6). Monthly immunoglobulin replacement decreases the incidence of infection and slows progression of chronic lung disease in common variable immunodeficiency.
Chronic granulomatous disease is caused by a defect in NADPH oxidase, resulting in an inability to kill bacteria and fungi such as Staphylococcus, Burkholderia, Nocardia, and Aspergillus species. Chronic infections can result in abscesses, pneumonia, bronchiectasis, and fibrosis (7). Long-term treatment with antibiotics and antifungal agents are mainstays of therapy, and a notable advance in curative therapy for chronic granulomatous disease is hematopoietic stem cell transplantation.
Marfan Syndrome and Ehlers–Danlos Syndrome
Inherited connective tissue disorders, including Marfan syndrome and Ehlers–Danlos syndrome, are associated with upper lobe–predominant emphysema, blebs, and spontaneous pneumothoraces. Skeletal abnormalities, including kyphoscoliosis and pectus deformities, are often present, resulting in restrictive lung disease (8). Although tall stature, joint hypermobility, and arachnodactyly are shared characteristics between both disorders, there are distinct differences. Aortic root disease is nearly exclusively seen in Marfan syndrome; whereas skin hyperextensibility and bruising is more common in Ehlers–Danlos syndrome.
Neuromuscular Disease–Related Respiratory Weakness
Congenital and acquired neuromuscular disease can result in respiratory weakness and progress to respiratory failure. Respiratory muscle weakness is secondary to the inability of the respiratory muscles to generate adequate pressure and flow during inspiration and expiration, which results in dyspnea (9). Orthopnea often occurs because of the decreased compliance of the chest wall and lungs, which worsens with reclining (attributable to the lack of gravity assisting in pulling the diaphragm down in the supine position).
Respiratory physiology measured by pulmonary function tests demonstrates reduced total lung capacity, reduced vital capacity, elevated residual volume, maximal inspiratory pressure less than –30 cm H2O, maximal expiratory pressure less than 40 cm H2O, and supine vital capacity declining more than 10% compared with upright spirometry values (10).
Table 7 summarizes key points described in the preceding section.
Table 7.
Key points: Rare and congenital lung disease
| • Ivacaftor has been shown to improve CFTR function in approximately 10% of patients with cystic fibrosis, resulting in improved lung function and decreased pulmonary exacerbations |
| • Primary ciliary dyskinesia is diagnosed by biopsy of the nasal epithelium |
| • Inherited connective tissue disorders, including Marfan and Ehlers–Danlos syndromes, are associated with upper lobe–predominant emphysema, blebs, and spontaneous pneumothorax |
Definition of abbreviation: CFTR = cystic fibrosis transmembrane conductance regulator.
References
- 1.Ratjen F, Döring G. Cystic fibrosis. Lancet. 2003;361:681–689. doi: 10.1016/S0140-6736(03)12567-6. [DOI] [PubMed] [Google Scholar]
- 2.Boyle MP. Adult cystic fibrosis. JAMA. 2007;298:1787–1793. doi: 10.1001/jama.298.15.1787. [DOI] [PubMed] [Google Scholar]
- 3.Ramsey BW, Davies J, McElvaney NG, Tullis E, Bell SC, Dřevínek P, Griese M, McKone EF, Wainwright CE, Konstan MW, et al. VX08-770-102 Study Group. A CFTR potentiator in patients with cystic fibrosis and the G551D mutation. N Engl J Med. 2011;365:1663–1672. doi: 10.1056/NEJMoa1105185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Knowles MR, Daniels LA, Davis SD, Zariwala MA, Leigh MW. Primary ciliary dyskinesia: recent advances in diagnostics, genetics, and characterization of clinical disease. Am J Respir Crit Care Med. 2013;188:913–922. doi: 10.1164/rccm.201301-0059CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O’Donnell AE, Barker AF, Ilowite JS, Fick RB. rhDNase Study Group. Treatment of idiopathic bronchiectasis with aerosolized recombinant human DNase I. Chest. 1998;113:1329–1334. doi: 10.1378/chest.113.5.1329. [DOI] [PubMed] [Google Scholar]
- 6.Busse PJ, Farzan S, Cunningham-Rundles C. Pulmonary complications of common variable immunodeficiency. Ann Allergy Asthma Immunol. 2007;98:1–8, quiz 8–11, 43. doi: 10.1016/S1081-1206(10)60853-8. [DOI] [PubMed] [Google Scholar]
- 7.Mahdaviani SA, Mohajerani SA, Rezaei N, Casanova JL, Mansouri SD, Velayati AA. Pulmonary manifestations of chronic granulomatous disease. Expert Rev Clin Immunol. 2013;9:153–160. doi: 10.1586/eci.12.98. [DOI] [PubMed] [Google Scholar]
- 8.Dinwiddie R, Sonnappa S. Systemic diseases and the lung. Paediatr Respir Rev. 2005;6:181–189. doi: 10.1016/j.prrv.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 9.Ambrosino N, Carpenè N, Gherardi M. Chronic respiratory care for neuromuscular diseases in adults. Eur Respir J. 2009;34:444–451. doi: 10.1183/09031936.00182208. [DOI] [PubMed] [Google Scholar]
- 10.Steier J, Kaul S, Seymour J, Jolley C, Rafferty G, Man W, Luo YM, Roughton M, Polkey MI, Moxham J. The value of multiple tests of respiratory muscle strength. Thorax. 2007;62:975–980. doi: 10.1136/thx.2006.072884. [DOI] [PMC free article] [PubMed] [Google Scholar]
Interstitial Lung Disease Diagnosis and Guidelines
Craig Rackley and Luke Neilans
Interstitial lung disease is an array of diffuse parenchymal lung diseases that can be difficult to differentiate and even more difficult to treat (Table 8). For these reasons, the American Thoracic Society (ATS) recommends cases be discussed at a multidisciplinary conference of experts to increase the diagnostic accuracy (1, 2).
Table 8.
Key points: Interstitial lung disease
| • Smoking-related interstitial disease includes respiratory bronchiolitis, desquamative interstitial pneumonia, and pulmonary Langerhans cell histiocytosis |
| • In patients with no clear identifiable cause of their interstitial disease, a diagnosis of idiopathic pulmonary fibrosis is made if they meet the ATS diagnostic criteria on imaging without a surgical biopsy or a definite/possible UIP pattern on HRCT with a biopsy showing definite or probable UIP |
| • Two new antifibrotic drugs, nintedanib and pirfenidone, have been shown to slow the progression of disease as measured by a reduced rate of decline in forced vital capacity |
Definition of abbreviations: ATS = American Thoracic Society; HRCT = high-resolution computed tomography; UIP = usual interstitial pneumonia.
Diagnosis
Diagnosis of an interstitial lung disease is made through a collaborative review of the clinical history, radiographs, and, if available, pathology. A major aspect of evaluating patients is the search for identifiable causes. This involves a thorough history and physical examination searching for possible exposures or associated symptoms to help guide further evaluation and management.
The ATS recommends serological evaluation for evidence of connective tissue disease with rheumatoid factor, anti-nuclear antibody titers, and anti–cyclic citrullinated peptide (1). In appropriate cases further testing for other serological markers of connective tissue diseases, such as extractable nuclear antigens, Scl70 (anti-topoisomerase 1), creatinine kinase, aldolase, or anti-neutrophil cytoplasmic antibody is considered.
Antisynthetase syndrome, characterized by the presence of anti–tRNA synthetase autoantibody, myositis, mechanic’s hands, arthritis, fever, and Raynaud’s phenomenon, is also associated with interstitial lung disease.
In patients with no clearly identifiable cause of their interstitial disease, a diagnosis of idiopathic pulmonary fibrosis is made if the ATS diagnostic criteria for a usual interstitial pneumonia (UIP) pattern on high-resolution computed tomography without a surgical biopsy, or a definite/possible UIP pattern on high-resolution computed tomography with a biopsy showing definite or probable UIP, are met (1).
In sarcoidosis, chest radiography, pulmonary function tests, blood work, urine analysis, electrocardiogram, ophthalmological examination, and tuberculin skin testing are routinely obtained as part of the initial evaluation to assess the severity and extent of disease.
Smoking-Related Interstitial Lung Disease
Smoking-related interstitial diseases include respiratory bronchiolitis-interstitial lung disease, desquamative interstitial pneumonia, and pulmonary Langerhans cell histiocytosis. Respiratory bronchiolitis is characterized by patchy centrilobular ground-glass opacities on high-resolution computed tomography and prominent collections of intraalveolar macrophages centered around the bronchiole and alveolar duct with mild septal thickening and fibrosis on pathology.
Compared with respiratory bronchiolitis, the chest computed tomography in desquamative interstitial pneumonia typically shows more diffuse ground-glass changes with occasional cysts and pathology demonstrates a marked increase in alveolar macrophages, septal thickening, and fibrosis.
Pulmonary Langerhans cell histiocytosis is typified by dense nodules and cysts with nodular sclerosing lesions containing Langerhans cells with mixed cellular infiltrates. Smoking cessation may halt progression of all three diseases (3).
Hypersensitivity Pneumonitis
Chronic hypersensitivity pneumonitis is a common interstitial disease related to exposures. Clinical history is the key to determining a cause, and ultimately eliminating the culprit antigen from the patient’s environment. Serological testing is available to help confirm the diagnosis if a suspect antigen is identified. However, in many instances no antigen can be identified.
Radiographic and pathological analyses are needed to make the diagnosis. The radiographic appearance of centrilobular ground-glass opacities or mosaic pattern is characteristic of chronic hypersensitivity pneumonitis.
As chronic hypersensitivity pneumonitis progresses, it can be difficult to differentiate from idiopathic pulmonary fibrosis radiographically, with septal thickening, traction bronchiectasis, and even honeycombing. Pathologically, chronic hypersensitivity pneumonitis is characterized by granulomatous interstitial bronchiolocentric pneumonitis (4).
Treatment
The mainstay of treatment for patients with connective tissue disease–associated interstitial disease is immunosuppression. Two new antifibrotic drugs, nintedanib and pirfenidone, have been shown to slow the progression of disease as measured by reduced rate of decline in FVC in idiopathic pulmonary fibrosis (5, 6).
References
- 1.Raghu G, Collard HR, Egan JJ, Martinez FJ, Behr J, Brown KK, Colby TV, Cordier JF, Flaherty KR, Lasky JA, et al. ATS/ERS/JRS/ALAT Committee on Idiopathic Pulmonary Fibrosis. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011;183:788–824. doi: 10.1164/rccm.2009-040GL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Travis WD, Costabel U, Hansell DM, King TE, Jr, Lynch DA, Nicholson AG, Ryerson CJ, Ryu JH, Selman M, Wells AU, et al. ATS/ERS Committee on Idiopathic Interstitial Pneumonias. An official American Thoracic Society/European Respiratory Society statement: update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med. 2013;188:733–748. doi: 10.1164/rccm.201308-1483ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Caminati A, Cavazza A, Sverzellati N, Harari S. An integrated approach in the diagnosis of smoking-related interstitial lung diseases. Eur Respir Rev. 2012;21:207–217. doi: 10.1183/09059180.00003112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Selman M, Pardo A, King TE., Jr Hypersensitivity pneumonitis: insights in diagnosis and pathobiology. Am J Respir Crit Care Med. 2012;186:314–324. doi: 10.1164/rccm.201203-0513CI. [DOI] [PubMed] [Google Scholar]
- 5.Richeldi L, du Bois RM, Raghu G, Azuma A, Brown KK, Costabel U, Cottin V, Flaherty KR, Hansell DM, Inoue Y, et al. INPULSIS Trial Investigators. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med. 2014;370:2071–2082. doi: 10.1056/NEJMoa1402584. [DOI] [PubMed] [Google Scholar]
- 6.King TE, Jr, Bradford WZ, Castro-Bernardini S, Fagan EA, Glaspole I, Glassberg MK, Gorina E, Hopkins PM, Kardatzke D, Lancaster L, et al. ASCEND Study Group. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med. 2014;370:2083–2092. doi: 10.1056/NEJMoa1402582. [DOI] [PubMed] [Google Scholar]
Pulmonary Hypertension
Josanna Rodriguez-Lopez and Hilary DuBrock
Pulmonary hypertension (PH) is defined as a mean pulmonary arterial pressure greater than or equal to 25 mm Hg measured at rest by right heart catheterization (Table 9). Elevations in pulmonary artery pressure can result from various mechanisms including high cardiac output, high pulmonary arterial wedge pressure, and/or a high pulmonary vascular resistance.
Table 9.
Key points: Pulmonary hypertension
| • Pulmonary arterial hypertension describes a subset of patients defined hemodynamically by a mean pulmonary artery pressure ≥ 25 mm Hg and pulmonary vascular resistance > 3 Wood’s units with wedge pressure < 16 mm Hg in the absence of secondary causes such as lung disease or chronic thromboembolic disease |
| • Although transthoracic echocardiography is the most common noninvasive screening test for pulmonary hypertension, right heart catheterization is still necessary to confirm the diagnosis |
| • Riociguat has been approved for the treatment of chronic thromboembolic pulmonary hypertension in patients who are not surgical candidates or who have persistent pulmonary hypertension after surgery |
Pulmonary arterial hypertension describes a subset of patients defined hemodynamically by a mean pulmonary artery pressure of or exceeding 25 mm Hg and pulmonary vascular resistance of 3 Wood’s units with wedge pressure less than 16 mm Hg in the absence of secondary causes such as lung disease or chronic thromboembolic disease (1).
Classification
Pulmonary hypertension is categorized into five groups with distinct mechanisms and therapeutic approaches (2). Group 1 includes idiopathic; heritable; drug- and toxin-induced; and associated with connective tissue disease, human immunodeficiency virus (HIV), portal hypertension, schistosomiasis, and congenital heart disease. Group 1′ includes pulmonary capillary hemangiomatosis and pulmonary veno-occlusive disease. Group 2 refers to PH associated with left-sided heart disease and is characterized by an elevated wedge pressure or left ventricular end diastolic pressure. This is the most common cause of pulmonary hypertension. Group 3 includes patients with PH secondary to lung disease or chronic hypoxia. Group 4 refers to patients with chronic thromboembolic pulmonary hypertension. Group 5 includes patients with pulmonary hypertension of unclear etiology or multifactorial mechanisms such as chronic hemolytic anemia and sarcoidosis.
Diagnosis
The diagnostic workup of pulmonary hypertension includes a focused history and physical examination, although signs and symptoms can be nonspecific. Transthoracic echocardiography is the most common noninvasive screening test. However, right heart catheterization is still necessary to confirm the diagnosis.
A diagnosis of group 1 PH requires exclusion of common causes of PH, such as heart and lung disease. If PH is suspected and common causes are ruled out, further workup with serological testing for connective tissue disease, HIV, and portal hypertension, and ventilation–perfusion scintigraphy to screen for chronic thromboembolic disease, should be performed.
Prognosis
Poor prognostic indicators in PH include connective tissue disease related PH or portopulmonary hypertension, symptoms at rest, elevated brain natriuretic peptide, the presence of a pericardial effusion, elevated mean right atrial pressure, and reduced cardiac index (3).
In sickle cell disease the mortality risk among patients with tricuspid regurgitation jet velocity greater than 2.5 m/s is 10 times greater than in those with velocities less than 2.5 m/s.
Treatment
Group 1 PAH is treated with targeted medications including prostacyclin analogs, endothelin receptor antagonists, phosphodiesterase inhibitors, and soluble guanylate cyclase stimulators (4). For patients with New York Heart Association class IV symptoms, intravenous prostacyclin is recommended as first-line therapy.
In patients with idiopathic PH, an acute vasodilator response at the time of catheterization (defined as a decrease in mean pulmonary artery pressure by 10–40 mm Hg without a decrease in cardiac output) is indicative of a therapeutic response to calcium channel blockers, although this degree of acute vasoreactivity is seen in only 6% (5).
Group 2 PH involves treatment of the left heart disease (e.g., diuretics, afterload reduction). Treatment of group 3 PH involves treatment of the lung/respiratory disease.
Patients with World Health Organization group 4 PH, or chronic thromboembolic pulmonary hypertension, should be referred to specialized centers with expertise in performing pulmonary thromboendarterectomy, as this may be curative. Riociguat has been approved for the treatment of nonsurgical patients or those with persistent PH after surgery (6).
References
- 1.Hoeper MM, Bogaard HJ, Condliffe R, Frantz R, Khanna D, Kurzyna M, Langleben D, Manes A, Satoh T, Torres F, et al. Definitions and diagnosis of pulmonary hypertension. J Am Coll Cardiol. 2013;62(25) Suppl:D42–D50. doi: 10.1016/j.jacc.2013.10.032. [DOI] [PubMed] [Google Scholar]
- 2.Simonneau G, Gatzoulis MA, Adatia I, Celermajer D, Denton C, Ghofrani A, Gomez Sanchez MA, Krishna Kumar R, Landzberg M, Machado RF, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2013;62(25) Suppl:D34–D41. doi: 10.1016/j.jacc.2013.10.029. [DOI] [PubMed] [Google Scholar]
- 3.Benza RL, Miller DP, Gomberg-Maitland M, Frantz RP, Foreman AJ, Coffey CS, Frost A, Barst RJ, Badesch DB, Elliott CG, et al. Predicting survival in pulmonary arterial hypertension: insights from the Registry to Evaluate Early and Long-Term Pulmonary Arterial Hypertension Disease Management (REVEAL) Circulation. 2010;122:164–172. doi: 10.1161/CIRCULATIONAHA.109.898122. [DOI] [PubMed] [Google Scholar]
- 4.Galiè N, Corris PA, Frost A, Girgis RE, Granton J, Jing ZC, Klepetko W, McGoon MD, McLaughlin VV, Preston IR, et al. Updated treatment algorithm of pulmonary arterial hypertension. J Am Coll Cardiol. 2013;62(25) Suppl:D60–D72. doi: 10.1016/j.jacc.2013.10.031. [DOI] [PubMed] [Google Scholar]
- 5.Sitbon O, Humbert M, Jaïs X, Ioos V, Hamid AM, Provencher S, Garcia G, Parent F, Hervé P, Simonneau G. Long-term response to calcium channel blockers in idiopathic pulmonary arterial hypertension. Circulation. 2005;111:3105–3111. doi: 10.1161/CIRCULATIONAHA.104.488486. [DOI] [PubMed] [Google Scholar]
- 6.Ghofrani HA, D’Armini AM, Grimminger F, Hoeper MM, Jansa P, Kim NH, Mayer E, Simonneau G, Wilkins MR, Fritsch A, et al. CHEST-1 Study Group. Riociguat for the treatment of chronic thromboembolic pulmonary hypertension. N Engl J Med. 2013;369:319–329. doi: 10.1056/NEJMoa1209657. [DOI] [PubMed] [Google Scholar]
Lung Transplantation
Cassie C. Kennedy and Diana J. Kelm
When candidates and donors are carefully selected, lung transplantation offers patients with end-stage lung disease the potential of increased survival and quality of life (1).
Candidate Referral and Selection
Known prognostic markers of the underlying disease guide timing of transplantation referral. Transplantation of patients with chronic obstructive lung disease can be guided by the BODE index score (body mass index [B], airflow obstruction [O], dyspnea [D], and exercise capacity [E]) (2).
In cystic fibrosis, those with an FEV1 less than 30%, FEV1 30% or greater with associated hypercapnia, or rapid decline in FEV1 should be referred for transplantation (2).
Reasons for referral for idiopathic pulmonary fibrosis include a decrease in FVC by more than 10% or in DlCO by greater than 15%, or an increase in level of dyspnea or fibrosis (2).
In patients with pulmonary arterial hypertension, progressive dyspnea, right heart failure, or a decline in 6-minute walk distance to less than 350 m, despite maximal therapy, should lead to referral (2).
Once referred, patients are carefully evaluated. According to guidelines, absolute contraindications to lung transplantation include the following: recent malignancy, a history of noncompliance, certain chronic infections, substance abuse in the past 6 months, morbid obesity, or untreatable psychiatric disorder (3).
Obesity, age greater than 65 years, malnutrition, colonization with virulent organisms, poor functional status, and severe osteoporosis are considered relative contraindications (3, 4). Caution should be used in the selection of patients for multiorgan transplantation or when considering transplantation for patients undergoing ventilation or extracorporeal membrane oxygenation.
Listing Process and Lung Allocation
Once patients are deemed fit for transplantation they are listed with specific details of their transplant requirements (including blood type and size) and ranked by lung allocation score (LAS).
The LAS is a composite score composed of numerous physiological and comorbid variables designed to give prioritization to patients with higher predicted waitlist mortality and transplantation benefit (5). The LAS uses physiological and comorbid variables (specifically age, height, weight, diagnosis, functional status, diabetes mellitus, assisted ventilation, supplemental oxygen, FVC percent predicted, mean pulmonary artery pressure and pulmonary capillary wedge pressure, Pco2 and Po2, 6-min walk, and serum creatinine).
Donation Criteria
Success of transplantation is dependent on careful screening of the donor. The standard lung donor criteria are as follows: age less than 55; ABO compatibility; clear chest radiograph; absence of chest trauma or surgery; absence of potentially pathological organisms on sputum Gram stain, aspiration, or purulent secretions at bronchoscopy; PaO2/FiO2 (fraction of inspired oxygen) ratio greater than 300 on positive end-expiratory pressure of 5 cm H2O and FiO2 100%; smoking history of less than 20 pack-years; and no evidence of sepsis (4). However, because of organ shortages and more recent research, extended lung donor criteria are used at times (5).
Posttransplantation Complications
Posttransplantation complications include both increased morbidity and mortality. The median survival is 5.7 years for adults (6). Posttransplantation complications can be graft-related, such as primary graft dysfunction, acute and chronic rejection (also called bronchiolitis obliterans and restrictive allograft syndrome), or anastomotic complications.
Primary graft dysfunction is associated with increased morbidity and mortality after lung transplantation and is assessed immediately posttransplantation and at 24, 48, and 72 hours with a grade of 0–3 based on the PaO2/FiO2 ratio and the chest radiograph.
Complications can also be secondary to transplant-related medications, such as malignancy, osteoporosis, infection, diabetes, and posttransplantation lymphoproliferative disease (7). Pretransplantation cytomegalovirus serology–negative recipients who receive organs from positive donors are at increased risk of developing posttransplantation lymphoproliferative disease after lung transplantation.
The key points regarding lung transplantation are listed in Table 10.
Table 10.
Key points: Lung transplantation
| • In patients with chronic obstructive lung disease, the timing of lung transplantation is guided by the BODE index score |
| • The LAS is a composite score composed of numerous physiological and comorbid variables designed to give prioritization to patients with higher predicted waitlist mortality and transplantation benefit |
| • Pretransplantation cytomegalovirus serostatus–negative recipients who receive organs from positive donors are at increased risk of developing posttransplantation lymphoproliferative disease after lung transplantation |
Definition of abbreviation: BODE = body mass index (B), airflow obstruction (O), dyspnea (D), and exercise capacity (E); LAS = lung allocation score.
References
- 1.Yusen RD. Survival and quality of life of patients undergoing lung transplant. Clin Chest Med. 2011;32:253–264. doi: 10.1016/j.ccm.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 2.Weill D, Benden C, Corris PA, Dark JH, Davis RD, Keshavjee S, Lederer DJ, Mulligan MJ, Patterson GA, Singer LG, et al. A consensus document for the selection of lung transplant candidates: 2014—an update from the Pulmonary Transplantation Council of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant. 2015;34:1–15. doi: 10.1016/j.healun.2014.06.014. [DOI] [PubMed] [Google Scholar]
- 3.Orens JB, Estenne M, Arcasoy S, Conte JV, Corris P, Egan JJ, Egan T, Keshavjee S, Knoop C, Kotloff R, et al. Pulmonary Scientific Council of the International Society for Heart and Lung Transplantation. International guidelines for the selection of lung transplant candidates: 2006 update—a consensus report from the Pulmonary Scientific Council of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant. 2006;25:745–755. doi: 10.1016/j.healun.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 4.Bhorade SM, Vigneswaran W, McCabe MA, Garrity ER. Liberalization of donor criteria may expand the donor pool without adverse consequence in lung transplantation. J Heart Lung Transplant. 2000;19:1199–1204. doi: 10.1016/s1053-2498(00)00215-1. [DOI] [PubMed] [Google Scholar]
- 5.Botha P. Extended donor criteria in lung transplantation. Curr Opin Organ Transplant. 2009;14:206–210. doi: 10.1097/mot.0b013e328326c834. [DOI] [PubMed] [Google Scholar]
- 6.Yusen RD, Edwards LB, Kucheryavaya AY, Benden C, Dipchand AI, Dobbels F, Goldfarb SB, Levvey BJ, Lund LH, Meiser B, et al. International Society for Heart and Lung Transplantation. The registry of the International Society for Heart and Lung Transplantation: thirty-first adult lung and heart–lung transplant report—2014; focus theme: retransplantation. J Heart Lung Transplant. 2014;33:1009–1024. doi: 10.1016/j.healun.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 7.Lyu DM, Zamora MR. Medical complications of lung transplantation. Proc Am Thorac Soc. 2009;6:101–107. doi: 10.1513/pats.200808-077GO. [DOI] [PubMed] [Google Scholar]
Footnotes
Supported by Pneum Rx and Allegro Diagnostics (G.C.M.).
Author disclosures are available with the text of this article at www.atsjournals.org.