Abstract
Rationale: Cross-sectional studies have linked intake of high-fructose corn syrup–sweetened beverages with asthma in schoolchildren.
Objectives: To examine associations of maternal prenatal and early childhood intake of sugar-sweetened beverages and fructose with current asthma in midchildhood (median age, 7.7 yr).
Methods: We assessed maternal pregnancy (first- and second-trimester average) and child (median age, 3.3 yr) intake of sugar-sweetened beverages and total fructose using food frequency questionnaires in 1,068 mother–child pairs from Project Viva, a prospective prebirth cohort. In a multivariable analysis, we examined associations of quartiles of maternal and child sugar-sweetened beverage, juice, and total fructose intake with child current asthma in midchildhood, assessed by questionnaire as ever having doctor-diagnosed asthma plus taking asthma medications or reporting wheezing in the past 12 months.
Results: Higher maternal pregnancy sugar-sweetened beverage consumption (mean, 0.6 servings/d; range, 0–5) was associated with younger maternal age, nonwhite race/ethnicity, lower education and income, and higher prepregnancy body mass index. Adjusting for prepregnancy body mass index and other covariates, comparing quartile 4 with quartile 1, higher maternal pregnancy intake of sugar-sweetened beverages (odds ratio, 1.70; 95% confidence interval, 1.08–2.67) and total fructose (odds ratio, 1.58; 95% confidence interval, 0.98–2.53) were associated with greater odds of midchildhood current asthma (prevalence, 19%). Higher early childhood fructose intake (quartile 4 vs. quartile 1) was also associated with midchildhood current asthma in models adjusted for maternal sugar-sweetened beverages (odds ratio, 1.79; 95% confidence interval, 1.07–2.97) and after additional adjustment for midchildhood body mass index z-score (odds ratio, 1.77; 95% confidence interval, 1.06–2.95).
Conclusions: Higher sugar-sweetened beverage and fructose intake during pregnancy and in early childhood was associated with childhood asthma development independent of adiposity.
Keywords: pregnancy, fructose, sugar-sweetened beverages, asthma, childhood
The rise in childhood asthma prevalence in the United States occurring from the early 1980s onward is likely multifactorial, with both poor nutrition and excess adiposity hypothesized to be among the important contributors (1–5). Concurrent with the rise in asthma has been increased caloric intake from added sugars, most notably in sugar-sweetened beverages, which has been linked to the obesity epidemic (6–9). Fructose, often in the form of high fructose corn syrup, is commonly added as a sweetener to sugar-sweetened beverages (e.g., fruit drinks and soda) (10–13). Natural sources of dietary fructose include fruits and their juices (10).
Many studies have demonstrated links between obesity or overweight and asthma, though the biological mechanisms of these associations and their implications for asthma therapy are still imperfectly understood (14). Recent studies suggest that in addition to influencing asthma through increasing the risk of obesity, high fructose intake may influence the risk of lung disease at least in part through distinct mechanisms. Independent of elevated body mass index, high intakes of sugar-sweetened beverages and fruit juices were cross-sectionally associated with higher asthma rates in 2- to 9-year-old children as well as with a higher prevalence of chronic obstructive pulmonary disease in adults in the 2003–2006 National Health and Nutrition Examination Survey. In addition to their adiposity-related inflammatory potential (15–21), beverages containing excess free fructose may have specific effects on the gut that may have downstream influences on the lung. Specifically, it has been hypothesized that excess free fructose increases intestinal formation and absorption of advanced glycation end-products, which may interact with the receptor of advanced glycation end-products, a potential mediator of obstructive lung disease development (22–26).
Few groups have studied longitudinal associations of early life exposure to fructose and its beverage sources with asthma risk. Independently of sugar intake in early childhood, higher maternal intake of free sugar during pregnancy was associated with increased risk of atopy and atopic asthma by ages 7–9 years in the Avon Longitudinal Study of Parents and Children (27). We hypothesized that during critical periods of lung and immune growth and development, higher maternal prenatal and early childhood intake of fructose and its beverage sources would be associated with increased asthma in midchildhood and that those associations would in part be independent of adiposity in the mother or child.
Methods
Study Design and Subjects
Between 1999 and 2002, we recruited women in early pregnancy into Project Viva from eight obstetric offices of Atrius Harvard Vanguard Medical Associates, a multispecialty group practice in eastern Massachusetts (22). Details of recruitment and retention are published elsewhere (22). Of the 2,128 women who delivered a live singleton infant, we excluded from this analysis 195 with no maternal pregnancy or early childhood exposure data and 865 with no midchildhood outcome data. Thus, our sample size for analysis was 1,068 mother–child pairs. Compared with the 1,068 participants in this analysis, the 1,060 nonparticipants were somewhat less likely to have college-educated mothers (58% vs. 71%) and to have annual household income exceeding $70,000 (54% vs. 62%), and mean maternal age was slightly younger (31.1 vs. 32.5 yr). Maternal prepregnancy body mass index (BMI; mean, 25.2 vs. 24.6 kg/m2) and intake of pregnancy sugar-sweetened beverages (mean, 0.7 vs. 0.6 servings/d), however, were similar.
After obtaining written informed consent, we performed in-person study visits with participating mothers at the end of the first and second trimesters of pregnancy and with mothers and children during the first few days after delivery. We conducted in-person visits with mothers and children in early childhood (median age, 3.3 yr) and midchildhood (median age, 7.7 yr). The institutional review board of Harvard Pilgrim Health Care approved this study protocol.
Maternal Prenatal and Child Dietary Assessment of Sugar-sweetened Beverage and Fructose Intake
We obtained data on consumption of beverages during pregnancy from semiquantitative food frequency questionnaires (FFQs) that expectant mothers completed after the first and second research visits, at mean (SD) gestational ages of 11.9 (3.5) and 29.2 (2.6) weeks. Each of the two FFQs was slightly modified for use in pregnancy from a commonly used adult FFQ from which sugar-sweetened beverage intake predicted a number of cardiometabolic outcomes (28–32). Participants endorsed categories of frequency of beverage consumption from “never/less than one per month” to a maximum of “two or more glasses per day” for some fruit juices, “four or more cans per day” for soda, and “six or more glasses per day” for water. The time referent for the first-trimester FFQ was “during this pregnancy” (i.e., from the woman’s last menstrual period until she completed the FFQ). For the second-trimester FFQ, the time referent was the previous 3 months. The FFQ included three questions on regular (sugary) soda intake, three questions on sugar-free soda, five questions on fruit juice, and one question on fruit drinks. We defined sugar-sweetened beverages as regular soda and fruit drinks.
We also assessed the children’s dietary intakes using an 88-item semiquantitative child FFQ previously validated among preschool-aged children, which was completed by the mothers when their children were in early childhood (median age, 3.3 yr) (33). Participants endorsed categories of frequency of beverage consumption from “never” to a maximum of “five or more times per day.” The time referent was “during the past month.” The FFQ included one question on regular (sugary) soda intake, one question on sugar-free soda, two questions on fruit juice (orange juice and other 100% juice), one question on fruit drinks, and one question on hot chocolate. We defined sugar-sweetened beverages as regular soda and fruit drinks. Using the Harvard nutrition composition database (derived from the U.S. Department of Agriculture and supplemented with information from manufacturers), we computed fructose intake by multiplying the frequency response of each item (foods and beverages) on the FFQ by the fructose content based on average serving size (34).
Midchildhood Outcome: Current Asthma
On the midchildhood questionnaire (median age, 7.7 yr), we used asthma questions from the International Study of Asthma and Allergies in Childhood (27). The main outcome was current asthma in midchildhood, defined as the mother’s affirmative response to her child’s ever having a doctor’s diagnosis of asthma, plus either report of wheezing or asthma medication use in the past 12 months, based on the midchildhood questionnaire. The comparison group had no asthma diagnosis ever and no wheezing or asthma medication use in the past 12 months.
In a supplementary analysis, we examined midchildhood blood levels of high-sensitivity C-reactive protein (hsCRP), interleukin 6 (IL-6), and tumor necrosis factor receptor 2 (TNFR2) among 562 participants. We used an immunoturbidimetric high-sensitivity assay on a Hitachi 911 analyzer to determine hsCRP concentrations (Roche Diagnostics). We measured plasma IL-6 by enzyme-linked immunosorbent assay (ELISA), and we measured TNFR2 by ELISA (R&D Systems).
Covariates
Using data derived from interviews and mailed questionnaires, we obtained information on maternal and child sociodemographics, including maternal age, race/ethnicity, education, pregnancy smoking status, household income, and child sex and race/ethnicity. At the midchildhood visit, trained research staff measured each child’s height to the nearest 0.1 cm with a calibrated stadiometer (Shorr Productions) and weight to the nearest 0.1 kg with a calibrated scale (Tanita model TBF-300A; Tanita Corporation of America, Inc.). We calculated age- and sex-specific BMI (in kg/m2) z-scores using U.S. national reference data (35).
Statistical Analysis
We used multivariable logistic regression models to examine associations of maternal pregnancy and early childhood sugar-sweetened beverage, juice, and total fructose intake (by quartiles) with current asthma in midchildhood. We also examined exposures as continuous variables. To assess the shape of the exposure–outcome associations, we fit generalized additive models with penalized splines for continuous exposures. Associations were similar for first- and second-trimester maternal diet, so we averaged the two trimesters for analyses of prenatal dietary exposures. We built multivariable logistic regression models in which we adjusted for maternal education, smoking during pregnancy, and prepregnancy BMI; household income; and child sex, race/ethnicity, and exact age at the time of the midchildhood assessment. We further adjusted models with early childhood exposures for the mother’s pregnancy sugar-sweetened beverage intake. Adding other potentially confounding variables, including parental asthma and maternal gestational weight gain, diet quality score, and vitamin D intake, did not materially change the observed associations, so we did not include them in our final models. In subsequent models, we also adjusted for midchildhood BMI z-score, a potential mediator. We also implemented mediation analysis via the mediation macro developed by VanderWeele (36) to examine the extent to which the exposure–outcome associations were mediated through BMI z-score (indirect effect). In a supplementary analysis, we used multivariable linear regression models, with the same exposures and covariates, and log-transformed hsCRP, IL-6, and TNFR2 as continuous outcomes.
To increase sample size and reduce bias due to missing data, we imputed missing covariates. Using a chained equation multiple imputation method (PROC MI in SAS software [SAS Institute]), we generated 50 imputed datasets including all Project Viva participants with live births (n = 2,128). The imputation model included all exposures, outcomes, and covariates under study, as well as additional potential predictors. In final analytic models, we combined imputed datasets using PROC MIANALYZE in SAS software. Participants with missing exposure or outcome data for a given exposure–outcome analysis were excluded from that analysis. We conducted all analyses using SAS version 9.4 software and R version 3.1.3 (R Foundation for Statistical Computing).
Results
Mean (SD) maternal age at enrollment was 32.5 (5.0) years, and mean (SD) prepregnancy BMI was 24.6 (5.1) kg/m2. Among the mothers, 10% smoked during pregnancy, 71% had at least a college degree, and 62% had household incomes greater than $70,000 per year. Among the children, 51% were female, and 32% were nonwhite. At midchildhood, 19% of children had current asthma, 12% were obese (BMI, ≥95th percentile for age and sex), and mean (SD) BMI z-score was 0.37 (0.99) (Table 1).
Table 1.
Characteristics of mother–child pairs (N = 1,068)
| Total (N = 1,068) | Maternal Sugar-sweetened Beverage Intake |
Maternal Total Fructose Intake |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Quartile 1 (n = 283) | Quartile 2 (n = 251) | Quartile 3 (n = 260) | Quartile 4 (n = 259) | Quartile 1 (n = 257) | Quartile 2 (n = 267) | Quartile 3 (n = 277) | Quartile 4 (n = 252) | ||
| Maternal characteristics | |||||||||
| Age, yr | 32.5 (5.0) | 34.0 (4.4) | 33.3 (4.7) | 32.2 (4.7) | 30.5 (5.4) | 33.5 (4.6) | 32.7 (4.9) | 32.6 (4.9) | 31.2 (5.2) |
| Education, % | |||||||||
| Less than college graduate | 306 (29) | 50 (18) | 68 (27) | 66 (25) | 114 (44) | 65 (25) | 75 (28) | 70 (25) | 88 (35) |
| College graduate or higher | 762 (71) | 233 (82) | 183 (73) | 194 (75) | 145 (56) | 192 (75) | 192 (72) | 207 (75) | 164 (65) |
| Household income, % | |||||||||
| ≤$70,000 | 402 (38) | 82 (29) | 91 (36) | 96 (37) | 125 (48) | 83 (32) | 96 (36) | 105 (38) | 110 (44) |
| >$70,000 | 666 (62) | 201 (71) | 160 (64) | 164 (63) | 134 (52) | 174 (68) | 171 (64) | 172 (62) | 142 (56) |
| Pregnancy smoking status, % | |||||||||
| Never | 750 (70) | 199 (70) | 183 (73) | 180 (69) | 176 (68) | 172 (67) | 174 (65) | 199 (72) | 193 (77) |
| Former | 214 (20) | 61 (22) | 55 (22) | 58 (22) | 40 (15) | 61 (24) | 65 (24) | 51 (19) | 37 (15) |
| During pregnancy | 104 (10) | 23 (8) | 13 (5) | 22 (8) | 43 (17) | 24 (9) | 28 (10) | 27 (10) | 22 (9) |
| Prepregnancy BMI, kg/m2 | 24.6 (5.1) | 23.7 (4.4) | 24.2 (4.5) | 25.0 (5.5) | 25.5 (5.6) | 24.8 (5.3) | 24.2 (4.8) | 24.6 (5.3) | 24.6 (4.8) |
| Pregnancy weight gain, kg | 15.6 (5.3) | 15.7 (4.8) | 16.2 (5.1) | 15.2 (5.2) | 15.1 (6.1) | 15.6 (5.3) | 15.5 (5.5) | 16.0 (5.0) | 15.2 (5.5) |
| Child characteristics | |||||||||
| Sex, % | |||||||||
| Male | 522 (49) | 134 (47) | 126 (50) | 123 (47) | 131 (51) | 126 (49) | 132 (49) | 124 (45) | 132 (52) |
| Female | 546 (51) | 149 (53) | 125 (50) | 137 (53) | 128 (49) | 131 (51) | 135 (51) | 153 (55) | 120 (48) |
| Race/ethnicity, % | |||||||||
| Black | 148 (14) | 22 (8) | 30 (12) | 36 (14) | 55 (21) | 20 (8) | 33 (12) | 47 (17) | 43 (17) |
| Hispanic | 39 (4) | 7 (2) | 7 (3) | 10 (4) | 14 (5) | 7 (3) | 4 (1) | 12 (4) | 15 (6) |
| White | 722 (68) | 202 (71) | 176 (70) | 183 (70) | 155 (60) | 190 (74) | 180 (67) | 191 (69) | 155 (62) |
| Other | 159 (15) | 52 (18) | 38 (15) | 31 (12) | 35 (14) | 40 (16) | 50 (19) | 27 (10) | 39 (15) |
|
Midchildhood (median age, 7.7 yr) |
|||||||||
| BMI z-score | 0.37 (0.99) | 0.30 (0.98) | 0.28 (0.87) | 0.40 (1.02) | 0.50 (1.06) | 0.38 (0.95) | 0.35 (0.99) | 0.36 (0.98) | 0.38 (1.03) |
| Current asthma, % | |||||||||
| No | 868 (81) | 240 (85) | 211 (84) | 218 (84) | 188 (73) | 220 (86) | 220 (82) | 226 (82) | 191 (76) |
| Yes | 200 (19) | 43 (15) | 40 (16) | 42 (16) | 71 (27) | 37 (14) | 47 (18) | 51 (18) | 61 (24) |
Definition of abbreviation: BMI = body mass index.
Data are presented as frequency (percent) or mean (SD).
Correlates of higher pregnancy sugar-sweetened beverage intake included younger maternal age, higher prepregnancy BMI, and smoking during pregnancy, as well as indicators of disadvantage/lower socioeconomic status, including lower education and household income. Higher pregnancy sugar-sweetened beverage intake was also correlated with child current asthma (15% in quartile 1 [Q1] vs. 27% in Q4) and BMI z-score (mean, 0.30 units in Q1 vs. 0.50 units in Q4) in midchildhood. Associations were similar for pregnancy fructose intake (Table 1).
Mothers consumed a mean (SD) of 32.5 (10.2) g/d of fructose in pregnancy, and children consumed 27.8 (11.6) g/d in early childhood. As illustrated in Figure E1 in the online supplement, the largest source of fructose-rich beverage intake was citrus juice for mothers (0.8 [0.7] serving/d; range, 0–4.4) and noncitrus juice for children (1.1 [1.1] servings/d; range, 0–5.0). Mothers reported more sugar-sweetened beverage intake in the form of soda and punch (0.6 [0.8] serving/d; range, 0–5.1) than children (0.2 [0.5] serving/d; range, 0–5.1).
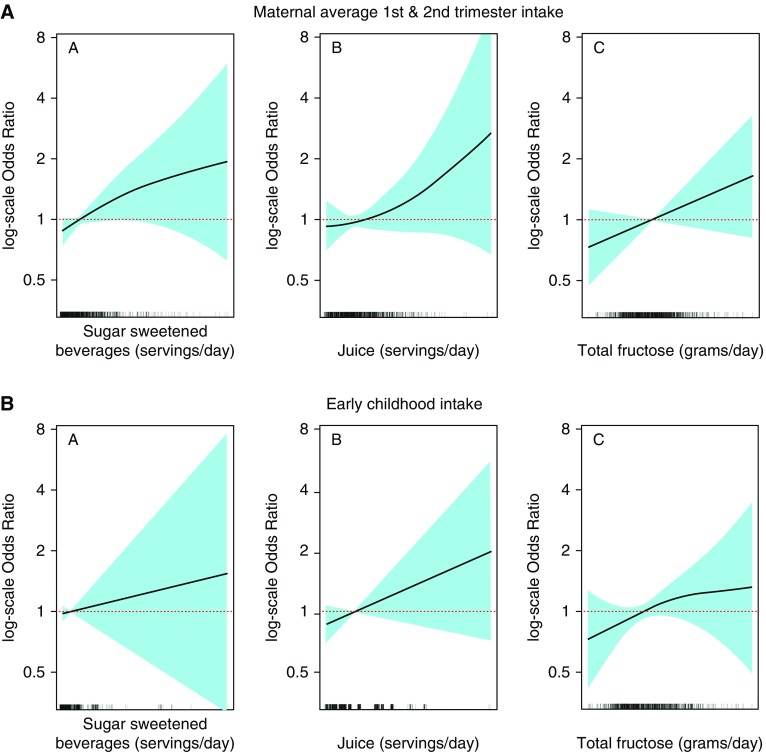
In logistic regression models adjusted for sociodemographic variables and maternal prepregnancy BMI, comparing Q4 with Q1, higher pregnancy intake of sugar-sweetened beverages (odds ratio [OR], 1.70; 95% confidence interval [CI], 1.08–2.67) and total fructose (OR, 1.58; 95% CI, 0.98–2.53) were associated with greater odds of midchildhood current asthma (Table 2). Associations of Q2 and Q3 versus Q1 were null, with effect estimates close to 1.0. Covariate-adjusted spline models suggested that most of the studied exposure–current asthma associations were fairly linear (Figures 1A and 1B).
Table 2.
Associations of maternal pregnancy and early childhood sugar-sweetened beverage, juice, and total fructose intake with midchildhood asthma
| Exposures |
N |
Model 0 |
Model 1 |
Model 2 |
|---|---|---|---|---|
| OR (95% CI) | OR (95% CI) | OR (95% CI) | ||
| Maternal prenatal | ||||
| Sugar-sweetened beverages | 1,053 | |||
| Continuous servings/d | 1.36 (1.13–1.63) | 1.20 (0.98–1.47) | 1.18 (0.96–1.45) | |
| Quartiles (vs. quartile 1) | ||||
| Quartile 2 | 1.06 (0.66–1.69) | 1.01 (0.62–1.63) | 1.02 (0.63–1.66) | |
| Quartile 3 | 1.08 (0.68–1.71) | 0.97 (0.60–1.56) | 0.96 (0.59–1.56) | |
| Quartile 4 | 2.11 (1.38–3.22) | 1.70 (1.08–2.67) | 1.68 (1.07–2.65) | |
| Juice | 1,053 | |||
| Continuous servings/d | 1.22 (1.04–1.43) | 1.11 (0.94–1.32) | 1.12 (0.95–1.33) | |
| Quartiles (vs. quartile 1) | ||||
| Quartile 2 | 0.83 (0.52–1.31) | 0.92 (0.57–1.48) | 0.90 (0.56–1.46) | |
| Quartile 3 | 0.95 (0.60–1.49) | 0.87 (0.54–1.40) | 0.89 (0.55–1.42) | |
| Quartile 4 | 1.40 (0.91–2.14) | 1.27 (0.81–1.99) | 1.31 (0.83–2.05) | |
| Total fructose | 1,053 | |||
| Continuous 15 g/d | 1.35 (1.08–1.69) | 1.19 (0.94–1.50) | 1.18 (0.94–1.50) | |
| Quartiles (vs. quartile 1) | ||||
| Quartile 2 | 1.27 (0.79–2.03) | 1.27 (0.78–2.06) | 1.27 (0.78–2.06) | |
| Quartile 3 | 1.34 (0.85–2.13) | 1.19 (0.73–1.93) | 1.20 (0.74–1.95) | |
| Quartile 4 | 1.90 (1.21–2.98) | 1.58 (0.98–2.53) | 1.60 (0.99–2.57) | |
| Early childhood | ||||
| Sugar-sweetened beverages | 924 | |||
| Continuous servings/d | 1.43 (1.09–1.87) | 1.09 (0.79–1.49) | 1.07 (0.78–1.48) | |
| Quartiles (vs. quartile 1) | ||||
| Quartile 2 | 1.11 (0.68–1.82) | 0.96 (0.58–1.61) | 0.96 (0.58–1.61) | |
| Quartile 3 | 0.99 (0.59–1.68) | 0.71 (0.40–1.24) | 0.73 (0.41–1.29) | |
| Quartile 4 | 1.79 (1.18–2.70) | 1.19 (0.73–1.93) | 1.20 (0.74–1.94) | |
| Juice | 924 | |||
| Continuous servings/d | 1.12 (1.00–1.26) | 1.10 (0.97–1.23) | 1.10 (0.98–1.24) | |
| Quartiles (vs. quartile 1) | ||||
| Quartile 2 | 1.19 (0.77–1.84) | 1.20 (0.76–1.89) | 1.24 (0.79–1.95) | |
| Quartile 3 | 0.81 (0.47–1.41) | 0.70 (0.39–1.25) | 0.69 (0.39–1.24) | |
| Quartile 4 | 1.50 (0.95–2.36) | 1.49 (0.93–2.40) | 1.53 (0.95–2.47) | |
| Total fructose | 924 | |||
| Continuous 15 g/d | 1.20 (0.97–1.48) | 1.16 (0.93–1.45) | 1.16 (0.93–1.45) | |
| Quartiles (vs. quartile 1) | ||||
| Quartile 2 | 1.62 (0.98–2.68) | 1.51 (0.90–2.54) | 1.48 (0.87–2.49) | |
| Quartile 3 | 1.42 (0.85–2.37) | 1.38 (0.81–2.36) | 1.34 (0.78–2.30) | |
| Quartile 4 | 1.93 (1.18–3.15) | 1.79 (1.07–2.97) | 1.77 (1.06–2.95) |
Definition of abbreviation: CI = confidence interval; OR = odds ratio.
Model 0 is unadjusted. Model 1 is adjusted for maternal education, smoking during pregnancy, and prepregnancy body mass index; household income; and child age, sex, and race/ethnicity. Early childhood exposures are also adjusted for mother’s sugar-sweetened beverage intake (first- and second-trimester average). Model 2 represents model 1 also adjusted for child body mass index z-score in midchildhood.
Figure 1.
General additive models (splines) illustrating associations of maternal pregnancy and early childhood sugar-sweetened beverage, juice, and total fructose intake with current asthma in midchildhood (logarithmic scale odds ratios and 95% confidence intervals), adjusted for maternal education, smoking during pregnancy, and prepregnancy body mass index; household income; and child age, sex, and race/ethnicity. Early childhood exposures were also adjusted for mother’s sugar-sweetened beverage intake (first and second trimester average).
In models adjusted for maternal sugar-sweetened beverage intake, comparing Q4 with Q1, higher early childhood intake of total fructose (OR, 1.79; 95% CI, 1.07–2.97) was associated with greater odds of midchildhood current asthma. These results were almost the same after also adjusting for midchildhood BMI z-score (OR, 1.77; 95% CI, 1.06–2.95). Results for early childhood sugar-sweetened beverage intake were null before (OR, 1.19; 95% CI, 0.73–1.93) and after (OR, 1.20; 95% CI, 0.74–1.94) adjusting for midchildhood BMI z-score (Table 2). As shown in Table 2, ORs for early childhood fructose and current asthma were 1.51, 1.38, and 1.79 for Q2, Q3, and Q4 versus Q1, respectively. The results from the Vanderweele mediation macro (36) confirmed that the exposure–outcome associations were not mediated through midchildhood BMI z-score. (ORs for the indirect effect were close to 1.0, and the CIs crossed 1.0.)
Table E1 shows associations of maternal pregnancy and early childhood intake of sugar-sweetened beverages, juice, and total fructose with hsCRP, IL-6, and TNFR2 levels (logarithmically transformed) in midchildhood. In multivariable linear regression models, comparing Q4 with Q1, higher early childhood intake of sugar-sweetened beverages was associated with higher levels of log-transformed hsCRP before (β = 0.35; 95% CI, −0.05, 0.74) and after (β = 0.36; 95% CI, −0.01, 0.74) adjusting for midchildhood BMI z-score, although CIs were wide and crossed 0. All other associations were null (Table E1). We also examined associations of maternal prenatal and child fructose intake with midchildhood plasma leptin and adiponectin, but both were null.
Discussion
Higher intake of fructose-containing beverages or total nutrient fructose in the prenatal and early childhood periods was associated with greater odds of later childhood asthma in our prebirth cohort. Whereas sugar-sweetened beverages as well as juices were significant sources of fructose for the mothers, the primary source of fructose in early childhood was fruit juice. The American Academy of Pediatrics recommends consumption of no more than 4 to 6 ounces (approximately one to two servings) per day of fruit juice for children 1 to 6 years old (37). In the highest quartile of fruit juice consumption in our cohort, children were drinking a mean of 3.9 servings (range, 3–10 servings) per day.
In addition to assessing the influence of sugar-sweetened beverage intake on asthma, we focused on dietary fructose intake because 1) it is a major contributor to total sugar intake (10); 2) for many pregnant women and most small children, sugar-sweetened beverages may not be the primary contributor to total fructose or sugar intake; and 3) there is biological evidence that fructose may have specific airway effects (see below). Higher intake of sugar-sweetened beverages and fructose may influence asthma, either through increasing adiposity, as well as adiposity-related pulmonary restriction and inflammation, or through adiposity-independent mechanisms (16). Using a mouse model, Singh and colleagues found that a high-fat or high-fructose diet led to reduced nitric oxide–related bronchodilation and increased oxo-nitrosative stress without evidence of inflammatory cell infiltrate or goblet cell metaplasia, supporting the hypothesis that a high-fructose diet could influence asthma through adiposity-independent mechanisms (26). Mediation analyses suggested that child BMI did not mediate the associations of prenatal or early childhood consumption of fructose with midchildhood risk of asthma.
Murine model studies support the hypothesis that there are direct as well as adiposity-mediated inflammatory effects of sugars, including fructose, on the airways as well as other target organs (16–21, 38). In a short overnight study of C57BL/6 mice, Kierstein and colleagues (38) suggested a direct effect of high sugar consumption, which increased susceptibility to allergic airway inflammation and activation of the innate immune system in part through impairment of carbohydrate recognition surfactant protein D, which is immunoprotective and prevents pulmonary inflammation (38). Dietary fructose has also been specifically and directly implicated in upregulation of lung inflammation (23) through the influences of excess free fructose on the receptor of advanced glycation end-products in the lung (39). This evidence is stronger, however, for inflammation related to chronic obstructive pulmonary disease than for asthma.
That is not to discount potential obesity-mediated effects. A short-term rodent model study of combined diet and pollution exposure conducted by Sun and colleagues (16) suggested an adiposity-mediated effect of fructose. Rats fed a high-fructose diet and exposed to ozone had higher macrophage infiltrates in adipose tissue with upregulation of proinflammatory genes and downregulation of antiinflammatory genes.
We further pursued evidence for the biological plausibility that the association we found between intake of sugar-sweetened beverages/fructose and asthma could relate to upregulation of adiposity-related innate cytokines and biomarkers of inflammation. We examined but did not find associations of maternal prenatal or early childhood intake of sugar-sweetened beverages, juice, and total fructose with peripheral blood levels of hsCRP, IL-6, and TNFR2 measured in the children at the midchildhood visit. Because the inflammatory influences of fructose may have occurred in the lung compartment, but not systemically, or may have occurred at an earlier point in time not captured by our measurements, the absence of association of prior intake of fructose with these systemic markers years later does not exclude the possibility that fructose caused innate cytokine-/adipokine-mediated inflammation specific to the airways (38).
A strength of Project Viva is our longitudinal study design, which included collection of detailed dietary data, both during pregnancy and in early childhood. These are life stages critical for immune system and lung development, and therefore for the early origins of asthma (40, 41). In contrast to our longitudinal study design, most previous studies of associations of sources of fructose intake with asthma have been cross-sectional, focused on older children and adults, and focused on soda or sugar-sweetened beverages without comprehensive dietary data on other sources of fructose, including juice or fruit consumption. In a nationally representative sample of 15,960 U.S. high school students, Park and colleagues (42) found that participants who consumed more soda (two or more servings per day) had greater odds of asthma than those who reported no soda intake (adjusted OR, 1.28; 95% CI, 1.02–1.62), and this association was independent of obesity (42). In an Australian study of people 16 years of age and older, Shi and colleagues (43) reported that higher sugar-sweetened beverage consumption (reported intake of ≥0.5 L/d) was associated with greater odds of asthma than in those who did not report sugar-sweetened beverage consumption (adjusted OR, 1.26; 95% CI, 1.01–1.58). DeChristopher and colleagues (23) conducted a more comprehensive cross-sectional evaluation of associations of fructose-containing beverages with asthma in children 2–9 years of age evaluated in the National Health and Nutrition Examination Survey during 2003–2006. Higher excess free fructose beverage intake (apple juice and sugar-sweetened beverages [nondiet soft drinks and fruit drinks]) was associated with greater odds of asthma. After controlling for other beverage intake, we observed that higher apple juice intake by itself (at least five times per week compared with once or less per month) was also associated with higher asthma prevalence (23).
One of the few longitudinal studies of maternal sugar-sweetened beverage ingestion and childhood asthma risk was conducted by Maslova and colleagues (44). In adjusted analyses in this study of 60,466 Danish mother-child pairs, the researchers did not find associations of maternal prenatal sugar-sweetened beverage intake with childhood asthma at age 7 years (OR, 1.07; 95% CI, 0.90–1.28). The null findings of this study may have been limited by the low consumption of sugar-sweetened beverages (44). Our study included a relatively wide range of sugar-sweetened beverage consumption and BMI. In addition, the relatively large sample size allowed us to evaluate independent associations of prenatal and early childhood intake of sugar-sweetened beverages, juice, and total fructose intake with midchildhood asthma.
Although there is diversity in income and socioeconomic status in Project Viva, a limitation of our cohort is that it is relatively advantaged. Our results may not be generalizable to more socioeconomically disadvantaged populations. Loss to follow-up, although regrettable, is common in cohort studies in early life. We observed some differences in baseline covariates between participants and those lost to follow-up, but we did not observe differences in maternal sugar-sweetened beverage intake. Furthermore, sugar-sweetened beverage intake was self-reported, and there may have been inaccuracy in assessment because of recall, social desirability, or other biases. Nevertheless, we anticipate that ranking was preserved within the cohort because relative disadvantage (lower income, less education) was associated with higher sugar-sweetened beverage intake. Higher parental and child intake of sugar-sweetened beverage intake, including intake of sweetened juices (45), has been described as a contributor to the higher prevalence of obesity in disadvantaged populations (6). Policy discussions are ongoing at the national level as well as at the local Boston level regarding how to reduce total sugary drink intake, in part by increasing access to affordable, healthier choices of drinks (1, 46). These discussions include consideration of the harms and benefits of fruit juice, which contains sugars that may be obesogenic as well as antioxidants and other healthy components (1, 46–48).
In conclusion, in our cohort, maternal prenatal sugar-sweetened beverage intake and early childhood total fructose intake were associated with asthma in midchildhood. Our findings contribute to the literature that should be considered when developing recommendations regarding consumption and availability of these drinks during pregnancy and early childhood. Further evaluation of potential mechanisms for influences of total fructose associated with asthma development is warranted, including further assessment of effects of fructose and fructose metabolites on airway inflammation or hyperreactivity that may be independent of obesity.
Supplementary Material
Footnotes
Supported by National Institutes of Health grants R01 HD034568, R01 AI102960, K24 HD069408, P30 DK092924, UG3OD023286, and F32HL124919-01 and in part by U.S. Environmental Protection Agency (EPA) grant RD-83479801, and National Institute of Environmental Health Sciences (NIEHS) center grant (P30 ES-000002). The contents are solely the responsibility of the grantee and do not necessarily represent the official views of the EPA. Furthermore, the EPA does not endorse the purchase of any commercial products or services mentioned in the paper.
Author Contributions: L.S.W., S.L.R.-S., E.O., and D.R.G.: contributed substantially to the conception or design of the work; S.L.R.-S.: analyzed the data; L.S.W. and S.L.R.-S.: drafted the work; and E.O., A.A.L., and D.R.G.: revised the manuscript critically for important intellectual content. All authors contributed to the interpretation of the data, revised the manuscript, and approved the final manuscript.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Akinbami LJ, Moorman JE, Bailey C, Zahran HS, King M, Johnson CA, et al. Trends in asthma prevalence, health care use, and mortality in the United States, 2001–2010. NCHS Data Brief. 2012;(94) [PubMed] [Google Scholar]
- 2.Akinbami LJ, Moorman JE, Garbe PL, Sondik EJ. Status of childhood asthma in the United States, 1980–2007. Pediatrics. 2009;123(Suppl 3):S131–S145. doi: 10.1542/peds.2008-2233C. [DOI] [PubMed] [Google Scholar]
- 3.Platts-Mills TA. The allergy epidemics: 1870–2010. J Allergy Clin Immunol. 2015;136:3–13. doi: 10.1016/j.jaci.2015.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stukus DR. Obesity and asthma: the chicken or the egg? J Allergy Clin Immunol. 2015;135:894–895. doi: 10.1016/j.jaci.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 5.Weinmayr G, Forastiere F, Büchele G, Jaensch A, Strachan DP, Nagel G ISAAC Phase Two Study Group. Overweight/obesity and respiratory and allergic disease in children: International Study of Asthma and Allergies in Childhood (ISAAC) Phase Two. PLoS One. 2014;9:e113996. doi: 10.1371/journal.pone.0113996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA. 2014;311:806–814. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Swinburn B, Sacks G, Ravussin E. Increased food energy supply is more than sufficient to explain the US epidemic of obesity. Am J Clin Nutr. 2009;90:1453–1456. doi: 10.3945/ajcn.2009.28595. [DOI] [PubMed] [Google Scholar]
- 8.Wang YC, Bleich SN, Gortmaker SL. Increasing caloric contribution from sugar-sweetened beverages and 100% fruit juices among US children and adolescents, 1988–2004. Pediatrics. 2008;121:e1604–e1614. doi: 10.1542/peds.2007-2834. [DOI] [PubMed] [Google Scholar]
- 9.Muñoz-Pareja M, Guallar-Castillón P, Mesas AE, López-García E, Rodríguez-Artalejo F. Obesity-related eating behaviors are associated with higher food energy density and higher consumption of sugary and alcoholic beverages: a cross-sectional study. PLoS One. 2013;8:e77137. doi: 10.1371/journal.pone.0077137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Park YK, Yetley EA. Intakes and food sources of fructose in the United States. Am J Clin Nutr. 1993;58(5 Suppl):737S–747S. doi: 10.1093/ajcn/58.5.737S. [DOI] [PubMed] [Google Scholar]
- 11.Bray GA, Popkin BM. Calorie-sweetened beverages and fructose: what have we learned 10 years later. Pediatr Obes. 2013;8:242–248. doi: 10.1111/j.2047-6310.2013.00171.x. [DOI] [PubMed] [Google Scholar]
- 12.Bray GA. Energy and fructose from beverages sweetened with sugar or high-fructose corn syrup pose a health risk for some people. Adv Nutr. 2013;4:220–225. doi: 10.3945/an.112.002816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guthrie JF, Morton JF. Food sources of added sweeteners in the diets of Americans. J Am Diet Assoc. 2000;100:43–51, quiz 49–50. doi: 10.1016/S0002-8223(00)00018-3. [DOI] [PubMed] [Google Scholar]
- 14.Litonjua AA, Gold DR. Asthma and obesity: common early-life influences in the inception of disease. J Allergy Clin Immunol. 2008;121:1075–1084, quiz 1085–1086. doi: 10.1016/j.jaci.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 15.Singh GM, Micha R, Khatibzadeh S, Lim S, Ezzati M, Mozaffarian D Global Burden of Diseases Nutrition and Chronic Diseases Expert Group (NutriCoDE) Estimated global, regional, and national disease burdens related to sugar-sweetened beverage consumption in 2010. Circulation. 2015;132:639–666. doi: 10.1161/CIRCULATIONAHA.114.010636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun L, Liu C, Xu X, Ying Z, Maiseyeu A, Wang A, et al. Ambient fine particulate matter and ozone exposures induce inflammation in epicardial and perirenal adipose tissues in rats fed a high fructose diet. Part Fibre Toxicol. 2013;10:43. doi: 10.1186/1743-8977-10-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Basaranoglu M, Basaranoglu G, Sabuncu T, Sentürk H. Fructose as a key player in the development of fatty liver disease. World J Gastroenterol. 2013;19:1166–1172. doi: 10.3748/wjg.v19.i8.1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Choi HK, Willett W, Curhan G. Fructose-rich beverages and risk of gout in women. JAMA. 2010;304:2270–2278. doi: 10.1001/jama.2010.1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cohen L, Curhan G, Forman J. Association of sweetened beverage intake with incident hypertension. J Gen Intern Med. 2012;27:1127–1134. doi: 10.1007/s11606-012-2069-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Koning L, Malik VS, Kellogg MD, Rimm EB, Willett WC, Hu FB. Sweetened beverage consumption, incident coronary heart disease, and biomarkers of risk in men. Circulation. 2012;125:1735–1741. doi: 10.1161/CIRCULATIONAHA.111.067017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Koning L, Malik VS, Rimm EB, Willett WC, Hu FB. Sugar-sweetened and artificially sweetened beverage consumption and risk of type 2 diabetes in men. Am J Clin Nutr. 2011;93:1321–1327. doi: 10.3945/ajcn.110.007922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.DeChristopher LR. Excess free fructose and childhood asthma. Eur J Clin Nutr. 2015;69:1371. doi: 10.1038/ejcn.2015.101. [DOI] [PubMed] [Google Scholar]
- 23.DeChristopher LR, Uribarri J, Tucker KL. Intakes of apple juice, fruit drinks and soda are associated with prevalent asthma in US children aged 2–9 years. Public Health Nutr. 2016;19:123–130. doi: 10.1017/S1368980015000865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.DeChristopher LR, Uribarri J, Tucker KL. Intake of high fructose corn syrup sweetened soft drinks is associated with prevalent chronic bronchitis in U.S. adults, ages 20–55 y. Nutr J. 2015;14:107. doi: 10.1186/s12937-015-0097-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.DeChristopher LR, Uribarri J, Tucker KL. The link between soda intake and asthma: science points to the high-fructose corn syrup, not the preservatives: a commentary. Nutr Diabetes. 2016;6:e234. doi: 10.1038/nutd.2016.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Singh VP, Aggarwal R, Singh S, Banik A, Ahmad T, Patnaik BR, et al. Metabolic syndrome is associated with increased oxo-nitrative stress and asthma-like changes in lungs. PLoS One. 2015;10:e0129850. doi: 10.1371/journal.pone.0129850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bédard A, Northstone K, Henderson AJ, Shaheen SO. Maternal intake of sugar during pregnancy and childhood respiratory and atopic outcomes. Eur Respir J. 2017;50:1700073. doi: 10.1183/13993003.00073-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen L, Hu FB, Yeung E, Willett W, Zhang C. Prospective study of pre-gravid sugar-sweetened beverage consumption and the risk of gestational diabetes mellitus. Diabetes Care. 2009;32:2236–2241. doi: 10.2337/dc09-0866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fawzi WW, Rifas-Shiman SL, Rich-Edwards JW, Willett WC, Gillman MW. Calibration of a semi-quantitative food frequency questionnaire in early pregnancy. Ann Epidemiol. 2004;14:754–762. doi: 10.1016/j.annepidem.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 30.Malik VS, Pan A, Willett WC, Hu FB. Sugar-sweetened beverages and weight gain in children and adults: a systematic review and meta-analysis. Am J Clin Nutr. 2013;98:1084–1102. doi: 10.3945/ajcn.113.058362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Malik VS, Popkin BM, Bray GA, Després JP, Willett WC, Hu FB. Sugar-sweetened beverages and risk of metabolic syndrome and type 2 diabetes: a meta-analysis. Diabetes Care. 2010;33:2477–2483. doi: 10.2337/dc10-1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Malik VS, Willett WC, Hu FB. Sugar-sweetened beverages and BMI in children and adolescents: reanalyses of a meta-analysis. Am J Clin Nutr. 2009;89:438–439, author reply 439–440. doi: 10.3945/ajcn.2008.26980. [DOI] [PubMed] [Google Scholar]
- 33.Blum RE, Wei EK, Rockett HR, Langeliers JD, Leppert J, Gardner JD, et al. Validation of a food frequency questionnaire in Native American and Caucasian children 1 to 5 years of age. Matern Child Health J. 1999;3:167–172. doi: 10.1023/a:1022350023163. [DOI] [PubMed] [Google Scholar]
- 34.U.S. Department of Agriculture, Agricultural Research Service. USDA National Nutrient Database for Standard Reference, release 16. 2003 [accessed 2017 Dec 19]. Available from: https://www.nal.usda.gov/fnic/food-composition.
- 35.Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, Flegal KM, Guo SS, Wei R, et al. CDC growth charts: United States. Adv Data. 2000;(314):1–27. [PubMed] [Google Scholar]
- 36.Valeri L, Vanderweele TJ. Mediation analysis allowing for exposure-mediator interactions and causal interpretation: theoretical assumptions and implementation with SAS and SPSS macros. Psychol Methods. 2013;18:137–150. doi: 10.1037/a0031034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Committee on Nutrition, American Academy of Pediatrics. The use and misuse of fruit juice in pediatrics. Pediatrics. 2001;107:1210–1213. doi: 10.1542/peds.107.5.1210. [DOI] [PubMed] [Google Scholar]
- 38.Kierstein S, Krytska K, Kierstein G, Hortobágyi L, Zhu X, Haczku A. Sugar consumption increases susceptibility to allergic airway inflammation and activates the innate immune system in the lung [abstract 754] J Allergy Clin Immunol. 2008;121(2 Suppl 1):S196. [Google Scholar]
- 39.Yonchuk JG, Silverman EK, Bowler RP, Agustí A, Lomas DA, Miller BE, et al. Circulating soluble receptor for advanced glycation end products (sRAGE) as a biomarker of emphysema and the RAGE axis in the lung. Am J Respir Crit Care Med. 2015;192:785–792. doi: 10.1164/rccm.201501-0137PP. [DOI] [PubMed] [Google Scholar]
- 40.Gluckman PD, Hanson MA, Cooper C, Thornburg KL. Effect of in utero and early-life conditions on adult health and disease. N Engl J Med. 2008;359:61–73. doi: 10.1056/NEJMra0708473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shi W, Bellusci S, Warburton D. Lung development and adult lung diseases. Chest. 2007;132:651–656. doi: 10.1378/chest.06-2663. [DOI] [PubMed] [Google Scholar]
- 42.Park S, Blanck HM, Sherry B, Jones SE, Pan L. Regular-soda intake independent of weight status is associated with asthma among US high school students. J Acad Nutr Diet. 2013;113:106–111. doi: 10.1016/j.jand.2012.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shi Z, Dal Grande E, Taylor AW, Gill TK, Adams R, Wittert GA. Association between soft drink consumption and asthma and chronic obstructive pulmonary disease among adults in Australia. Respirology. 2012;17:363–369. doi: 10.1111/j.1440-1843.2011.02115.x. [DOI] [PubMed] [Google Scholar]
- 44.Maslova E, Strøm M, Olsen SF, Halldorsson TI. Consumption of artificially-sweetened soft drinks in pregnancy and risk of child asthma and allergic rhinitis. PLoS One. 2013;8:e57261. doi: 10.1371/journal.pone.0057261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ogden CL, Kit BK, Carroll MD, Park S. Consumption of sugar drinks in the United States, 2005–2008. NCHS Data Brief. 2011;(71) [PubMed] [Google Scholar]
- 46.Akinbami LJ, Moorman JE, Simon AE, Schoendorf KC. Trends in racial disparities for asthma outcomes among children 0 to 17 years, 2001–2010. J Allergy Clin Immunol. 2014;134:547–553.e5. doi: 10.1016/j.jaci.2014.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Han YY, Forno E, Holguin F, Celedón JC. Diet and asthma: an update. Curr Opin Allergy Clin Immunol. 2015;15:369–374. doi: 10.1097/ACI.0000000000000179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim JH, Ellwood PE, Asher MI. Diet and asthma: looking back, moving forward. Respir Res. 2009;10:49. doi: 10.1186/1465-9921-10-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.