Short abstract
Muscle pain is a common condition that relates to various pathologies. Muscle overuse induces muscle pain, and neutrophils are key players in pain production. Neutrophils also play a central role in chronic pain by secreting interleukin (IL)-18. The aim of this study was to investigate the involvement of neutrophils and IL-18 in a mouse model of muscle pain. The right hind leg muscles of BALB/c mice were stimulated electrically to induce excessive muscle contraction. The left hind leg muscles were not stimulated. The pressure pain threshold, number of neutrophils, and IL-18 levels were investigated. Furthermore, the effects of the IL-18-binding protein and Brilliant Blue G on pain were investigated. In stimulated muscles, pressure pain thresholds decreased, and neutrophil and IL-18 levels increased compared with that in non-stimulated muscles. The administration of IL-18-binding protein and Brilliant Blue G attenuated hyperalgesia caused by excessive muscle contraction. These results suggest that increased IL-18 secretion from larger numbers of neutrophils elicits mechanical hyperalgesia.
Keywords: Muscle pain, neutrophil, interleukin-18, electrical stimulation, mechanical hyperalgesia
Introduction
Musculoskeletal disorders are the most prevalent causes of chronic health problems, disabilities, and health care utilization.1 Among these health conditions, muscle pain is a common condition2 that relates to various pathologies. Muscle pain may manifest as neck and shoulder pain,3 non-specific low back pain,4 and myofascial pain syndrome (MPS).5–11 Neck and shoulder pain occurs in approximately 30% of the working population12,13 and is a risk factor for long-term absenteeism.12,13 Lower back pain has a lifetime prevalence of as high as 84% and causes disabilities of 11%–12% in the general population.14 MPS is very common in the general population, and its incidence may be as high as 54% in women and 45% in men.9 Furthermore, MPS is a primary source of pain in between 30% and 85% of patients presenting at a primary care setting or pain clinic.6–8 Although numerous treatment methods, such as pharmacological treatments, stretching, and massage, have been developed to alleviate these diseases, no single successful strategy is available as the underlying pathogenic mechanisms have not been fully determined.5,6,10,15,16
Muscle overuse, especially with eccentric contraction, induces pain during exercise.17,18 Muscle fiber destruction due to muscle overuse induces the release of adenosine triphosphate (ATP).2,19 Activation of specific receptors (so-called nociceptors) by ATP and low pH are particularly important in generating muscle pain.2,19 Shinoda et al. reported the involvement of ATP in a masseter muscle pain model in rats.20 Furthermore, sustained muscle contraction and chronic muscle ischemia induce a decrease in pH in affected tissues.2,19
Inflammatory responses to exercise-induced muscle damage may also produce muscle pain.17 Neutrophils and macrophages invade skeletal muscles and are assumed to produce proinflammatory cytokines after exercise-induced muscle damage.17,21 Neutrophils, which first invade injured skeletal muscles after exercise, are key players in innate immunity and influence inflammatory and immune reactions through the production of numerous cytokines such as interleukin (IL)-1β, tumor necrosis factor alpha (TNF-α), and interferon gamma (IFN-γ).22 Dessem et al. reported an increased number of neutrophils in muscles presenting with mechanical hyperalgesia after eccentric contraction.23
IL-18 is a proinflammatory cytokine that was first described in 1989. Recent findings have revealed that neutrophils are a source of IL-18.22,24 The activity of IL-18 is balanced by the naturally occurring IL-18 binding protein (IL-18BP), which shows high affinity for IL-18 in humans.25 IL-18 has been implicated in numerous diseases (e.g. autoimmune diseases, metabolic syndromes, inflammatory bowel disease, sepsis, and acute kidney injury).25 Recombinant IL-18BP has been investigated as a therapeutic cytokine in clinical trials of rheumatoid arthritis and plaque psoriasis.26 Previous investigators reported a relationship between IL-18 and chronic pain.27,28 For example, Vasudeva et al. reported that IL-18 functions as a central node associated with neuropathic pain in animal models.27 However, few reports have described the relationship between IL-18 and muscle pain. Eriksson et al. reported that elevated serum IL-18 levels were found in patients with Sjögren’s syndrome and myalgia, compared with IL-18 levels in patients with Sjögren’s syndrome without myalgia.29
The aim of the present study was to investigate the roles of the increased numbers of neutrophils and IL-18 secretion from these cells in mechanical hyperalgesia induced by repeated, excessive muscle contractions.
Materials and methods
Experimental animals
Male, five- to seven-week-old BALB/c mice (body weight 20–23 g) were obtained from Japan CLEA (Tokyo, Japan). The mice were housed under a 12:12-h light–dark cycle at 23 ± 1°C. All aspects of handling, care, and animal use were approved by the Animal Research Committee of Tohoku University (approval number: 2016MdA-240).
Repeated electrical stimulation of triceps surae muscles
Repeated electrical stimulation was used to induce excessive muscle contraction. After each mouse was anesthetized with an intraperitoneal injection of medetomidine (ZENOAQ, Fukushima, Japan, 0.3 mg/kg), midazolam (SANDZ, Tokyo, Japan, 4.0 mg/kg), and butorphanol (Meiji Seika Pharma Co, Tokyo, Japan, 5.0 mg/kg), two needle electrodes (Single-Stranded Stainless-Steel Wire, A-M System, Sequim, WA, USA) were transcutaneously applied into the triceps surae muscle of the right hind leg. Needle electrodes were placed on the proximal and distal ends of the muscle. After placing the needle electrodes, electrical stimulation of the muscle was performed at 10 Hz with a 10-V amplitude and a 100-µs pulse width for 30 min using a STG4004 multichannel system (MCS GmbH, Reutlingen, Germany), as previously described.20 During electrical stimulation for 30 min, the right hind leg was immobilized with the ankle joint in dorsal flexion so that the triceps surae muscle was fully extended to induce isometric contraction. A fused tetanic contraction was evoked. The electrical stimulation was performed for seven days per week. The needle electrodes were also applied to the contralateral triceps surae muscle without electrical stimulation or immobilization.
Assessment of mechanical nociceptive thresholds
The pressure pain threshold (PPT) was assessed using the Randall–Selitto test (MK-201D Pressure Analgesy-Meter, Muromachi Kikai Co., Tokyo, Japan).30 This test employed a cone-shaped plastic tip (tip diameter: 2.6 mm) attached to a scale with a display. A linearly increasing pressure (10 mm Hg/s) was applied to the lateral surface of the triceps surae muscle. Skin over the triceps surae muscle was carefully shaved bilaterally at the beginning of the experiment in order to find the muscle more easily. Mice were loosely wrapped in a towel to calm them down and were treated gently during the experiment. The PPT was defined as the amount of pressure (mm Hg) required to elicit pain-related behaviors such as vocalization, struggling, and leg withdrawal.20,31,32 PPT assessment of both hind legs was performed every day for 13 days after initiation of the electrical stimulation. All experiments investigating mechanical hyperalgesia were performed in the morning as circadian rhythm affects pain sensitivity. Experiments investigating secondary hyperalgesia were performed in the plantar surface of the foot. The cut-off value of the PPT was 300 mm Hg.31 Assessment of the PPT data was performed by a blinded investigator to avoid bias.
Local effect of IL-18 on hyperalgesia
To confirm the local effects of IL-18, recombinant mouse IL-18 protein (B004–5, 50 ng; Medical & Biological Laboratories Co., Ltd., Nagoya, Japan) dissolved in saline (rmIL-18 group) or saline alone (saline group) was administrated to the triceps surae muscles, as previously described.33 The volume of injected solution was 25 μl in each group. At 1, 2, 4, 24, and 48 h post-injection, PPTs were assessed as described above.
Tissue preparation
On day 7 after the initiation of electrical stimulation, mice were sacrificed by cervical dislocation and the triceps surae muscles were obtained. Specimens for quantitative real-time polymerase chain reaction (qRT-PCR) analysis and enzyme-linked immunosorbent assays (ELISAs) were frozen in liquid nitrogen and stored at –80°C. For immunohistochemical staining, the specimens were immersed in 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4) overnight at 4°C. After dehydration through a graded series of ethanol solutions, the specimens were embedded in paraffin. The embedded tissues were cut into 5-μm axial sections. For fluorescence activated cell sorting (FACS) analysis, the specimens were immersed in 1% phosphate-buffered saline (PBS) and analyzed the same day.
qRT-PCR analysis
Total RNA was extracted using the Trizol reagent (Invitrogen, Carlsbad, CA, USA). cDNA was prepared using a Transcriptor First Strand cDNA Synthesis Kit (Roche Diagnostics, Basel, Switzerland) and primers with the following sequences: IL-18: F5′-TGG TGG GGG TTC TCT GTG GTT-3′ and R5′-TTG AGG CGG CTT TCT TTG TCC-3′; EF1α1 (internal control primer): F5′-TCG CTT TGC TGT TCG TGA C-3′ and R5′-TGG GGT GGC AGG TGT TAG-3′. Relative expression levels of each mRNA were calculated as a function of EF1α1 expression, as previously described.34
ELISA experiments
Tissue samples were disrupted and homogenized using lysis buffer composed of bovine serum albumin (BSA; 100 µg/ml, A4503, Sigma–Aldrich, St. Louis, MO, USA), Triton X-100 (0.1%, Wako Pure Chemicals Industries, Osaka, Japan), 1 M HEPES (1%, 533–08083, Wako Pure Chemicals Industries), protease inhibitor (1%, P8340, Sigma–Aldrich), and distilled water (DW). After homogenization, samples were centrifuged for 10 min at 9730 × g and 4°C, and the supernatant was stored at −80°C. IL-18 expression levels were analyzed with a Bio-Plex Multiplex Immunoassay System (Bio-Rad, Hercules, CA, USA) and a Bio-Plex Pro Mouse Cytokine 23-plex Assay (Bio-Rad), according to the manufacturer’s instructions.
FACS analysis
Tissue samples were transferred to sterile Dulbecco’s modified Eagle’s medium (Wako Pure Chemicals Industries) supplemented with 1% penicillin–streptomycin. Tissues were minced and digested with 0.2% collagenase (Wako Pure Chemicals Industries) and 0.1% DNase I (Sigma-Aldrich) for 1 h at 37°C. PBS was added to the digested muscle tissue samples, which were then filtered through a 70-μm cell strainer (BD Biosciences, Franklin Lakes, NJ, USA) and centrifuged at 700 × g for 20 min at 4°C. Pellets were resuspended in 1 ml of staining solution composed of 1% BSA (Sigma–Aldrich) in PBS, and then incubated with an Fc receptor-blocking solution (TruStain fcX, 1:50 in staining buffer; BioLegend, San Diego, CA, USA) for 10 min. The samples were then labeled with the following monoclonal antibodies (all at a 1:20 dilution): fluorescein isothiocyanate-conjugated anti-CD45 (clone 30-F11, BioLegend), allophycocyanin (APC)/Cy7-conjugated anti-CD11b (clone M1/70, BioLegend), peridinin chlorophyll a protein/Cy5.5-conjugated anti-Ly6G (clone 1A8, BioLegend), phycoerythrin-conjugated anti-Siglec F (clone E50–2440, BD Biosciences), or APC-conjugated anti-F4/80 (clone BM8, BioLegend). After a 45-min incubation on ice, the cell suspension was washed with staining solution and centrifuged twice for 5 min at 700 × g. Neutrophils were defined as single live mononuclear CD45+CD11b+Ly6G+SiglecF−F4/80− cells.35–37 FACS was performed on a FACS ARIA II flow cytometer (BD Biosciences), and data were analyzed with FlowJo software (Tree Star, Ashland, OR, USA). The sorting gate was set using negative-control cells treated with the Fc receptor-blocking solution.
Immunohistochemistry
Tissue sections were deparaffinized and washed in PBS. Subsequently, they were incubated with Target Retrieval Solution (Agilent Technologies, Santa Clara, CA, USA) for 60 min at 100°C to induce antigen retrieval. After washing in DW and PBS, endogenous immunoglobulins were blocked by incubation with 10% normal goat serum (Nichirei Biosciences Inc., Tokyo, Japan) for 60 min. The slides were washed again in PBS and incubated with a polyclonal rabbit anti-mouse IL-18 antibody (ab71495, Abcam plc, Cambridge, UK, dilution 1:500) and a monoclonal rat anti-mouse granulocyte receptor-1 (Gr-1) antibody (RB6–8C5, BioLegend, dilution 1:500) in PBS overnight at 4°C, and then rinsed in PBS. Subsequently, the slides were incubated for 60 min in PBS with an Alexa Fluor 488-conjugated goat anti-rabbit IgG (A-11034, Life Technologies, Carlsbad, CA, USA, dilution 1:750) for IL-18 and an Alexa Fluor 555-cojugated goat anti-rat IgG (A-21434, Life Technologies, dilution 1:750) for Gr-1 at room temperature, after which they were rinsed in PBS. Finally, the slides were incubated with 4,6-diamidino-2-phenylindole (Sigma–Aldrich, dilution 1:500) for 10 min at 25°C for nuclear staining. Images were captured with a fluorescence microscope (BZ-9000 BIOREVO, KEYENCE, Osaka, Japan). The images were analyzed using Adobe Photoshop (Adobe System Inc., San Jose, CA, USA). The localization of Gr-1-positive cells (neutrophils) was evaluated by two blinded investigators to avoid bias. Two animals were used for immunohistochemistry, and two slides/animal were analyzed. After confirming reproducibility, representative images were presented.
Assessment of the systemic effect of BBG on hyperalgesia
Brilliant Blue G (BBG) is a selective P2X7R antagonist that decreases secretion of mature IL-18 by attenuating inflammasome activation in various cell types, including neutrophils.38,39 BBG was administrated to mice to test the effects of decreased mature IL-18 secretion on hyperalgesia. During repeated electrical stimulation of the triceps surae muscles, BBG dissolved in 0.9% saline (45.5 mg/kg, 0.3 ml/body) was injected intraperitoneally every 48 h (BBG group), as previously described.40 Control animals received an equivalent volume of 0.9% saline (saline group). The PPTs were assessed by performing the Randall–Selitto test on day 7. Measurement of IL-18 levels by ELISA was performed as described above.
Assessment of the local effect of IL-18BP on hyperalgesia
To test the local effects of IL-18BP on hyperalgesia, IL-18BP (122-BP-100, R&D Systems, Inc., Minneapolis, MN, USA, 1 μg) dissolved in saline (100 μl) or saline alone (100 μl) was administered to the triceps surae muscles, as previously described.41 During repeated electrical stimulation, mice were administered either IL-18BP (IL-18BP group) or saline (saline group) in the triceps surae muscles every day of stimulation. PPTs were assessed in the same manner.
Statistical analysis
Statistical analyses were performed using SPSS Statistics 24 (IBM, Armonk, NY, USA). Analysis of PPT time-course data was performed by two-way analysis of variance (ANOVA) with repeated measures with Tukey’s post hoc multiple-comparison test. To compare data from four groups from a single day (PPTs and ELISA), one-way ANOVA with Tukey’s post hoc, multiple-comparison test was used for the analysis. ELISA data between two groups were analyzed with the Mann–Whitney test. FACS and qRT-PCR data were analyzed using the Wilcoxon signed-rank test. Data were expressed as the mean ± standard error of the mean. A p value of <0.05 was considered to represent statistical significance.
Results
Induction of recombinant IL-18 protein production by hyperalgesia
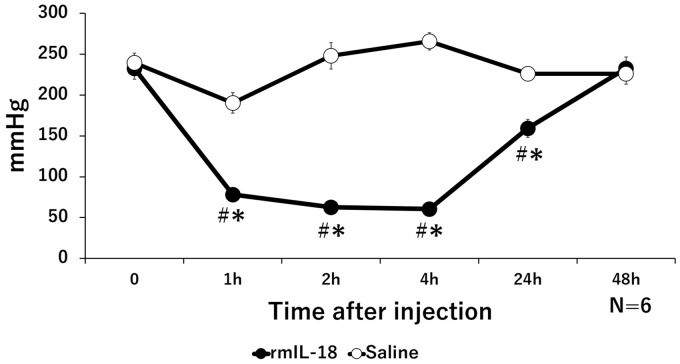
The PPTs of the recombinant IL-18 (rmIL-18) group significantly decreased from 1 to 24 h after administration, compared with that before administration. PPTs recovered by 48-h post-administration. In the saline group, no significant changes were observed during between 1 and 24 h after administration compared with that before administration. Significant differences were observed between the rmIL-18 group and saline group between 1 and 24 h after administration (Figure 1).
Figure 1.
Change in pressure pain thresholds (PPTs) induced by local administration of recombinant IL-18 protein (rmIL-18). Time course of PPT values in the rmIL-18 group and saline group. The PPTs of the rmIL-18 group significantly decreased compared with those of the saline group from 1 to 24 h after administration; *p < 0.05, significantly different from the saline group; #p < 0.05, significantly different from the pre-injection values.
Excessive contraction caused by repeated electrical stimulation induced mechanical hyperalgesia and elevated IL-18 production in muscles
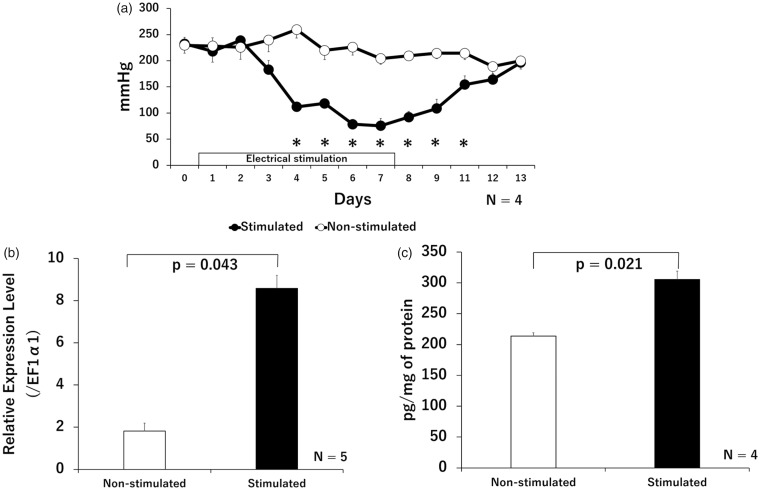
The PPTs of the stimulated muscles began to decrease on day 3, and significantly decreased during days 4 to 11 compared with that of non-stimulated contralateral muscle. Reduced PPTs in the stimulated muscles recovered after the electrical stimulation was completed, and no significant difference was observed in PPTs between stimulated and non-stimulated muscles after day 12. No significant changes were observed in the PPTs of non-stimulated muscles, during the experimental period compared with the values before electrical stimulation (Figure 2(a)). There was no evidence for secondary hyperalgesia during the experiments. IL-18 induction in the stimulated muscles significantly increased compared with that in non-stimulated muscles, as determined by qRT-PCR (Figure 2(b)) or ELISA (Figure 2(c)).
Figure 2.
Changes in the PPTs and IL-18 levels in stimulated and non-stimulated muscles. Time course of PPT changes (a). The PPTs of stimulated muscles significantly decreased compared with that of non-stimulated contralateral muscles, during days 4 to 11 after initiating electrical stimulation; *p < 0.05, significantly different from non-stimulated muscle. Relative gene expression levels of IL-18 (b) and quantities of IL-18 (c) in muscle tissues, with or without electrical stimulation at day 7; Relative IL-18 mRNA expression (determined by qRT-PCR) and protein expression (determined by ELISA) increased significantly in stimulated muscles. Expression of the IL-18 gene is shown ratiometrically, relative to EF1α1 expression. The concentrations of protein of all the samples used for ELISA were equalized to 1.3 mg/ml.
The number of neutrophils increased in hyperalgesic muscles
The number of neutrophils from stimulated muscles (21%) significantly increased compared with that in non-stimulated muscles (14%), as determined by FACS (Figure 3(a), (c), and (d)). However, the number of macrophages did not increase in the stimulated muscles (Figure 3(b)). Immunohistochemical staining revealed an increased number of Gr-1-positive cells (neutrophils) in stimulated muscle fibers compared with that in non-stimulated Gr-1-positive cells. Most of these cells were also positive for IL-18 (Figure 3(e) to (j)).
Figure 3.
Changes in the number of neutrophils and macrophages in stimulated and non-stimulated muscles. The percentage of the number of neutrophils (a, c, and d) and macrophages (b), and immunohistochemical staining of IL-18 (green) and Gr-1 (red) in stimulated and non-stimulated muscle at day 7 are shown (e to j). The number of neutrophils increased significantly in stimulated muscles. The number of GR-1-positive cells (neutrophils) increased; most of these cells were also positive for IL-18 in stimulated muscles (e to j). The percentage of the number of neutrophils and macrophages are shown as a ratio of the number of CD45-positive cells; scale bar = 50 μm.
BBG and IL-18BP attenuated hyperalgesia in excessively contracted muscles
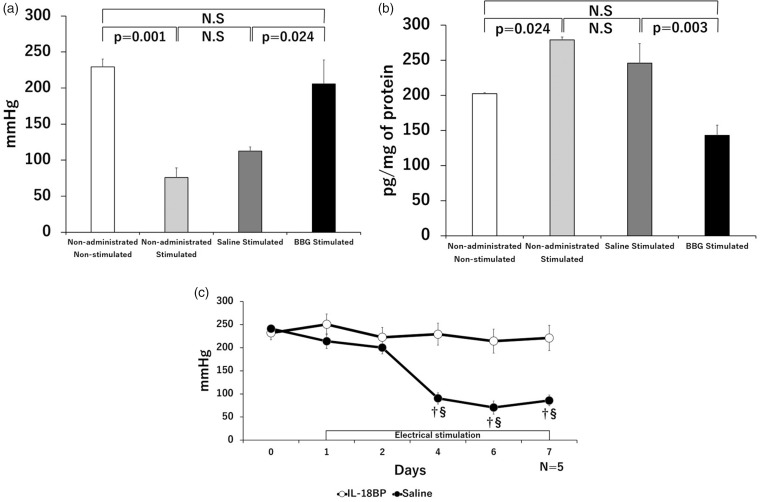
In the BBG group, the PPTs significantly increased and IL-18 levels significantly decreased compared with those in the saline group (Figure 4(a) and (b)). No significant differences were observed in the PPTs and IL-18 levels between the saline group and the stimulated muscles of non-treated mice or between the BBG group and the non-stimulated muscles of non-treated mice. In the PPTs of the IL-18BP group, no significant changes were found during the experimental period versus before the electrical stimulation. The PPTs of the saline group significantly decreased after day 4 compared those before electrical stimulations. Significant differences were observed between the IL-18BP and saline groups from four to seven days after initiating electrical stimulation (Figure 4(c)).
Figure 4.
Changes in PPTs and the amount of IL-18 produced after administration of Brilliant Blue G (BBG) and IL-18 binding protein (IL-18BP). The PPTs (a) and quantities of IL-18 (b) in non-stimulated muscles, stimulated muscles of non-treated mice, the saline group, and the BBG group seven days after initiating electrical stimulation are shown. The PPTs increased, and the amount of IL-18 decreased significantly in the BBG group compared with that in the saline group. No significant differences were observed between the saline group and the stimulated muscles of non-treated mice. Time course of the PPT changes in the IL-18BP group and the saline group (c). In the IL-18BP group, PPTs significantly increased compared with those in the saline group; †p < 0.05, significantly different from the IL-18BP group; §p < 0.05, significantly different from the pre-stimulation values (day 0). The protein concentrations of protein of all samples used for ELISA were equalized to 1.3 mg/ml.
Discussion
Data generated in the present study revealed the following: (1) The administration of rmIL-18 administration into the triceps surae muscles induced hyperalgesia, (2) excessive muscle contraction by electrical stimulation induced mechanical hyperalgesia and IL-18 elevation in the muscle, (3) the number of neutrophils increased after electrical stimulation; these neutrophils secreted IL-18 in painful muscles, and (4) intraperitoneal BBG administration and local IL-18BP administration attenuated hyperalgesia caused by excessive muscle contraction.
Although few reports have shown an association between IL-18 and muscle pain, previous studies reported an association with chronic pain.27,28,33 Pilat et al. reported that elevation of IL-18 levels in the spinal cord and intrathecal IL-18BP administration attenuated neuropathic pain in rats.28 Verri et al. reported that intraplantar administration of rmIL-18 induced mechanical hyperalgesia in the planters of mice.33 These data agree closely with our findings.
It has been reported that muscle contraction in response to electrical stimulation induces mechanical hyperalgesia.20,42 This previously reported experimental protocol was followed in experiments using a similar pain model for the rat masseter muscle,20 and the stimuli were set so as to avoid inducing motion or contractions of abdominal muscle. Shinoda et al. reported that repeated muscle contraction (15 days) produced mechanical hyperalgesia 6–15 days following the initiation of contraction in the masseter muscle pain model in rats; these findings were similar to the results of this study. Therefore, the present mouse model is suitable for investigation of triceps surae muscle pain induced by repeated excessive contraction. In order to induce muscle pain, mice were subjected to seven days of contractions in this study. The duration of our experiments was shorter than that in previous studies as hyperalgesia of the muscle was observed at day 4. Although the needle electrodes were also inserted into the contralateral triceps surae muscle without electrical stimulation, hyperalgesia of the muscle was not observed. This finding demonstrates that the observed muscle hyperalgesia was caused by stimulation and not possible injury resulting from repetitive insertion of the needle.
Neutrophils, which immediately migrate to damaged tissues following injury, are involved in inflammatory responses.17 Infiltration of neutrophils into damaged muscles within several hours of exercise has been demonstrated in various species of mice and rats, as well as in humans.23,43,44 After infiltration into damaged muscles, neutrophils phagocytose necrotic tissue45 and secrete proinflammatory cytokines.17 Kanda et al. reported an increased number of circulating neutrophils and their migration to damaged muscle tissues after eccentric exercise, within several hours in humans.43 Dessem et al. and Pizza et al. reported an increase in neutrophil counts in muscle tissues at one to four days after eccentric contraction with electrical stimulation in rat and mouse models, respectively.23,44 Furthermore, the relationship between neutrophil invasion and muscle pain has been reported. Schiavuzzo et al. reported that pre-treatment with fucoidan (a leukocyte-adhesion inhibitor) attenuated hyperalgesia induced by local administration of α, β-meATP (a P2X3 agonist) in a rat model.46 These reports support the present observation of increased neutrophils in painful muscles. However, some reports indicate that neutrophils are present for up to 24 h after exercise, and that macrophages subsequently replace the neutrophils.17,23 Although the muscle tissues were harvested after repeated stimulation on day 7, we did not observe that the number of macrophages increased in stimulated muscles versus non-stimulated muscles. These results may be explained by the fact that the muscle tissues were harvested approximately 24 h after the last stimulation.
Although it has been reported that IL-18 is produced by macrophages, dendritic cells, and epithelial cells,25,47 recent reports have shown that neutrophils may represent a source of IL-18.22,24,48 Robertson et al. reported the constitutive expression of IL-18 in human neutrophils obtained from healthy volunteers and patients with rheumatoid arthritis.24 Spörri et al. reported that neutrophils produced mature IL-18 in a Legionella pneumophilia-infection mouse model.48 These reports support our finding that neutrophils represent a source of IL-18 in painful muscles. Immunohistochemical experiments indicated both neutrophils and muscle fibers were also IL-18-positive in stimulated muscles, which suggest that IL-18 was secreted from neutrophils in the affected muscle cells.
No previous reports have shown that BBG attenuates pain or suppresses IL-18 production in a model of muscle pain. However, numerous studies have shown that BBG decreases IL-18 secretion by blocking P2X7R in various diseases such as graft-versus-host disease,38 subarachnoid hemorrhage,39 and sleep apnea syndrome.49 Furthermore, several reports have shown that BBG attenuates pain in various diseases such as bone cancer,50 neuropathic pain,51 and chronic pancreatitis.52 These reports support our results that intraperitoneal BBG administration attenuated hyperalgesia and IL-18 induction by excessive muscle contraction. Our results showed that the saline group tended to have higher PPTs and lower IL-18 levels than the non-treated group. These trends may have occurred due to washing out or dilution of IL-18 in muscles by intraperitoneally injected saline. Although no studies have investigated the treatment of muscle pain by administration of IL-18BP to peripheral tissues, Min reported that subcutaneous IL-18BP administration attenuated acute graft-versus-host disease responses.41 This report supports the present finding that IL-18BP administration to the triceps surae muscles attenuated hyperalgesia following excessive contraction. Our data from experiments conducted using BBG showed that systemic IL-18 inhibition attenuated muscle hyperalgesia induced by excessive contraction. However, we also found that local administration of IL-18BP to the triceps surae muscles attenuated hyperalgesia. The PPTs of the IL-18BP group (local administration) were almost the same as those of the BBG group (intraperitoneal administration) at day 7 post-initiation of electrical stimulation. These results suggest that increased IL-18 levels affect peripheral tissues, causing muscle hyperalgesia.
Muscle overuse induces ATP release, which generates muscle pain.2,19 A few reports have described the relationship between neutrophils, IL-18, and ATP. Perregaux et al. and Eltom et al. reported that ATP enhanced IL-18 production in human blood samples and cigarette smoke-exposed mice, respectively.53,54 However, it is not known whether neutrophils produce IL-18 after stimulation by ATP, and therefore whether IL-18 secretion from neutrophils is due to ATP stimulation following muscle contraction. Elton et al. reported that IL-18 enhances the transmigration of neutrophils.54 These findings may explain the persistence of muscle pain.
Our study has the following limitations: (1) evaluation of IL-18 levels was performed only in muscle tissues, without additional testing of sensory neurons and the central nervous system; (2) evaluation of ATP production was not performed; (3) assessment of the PPT using the Randall–Selitto test was performed by only one investigator; (4) evaluation of other inflammatory cytokines such as IL-1β, TNF-α, and IFN-γ was not performed.
Conclusions
The results of our study suggest that increased neutrophil numbers and IL-18 secretion from neutrophils produce mechanical hyperalgesia induced by repeated excessive muscle contraction. To our knowledge, this report is the first to demonstrate the relationship between IL-18 and muscle pain induced by repeated excessive contraction. These findings reveal a potential therapeutic target for treating various muscle pains, including neck and shoulder pain, lower back pain, and MPS.
Acknowledgment
The authors thank Natsumi Emoto for technical advice and data collection.
Authors' Note
Masamichi Shinoda is currently affiliated to Department of Physiology,Nihon University School of Dentistry, Tokyo, Japan.
Author Contributions
SY performed experiments and wrote the article. YH and MT supervised the research design and writing of the article. MS and MK supervised the research design and collection of data. MK, HH, and CC participated in data collection. TY, YS, NI, TS, and YY gave advice regarding the experimental methods used. KS and EI provided advice regarding research design.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD
Shinichirou Yoshida http://orcid.org/0000-0003-1110-2363
References
- 1.Dannecker EA, Gagnon CM, Jump RL, et al. Self-care behaviors for muscle pain. J Pain 2004; 5: 521–527. [DOI] [PubMed] [Google Scholar]
- 2.Mense S. Muscle pain: mechanisms and clinical significance. Dtsch Arztebl Int 2008; 105: 214–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hanvold TN, Wærsted M, Mengshoel AM, et al. The effect of work-related sustained trapezius muscle activity on the development of neck and shoulder pain among young adults. Scand J Work Environ Health 2013; 39: 390–400. [DOI] [PubMed] [Google Scholar]
- 4.van Dieën JH Selen LP andCholewicki J.. Trunk muscle activation in low-back pain patients, an analysis of the literature. J Electromyogr Kinesiol 2003; 13: 333–351. [DOI] [PubMed] [Google Scholar]
- 5.Kalichman L andVulfsons S.. Dry needling in the management of musculoskeletal pain. J Am Board Fam Med 2010; 23: 640–646. [DOI] [PubMed] [Google Scholar]
- 6.Borg-Stein J andSimons DG.. Focused review: myofascial pain. Arch Phys Med Rehabil 2002; 83: S40–S49. [DOI] [PubMed] [Google Scholar]
- 7.Skootsky SA Jaeger B andOye RK.. Prevalence of myofascial pain in general internal medicine practice. West J Med 1989; 151: 157–160. [PMC free article] [PubMed] [Google Scholar]
- 8.Fishbain DA, Goldberg M, Meagher BR, et al. Male and female chronic pain patients categorized by DSM-III psychiatric diagnostic criteria. Pain 1986; 26: 181–197. [DOI] [PubMed] [Google Scholar]
- 9.Vázquez-Delgado E Cascos-Romero J andGay-Escoda C.. Myofascial pain syndrome associated with trigger points: a literature review. (I): Epidemiology, clinical treatment and etiopathogeny. Med Oral Patol Oral Cir Bucal 2009; 14: e494–e498. [DOI] [PubMed] [Google Scholar]
- 10.Mata Diz JB, de Souza JR, Leopoldino AA, et al. Exercise, especially combined stretching and strengthening exercise, reduces myofascial pain: a systematic review. J Physiother 2017; 63: 17–22. [DOI] [PubMed] [Google Scholar]
- 11.Xu YM Ge HY andArendt-Nielsen L.. Sustained nociceptive mechanical stimulation of latent myofascial trigger point induces central sensitization in healthy subjects. J Pain 2010; 11: 1348–1355. [DOI] [PubMed] [Google Scholar]
- 12.Andersen LL, Mortensen OS, Hansen JV, et al. A prospective cohort study on severe pain as a risk factor for long-term sickness absence in blue- and white-collar workers. Occup Environ Med 2011; 68: 590–592. [DOI] [PubMed] [Google Scholar]
- 13.Buckle PW andDevereux JJ.. The nature of work-related neck and upper limb musculoskeletal disorders. Appl Ergon 2002; 33: 207–217. [DOI] [PubMed] [Google Scholar]
- 14.Balagué F, Mannion AF, Pellisé F, et al. Non-specific low back pain. Lancet 2012; 379: 4482–4491. [DOI] [PubMed] [Google Scholar]
- 15.Andersen CH, Andersen LL, Zebis MK, et al. Effect of scapular function training on chronic pain in the neck/shoulder region: a randomized controlled trial. J Occup Rehabil 2014; 24: 316–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kamali F, Panahi F, Ebrahimi S, et al. Comparison between massage and routine physical therapy in women with sub acute and chronic nonspecific low back pain. BMR 2014; 27: 475–480. [DOI] [PubMed] [Google Scholar]
- 17.Peake J Nosaka K andSuzuki K.. Characterization of inflammatory responses to eccentric exercise in humans. Exerc Immunol Rev 2005; 11: 64–85. [PubMed] [Google Scholar]
- 18.Dannecker EA andKoltyn KF.. Pain during and within hours after exercise in healthy adults. Sports Med 2014; 44: 921–942. [DOI] [PubMed] [Google Scholar]
- 19.Mastaglia FL. The relationship between muscle pain and fatigue. Neuromuscul Disord 2012; 22: S178–S180. [DOI] [PubMed] [Google Scholar]
- 20.Shinoda M Ozaki N andSugiura Y.. Involvement of ATP and its receptors on nociception in rat model of masseter muscle pain. Pain 2008; 134: 148–157. [DOI] [PubMed] [Google Scholar]
- 21.Kawanishi N, Mizokami T, Niihara H, et al. Neutrophil depletion attenuates muscle injury after exhaustive exercise. Med Sci Sports Exerc 2016; 48: 1917–1924. [DOI] [PubMed] [Google Scholar]
- 22.Fortin CF Ear T andMcDonald PP.. Autocrine role of endogenous interleukin-18 on inflammatory cytokine generation by human neutrophils. Faseb J 2009; 23: 194–203. [DOI] [PubMed] [Google Scholar]
- 23.Dessem D, Ambalavanar R, Evancho M, et al. Eccentric muscle contraction and stretching evoke mechanical hyperalgesia and modulate CGRP and P2X(3) expression in a functionally relevant manner. Pain 2010; 149: 284–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Robertson SE, Young JD, Kitson S, et al. processing of IL-18 in human neutrophils. Eur J Immunol 2006; 36: 722–731. [DOI] [PubMed] [Google Scholar]
- 25.Dinarello CA, Novick D, Kim S, et al. Interleukin-18 and IL-18 binding protein. Front Immunol 2013; 4: 289. [DOI] [PMC free article] [PubMed]
- 26.Tak PP Bacchi M andBertolino M.. Pharmacokinetics of IL-18 binding protein in healthy volunteers and subjects with rheumatoid arthritis or plaque psoriasis. Eur J Drug Metab Pharmacokinet 2006; 31: 109–116. [DOI] [PubMed] [Google Scholar]
- 27.Vasudeva K, Vodovotz Y, Azhar N, et al. In vivo and systems biology studies implicate IL-18 as a central mediator in chronic pain. J Neuroimmunol 2015; 15; 283: 43–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pilat D, Piotrowska A, Rojewska E, et al. Blockade of IL-18 signaling diminished neuropathic pain and enhanced the efficacy of morphine and buprenorphine. Mol Cell Neurosci 2016; 71: 114–124. [DOI] [PubMed] [Google Scholar]
- 29.Eriksson P, Andersson C, Ekerfelt C, et al. Sjögren’s syndrome with myalgia is associated with subnormal secretion of cytokines by peripheral blood mononuclear cells. J Rheumatol 2004; 31: 729–735. [PubMed] [Google Scholar]
- 30.Randall LO andSelitto JJ.. A method for measurement of analgesic activity on inflamed tissue. Arch Int Pharmacodyn Ther 1957; 111: 409–419. [PubMed] [Google Scholar]
- 31.Ide S, Minami M, Ishihara K, et al. Abolished thermal and mechanical antinociception but retained visceral chemical antinociception induced by butorphanol in mu-opioid receptor knockout mice. Neuropharmacology 2008; 54: 1182–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.TenBroek EM, Yunker L, Nies MF, et al. Randomized controlled studies on the efficacy of antiarthritic agents in inhibiting cartilage degeneration and pain associated with progression of osteoarthritis in the rat. Arthritis Res Ther 2016; 18: 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Verri WA, Jr, Cunha TM, Parada CA, et al. Antigen-induced inflammatory mechanical hypernociception in mice is mediated by IL-18. Brain Behav Immun 2007; 21: 535–543. [DOI] [PubMed] [Google Scholar]
- 34.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 2001; 29: e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Swamydas M, Luo Y, Dorf ME, et al. Isolation of mouse neutrophils. Curr Protoc Immunol 2015; 110: 3.20.1–3.20.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gordon S, Hamann J, Lin HH, et al. F4/80 and the related adhesion-GPCRs. Eur J Immunol 2011; 41: 2472–2476. [DOI] [PubMed] [Google Scholar]
- 37.Percopo CM, Brenner TA, Ma M, et al. SiglecF+Gr1hi eosinophils are a distinct subpopulation within the lungs of allergen-challenged mice. J Leukoc Biol 2017; 101: 321–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhong X, Zhu F, Qiao J, et al. The impact of P2X7 receptor antagonist, Brilliant Blue G on graft-versus-host disease in mice after allogeneic hematopoietic stem cell transplantation. Cell Immunol 2016; 310: 71–77. [DOI] [PubMed] [Google Scholar]
- 39.Chen S, Ma Q, Krafft PR, et al. P2X7R/cryopyrin inflammasome axis inhibition reduces neuroinflammation after SAH. Neurobiol Dis 2013; 58: 296–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Díaz-Hernández M, Díez-Zaera M, Sánchez-Nogueiro J, et al. Altered P2X7-receptor level and function in mouse cmodels of Huntington’s disease and therapeutic efficacy of antagonist administration. Faseb J 2009; 23: 1893–1906. [DOI] [PubMed] [Google Scholar]
- 41.Min XU. Effect and mechanism of IL-18BP on acute graft-versus-host disease. Chin J Immunol 2006; 7: 615–618 (in Chinese). [Google Scholar]
- 42.Alvarez P Levine JD andGreen PG.. Eccentric exercise induces chronic alterations in musculoskeletal nociception in the rat. Eur J Neurosci 2010; 32: 819–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kanda K, Sugama K, Hayashida H, et al. Eccentric exercise-induced delayed-onset muscle soreness and changes in markers of muscle damage and inflammation. Exerc Immunol Rev 2013; 19: 72–85. [PubMed] [Google Scholar]
- 44.Pizza FX, Peterson JM, Baas JH, et al. Neutrophils contribute to muscle injury and impair its resolution after lengthening contractions in mice. J Physiol 2005; 562: 899–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Toumi H andBest TM.. The inflammatory response: friend or enemy for muscle injury? Br J Sports Med 2003; 37: 284–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schiavuzzo JG, Teixeira JM, Melo B, et al. Muscle hyperalgesia induced by peripheral P2X3 receptors is modulated by inflammatory mediators. Neuroscience 2015; 285: 24–33. [DOI] [PubMed] [Google Scholar]
- 47.Stoll S, Jonuleit H, Schmitt E, et al. Production of functional IL-18 by different subtypes of murine and human dendritic cells (DC): DC-derived IL-18 enhances IL-12-dependent Th1 development. Eur J Immunol 1998; 28: 3231–3239. [DOI] [PubMed] [Google Scholar]
- 48.Spörri R, Joller N, Hilbi H, et al. A novel role for neutrophils as critical activators of NK cells. J Immunol 2008; 181: 7121–7130. [DOI] [PubMed] [Google Scholar]
- 49.Deng Y, Guo XL, Yuan X, et al. P2X7 Receptor antagonism attenuates the intermittent hypoxia-induced spatial deficits in a murine model of sleep apnea via inhibiting neuroinflammation and oxidative stress. Chin Med J 2015; 128: 2168–2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Huang ZX, Lu ZJ, Ma WQ, et al. Involvement of RVM-expressed P2X7 receptor in bone cancer pain: mechanism of descending facilitation. Pain 2014; 155: 783–791. [DOI] [PubMed] [Google Scholar]
- 51.Di Cesare Mannelli L, Marcoli M, Micheli L, et al. Oxaliplatin evokes P2X7-dependent glutamate release in the cerebral cortex: a pain mechanism mediated by Pannexin 1. Neuropharmacology 2015; 97: 133–141. [DOI] [PubMed] [Google Scholar]
- 52.Liu PY, Lee IH, Tan PH, et al. P2X7 receptor mediates spinal microglia activation of visceral hyperalgesia in a rat model of chronic pancreatitis. Cell Mol Gastroenterol Hepatol 2015; 1: 710–720.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Perregaux DG, McNiff P, Laliberte R, et al. ATP acts as an agonist to promote stimulus-induced secretion of IL-1 beta and IL-18 in human blood. J Immunol 2000; 165: 4615–4623. [DOI] [PubMed] [Google Scholar]
- 54.Eltom S, Belvisi MG, Stevenson CS, et al. Role of the inflammasome-caspase1/11-IL-1/18 axis in cigarette smoke driven airway inflammation: an insight into the pathogenesis of COPD. PLoS One 2014; 9: e112829. [DOI] [PMC free article] [PubMed] [Google Scholar]