Abstract
Spinal muscular atrophy (SMA) is a progressive, recessively inherited neuromuscular disease, characterized by the degeneration of lower motor neurons in the spinal cord and brainstem, which leads to weakness and muscle atrophy. SMA currently represents the most common genetic cause of infant death. SMA is caused by the lack of survival motor neuron (SMN) protein due to mutations, which are often deletions, in the SMN1 gene. In the absence of treatments able to modify the disease course, a considerable burden falls on patients and their families. Greater knowledge of the molecular basis of SMA pathogenesis has fuelled the development of potential therapeutic approaches, which are illustrated here. Nusinersen, a modified antisense oligonucleotide that modulates the splicing of the SMN2 mRNA transcript, is the first approved drug for all types of SMA. Moreover, the first gene therapy clinical trial using adeno-associated virus (AAV) vectors encoding SMN reported positive results in survival and motor milestones achievement. In addition, other strategies are in the pipeline, including modulation of SMN2 transcripts, neuroprotection, and targeting an increasing number of other peripheral targets, including the skeletal muscle. Based on this premise, it is reasonable to expect that therapeutic approaches aimed at treating SMA will soon be changed, and improved, in a meaningful way. We discuss the challenges with regard to the development of novel treatments for patients with SMA, and depict the current and future scenarios as the field enters into a new era of promising effective treatments.
Keywords: antisense oligonucleotides, gene therapy, neuromuscular disease, spinal muscular atrophy
Introduction
Spinal muscular atrophy (SMA) is a devastating autosomal recessive neuromuscular disease characterized by motor neuron degeneration in the brain stem and spinal cord, resulting in progressive muscle weakness and atrophy.1 SMA occurs in approximately 1 in 10,000 newborns, and represents the most common hereditary disease-causing childhood death to date.2 This disease arises from mutations in the survival motor neuron 1 (SMN1) gene. These mutations, that are often deletions, lead to the deficiency of the ubiquitous SMN protein.3 The human genome contains a SMN1 paralogous gene, SMN2, which produces a truncated unstable protein (SMNΔ7) due to alternative splicing which excludes exon 7 from the final transcript. Therefore, the low level (approximately 10%) of full-length functional SMN protein produced only partially compensates for the lack of SMN1.4 All patients with SMA have at least one copy of the SMN2 gene. They are classified as having SMA type 1–4 (SMA1–SMA4) on the basis of their age of onset and their highest motor milestones, and the number of SMN2 copies inversely correlates with the clinical severity of the disease phenotype.5
The SMA field has been revolutionized during recent months following therapeutic advances that have led to the approval of the first therapy regimen for SMA. In addition, progress has been made on other specific approaches, such as gene therapy and splicing modifier molecules. In this study, we discuss the progress and challenges related to the implementation of novel therapies for all patients with SMA.
Therapeutic developments
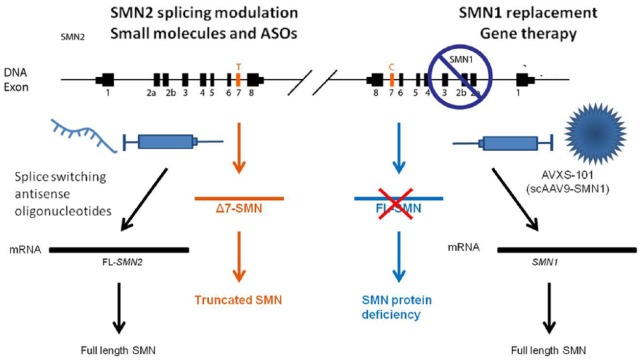
The pipeline of therapies for SMA encompasses strategies that are focused on increasing SMN levels by modulating SMN2 transcription or splicing, promoting full-length SMN protein production by antisense oligonucleotides (ASOs) or small molecules as well as SMN1 gene replacement with gene therapy (Figure 1). Other therapeutic options include SMN independent approaches that are centred on previously defined targets downstream of SMN, neuroprotective approaches (with small molecules or stem cells), or approaches that focus on improving muscle strength and function. Translational research keeps moving forward, and recent clinical trials have demonstrated the safety and efficacy of the newest approaches (Table 1). In addition to these promising results, several clinical trials based on repurposed drugs, such as valproic acid, acetyl-L-carnitine, phenylbutyrate hydroxyurea, riluzole and somatotropin, have failed to yield the expected results, despite promising preclinical data.6,7 Nevertheless, these negative reports provide relevant information about clinical trial design, which represents an essential step in moving therapeutic compounds towards regulatory approval. Furthermore, these negative reports also provide information about the reliability and feasibility of specific outcome measures and the identification of patient populations, which highlights the importance of patient stratification to ensure the efficacy of the compounds.
Figure 1.
Genetics and therapeutic developments of spinal muscular atrophy (SMA).
SMA is caused by mutations, in the survival motor neuron 1 (SMN1) gene that mainly produces full-length SMN that is essential for motor neurons (below right). The human genome harbours a paralogous SMN1 gene, SMN2 (on the left), that differs only by a few nucleotides, and in particular by a C to T transition in exon 7. This base change causes the skipping of exon 7 in most SMN2 transcripts and generates a truncated unstable protein (SMNΔ7) with low levels (approximately 10%) of full-length, functional SMN protein produced (below left). Increasing the full-length SMN protein levels by promoting the inclusion of exon 7 in SMN2 mRNA, that is, with oligonucleotides (on the left) or transferring a wild-type copy of SMN1 through gene therapy (on the right), represents a promising therapeutic approach for SMA. ASO, antisense oligonucleotide.
Table 1.
List of SMA clinical trials.
| ClinicalTrials.gov identifier | Study | Drug | Phase | Type/status | Sponsor | Description |
|---|---|---|---|---|---|---|
| NCT01703988 | An open-label safety, tolerability and dose-range finding study of multiple doses of nusinersen (ISIS 396443) in participants with SMA | Nusinersen (ISIS 396443) | I/II | Interventional/completed | Biogen | Administration of multiple doses of nusinersen (6 mg and 12 mg dose equivalents) into the spinal fluid of patients with SMA type 1 (SMA1), two or three times over the duration of the trial |
| NCT02193074 | A study to assess the efficacy and safety of nusinersen (ISIS 396443) in infants with SMA (ENDEAR) | Nusinersen | III | Interventional/completed | Biogen | Intrathecal administration of nusinersen (12 mg) to infants under 7 months of age with SMA1 |
| NCT02594124 | A study for participants with SMA who previously participated in nusinersen (ISIS 396443) investigational studies (SHINE) | Nusinersen | III | Interventional/enrolling participants by invitation only | Biogen | Intrathecal administration of nusinersen to patients with SMA who previously participated in investigational studies of nusinersen |
| NCT02292537 | A study to assess the efficacy and safety of nusinersen (ISIS 396443) in participants with later-onset SMA (CHERISH) | Nusinersen | III | Interventional/completed | Biogen | Intrathecal administration of nusinersen to participants with later-onset SMA |
| NCT02386553 | A study of multiple doses of nusinersen (ISIS 396443) delivered to infants with genetically diagnosed and presymptomatic SMA (NURTURE) | Nusinersen | II | Interventional/ongoing, not recruiting participants | Biogen | Intrathecal administration of multiple doses of nusinersen in infants with genetically diagnosed and presymptomatic SMA |
| NCT02240355 | A study of RO6885247 in adult and paediatric patients with SMA (MOONFISH) | RO6885247 | I | Interventional/terminated | Hoffmann-La Roche | Oral administration of RO6885247 to adult and paediatric patients with SMA |
| NCT02633709 | A study to investigate the safety, tolerability, pharmacokinetics and pharmacodynamics of RO7034067 (RG7916) given by mouth in healthy volunteers | RO7034067 | I | Interventional/completed | Hoffmann-La Roche | Oral administration of RO7034067 in healthy subjects to investigate safety, tolerability, pharmacokinetics and pharmacodynamics |
| NCT02908685 | A study to investigate the safety, tolerability, pharmacokinetics, pharmacodynamics and efficacy of RO7034067 in participants with type 2 and 3 spinal SMA (Sunfish) | RO7034067 | II | Interventional/currently recruiting participants | Hoffmann-La Roche | Oral administration of RO7034067 in adult and paediatric patients with SMA2 and SMA3. The two-part study consists of an exploratory dose-finding part for 12 weeks and a confirmatory part for 24 months |
| NCT02913482 | A study to investigate the safety, tolerability, pharmacokinetics, pharmacodynamics and efficacy of RO7034067 in infants with type 1 SMA (Firefish) | RO7034067 | II | Interventional/currently recruiting participants | Hoffmann-La Roche | Oral administration of RO7034067 in infants with SMA1. The two-part study consists of an exploratory dose-finding part and a confirmatory part for 24 months at the dose selected in part 1 |
| NCT03032172 | A study of RO7034067 in adult and paediatric participants with SMA (Jewelfish) | RO7034067 | II | Interventional/currently recruiting participants | Hoffmann-La Roche | Oral administration of RO7034067 in adults and children with SMA2 and SMA3 previously treated with a SMN2-targeting therapy |
| NCT02268552 | An open-label study of LMI070 in type 1 SMA | LMI070 | I/II | Interventional/ongoing, not recruiting participants | Novartis Pharmaceuticals | Oral administration of LMI070 in infants with SMA1 for 13 weeks |
| NCT02122952 | Gene transfer clinical trial for type 1 SMA | AVXS-101 | I | Interventional/ongoing, not recruiting participants | AveXis, Inc. | Intravenous delivery of AVXS-101 as a treatment for SMA1 |
| NCT01302600 | Safety and efficacy of olesoxime (TRO19622) in patients with SMA aged 3–25 years | Olesoxime | II | Interventional/completed | Hoffmann-La Roche | Administration of liquid suspension formulation (10 mg/kg) of olesoxime once a day with food at dinner to nonambulant 3–25-year-old patients with SMA2 or SMA3 |
| NCT02628743 | A study to evaluate long term safety, tolerability, and effectiveness of olesoxime in participants with SMA | Olesoxime | II | Interventional/ongoing, not recruiting participants | Hoffmann-La Roche | Administration of 10 mg/kg suspension of olesoxime once a day either orally or via a nasogastric or gastrostomy tube in patients with SMA who participated in previous TRO19622 studies |
| NCT02644668 | A study of CK-2127107 in patients with SMA | CK-2127107 | II | Interventional/currently recruiting participants | Cytokinetics | Oral administration of multiple doses of CK-2127107 to ambulant and nonambulant patients with SMA2, SMA3 and SMA4 |
SMA, spinal muscular atrophy.
Mechanism: increase SMN levels
Mutations in the SMN1 gene that reduce the expression of SMN protein have been identified as the genetic causes underlying SMA. Therefore, one of the most promising strategies investigated for SMA treatment is to increase the levels of full-length SMN.
Strategy: SMN2 splicing modulation
The ASO nusinersen
ASOs are synthetic short strands of chemically modified nucleic acids that are specifically designed to target and bind to RNA to affect RNA activation or alter the exon splicing. The two most commonly used chemically modified ASOs are phosphorothioate oligonucleotides and morpholino oligonucleotides. They can be designed to promote exon exclusion, such as exon 51 skipping in Duchenne muscular dystrophy, or enhance the inclusion of an exon that would be otherwise excluded, such as exon 7 in SMN2. This gene differs from SMN1 by only a few nucleotides and, in particular, by a C to T transition in exon 7. This single-base change causes the skipping of exon 7 in most SMN2 transcripts, generating a truncated unstable protein (SMNΔ7) with approximately 10% of the full-length, functional SMN protein produced,4 which only partially compensates for the lack of SMN1. All patients with SMA have at least one copy of the SMN2 gene. The ASOs have huge potential in SMA as they offer the possibility of promoting the inclusion of exon 7 in the SMN2 gene, a strategy that is applicable to all patients. Most of the oligonucleotides under investigation target the intronic splicing silencer N1 (ISS-N1) motif in SMN2 pre-mRNA, thus fostering the inclusion of exon 7 into the SMN protein. This approach has been safe and effective under in vitro and in vivo conditions using SMA rodent models and rescuing the SMA phenotype.8–12 ASOs have very limited ability to cross the blood–brain barrier (BBB), and they can be administered by repeated intrathecal injection (lumbar puncture) in the cerebrospinal fluid (CSF).
Nusinersen (previously also known as IONIS–SMNRX, with the commercial name Spinraza, Biogen Weston, MA, USA) is the first treatment for SMA that has been approved by the US Food and Drug Administration (FDA) and European Medicines Agency (EMA). It received approval by the FDA on 23 December 2016 and by the EMA Committee for Medicinal Products for Human Use on 21 April 2017.
Nusinersen is a modified 2’-O-methoxyethyl phosphorothioate ASO that binds to the ISS-N1 region in the intron 7 sequence and blocks access to the sites of negative regulators. This binding modulates the splicing of the SMN2 mRNA transcript, increasing the inclusion of exon. Nusinersen has been demonstrated to increase the production of full-length SMN protein both in vitro and in transgenic animal models of SMA.9
Based on these positive results, clinical trials have been conducted in both type 1 and later-onset SMA. A safety, tolerability and dose-range-finding phase II open-label study of multiple doses of nusinersen (6 mg and 12 mg dose equivalents) in patients with SMA1 (n = 20) has been carried out (CS3A) [ClinicalTrials.gov identifier: NCT01703988] and recently published.13 In this trial, infants with a homozygous deletion or mutation of the SMN1 gene were enrolled at the age of onset of their SMA symptoms, between 3 weeks and 7 months of age. An interim analysis of the data showed that treatment with 12 mg nusinersen resulted in significant mean improvements in developmental motor milestones, including sitting and walking, assessed according to the Hammersmith Infant Neurological Examination (HINE) Section 2 total score, and motor function, determined by the Children’s Hospital of Philadelphia Infant Test of Neuromuscular Disorders (CHOP-INTEND) total score. An interim analysis also showed that the Kaplan–Meier survival curve of patients with two SMN2 gene copies (n = 17) significantly differed from a natural history case series (p = 0.0014). The collected autopsies of three affected infants who received treatment showed an increase in full-length SMN2 transcripts and SMN protein in spinal cord motor neurons compared with patients with SMA who were untreated.13 Intrathecal injection allowed the distribution of nusinersen from the CSF throughout the central nervous system (CNS) into motor neurons, vascular endothelial cells and glial cells. Overall, this study showed a good safety profile and presented encouraging efficacy data that supported the continued development of nusinersen to treat SMA.
A subsequent randomized, double-blind, sham-controlled, multinational, phase III study, ENDEAR [ClinicalTrials.gov identifier: NCT02193074] was conducted to assess the efficacy and safety of nusinersen in infants with SMA1 at 6 months or younger who received nusinersen (12 mg) or a sham procedure. The primary endpoints were a motor milestone achievement, based on the HINE, and survival without an event (time to death or use of permanent assisted ventilation). In the interim analysis, infants in the nusinersen group were significantly more likely to reach a motor milestone [21 of 51 infants (41%)] than infants in the placebo group [0 of 27 (0%), p < 0.001]. Based on the positive results of the interim analysis, which exhibited clinically and statistically significant gains across multiple efficacy endpoints, the study was terminated early. The final analysis showed a significantly higher likelihood of motor milestone achievement in infants treated with nusinersen [37 of 73 infants (51%) than in the control group (0 of 37, 0%)]. However, of infants who reached motor milestones only 8% were able to sit independently, and just 1% were able to stand. Upon completion of the trial, 39% of the infants in the nusinersen group and 68% in the control group had died or needed permanent assisted ventilation. Patients who started treatment within 13 weeks after the onset of disease showed the best response to treatment, with similar incidence and severity of adverse events in both treatment and placebo groups.14
Following discontinuation of the ENDEAR study, participants were given the possibility to join an open-label phase III extension study SHINE [ClinicalTrials.gov identifier: NCT02594124] to gather more information on the long-term safety and the tolerability of repeated doses of nusinersen. The positive results of the interim analysis also included a new drug application to the FDA, and the drug was subsequently approved for commercialization for all SMA types.
The nusinersen phase III programme includes the CHERISH study [ClinicalTrials.gov identifier: NCT02292537], which is a randomized, double-blind, multinational trial in which patients with later-onset SMA (type 2 phenotype) received either nusinersen or a sham procedure. Based on the positive results of the interim analysis, CHERISH was stopped, which allowed the participants to transfer to SHINE.
Two additional phase II programmes have been carried out to collect additional data on nusinersen. EMBRACE involves a small group of infants or patients with late-onset SMA not recruited for ENDEAR or CHERISH due to age and the other criteria requested [ClinicalTrials.gov identifier: NCT02462759].
Another very interesting study is NURTURE [ClinicalTrials.gov identifier: NCT02386553], an ongoing 30-month, open-label, multinational, phase II clinical trial in presymptomatic patients up to 6 weeks of age after their first dose of nusinersen. The primary aim of this study is to determine whether treatment with nusinersen could prevent or delay the development of SMA symptoms [ClinicalTrials.gov identifier: NCT02386553]. Interim analysis of the data,15 which have been presented at the World Muscle Society Congresses (2016, 2017) and the American Academy of Neurology (2017), support the potential benefit of early treatment with nusinersen in SMA.
The clinically approved use of nusinersen consists of periodical intrathecal administration patients with SMA, and the recommended dose is 12 mg injected by lumbar puncture (https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/209531lbl.pdf). At the beginning of the treatment, three loading doses should be administered with each dose 14 days apart and a fourth loading dose should be provided 30 days after the third dose. The maintenance treatment schedule is one dose every 4 months thereafter. Repeated lumbar punctures have seemed well tolerated in nusinersen trials, even if doing this procedure for many years can pose some challenges in terms of patient tolerability. Scoliosis and related surgery, advanced disease with respiratory problems, and the need for sedation represent the current challenges for the wide application of this approach. The use of intrathecal pumps that deliver the compound could represent an alternative to repeated injections.
The development of ASO-based therapy is an important advance to identify effective therapeutic treatments for SMA, but efficient delivery remains a major challenge. Further advances in this approach provide for the development of peptide-mediated oligonucleotides to enable systemic therapy to overcome the difficulties with CNS delivery via repeated intrathecal injections and allow the targeting of other systemic organs. The efficacy of this approach has recently been demonstrated in rodent models.16,17
Small molecules
Orally bioavailable small molecules that specifically target the SMN2 gene and modulate its splicing are under development for the treatment of SMA, and are currently entering early phase clinical trials. Oral administration makes these drugs more tolerable than ASOs, as it overcomes the need for repeated lumbar puncture; moreover, oral administration enables targeting not only the CNS but also other organs. The major concern related to this approach is the risk of off-target effects given that small molecules, unlike ASOs and gene therapy, are not completely specific and can, in principle, affect the expression of other genes.
To overcome these issues, it has been proposed that small molecules could be designed with high specificity for exon 7 inclusion, and a high-throughput in vitro screening identified RG7800 and RG7916 as potential candidates. Treatment of mice genetically modified to carry human SMN2, resulting in severe SMA1, with either of these compounds led to a significant increase in SMN protein levels, motor function and survival (from 18 days to >150 days).18 Their mechanism of action is based on the direct interaction with two distinct sites in the SMN2 pre-mRNA: a thin bond stabilizes a yet unidentified ribonucleoprotein (RNP) complex, ensuring the specific action of these small molecules for SMN2 over other genes.19 These compounds are now under investigation in clinical studies.
RG7800
RG7800 was tested in a phase I multicentre, randomized, double-blind, placebo-controlled clinical trial (Moonfish) [ClinicalTrials.gov identifier: NCT02240355] that investigated the safety, tolerability, pharmacokinetics and pharmacodynamics of 12 weeks of treatment in adult and paediatric patients with SMA. Clinical data from the study showed significant dose-dependent increases in the level of full-length SMN2 mRNA after a single dose, which demonstrated the proof of mechanism based on the expected pharmacodynamic effect. Following long-term preclinical animal tests, data have shown an unexpected safety finding that was not observed in humans, namely, eye toxicity, which was identified through a long-term (39-week) treatment study in monkeys exposed to RG7800 [ClinicalTrials.gov identifier: NCT02240355]. As a safety precaution, the study was ended early after recruiting the first cohort of patients to evaluate this finding and confirm the next steps for the study.
RG7916
Thus, another compound RG7916 was moved to the clinical stage. After completion of a phase I study [ClinicalTrials.gov identifier: NCT02633709] in August 2016 that helped to establish the optimal treatment dose by investigating the safety and tolerability of RG7916 dose escalation in healthy subjects, RG7916 is being tested in phase II clinical trials in Europe in patients with SMA. The Sunfish study [ClinicalTrials.gov identifier: NCT02908685], which is currently recruiting participants, aims to assess the safety, tolerability and effectiveness of RG7916 in patients with SMA2 and SMA3. The open-label (without placebo) Firefish [ClinicalTrials.gov identifier: NCT02913482] trial will assess the effectiveness of RG7916 in patients with SMA1 (1–7 months old) with two copies of SMN2. The Jewelfish phase II study [ClinicalTrials.gov identifier: NCT03032172] will assess RG7916 treatment in adults and children with SMA2 or SMA3, who were previously treated with an SMN2-targeting therapy and received once daily doses of RG7916 for 2 years.
LM1070
LM1070 (Novartis, Basilea, Svizzera) is another small molecule that may promote exon 7 inclusion in SMN2. This compound was shown to increase life expectancy in SMA model mice and demonstrates that a sequence-selective small-molecule based approach is feasible for increasing the functional SMN protein level through modifying SMN2 splicing.20 In April 2015, an open-label, multi-part, phase I/II study of oral LMI070 (branaplam) administration to patients with SMA1 [ClinicalTrials.gov identifier: NCT02268552] was initiated to evaluate the efficacy, safety, tolerability, pharmacokinetics and pharmacodynamics of 13 weeks of treatment, as well as to assess the optimal dose regimen. Since results obtained from chronic preclinical toxicology studies that were conducted in animals in parallel with the trial showed unexpected toxicities to the peripheral nerves and spinal cord, testes and blood vessels in the kidney, enrolment was discontinued in mid 2016, and the patients who were already enrolled are being closely monitored [ClinicalTrials.gov identifier: NCT02268552] to decide the appropriate next steps.
Strategy: SMN gene replacement
Gene therapy
The gene therapy approach is based on the transfer of a healthy copy of the desired gene into cells, which usually occurs through the use of viral vectors and represents an extremely promising option to treat monogenic diseases. The root cause of SMA, the loss of the SMN1 gene, could be directly corrected by delivering a wild-type copy of the faulty gene. Viral SMN1 gene delivery has been remarkably effective in preclinical studies, thanks to the use of a last generation adeno-associated viral vector (AAV). AAVs are small nonpathogen viruses that can efficiently transfect the CNS as they can target motor neurons and astrocytes. In particular, AAV serotype 9 can be delivered systemically or through intrathecal injection since it is able to cross the BBB, which allows for noninvasive administration of the therapy.21
In 2010, Brian Kaspar’s group demonstrated that intravenous injection of a self-complementary (sc)AAV9 encoding SMN1 could completely rescue a mouse model of severe SMA. After treatment, its motor function and neurophysiology were improved, and mouse survival was extended from 2 weeks to beyond 250 days.22 These data were independently replicated by other laboratories, as well as in large animal disease models by the Kaspar group and collaborators.
Based on these preclinical findings, another AAV-based treatment, scAAV9 carrying the SMN gene (AVXS-101; AveXis), has been developed to restore SMN protein expression by reintroducing SMN1 into cells. An open-label, single-site, phase I–II clinical trial [ClinicalTrials.gov identifier: NCT02122952] evaluating this was completed in January 2017, and the results were published in November 2017.23 In this study, the AVXS-101 under the control of a hybrid CMV enhancer/chicken-β-actin promoter was administered to a total of 15 infants with SMA1 who were 6 months old or younger (mean age 3.4 months in the higher dose group). Patients were divided into two cohorts that received either 6.7e13 vg/kg of AVXS-101 (n = 3) or 2.0e14 vg/kg of AVXS-101 (n = 12), delivered as a single intravenous administration. The primary outcome was safety, and secondary outcome was the time between birth and death or time to requirement for assisted ventilation for more than 16 h per day. The exploratory analysis evaluated scores obtained by the two cohorts based on the CHOP INTEND scale of motor function (ranging from 0 to 64, with higher scores indicating better function), as well as motor milestones achieved in the high-dose cohort compared with motor milestones achieved in historical cohorts, reflecting the natural history of the disease. By the end of the cutoff date (end 7 August 2017, actual start date 5 May 2014), all 15 patients treated with AVXS-101 were alive and event free, whereas only 8% of infants in historical cohorts were alive at a similar time point. In the high-dose cohort, the AVXS-101 gene therapy improved CHOP INTEND scale scores from baseline (9.8-point increase at 1 month and 15.4-point increase at 3 months, mean baseline score 16 in low-dose group and 28 in high-dose group), indicating improved motor function. However, in the historical cohort, the CHOP INTEND scores declined: 11 of the 12 infants in the high-dose cohort were able to sit without any support, 9 could roll over, 11 could feed orally and speak, and 2 walked independently. Four patients showed an increase in serum aminotransferase levels, which was controlled by the administration of oral prednisolone 1 mg/kg per 30 days. In summary, the one-time intravenous infusion of AAV vector containing DNA coding for SMN in patients with SMA1 prolonged survival, improved motor function and facilitated motor milestones achievement in comparison with the historical cohorts. Further studies designed to confirm the long-term safety and efficacy of this approach in patients with SMA1 are needed.
Theoretically, gene therapy appears to be one of the most rational and curative approaches to SMA. The most important advantage of scAAV9-mediated therapy is the possibility of achieving meaningful clinical benefits by only a single intravenous infusion. Possible safety concerns of scAAV9 therapies are related to the effective long-term expression of the vector; although the continued expression of AAVs has been demonstrated over decades for other diseases, it is not completely established.24 In any case, no decrease of the therapeutic effect or clinical worsening have been reported in the first 2 years of the SMA gene therapy study.23
Mechanism: SMN independent
Strategy: neuroprotection
SMA specifically affects lower motor neurons in the spinal cord, leading to muscle waste and atrophy. Neuroprotection aims to restore motor neuron function and prevent symptoms from worsening. This approach is hypothesized to have a relevant clinical benefit in patients with SMA and may be a successful approach for treating SMA when used in combination with genetic therapies.
Olesoxime
The main neuroprotective strategy for SMA that is currently under consideration focuses on enhancing motor neuron survival and function. Among these approaches, olesoxime (TRO19622; Roche, Basilea, Svizzera; initially developed by the Trophos, Marseille, France) is a cholesterol-like compound that entered clinical trials for SMA following the demonstration of its neuroprotective properties in cell culture and in SMA model mice.25 Preclinical studies suggest that olesoxime can preserve mitochondrial function by directly interacting with mitochondrial membranes and decreasing their fluidity in cell and animal models. The first phase II trial [ClinicalTrials.gov identifier: NCT01302600], a multicentre, randomized, adaptive, double-blind, placebo-controlled study involving patients with SMA aged 3–25 years, was completed in October 2013. Although the clinical evidence did not establish a risk–benefit profile for this treatment approach, comparison between symptomatic patients with SMA2 and SMA3 treated with olesoxime and individuals treated with a placebo in the maintenance of motor function suggested that olesoxime may slow the typical decline associated with SMA over a period of 24 months.15,25 However, additional efficacy evidence has been requested by the FDA and EMA to determine whether this is a clinically meaningful effect. An open-label phase II study [ClinicalTrials.gov identifier: NCT02628743] is currently enrolling patients who participated in previous studies to evaluate the long-term safety, tolerability and effectiveness of olesoxime. This study is expected to be completed at the end of 2020.
Strategy: muscle activators
In addition to SMN-restoring strategies, promoting motor neuron survival and neural circuit, muscle and neuromuscular junctions represent promising targets to treat SMA. Neuromuscular junctions are significantly altered in SMA, manifesting as immaturity (such as reduced size of acetylcholine receptor clusters), denervation and neurofilament accumulation, which are associated with impaired synaptic functions.26 Thus, these symptoms represent an important target for correcting the SMA phenotype. Furthermore, muscle protection in SMA aims to counter muscle atrophy and increase muscle mass, thus slowing or halting disease progression.
SMN-independent therapeutic approaches include the employment of small molecules to enhance the ability of muscles to contract and increase muscle mass. Some of these agents are being introduced in clinical trials and for amelioration of the physical performance of patients with SMA.
CK-2127107
CK-2127107 (2-aminoalkyl-5-N-heteroarylpyrimidine; Cytokinetics, South San Francisco, California, USA) is a skeletal muscle troponin activator that slows the rate of calcium release from the regulatory troponin complex in fast skeletal muscles, and sensitizes the sarcomere to calcium, which increases the contractile response to nerve signalling and improves muscle function and physical performance in neuromuscular patients. After a phase I clinical trial, a second study was conducted with the primary aim of assessing the pharmacodynamic effects of the therapy. This study [ClinicalTrials.gov identifier: NCT02644668] is a double-blind, randomized, placebo-controlled study and will enrol 72 ambulant and nonambulant patients with SMA2, SMA3 and SMA4 in the USA and Canada.
Muscle exercise
Additionally, exercise has been linked with increased motor neuron survival, neuromuscular junction protection and improved neuromuscular excitability properties in SMA-like mice. Furthermore, exercise-induced neuroprotection accompanies metabolic and behavioural changes.27 Studies focused on the beneficial effects of physical activity in patients with SMA have assessed feasibility, safety and tolerability of resistance training, as well as the ability of aerobic training to improve oxidative capacity.28,29 Further efforts are needed to plan patient therapy.
Other compounds acting on muscle
Given the role of the small GTPase RhoA and its effector Rho kinase (ROCK) in cytoskeleton organization,30 RhoA and ROCK have been suggested to contribute to the pathophysiology of motor neuron diseases; indeed, enhanced RhoA and ROCK activation has been associated with SMA pathogenesis in the spinal cord of SMA model mice. Administration of ROCK inhibitors in SMA mice can improve the lifespan and phenotype of SMA mice without any effects on motor neuron survival or on SMN protein expression levels. Several lines of evidence suggest that cell types other than motor neurons are involved in SMA pathogenesis. The therapeutic effects of ROCK inhibitors, such as fasudil and Y–27632, on SMA mouse models could, therefore, be imputed for the effects of this treatment on motor neurons as well as on other non-neuronal cell types.31,32
Another compound, the antioxidant flavonoid quercetin, has been shown to effectively ameliorate neuromuscular pathology in SMA mouse models by inhibiting β-catenin signalling, which represents an attractive therapeutic target for the treatment of SMA.33 The indirect stabilization of SMN has been achieved by pharmacologically activating MAPK14 or the p38 pathway with BAY 55–9837, which is an agonist of the vasoactive intestinal peptide receptor 2 (VPAC2), and the disease phenotype of SMA mice treated with this compound has been observed.34 Moreover, the decreased activity of compounds activating the mechanistic target of rapamycin (mTOR) cellular pathway has been related to SMA.35 Given that the increase of micro-RNA-183 (miRNA-183) reduces mTOR activity in SMA cells, an approach that focused on the blocking of this miRNA represents another possible target for a novel therapeutic intervention in SMA, and warrants further examination in mouse models. In addition, RNA sequencing of motor neurons may identify novel downstream targets of splicing alterations.
Another therapeutic strategy that aimed to stabilize the endogenous SMN protein is STL-182, which is an orally available small molecule that showed promising preclinical efficacy in mouse SMA models in which it restored neuromuscular function.36
Strategy: stem cells
Stem-cell-based therapies might have notable therapeutic benefits, such as protecting unaffected motor neuron function, modulating the toxicity of the environment and replacing both neuronal and non-neuronal cell populations. Our research group has previously demonstrated that transplantation of neural stem cells or motor neuron precursors directly into the spinal cord or intrathecally into the CSF ameliorates the phenotype of SMA mice.37–41 We focused our research on the identification of specific neuronal stem cell subpopulations characterized by high migratory capacity and engraftment ability, but studies using stem cell therapies are still experimental. In the future, stem cells may represent a complementary therapeutic approach for SMA along with SMN restoration because their progression in clinical trials requires a complete understanding of the mechanisms of action underlying the biology of their therapeutic benefits as well as robust preclinical studies.
Discussion
In the past few years, considerable advancements have been made in understanding the pathophysiology of SMA and, along with progress in clinical management, this research has created opportunities to perform successful clinical trials with progress from proof-of-concept ideas to reality, and these approaches offer hope to the broader SMA community.
Despite this, several questions remain unanswered. One of the main questions concerns the most suitable timing for intervention for these approaches: is there a moment when the pathology becomes irreversible, preventing the possibility of obtaining a therapeutic effect with treatment in all subtypes of SMA?
The natural history of SMA implies that motor neurons are lost early, and the disease severity is closely related to the number of remaining motor neurons. However, the therapy can also be effective in the symptomatic stage, when muscle weakness results from not only motor neuron loss but also dysfunctional motor neurons losing contact with muscles, an aspect that can be repaired when SMN is restored. Insights from rodent models and current clinical trials suggest that the timing of therapy administration plays a crucial role in attaining the maximal response from the treatment, but it is necessary to determine whether intervening at an advanced stage of the disease can benefit a patient. For patients with an advanced disease, an SMN-independent approach will become increasingly important. Overall, all the preclinical and clinical data suggest ‘the earlier, the better’ for treatment, and the tipping point is represented by a prompt identification of clinical manifestations and an early diagnosis. Presymptomatic diagnosis will enable clinicians to predict SMA disease onset and ultimately prevent its progression or even its occurrence.
Furthermore, it is necessary to establish the long-term effects of the therapeutic approaches, particularly in growing children. It is crucial to assess whether there is a capping effect or continuous improvement, especially in the early phases, to determine whether the obtained results become permanent as children grow. Until now, data obtained from the nusinersen/gene therapy trials suggest continuous positive amelioration, especially in responsive patients, but it is not clear whether the drug treatment can be discontinued. Although SMN is required more in the developmental stages, it is also important during adolescence and adulthood. Since adult-onset SMA4 is more likely due to a degeneration process by a slight reduction of SMN protein, it is possible that therapy must be continued for the patient’s entire life, even if the level of SMN required after the developmental stages is lower.
Further questions concern the exact magnitude of the benefits that SMN upregulation therapy can achieve in older patients with a longer disease duration. All the patients treated up until now in the nusinersen/gene therapy trials are less than 12 years old, and the definition of clinically meaningful effects can vary among different patients’ severity types based on improvement of motor functions as well as maintenance of those improvements. Programmes that monitor the long-term impact of these therapies are required, and they should include assessments of cognition, growth and involuntary functionality. It is necessary to determine whether the proposed approaches can reduce the disease progression, and improve patient function and survival.
SMN is a ubiquitously expressed protein and the idea that SMA disease is not restricted to motor neurons is emerging.41,42 Evidence of the involvement of skeletal muscle, motor circuits and multiple subpopulations of neurons, as well as other cell types is increasingly being provided. This matter raises the issue of the efficacy of therapies that target mainly motor neurons rather than systemic organ dysfunction as well as the possible onset of unexpected multiorgan problems if individuals lack SMN, which may become an issue over time. Additional studies to determine the best routes of drug administration and tissue distribution will be crucial. Intrathecal administration targets mainly the CNS, which does not cover the systemic organs. Additionally, the different motor units can respond in different ways to the treatment, and preliminary data in clinical trials seem to suggest that appendicular motor units respond better than axial/bulbar motor units to SMN upregulation; this aspect should also be considered for patient clinical care.
Conclusion and future directions
The approval of an effective therapy for SMA is drastically changing clinical trial design. The ethical issue of including the placebo arm has already been removed from SMA1 trials, and a setting of clinical trials with the patients treated with the standard of care drug plus the novel investigated compound should now take place. Thus, advancement in the second generation of therapeutics requires innovative strategies in clinical trial design. Moreover, new issues in the clinical applications of these treatments are emerging given the complexity of administering this intrathecal treatment with expanding access to the treatment (establishing specialized clinical teams can be necessary) and the treatment costs and thereby associated problems with reimbursement.
In a field that is constantly moving forward, it is important to guarantee access to novel, disease-modifying treatments and develop new treatments tailored for patients with SMA of any age of onset and severity. It is reasonable to consider that combined therapies providing SMN-targeted and SMN-independent treatments, which aim to preserve neuromuscular function and prevent additional systemic pathologies, represent the best approaches to treating SMA.
Footnotes
Funding: SC received the Joint Programme Neurodegenerative Disease (JPND) research grant DAMNDPATHS (2014).
Conflict of interest statement: The authors declare that there is no conflict of interest.
Contributor Information
Valeria Parente, Dino Ferrari Centre, Neuroscience Section, Department of Pathophysiology and Transplantation (DEPT), University of Milan, Neurology Unit, IRCCS Foundation Ca’ Granda Ospedale Maggiore Policlinico, Milan, Italy.
Stefania Corti, Neuroscience Section, Department of Pathophysiology and Transplantation (DEPT), University of Milan, Neurology Unit, IRCCS Foundation Ca’ Granda Ospedale Maggiore Policlinico, Via Francesco Sforza 35, 20122 Milan, Italy.
References
- 1. Oskoui M, Darras BT, De Vivo DC. Spinal muscular atrophy: 125 years later and on the verge of a cure. In: Sumner CJ, Paushkin S, Ko CPN. (eds) Spinal muscular atrophy. Disease mechanisms and therapy. 1st ed. London, UK: Academic Press, 2017, pp.3–17. [Google Scholar]
- 2. Sugarman EA, Nagan N, Zhu H, et al. Pan-ethnic carrier screening and prenatal diagnosis for spinal muscular atrophy: clinical laboratory analysis of >72,400 specimens. Eur J Hum Genet 2012; 20: 27–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lefebvre S, Burglen L, Reboullet S, et al. Identification and characterization of a spinal muscular atrophy-determining gene. Cell 1995; 80: 155–165. [DOI] [PubMed] [Google Scholar]
- 4. Lorson CL, Hahnen E, Androphy EJ, et al. A single nucleotide in the SMN gene regulates splicing and is responsible for spinal muscular atrophy. Proc Natl Acad Sci USA 1999; 96: 6307–6311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Monani UR, Lorson CL, Parsons DW, et al. A single nucleotide difference that alters splicing patterns distinguishes the SMA gene SMN1 from the copy gene SMN2. Hum Mol Genet 1999; 8: 1177–1183. [DOI] [PubMed] [Google Scholar]
- 6. Kirschner J, Schorling D, Hauschke D, et al. Somatropin treatment of spinal muscular atrophy: a placebo-controlled, double-blind crossover pilot study. Neuromuscul Disord 2014; 24: 134–142. [DOI] [PubMed] [Google Scholar]
- 7. Wadman RI, Bosboom WM, van der Pol WL, et al. Drug treatment for spinal muscular atrophy type I. Cochrane Database Syst Rev 2012; 4: CD006281. [DOI] [PubMed] [Google Scholar]
- 8. Zanetta C, Riboldi G, Nizzardo M, et al. Molecular, genetic and stem cell-mediated therapeutic strategies for spinal muscular atrophy (SMA). J Cell Mol Med 2014; 18: 187–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hua Y, Sahashi K, Hung G, et al. Antisense correction of SMN2 splicing in the CNS rescues necrosis in a type 3 SMA mouse model. Genes Dev 2010; 24: 1634–1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hua Y, Sahashi K, Rigo F, et al. Peripheral SMN restoration is essential for long-term rescue of a severe spinal muscular atrophy mouse model. Nature 2011; 478: 123–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nizzardo M, Simone C, Salani S, et al. Effect of combined systemic and local morpholino treatment on the spinal muscular atrophy delta7 mouse model phenotype. Clin Ther 2014; 36: 340–356. [DOI] [PubMed] [Google Scholar]
- 12. Porensky PN, Mitrpant C, McGovern VL, et al. A single administration of morpholino antisense oligomer rescues spinal muscular atrophy in mouse. Hum Mol Genet 2012; 21: 1625–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Finkel RS, Chiriboga CA, Vajsar J, et al. Treatment of infantile-onset spinal muscular atrophy with nusinersen: a phase 2, open-label, dose-escalation study. Lancet 2016; 388: 3017–3026. [DOI] [PubMed] [Google Scholar]
- 14. Finkel RS, Mercuri E, Darras BT, et al. Nusinersen versus sham control in infantile-onset spinal muscular atrophy. N Engl J Med 2017; 377: 1723–1732. [DOI] [PubMed] [Google Scholar]
- 15. Bertini E, Dessaud E, Mercuri E, et al. Safety and efficacy of olesoxime in patients with type 2 or non-ambulatory type 3 spinal muscular atrophy: a randomised, double-blind, placebo-controlled phase 2 trial. Lancet Neurol 2017; 16: 513–522. [DOI] [PubMed] [Google Scholar]
- 16. Rizzuti M, Nizzardo M, Zanetta C, et al. Therapeutic applications of the cell-penetrating HIV-1 tat peptide. Drug Discov Today 2015; 20: 76–85. [DOI] [PubMed] [Google Scholar]
- 17. Hammond SM, Hazell G, Shabanpoor F, et al. Systemic peptide-mediated oligonucleotide therapy improves long-term survival in spinal muscular atrophy. Proc Natl Acad Sci USA 2016; 113: 10962–10967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Naryshkin NA, Weetall M, Dakka A, et al. Motor neuron disease. SMN2 splicing modifiers improve motor function and longevity in mice with spinal muscular atrophy. Science 2014; 345: 688–693. [DOI] [PubMed] [Google Scholar]
- 19. Sivaramakrishnan M, McCarthy KD, Campagne S, et al. Binding to SMN2 pre-mRNA-protein complex elicits specificity for small molecule splicing modifiers. Nat Commun 2017; 8: 1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Palacino J, Swalley SE, Song C, et al. SMN2 splice modulators enhance U1-pre-mRNA association and rescue SMA mice. Nat Chem Biol 2015; 11: 511–517. [DOI] [PubMed] [Google Scholar]
- 21. Schuster DJ, Dykstra JA, Riedl MS, et al. Biodistribution of adeno-associated virus serotype 9 (AAV9) vector after intrathecal and intravenous delivery in mouse. Front Neuroanat 2014; 8: 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Foust KD, Wang X, McGovern VL, et al. Rescue of the spinal muscular atrophy phenotype in a mouse model by early postnatal delivery of SMN. Nat Biotechnol 2010; 28: 271–274. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 23. Mendell JR, Al-Zaidy S, Shell R, et al. Single-dose gene-replacement therapy for spinal muscular atrophy. N Engl J Med 2017; 377: 1713–1722. [DOI] [PubMed] [Google Scholar]
- 24. High KA, Anguela XM. Adeno-associated viral vectors for the treatment of hemophilia. Hum Mol Genet 2016; 25: R36–R41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dessaud E, André C, Scherrer B, et al. G.O.19: results of a phase II study to assess safety and efficacy of olesoxime (TRO19622) in 3–25 years old spinal muscular atrophy patients. Neuromuscular Disord 2014; 24: 920–921. [Google Scholar]
- 26. Boido M, Vercelli A. Neuromuscular junctions as key contributors and therapeutic targets in spinal muscular atrophy. Front Neuroanat 2016; 10: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chali F, Desseille C, Houdebine L, et al. Long-term exercise-specific neuroprotection in spinal muscular atrophy-like mice. J Physiol 2016; 594: 1931–1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Madsen KL, Hansen RS, Preisler N, et al. Training improves oxidative capacity, but not function, in spinal muscular atrophy type III. Muscle Nerve 2015; 52: 240–244. [DOI] [PubMed] [Google Scholar]
- 29. Lewelt A, Krosschell KJ, Stoddard GJ, et al. Resistance strength training exercise in children with spinal muscular atrophy. Muscle Nerve 2015; 52: 559–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Coque E, Raoul C, Bowerman M. ROCK inhibition as a therapy for spinal muscular atrophy: understanding the repercussions on multiple cellular targets. Front Neurosci 2014; 8: 271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bowerman M, Beauvais A, Anderson CL, et al. Rho-kinase inactivation prolongs survival of an intermediate SMA mouse model. Hum Mol Genet 2010; 19: 1468–1478. [DOI] [PubMed] [Google Scholar]
- 32. Bowerman M, Murray LM, Boyer JG, et al. Fasudil improves survival and promotes skeletal muscle development in a mouse model of spinal muscular atrophy. BMC Med 2012; 10: 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wishart TM, Mutsaers CA, Riessland M, et al. Dysregulation of ubiquitin homeostasis and β-catenin signaling promote spinal muscular atrophy. J Clin Invest 2014; 124: 1821–1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hadwen J, MacKenzie D, Shamim F, et al. VPAC2 receptor agonist BAY 55-9837 increases SMN protein levels and moderates disease phenotype in severe spinal muscular atrophy mouse models. Orphanet J Rare Dis 2014; 9: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kye MJ, Niederst ED, Wertz MH, et al. SMN regulates axonal local translation via miR-183/mTOR pathway. Hum Mol Genet 2014; 23: 6318–6331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Calder AN, Androphy EJ, Hodgetts KJ. Small molecules in development for the treatment of spinal muscular atrophy. J Med Chem 2016; 59: 10067–10083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Corti S, Locatelli F, Papadimitriou D, et al. Transplanted ALDHhiSSClo neural stem cells generate motor neurons and delay disease progression of NMD mice, an animal model of SMARD1. Hum Mol Genet 2006; 15: 167–187. [DOI] [PubMed] [Google Scholar]
- 38. Corti S, Nizzardo M, Nardini M, et al. Neural stem cell transplantation can ameliorate the phenotype of a mouse model of spinal muscular atrophy. J Clin Invest 2008; 118: 3316–3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Corti S, Nizzardo M, Nardini M, et al. Embryonic stem cell-derived neural stem cells improve spinal muscular atrophy phenotype in mice. Brain 2010; 133: 465–481. [DOI] [PubMed] [Google Scholar]
- 40. Corti S, Nizzardo M, Simone C, et al. Genetic correction of human induced pluripotent stem cells from patients with spinal muscular atrophy. Sci Transl Med 2012; 4: 165ra162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Faravelli I, Nizzardo M, Comi GP, et al. Spinal muscular atrophy – recent therapeutic advances for an old challenge. Nat Rev Neurol 2015; 11: 351–359. [DOI] [PubMed] [Google Scholar]
- 42. Simone C, Ramirez A, Bucchia M, et al. Is spinal muscular atrophy a disease of the motor neurons only: pathogenesis and therapeutic implications? Cell Mol Life Sci 2016; 73: 1003–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]