Abstract
Tissue engineering appears promising as an alternative technique for esophageal replacement. Mesenchymal stem cells (MSCs) could be of interest for esophageal regeneration. Evaluation of the ability of an acellular matrix seeded with autologous MSCs to promote tissue remodeling toward an esophageal phenotype after circumferential replacement of the esophagus in a mini pig model. A 3 cm long circumferential replacement of the abdominal esophagus was performed with an MSC-seeded matrix (MSC group, n = 10) versus a matrix alone (control group, n = 10), which has previously been matured into the great omentum. The graft area was covered with an esophageal removable stent. A comparative histological analysis of the graft area after animals were euthanized sequentially is the primary outcome of the study. Histological findings after maturation, overall animal survival, and postoperative morbidity were also compared between groups. At postoperative day 45 (POD 45), a mature squamous epithelium covering the entire surface of the graft area was observed in all the MSC group specimens but in none of the control group before POD 95. Starting at POD 45, desmin positive cells were seen in the graft area in the MSC group but never in the control group. There were no differences between groups in the incidence of surgical complications and postoperative death. In this model, MSCs accelerate the mature re-epitheliazation and early initiation of muscle cell colonization. Further studies will focus on the use of cell tracking tools in order to analyze the becoming of these cells and the mechanisms involved in this tissue regeneration.
Keywords: tissue engineering, esophagus, mesenchymal stem cells, acellular matrix, esophageal stenting, translational research, large animal model
Introduction
After esophagectomy for benign or malignant lesions, esophageal replacement is usually performed with a gastric or a colonic substitute. Although these surgical techniques are effective in allowing nutritional autonomy in most patients, they carry substantial morbidity and mortality. Long-term quality of life is frequently impaired by reflux, delayed conduit emptying, or dumping syndrome.1,2 For limited benign lesions such as strictures refractory to an endoscopic dilatation or a long-gap esophageal atresia, these techniques require the replacement of the entire esophagus. Currently, repeated failed reconstructions result in a therapeutic deadlock, requiring lifelong feeding jejunostomy.
Tissue engineering (TE) appears as a promising alternative technique for esophageal replacement.3,4 TE aims at manufacturing constructs with matrices and stem cells to promote in vivo regeneration of native tissues or organs. This approach creates a substitute tailored to the exact length of the esophageal defect, while preserving intra-abdominal conduits. As a result, it limits the operative risk and the functional disorders seen during conventional esophageal replacement.
We previously showed in a pig model that full thickness circumferential replacement of the cervical esophagus, with a hybrid substitute composed of an acellular matrix cellularized with porcine skeletal myoblasts and an amniotic membrane cellularized with oral epithelial cells, allowed recovery of nutritional autonomy and tissue remodeling toward an esophageal phenotype.5 However, the complexity of this technique, the delay for tissue remodeling, and the high fibrotic retraction lead us to modify substantially our experimental approach.
Mesenchymal stem cells (MSCs) have already been evaluated for urothelial,6,7 myocardial,8 and bone9 regeneration with promising results. These cells could be of interest for esophageal regeneration due to their multipotency and their anti-inflammatory, proangiogenic, chemoattractive, and immunomodulatory properties.10–13
The aim of this study is to evaluate the ability of an acellular matrix, seeded with autologous MSCs, to promote tissue remodeling toward an esophageal phenotype after a circumferential replacement of the abdominal esophagus in a mini pig model.
Materials and Methods
Animal Model
Twenty Göttingen male adult mini pigs, weighing 35 to 45 kg, issued from the same breeding (S.A.S. Pannier, Wylder, France) were used. All animals received care in accordance with French regulations and institutional guidelines for animal research. The experimental protocol was approved by the scientific committee of our institution, the ethics committee of animal experimentation n° 34, and the Ministry of National Education, Higher Education and Research (agreement number: APAFIS#2397-2015101900217536 v4).
Anesthesia
All surgical procedures were performed under general anesthesia following 24 h of fasting. Next, an intramuscular injection of 2 mg/kg of azaperone, 20 µg/kg of atropine and 10 mg/kg of ketamine (Ketamine Virbac®; Virbac, Carros, France) was administered. Tracheal intubation was performed subsequently with 8 mg/kg of intravenous propofol (Diprivan 1%®; AstraZeneca, Rueil-Malmaison, France). During surgery, all animals were perfused with a balanced crystalloid solution (500 mL/h). Anesthesia was maintained by inhalation of isoflurane 1% and additional shots of propofol. Mechanical ventilatory parameters were set to a total volume of 10 mL/kg, with a respiratory rate of 20/min and 60% of fraction of inspired oxygen. One intravenous ampoule of 0.5 g sulbactam /1 g ampicillin was given before surgery as prophylactic antibiotic therapy. Analgesia was provided by an intramuscular injection of 13 mg of nalbuphine (Nalbuphine®; Merck, Paris, France) before surgery.
Substitute Construction
Bone marrow (BM)-derived MSC isolation and characterization
BM-MSCs were isolated as previously described.14 Twenty milliliters of BM were obtained from an aspiration of a posterior iliac crest under sterile conditions. The mononuclear cells were isolated by density centrifugation over a Ficoll gradient, then plated in MSC medium: α-minimum essential medium (MEM)-GlutaMax-I (Gibco Life Technologies™, Netherlands), 10% fetal bovine serum (FBS; Hyclone Thermo Scientific, South American Origin, Logan, Utah, USA), 1% antibiotic–antimycotic (ATB-ATM) streptomycin, Pénicillin G-Amphotericin B (D. Dutscher, Issy-les-Moulineaux, France), and 1 ng/mL basic fibroblast growth factor (bFGF; R&D Systems, Minneapolis, MN, USA). The MSCs were isolated on the basis of their ability to adhere to the culture plates and the specificity of the medium used. After 15 d of expansion (passage 2) and before being seeded, the MSCs were systematically characterized by their phenotype of BM-MSCs and their ability for osteogenic and adipogenic differentiation15 as described below.
The phenotype was obtained by immunostaining with the following antibodies: mouse antihuman CD34-PE (BD Biosciences, San Jose, CA, USA), mouse antihuman CD90-PE (BD Biosciences, Pharmingen, San Diego, CA, USA), mouse antihuman CD105-PE (Abcam, Cambridge, UK), mouse antipig CD45 Clone MAC323 (BioRad, Oxford, UK) with secondary antibody donkey antimouse conjugated DyLight® 650 (Thermo Fischer Scientific Pierce Biotechnology, Rockford, IL, USA), mouse antipig CD29 (BD Biosciences, Pharmingen, San Diego, CA, USA) with secondary antibody donkey antimouse conjugated DyLight® 650 (Thermo Fischer Scientific Pierce Biotechnology, Rockford, IL, USA), and rat antipig CD44 (BioRad, Oxford, UK) with secondary antibody donkey antirat conjugated DyLight® 650 (Thermo Fischer Scientific Pierce Biotechnology).16,17 Isotype antibody or omission of the primary antibody was used as negative control. Data were acquired and analyzed on a 5-parameter flow cytometer (FACS Calibur, Becton Dickinson, San Jose, CA, USA) with the CellQuestPro software 4.0.2 (Becton Dickinson).
To induce osteogenic differentiation, a sample of the expanded cells was plated onto 8-well plates (2 × 104 cells/cm2). At 70% to 80% confluence, the MSC medium was replaced by an osteogenic medium composed of Dulbecco’s modified Eagle’s medium (DMEM) low glucose 1 g/l GlutaMax-II (Gibco Life Technologies™), 10% FBS, 1% ATB-ATM, ascorbic acid 50 µg/mL (Laroscorbine, Bayer, France), dexamethasone 10−7 M (Merck, Darmstadt, Germany), and 3 mM inorganic phosphate (Sigma Aldrich, Saint-Quentin-Fallavier, France). The medium was renewed twice a week. After 2 wk, osteogenic differentiation was assessed by staining with 2% of Alizarin Red S.
Likewise, the adipogenic differentiation was provided by culture of a sample of the expanded cells in an adipogenic medium composed of DMEM low glucose 1 g/l GlutaMax-II (Gibco Life Technologies), 20% FBS, 1% ATB-ATM, Indomethacin 60 µM (Sigma Aldrich, France), Dexamethasone 10−6 M (Merck), and 0.5 mM of 3-isobutyl-1-methylxanthine (Sigma Aldrich, Saint-Quentin-Fallavier, France). The medium was replaced twice a week. After 2 wk of culture, the presence of intracellular lipid droplets indicating adipogenic differentiation was confirmed by oil red o (Bio Optica, Milan, Italy) staining.
MSC seeding
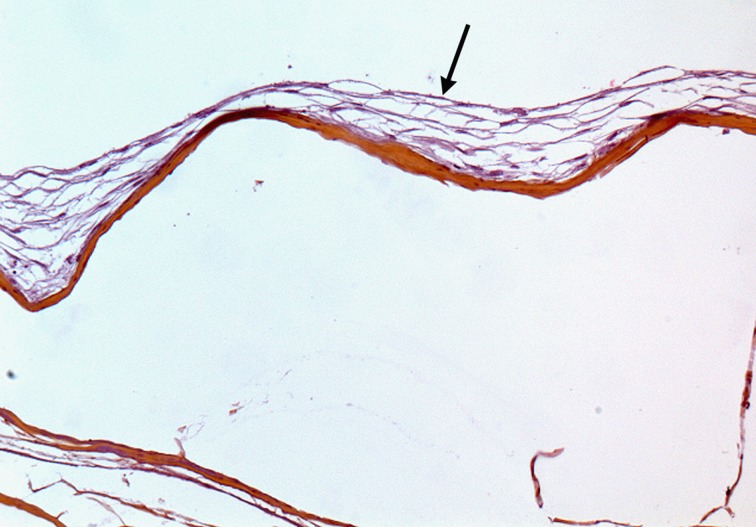
The natural acellular matrix used was the Biodesign® 4 Layer Tissue Graft 7 × 10 cm (Cook Medical, Charenton Le Pont, France) produced from the porcine small intestinal submucosa (SIS). MSCs were seeded onto the matrix at the density of 500,000 MSCs/cm2 and incubated in a humidified condition at 37 °C for 7 d. The MSC culture medium was renewed twice a week. At the end of the 7 d MSC seeding period, a sample of SIS seeded with MSCs was analyzed with hematoxylin–eosin–saffron (HES) staining to control the presence of living MSCs on the SIS matrix before implantation of the seeded substitute for maturation ( Fig. 1 ).
Fig. 1.
Histological analysis of mesenchymal stem cells seeded on small intestinal submucosa (SIS) at day 7: Multilayered spindle cell coat (arrow) at the surface of the SIS matrix with no cell colonization within the matrix. Hematoxylin–eosin–saffron stain. Original ×10.
The optimal seeding conditions of MSCs on the SIS matrix had been previously assessed comparing 3 densities of seeding (100,000 MSC/cm2; 500,000 MSC/cm2; and 1,000,000 MSC/cm2) and 3 durations of culture (7 d, 14 d, and 21 d). Cell viability and proliferation was assessed using the 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H- tetrazolium assay and histological examination with HES staining.
Study Design: Experimental Protocol
Twenty 3 cm long circumferential replacements of the abdominal esophagus were performed. Animals were divided into 2 groups undergoing transplantation with MSC seeded matrices (MSC group, n = 10) or matrices alone (control group, n = 10). Before esophageal replacement, the substitute was implanted into the greater omentum for a 14-d maturation period. After esophageal replacement, the graft area was systematically covered during 3 mo with a Polyflex™ esophageal removable stent (Boston Scientific, Paris, France) to prevent early anastomotic leakage and stricture of the graft area. The primary outcome was the comparative histological analysis of the graft area after animals were euthanized sequentially. Histologic findings after maturation into the greater omentum, overall animal survival, and postoperative morbidity were also compared between the 2 groups.
In Vivo Maturation of the Biomaterials
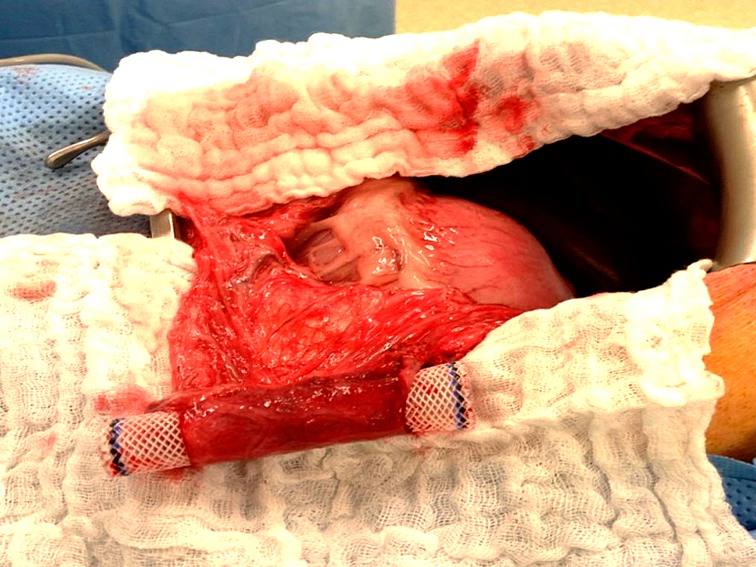
In order to create a tubular structure, the substitute was wrapped around the esophageal stent and set to itself with 3 sutures of absorbable sutures (Vicryl 3/0). The tube-shaped substitute was placed through a midline laparotomy into the bottom-right corner of the greater omentum. An omental wrapping around the biomaterial was performed with absorbable sutures (Vicryl 3/0) in order to avoid its migration. This step of in vivo maturation lasted 2 wk ( Fig. 2 ).
Fig. 2.
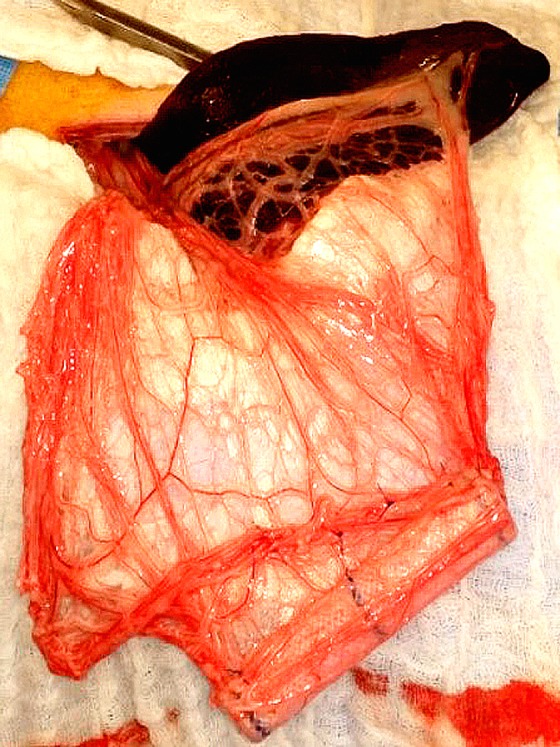
In vivo maturation. The tube-shaped substitute is fixed into the greater omentum with absorbable sutures.
Esophageal Replacement
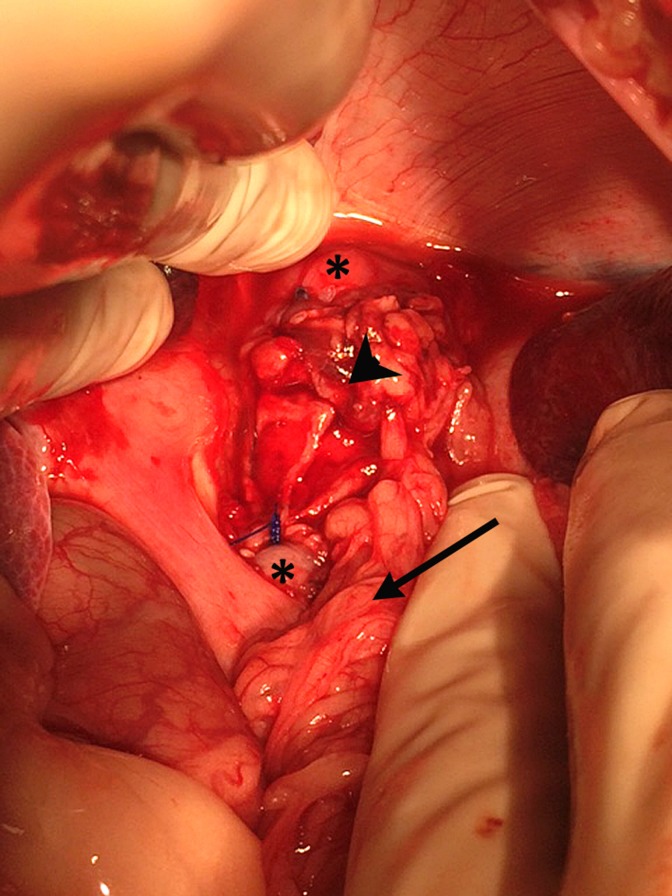
Two weeks after omental implantation, the animals were reoperated by a midline incision. Both ends of the maturated biomaterial were resected and fixed in 4% paraformaldehyde (PFA) for histological analysis, exposing the esophageal stent which was removed ( Fig. 3 ). Afterward, the abdominal esophagus was dissected between the diaphragm pillars allowing the preservation of the vagus nerves. A full thickness circumferential 3 cm long esophageal resection was performed 2 cm above the esophagogastric junction. The maturated substitute was taken up to the defected area as an omental pedicle flap. Interposition of the substitute was performed by running nonabsorbable sutures (Prolene 3/0; Fig. 4 ). The removable esophageal stent was placed under fluoroscopic guidance. Adequate positioning of the stent (i.e., above the cardia, covering both anastomoses) was controlled endoscopically. Last, the stent was fixed to the native esophagus with transfixing nonabsorbable stitches (Prolene 2/0) in order to reduce the risk of further stent migration.
Fig. 3.
In vivo maturation. The substitute after a 2-wk maturation period with its omental pedicle.
Fig. 4.
Esophageal replacement by the substitute: Operating view showing the native esophagus (asterisk), the substitute (arrowhead), and the omental pedicle (arrow).
Postoperative Care
Intravenous antibiotic therapy (sulbactam = 0.5 g/ampicillin = 1 g) was administrated after surgery and then replaced by amoxicillin 500 mg × 2/d for 3 d (orally). Analgesia was provided by intramuscular injection of 13 mg of nalbuphine × 2/d (Nalbuphine) until postoperative day 5 (POD 5). Proton pump inhibitor (esomeprazole 40 mg) was introduced postoperatively and maintained for 1 mo. Liquid hypercaloric and hyperprotidic feeding was initiated on POD 2. Following the first week, semiliquid food (flour mixed with water) was given.
Clinical Follow-Up and Management of Complications
Postoperative monitoring was performed daily, assessing the respiratory and dietary conditions as well as the occurrence of surgical complications. In case of coughing, food refusal or vomiting suggesting stricture occurrence due to stent migration, an endoscopy was performed. In case of stricture, a new esophageal stent was inserted after balloon dilatation. In case of an anastomotic leakage, the stent was repositioned to cover the fistula and an antibiotic therapy by Benzylpenicilline procaïne (Duphapen®, Pfizer, Paris, France) was given for 7 d. After 3 mo, the esophageal stent was removed endoscopically. After this end point, in case of stricture, it was treated once by balloon dilatation without restenting.
Euthanasia
Euthanasia was performed in case of obvious pain observed clinically despite suitable analgesia, extended food or drink refusal despite suitable treatment, esophageal obstruction preventing from performing endoscopic dilatation, perforation occurring during dilatation, or more than 3 stricture recurrences. In the absence of postoperative complication leading to euthanasia, sequential sacrifices were planned 3, 6, and 12 mo after surgery. Euthanasia was performed by intravenous injection of 0.7 mL/kg of pentobarbital (Dolethal®).
Outcomes
Primary outcome: Graft area analysis
Following euthanasia, graft area and surrounding tissues were resected and fixed in 4% PFA. Macroscopic analysis showed graft appearance and consistency. For microscopic examination, 4 μm paraffin sections were stained with HES. Qualitative and quantitative analysis of the cell infiltrate, vascularization, epithelialization, and muscular regeneration were recorded blindly in the graft area for a comparative analysis between the 2 groups. For immunohistochemistry analysis, antigen retrieval was performed by heating the tissue section in 0.1 M citrate buffer, pH 6.0. Immunostaining was performed in a Ventana processor (Ventana, Illkirsh, France), with antidesmin (Desmine D33; 1/100; Dako, Les Ulis, France).
Secondary outcomes
Histological analysis with HES staining of samples taken at the end of the 14 d maturation period was performed in the 2 groups. The overall survival and postoperative morbidity within 30 d, particularly stricture occurrence, were assessed.
Statistical Analysis
Variables were expressed as mean ± standard deviation, median (range), and frequency. Comparisons between groups were analyzed with nonparametric tests as appropriate: the Fisher’s exact test for proportions and the Mann–Whitney U test for medians. Survivals were compared with the log rank test. The statistical significance was defined as P < 0.05.
Results
Characterization of MSCs
All cell isolations proceeded successfully. As expected, BM porcine MSCs expressed high levels of CD29 (98.3% ± 1.3), CD44 (97% ± 2.1), CD90 (99.1% ± 1.8), and CD105 (88.92% ± 1.7) and were found to be negative for expressions of the hematopoietic markers CD34 (0%) and CD45 (0.7% ± 2.5). Furthermore, MSCs differentiated into adipogenic (defined by the accumulation of lipid-rich vacuoles) and osteogenic (characterized by ALkaline Phosphatase (ALP) activity and deposition of a calcium-rich mineralized extracellular matrix) lineages.
Histological Examinations of the Substitute before In Vivo Implantation
In all samples, HES staining showed a multilayered fusiform cellular coat at the surface of the matrix after 7 d of MSC culture. However, no cell colonization was observed in the matrix (Fig. 1).
Substitute Analysis after In Vivo Maturation and before Esophageal Replacement
Macroscopic examination, after 14 d of maturation in the greater omentum, showed a high thickness and neovascularization heterogeneity of the substitute between animals in both groups.
Histologically, no difference between groups was observed when considering blood vessel density and mononuclear cell infiltrate. However, numerous cells into the thickness of the matrix were systematically observed in the MSC group, but only in 20% of the samples of the control group (P = 0.001). When observed, these cells were quantitatively less numerous in the control group than in the MSC group (P = 0.007).
Clinical Outcome
There were no differences between the 2 groups in the incidence of surgical complications and postoperative death. One animal (control group) had to be euthanized following esophageal replacement because of tracheal perforation during intubation. No anastomotic leakage was recorded. All animals experienced at least 1 episode of stent migration revealed by a stricture formation. In each case, dilatation and stent repositioning were successfully performed. However, repeated stent migration and subsequent stricture formation was the main cause of euthanasia. Median survival was of 45 d (22 to 119) in the MSC group versus 66 (0 to 98) d in the control group (P = 0.43; Table 1 ).
Table 1.
Outcomes after Circumferential Esophageal Replacement.
| Group | Survival Time (Days) | Mature Re-epithelialization | Muscular Regeneration |
|---|---|---|---|
| Control | 0 | − | − |
| Control | 36 | − | − |
| Control | 36 | − | − |
| Control | 45 | − | − |
| Control | 64 | − | − |
| Control | 68 | − | − |
| Control | 73 | − | − |
| Control | 77 | − | − |
| Control | 77 | − | − |
| Control | 98a | + | − |
| MSC | 22 | − | − |
| MSC | 23 | − | − |
| MSC | 26 | − | − |
| MSC | 29 | − | − |
| MSC | 40 | − | − |
| MSC | 50 | + | + |
| MSC | 53 | + | + |
| MSC | 65 | + | + |
| MSC | 78 | + | + |
| MSC | 119a | + | + |
Abbreviation: MSC = mesenchymal stem cell.
aAfter stent removal at 3 mo.
Graft Area Analysis
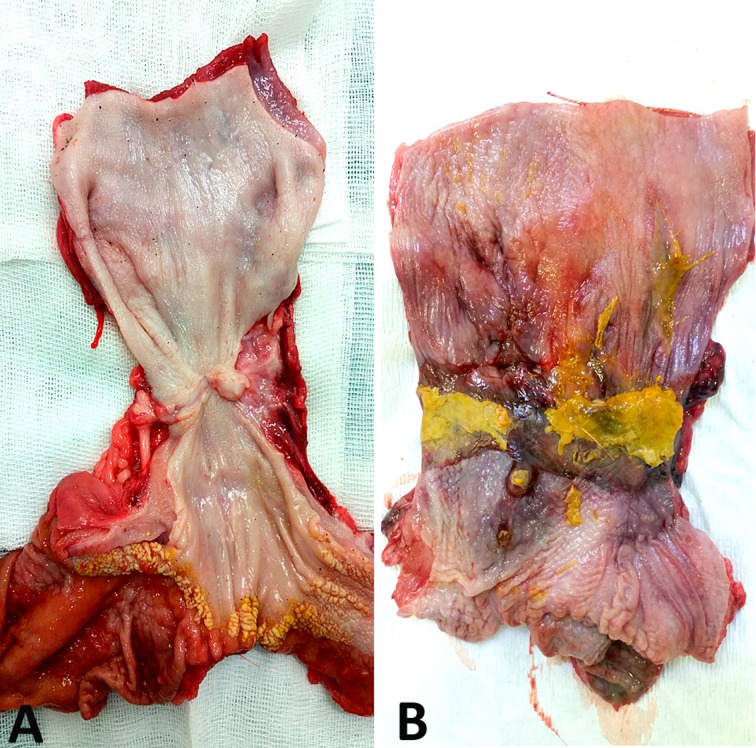
Macroscopic examination of the graft areas showed no differences between groups before POD 45. All areas appeared ulcerated and were covered with fibrin. From POD 45, a continuous mucosa was observed in the MSC group, while all control specimens showed large epithelial ulcerations until POD 98 ( Fig. 5 ).
Fig. 5.
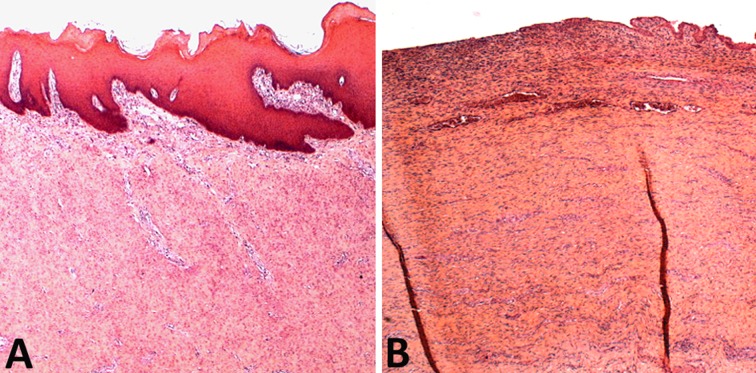
Macroscopic appearance at 2 mo showing a smooth mucosa in the mesenchymal stem cell group (A) and large epithelial ulceration in the control group (B).
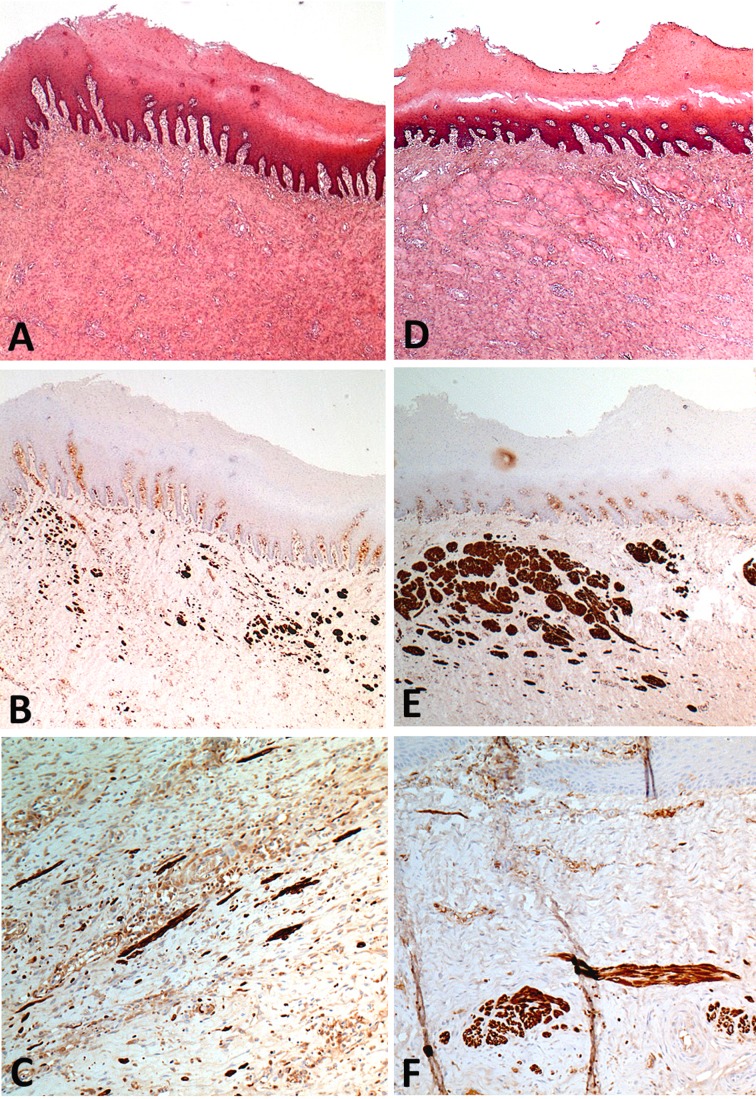
Before POD 45, microscopic examination showed no difference between the 2 groups. A superficial inflammatory infiltrate, with a deeper fibrotic reaction characterized by fusiform cells surrounded by connective tissue framework, was observed. No mature epithelialization of the graft area was observed, but an immature epithelium originating from the edges of the substitute, in connection with the native esophagus was seen. From POD 45, a mature stratified squamous epithelium covering the entire surface of the graft area was observed in all the MSC group specimens, but in none of the control group before POD 95 (Fig. 6). Starting POD 45, desmin positive cells were observed in the graft area, far from the edges of the native esophagus and without connection to it in the MSC group. With time, these cells organized into bundles or remained isolated ( Fig. 7 ). Desmin positive cells were never seen, at any time point, in the control group.
Fig. 6.
Comparative histological analysis of the epithelialization of the graft area at 2 mo. (A) Mature squamous epithelium and no inflammation in the mesenchymal stem cell group. (B) Ulceration and inflammation without epithelialization in the control group. Hematoxylin–eosin–saffron stain. Original ×2.5.
Fig. 7.
Histological analysis of the grafted areas in mesenchymal stem cell group showing muscle cells below the squamous epithelium and presenting as scattered cells (A–C) or cell bundles (D–F). (A) Scattered cells were inconspicuous at hematoxylin–eosin–saffron stain (HES) and (B, C) highlighted by desmin immunohistochemistry. Cell bundles were occasionally observed below the squamous epithelium at HES and desmin labeling (D–F). A and D: HES; original ×2.5. B and E: Desmin immunohistochemistry; original ×2.5. C and F: Desmin immunohistochemistry; original ×10. A, B, D, and E: Postoperative day (POD) 50. C: POD 78. F: POD 119.
Discussion
The present work follows the exploration by our team of other esophageal substitutes, an aortic allograft first18 and then a hybrid substitute composed of an SIS matrix seeded with myoblasts (muscle progenitor cells) and covered with a human amniotic membrane carrying autologous epithelial cells.5 Although the latter approach has yielded encouraging results, the importance of the inflammation in the graft area and the complexity of the approach (multiple cells and matrices) were not satisfactory. We use here MSCs, whose differentiation potential, proangiogenic, immunomodulating, and anti-inflammatory properties represent a promising therapeutic tool for TE, as it has been shown for bone,9 myocardial8 and urinary tract6,7 regeneration.
Specifically, our objectives were to analyze the tissue remodeling after full thickness circumferential replacement of the abdominal esophagus by the SIS matrix seeded with autologous MSCs, compared to a control group receiving a noncellularized matrix. Our main finding is that muscle regeneration is initiated in the presence of MSCs, association that was never observed in the absence of MSCs. Furthermore, the presence of MSCs was associated with an earlier re-epithelialization of the graft area, when compared to the control group.
Arguments from different animal models and from our own observations suggest that re-epithelialization of the graft area comes from the banks of the native esophagus, especially because it appears in continuity with the native epithelium.5,1 8,19 Histological analysis showed a mature epithelium covering the whole graft area from POD 53 in the presence of MSCs, and only at POD 98 in the control group, corroborating the data of the macroscopic analysis. This observation could be explained by the secretion of growth factors by MSCs intervening in wound healing, such as transforming growth factor-β, bFGF, and platelet-derived growth factor–BB.20 Although we have not been able to show it, it has been reported that re-epithelialization has an anti-inflammatory and antifibrotic effect.21–23 Finally, epithelial cells could help muscle cell colonization via a paracrine effect.24
The appearance of muscle cells into the graft area was observed as soon as POD 53 in the presence of MSCs, while no muscle cell was ever observed in control animals (>POD 90). To date, only circumferential esophageal replacement with a matrix seeded with muscle cells has allowed muscle regeneration. Nakase et al. reported the presence of a smooth muscle layer 70 d after esophageal replacement by a substitute composed of a synthetic matrix seeded with muscle cells and keratinocytes cultured on an amniotic membrane.23 With a similar hybrid substitute comprising myoblasts, we have observed muscle cells 7 mo after replacement and eventually a muscular layer at 12 mo.5 In the present work, the early onset of muscle cells was always seen in the graft area, with no connection to the banks of the native esophagus, suggesting that these cells are not an emanation of the native muscular layer. The absence of muscle cell colonization in the control group at the latest time points confirms this assumption. Thus, muscle regeneration appears to depend on seeded MSCs in this instance. Tan et al. have already shown in dogs in an esophagoplasty patch model that MSCs seeded on SIS accelerated muscle regeneration.25 However, the mechanism of action remains unclear. In our model, the degree of MSC survival and implantation at the graft site as well as the reality of MSC differentiation into muscle cells is unknown. To get answers, it would be interesting to mark MSCs in vitro with green fluorescent protein (GFP), for example, prior to transplantation.26 However, it has been demonstrated that MSCs affect tissue repair largely via a paracrine effect and stimulation of host cells by the production of abundant growth factors and cytokines.27,28 Recent studies indicate that MSCs also produce extracellular vesicles (exosomes and microvesicles) which carry mRNAs, microRNAs, and proteins. Extracellular vesicles function via horizontal transfer of their components to alter the activity of target cells.29
Without stent calibration of the graft area, circumferential esophageal replacements by a TE construct systematically lead to the formation of a fibrous stricture in 2 to 3 wk except in the experience of Nakase et al.23 We ourselves have stressed the importance of this prosthetic calibration to ensure prolonged animal survival.30 Currently, the limiting factor of our technique is the associated morbidity, with a high rate of prosthesis migration, which was the leading cause of euthanasia of our animals and no extensive follow-up. Intra-abdominal location of the esophageal replacement seems to be a risk factor for this complication, probably because of the location of the prosthesis near the gastroesophageal junction and the hyperperistalsis of the region. We chose this location to avoid compromising vascularization of the substitute by some section of omental vessels, necessary for the ascent of the substitute up to the thorax or to the neck. Unlike in humans, epiploplasty in pigs regularly induces ischemia of the tip of the great omentum, probably causing massive cell loss in the substitute.
Substantial variations in the macroscopic aspect of the substitute were observed after maturation in the greater omentum among the same group of animals. This variability is inherent to the MSCs which are a heterogeneous cell population for a given individual, whose phenotypic expression, cell doubling time and differentiation capacity are known to vary from one individual to another. Variable survival rates of cells after implantation and this great variability in the biological response are factors limiting the use of cells of interest. MSCs secrete factors with chemoattractant and anti-inflammatory properties that recruit in vivo host cells in order to induce optimized tissue regeneration and to reduce inflammation at the origin of deleterious scarring.20 A better understanding of these factors will eventually help to skip cellular implantation. It is thus interesting to consider use of a matrix carrying active molecules identified by the study of the MSC secretome. These “smart matrices,” compared with cellularized matrices, would enable standardized production and reproducible in vivo responses.
The first case report of successful esophageal circumferential replacement by TE in a human being has been recently published.31 An extracellular matrix coated with platelet-rich plasma was used to bridge a posttraumatic circumferential esophageal defect in a 24-y-old man under a compassionate ground. The patient recovered a functional cervical esophagus allowing nutritional autonomy. However, the duration of the stenting of the neoesophageal lumen (3.5 y) questions the efficacy of this approach on a mechanistic level.
In all cases, the application of existing clinical techniques for replacement of other organs by tissue engineering, in combination with the multiplication of translational research protocols for esophageal replacement in large animals, should soon pave the way for health agencies to authorize clinical trials.
In conclusion, we created a new viable model to accelerate the mature re-epitheliazation and early initiation of muscle cell colonization. Further studies will focus on the use of cell tracking tools and on the improvement of animal models in order to allow a longer follow-up.
Footnotes
Authors’ Note: The content of this article has been previously presented in a Poster presentation at the Société Française de Chirurgie Digestive (SFCD 2015, 11th Annual Congress), Poster P08, 25–27 November, Paris, France.
Ethical Approval: The experimental protocol was approved by the scientific committee of our institution, the ethics committee of animal experimentation n_ 34, and the Ministry of National Education, Higher Education and Research (agreement number: APAFIS#2397-2015101900217536 v4).
Statement of Human and Animal Rights: All animals received care in accordance with French regulations and institutional guidelines for animal research.
Statement of Informed Consent: There are no human subjects in this article and informed consent is not applicable.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Fondation de l’Avenir (ETO-579), the Association Française de l’Atrésie de l’Œsophage (AFAO), the Fondation pour la Recherche Médicale (DBS20140930773), the Agence de Biomédecine, and the Académie Nationale de Médecine.
References
- 1. Poghosyan T, Gaujoux S, Chirica M, Munoz-Bongrand N, Sarfati E, Cattan P. Functional disorders and quality of life after esophagectomy and gastric tube reconstruction for cancer. J Visc Surg. 2011;148(5):327–335. [DOI] [PubMed] [Google Scholar]
- 2. Chirica M, Veyrie N, Munoz-Bongrand N, Zohar S, Halimi B, Celerier M, Cattan P, Sarfati E. Late morbidity after colon interposition for corrosive esophageal injury: risk factors, management, and outcome. A 20-years experience. Ann Surg. 2010;252(2):271–280. [DOI] [PubMed] [Google Scholar]
- 3. Poghosyan T, Catry J, Luong-Nguyen M, Bruneval P, Domet T, Arakelian L, Sfeir R, Michaud L, Vanneaux V, Gottrand F, et al. Esophageal tissue engineering: current status and perspectives. J Visc Surg. 2016;153(1):21–29. [DOI] [PubMed] [Google Scholar]
- 4. Saxena AK. Esophagus tissue engineering: designing and crafting the components for the “hybrid construct” approach. Eur J Pediatr Surg. 2014;24(3):246–262. [DOI] [PubMed] [Google Scholar]
- 5. Poghosyan T, Sfeir R, Michaud L, Bruneval P, Domet T, Vanneaux V, Luong-Nguyen M, Gaujoux S, Gottrand F, Larghero J, et al. Circumferential esophageal replacement using a tube-shaped tissue-engineered substitute: An experimental study in minipigs. Surgery. 2015;158(1):266–277. [DOI] [PubMed] [Google Scholar]
- 6. Sharma AK, Bury MI, Marks AJ, Fuller NJ, Meisner JW, Tapaskar N, Halliday LC, Matoka DJ, Cheng EY. A nonhuman primate model for urinary bladder regeneration using autologous sources of bone marrow-derived mesenchymal stem cells. Stem Cells. 2011;29(2):241–250. [DOI] [PubMed] [Google Scholar]
- 7. Li CL, Liao WB, Yang SX, Song C, Li YW, Xiong YH, Chen L. Urethral reconstruction using bone marrow mesenchymal stem cell- and smooth muscle cell-seeded bladder acellular matrix. Transplant Proc. 2013;45(9):3402–3407. [DOI] [PubMed] [Google Scholar]
- 8. Ishikane S, Hosoda H, Yamahara K, Akitake Y, Kyoungsook J, Mishima K, Iwasaki K, Fujiwara M, Miyazato M, Kangawa K, et al. Allogeneic transplantation of fetal membrane-derived mesenchymal stem cell sheets increases neovascularization and improves cardiac function after myocardial infarction in rats. Transplantation. 2013;96(8):697–706. [DOI] [PubMed] [Google Scholar]
- 9. Liu C, Tan X, Luo J, Liu H, Hu M, Yue W. Reconstruction of beagle hemi-mandibular defects with allogenic mandibular scaffolds and autologous mesenchymal stem cells. PLoS One. 2014;9(8):105733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dezawa M, Ishikawa H, Itokazu Y, Yoshihara T, Hoshino M, Takeda S, Ide C, Nabeshima Y. Bone marrow stromal cells generate muscle cells and repair muscle degeneration. Science. 2005;309(5732):314–317. [DOI] [PubMed] [Google Scholar]
- 11. Baiguera S, Jungebluth P, Mazzanti B, Macchiarini P. Mesenchymal stromal cells for tissue-engineered tissue and organ replacements. Transpl Int. 2012;25(4):369–382. [DOI] [PubMed] [Google Scholar]
- 12. Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol. 2008;8(9):726–736. [DOI] [PubMed] [Google Scholar]
- 13. D’souza N, Rossignoli F, Golinelli G, Grisendi G, Spano C, Candini O, Osturu S, Catani F, Paolucci P, Horwitz EM, et al. Mesenchymal stem/stromal cells as a delivery platform in cell and gene therapies. BMC Med. 2015;13(1):186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Comite P, Cobianchi L, Avanzini MA, Zonta S, Mantelli M, Achille V, De Martino M, Cansolino L, Ferrari C, Alessiani M, et al. Isolation and ex vivo expansion of bone marrow-derived porcine mesenchymal stromal cells: potential for application in an experimental model of solid organ transplantation in large animals. Transplant Proc. 2010;42(4):1341–1343. [DOI] [PubMed] [Google Scholar]
- 15. Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop Dj, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315–317. [DOI] [PubMed] [Google Scholar]
- 16. Casado JG, Gomez-Mauricio G, Alvarez V, Mijares J, Tarazona R, Bernad A, Sanchez-Margallo FM. Comparative phenotypic and molecular characterization of porcine mesenchymal stem cells from different sources for translational studies in a large animal model. Vet Immunol Immunopathol. 2012;147(1-2):104–112. [DOI] [PubMed] [Google Scholar]
- 17. Stramandinoli-Zanicotti RT, Carvalho AL, Rebelatto CL, Sassi LM, Torres MF, Senegaglia AC, Boldrinileite LM, Correa-Dominguez A, Kuligovsky C, Brofman PR. Brazilian minipig as a large-animal model for basic research and stem cell-based tissue engineering. Characterization and in vitro differentiation of bone marrow-derived mesenchymal stem cells. J Appl Oral Sci. 2014;22(3):218–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gaujoux S, Le Balleur Y, Bruneval P, Larghero J, Lecourt S, Domet T, Lambert B, Zohar S, Prat F, Cattan P. Esophageal replacement by allogenic aorta in a porcine model. Surgery. 2010;148(1):39–47. [DOI] [PubMed] [Google Scholar]
- 19. Maghsoudlou P, Eaton S, De Coppi P. Tissue engineering of the esophagus. Semin Pediatr Surg. 2014;23(3):127–134. [DOI] [PubMed] [Google Scholar]
- 20. Konala VB, Mamidi MK, Bhonde R, Das AK, Pochampally R, Pal R. The current landscape of the mesenchymal stromal cell secretome: a new paradigm for cell-free regeneration. Cytotherapy. 2016;18(1):13–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wei RQ, Tan B, Tan MY, Luo JC, Deng L, Chen XH, Li XQ, Zuo X, Zhi W, Yang P, et al. Grafts of porcine small intestinal submucosa with cultured autologous oral mucosal epithelial cells for esophageal repair in a canine model. Exp Biol Med. 2009;234(4):453–461. [DOI] [PubMed] [Google Scholar]
- 22. Ohki T, Yamato M, Ota M, Takagi R, Murakami D, Kondo M, Sasaki R, Namiki H, Okano T, Yamamoto M. Prevention of esophageal stricture after endoscopic submucosal dissection using tissue-engineered cell sheets. Gastroenterology. 2012;143(3):582–588. [DOI] [PubMed] [Google Scholar]
- 23. Nakase Y, Nakamura T, Kin S, Nakashima S, Yoshikawa T, Kuriu Y, Sakakura C, Yamagishi H, Hamuro J, Ikada Y, et al. Intrathoracic esophageal replacement by in situ tissue-engineered esophagus. J Thorac Cardiovasc Surg. 2008;136(4):850–859. [DOI] [PubMed] [Google Scholar]
- 24. Marzaro M, Vigolo S, Oselladore B, Conconi MT, Ribatti D, Giuliani S, Nico B, Perrino G, Nussdorfer GG, Parnigotto PP. In vitro and in vivo proposal of an artificial esophagus. J Biomed Mater Res A. 2006;77(4):795–801. [DOI] [PubMed] [Google Scholar]
- 25. Tan B, Wei RQ, Tan MY, Luo JC, Deng L, Chen XH, Hou JL, Li XQ, Yang ZM, Xie HQ. Tissue engineered esophagus by mesenchymal stem cell seeding for esophageal repair in a canine model. J Surg Res. 2013;182(1):40–48. [DOI] [PubMed] [Google Scholar]
- 26. Chen WH, Liu HY, Lo WC, Wu SC, Chi CH, Chang HY, Hsiao SH, Wu CH, Chiu WT, Chen BJ, et al. Intervertebral disc regeneration in an ex vivo culture system using mesenchymal stem cells and platelet-rich plasma. Biomaterials. 2009;30(29):5523–5533. [DOI] [PubMed] [Google Scholar]
- 27. Fontaine MJ, Shih H, Schäfer R, Pittenger MF. Unraveling the mesenchymal stromal cells’ paracrine immunomodulatory effects. Transfus Med Rev. 2016;30(1):37–43. [DOI] [PubMed] [Google Scholar]
- 28. Galipeau J, Krampera M, Barrett J, Dazzi F, Deans RJ, DeBruijn J, Dominici M, Fibbe WE, Gee AP, Gimble JM, et al. International Society for Cellular Therapy perspective on immune functional assays for mesenchymal stromal cells as potency release criterion for advanced phase clinical trials. Cytotherapy. 2016;18(2):151–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Phinney DG, Pittenger MF. Concise review: MSC-derived exosomes for cell-free therapy. Stem Cells. 2017;35(4):851–858. [DOI] [PubMed] [Google Scholar]
- 30. Le Baleur Y, Gaujoux S, Bruneval P, Lambert B, Larghero J, Cattan P, Prat F. Self-expanding removable plastic stents for the protection of surgical anastomoses after esophageal replacement in a porcine model. Gastrointest Endosc. 2010;72(4):790–795. [DOI] [PubMed] [Google Scholar]
- 31. Dua KS, Hogan WJ, Aadam AA, Gasparri M. In-vivo oesophageal regeneration in a human being by use of a non-biological scaffold and extracellular matrix. Lancet. 2016;388(10039):55–61. [DOI] [PubMed] [Google Scholar]