Abstract
Background
Patients with ulcerative colitis often report fatigue.
Objectives
To investigate prevalence of and risk factors for fatigue in patients with ulcerative colitis with active disease and during deep remission.
Methods
In this cross-sectional study, disease activity was evaluated with endoscopy and calprotectin, and patients were classified as having active disease (n = 133) or being in deep remission (n = 155). Blood samples were analysed to assess anaemia, iron deficiency and systemic immune activity. Patients completed questionnaires to assess fatigue, psychological distress, gastrointestinal symptoms and quality of life.
Results
The prevalence of high fatigue (general fatigue ≥ 13, Multidimensional Fatigue Inventory) was 40% in the full study population. Among patients with high fatigue, female gender and iron deficiency were more prevalent, and these patients had more severe disease activity and reported higher levels of anxiety, depression and decreased quality of life compared with patients with no/mild fatigue. A logistic regression analysis identified probable psychiatric disorder (odds ratio (OR) (confidence interval) 6.1 (3.1–12.2)), iron deficiency (OR 2.5 (1.2–5.1)), active disease (OR 2.2 (1.2–3.9)) and female gender (OR 2.1 (1.1–3.7)) as independent risk factors for high fatigue. Similar results were found concerning psychological distress, gender and quality of life, but immune markers did not differ in patients in deep remission with high vs. no/mild fatigue.
Conclusions
Probable psychiatric disorder, iron deficiency, active disease and female gender are independent risk factors for high fatigue in patients with ulcerative colitis. Low-grade immune activity does not seem to be the cause of fatigue among patients in deep remission.
Keywords: Ulcerative colitis, inflammatory bowel disease, fatigue, psychiatric disease, iron deficiency, inflammation
Introduction
Fatigue is a common clinical feature among patients with chronic inflammatory diseases, including inflammatory bowel disease (IBD). The prevalence of fatigue has been reported to be 21–41% in patients with IBD with inactive disease1–6 and as high as 70–75% in patients with active disease.1,5 Fatigue is associated with reduced health-related quality of life, and effective management options are lacking.3–5,7–9 Moreover, fatigue is a complex symptom with both physical and mental components.10 Disease activity, psychological distress, perceived stress, quality of sleep and female gender have all been correlated with high levels of fatigue,1,4–6,11–13 while factors important for fatigue among IBD patients with inactive disease are not fully understood. Further, anaemia and iron deficiency have been suggested as important contributing factors to fatigue. However, existing data concerning the association between anaemia and fatigue in IBD are conflicting,2,4,6,13 and previous studies have reported no correlation between fatigue and iron deficiency in IBD patients,6,12,13 but the measurement and definition of iron deficiency in patients with IBD are problematic. Serological markers for iron deficiency, such as ferritin and transferrin saturation, are affected by inflammation. Therefore, use of several markers in combination has been suggested to provide the most reliable assessment of iron deficiency in IBD,14,15 which has been taken into account in this study.
Low-grade immune activity might also be a factor of importance for fatigue in IBD, since patients with different chronic inflammatory conditions report high levels of fatigue.3,16 Chronic fatigue syndrome is a multidimensional condition characterized by chronic, disabling fatigue and various other somatic symptoms.17 The pathogenesis and pathophysiology of this syndrome are unknown, but post-infectious immune alterations have been proposed as a plausible explanation.17 A systematic review reported that 51% of patients with chronic fatigue syndrome have co-existing irritable bowel syndrome (IBS),18 and post-infectious low-grade immune activity is considered to be a potential contributing factor to symptom generation in IBS.19 Among patients with IBS, fatigue is a common symptom, which is associated with gastrointestinal symptom severity and psychological distress.20 Further, IBS-like symptoms during remission among patients with IBD are common21,22 and have been associated with higher levels of fatigue, suggesting possible common pathophysiological pathways.23,24 Low-grade immune activity during remission has not been studied previously as a potential risk factor for fatigue among patients with ulcerative colitis (UC).
We hypothesized that disease activity, psychological distress, anaemia and iron deficiency are all associated with fatigue in patients with UC. Further, we also hypothesized that low-grade immune activity and residual gastrointestinal symptoms contribute to fatigue among patients with UC in deep remission. Therefore, our aim was to investigate the prevalence of high fatigue in a large cohort of patients with UC with active disease and in deep remission, and to determine factors associated with fatigue in UC.
Materials and methods
Study design
Patients with UC (n = 298) were recruited from four specialized IBD clinics in the Västra Götaland Region, Sweden. The criteria for inclusion were verified diagnosis of UC in patients between 18 and 75 years old according to Lennard-Jones criteria.25 Patients with malignancy within the previous 5 years, difficulties in understanding the Swedish language, abuse of alcohol or drugs, previous surgery with resection of colon or small intestine, celiac disease, or significant heart, lung, kidney, neurological, rheumatic or psychiatric disease were excluded. The study was approved by the Regional Ethical Review Board in Gothenburg, and the subjects gave verbal and written informed consent before participation.
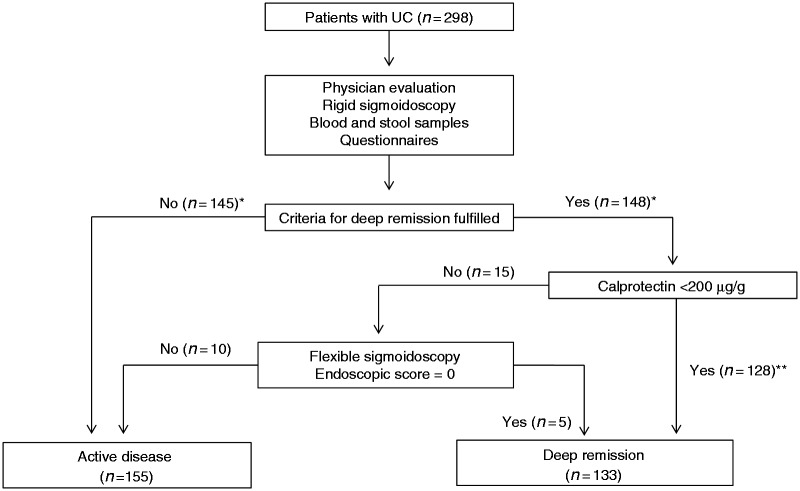
The patients were recruited to the study at a regular outpatient-clinic visit between September 2012 and April 2014. Blood and stool samples were collected and stored at −80℃ and −20℃, respectively. The patients completed self-administrated questionnaires and were examined by a physician. The examination included a rigid sigmoidoscopy. Patients with faecal calprotectin >200 µg/g and normal rectal mucosa at the initial endoscopic investigation were further examined with a flexible sigmoidoscopy within 2 weeks to determine inflammatory activity of the colonic mucosa (Figure 1).
Figure 1.
Study flow chart. 298 patients with ulcerative colitis were thoroughly investigated to rule out ongoing macroscopic mucosal inflammation among the patients classified as deep remission. *Four patients did not complete the MFI questionnaire and one patient was excluded due to celiac disease. **Five patients did not provide stool samples and were excluded.
Inflammatory activity
Clinical disease activity
At the study visit, the disease activity was evaluated using the Mayo score.26 The Mayo score contains four variables: stool frequency, rectal bleeding, endoscopic finding and the physician’s global assessment. Each variable is graded from 0 to 3, and the maximum total score is 12, with higher score indicating more severe disease. The initial endoscopic score, the physician’s global assessment and the total Mayo score were adjusted in the patients who underwent flexible sigmoidoscopy if macroscopic inflammation was detected (Figure 1). Disease activity was graded as follows using the Mayo score: severe disease 10–12 points, moderate disease 6–9 points, mild disease 3–5 points and remission 0–2 points with no subscore >1.26 The patients were classified as having active disease or being in deep remission. To the best of our knowledge, criteria for the term “deep remission” have not yet been established.27 In this study, deep remission was defined as a relapse-free period of 3 months prior to inclusion and a total Mayo score ≤ 2, provided the scores for physician’s global assessment, rectal bleeding and the endoscopic subscore were all 0. The colonic extent of the disease was determined according to the Montreal classification,28 and the most proximal extent of macroscopic inflammation since diagnosis was recorded.
Inflammatory biomarkers
Faecal calprotectin was used as a marker for mucosal inflammation 29 and was analysed using a sandwich enzyme-linked immunosorbent assay (Calprotectin ELISA; Bühlmann Laboratories AG, Basel, Switzerland) with a monoclonal capture antibody specific for calprotectin, according to the manufacturer’s instructions. The inter-assay coefficient of variation was <15% and the sensitivity of the assay was <10 µg/g. In the general population, the normal level is <50 µg/g. Furthermore, high sensitive C-reactive peptide (hs-CRP), a systemic inflammatory marker, was analysed by enzyme-linked immunosorbent assay (ELISA) as a routine test at the clinical immunology laboratory at Sahlgrenska University Hospital. Additionally, as markers for systemic immune activity, the levels of interleukin (IL) 12p70 and interferon (IFN)-γ (reflecting T helper 1 cell-mediated activity), IL-4, IL-13 and IL-10 (reflecting T helper 2 cell-mediated activity), and IL-17A (reflecting T helper 17 cell-mediated activity) were measured. Further, IL-1β, IL-6, IL-8 and tumour necrosis factor (TNF)-α (mainly reflecting activity of the innate immune system) in serum samples were measured. Samples were measured using the Meso Scale Discovery® (MSD) platform (MSD, Rockville, MD). All analyses were performed according to the manufacturers’ instructions.
Anaemia and iron deficiency
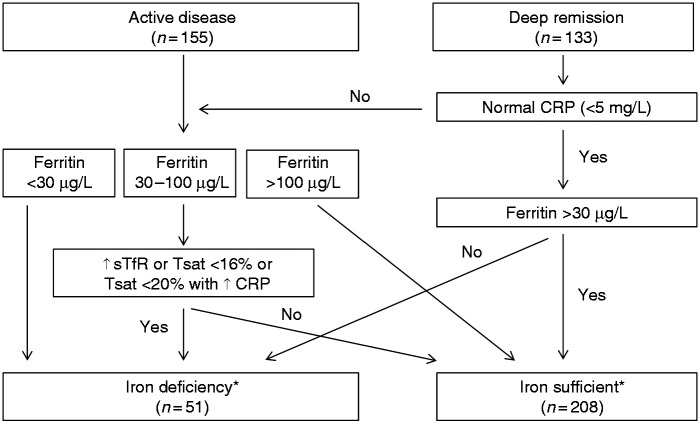
Anaemia was defined as haemoglobin < 12.0 g/dL (female) or <13.0 g/dL (male) in accordance with European Crohn’s and Colitis Organisation (ECCO) guidelines 2015.14 To assess iron deficiency, levels of ferritin, transferrin saturation and soluble transferrin receptor were analysed in serum as a routine test at the clinical chemistry laboratory at Sahlgrenska University Hospital. The definition of iron deficiency was determined in accordance with current ECCO guidelines14 (Figure 2).
Figure 2.
Iron status in the patients according to the ECCO guidelines 2015.14 CRP: C-reactive peptide; ↑ sTfR: elevated soluble transferrin receptor; Tsat: Transferrin saturation. *29 patients were not able to classify due to missing data.
Questionnaires
Fatigue
The level of fatigue was measured by the Multidimensional Fatigue Inventory (MFI). The MFI consists of 20 statements, which cover different aspects of fatigue. The results are expressed as five domains: General fatigue, Physical fatigue, Reduced activity, Reduced motivation and Mental fatigue, with scores ranging from 4 to 20. General fatigue is the domain recommended for use as an indicator for fatigue when only one score is desired.10 Similarly to several previous studies, ‘high fatigue’ was defined as a General fatigue score ≥ 13,1,5,6 which is based on the 95th percentile of the score in a group of healthy controls.3
Psychological distress
The Hospital Anxiety and Depression scale (HAD) was used to assess psychological distress. The HAD scale consists of 14 items, each using a Likert scale (0–3), with subscales for anxiety (7 items) and depression (7 items). The results are expressed as a total score (min–max: 0–42) in this study. The higher the scores, the more severe are the symptoms of anxiety and depression.30 Further, patients can be classified as having a probable psychiatric disorder (total HAD ≥ 13) or no/possible psychiatric disorder (total HAD < 13).31
Quality of life
Health-related quality of life was measured using the Inflammatory Bowel Disease Questionnaire (IBDQ), which consists of 32 questions, each using a Likert scale (1–7). The results are expressed as a total score, range 32–234, with higher values indicating better quality of life.32,33
Gastrointestinal (GI) symptoms
The severity of GI symptoms was assessed using the Gastrointestinal Symptom Rating Scale (GSRS). The GSRS contains 15 questions and uses a Likert scale (1–7). The higher the score, the more pronounced are the symptoms. The results can be expressed as a total score or be divided into subdomains (Diarrhoea, Constipation, Abdominal pain, Indigestion and Reflux).34,35 In this study, the overall severity of GI symptoms is reported as a total GSRS score.
The presence of IBS-like symptoms during deep remission was determined using the Rome III Diagnostic Questionnaire.36 To fulfil the Rome III criteria for IBS, the patients had to have abdominal discomfort or pain combined with two of the following three features: (1) relieved with defecation, (2) onset associated with a change in stool frequency, and (3) onset associated with a change in form (appearance) of stool. The symptoms should have been present for at least 2–3 days per month during the previous 3 months, and the symptoms should have been present for more than 6 months.37
Data analysis
The study population was first investigated to determine the prevalence of high fatigue and characterize the type of fatigue using the different MFI domains. To investigate the patients with high fatigue and identify possible risk factors for high fatigue, the UC patients with high fatigue (MFI General fatigue score ≥ 13) were compared with patients with mild/no fatigue (MFI General fatigue score < 13) concerning demographic data, disease characteristics, inflammatory disease activity, anaemia, iron deficiency, psychological distress and quality of life. Independent risk factors for high fatigue were then identified using a multivariate logistic regression analysis. To characterize the fatigue among the patients with and without these risk factors, each MFI domain was further analysed. Thereafter, the UC patients in deep remission with high vs. mild/no fatigue were compared concerning age, gender, anaemia, iron deficiency, psychological distress, quality of life, residual gastrointestinal symptoms (including prevalence of IBS-like symptoms) and low-grade inflammatory activity.
Statistical analysis
Continuous data are expressed as mean values with standard deviation (SD) for normal distributed data (Mayo score, MFI, HAD, IBDQ, GSRS, age and disease duration) and median values with interquartile range (IQR) for non-normally distributed data (calprotectin, hs-CRP and cytokines, where data were strongly skewed). Categorical variables are expressed as number of patients and percentage. Comparisons between two groups were performed with Student’s t-test for normally distributed continuous data, Mann–Whitney U test for non-normally distributed continuous data, and Fisher’s exact test (2-sided) for categorical data. In order to determine factors independently associated with high fatigue (‘risk factors’), we performed binary logistic regression analyses in the cohort with high fatigue (MFI General fatigue ≥ 13) as the dependent variable, and factors univariately associated with high fatigue as independent variables. A p-value < .05 was considered to be statistically significant. All statistical analyses were performed using the IBM SPSS Statistics 19 software (SPSS Inc., Chicago, IL, USA).
Results
Characterization of the study population
Demographic data and disease activity
In total, 288 patients with UC were included in the study and were classified as having active disease (n = 155) or being in deep remission (n = 133) (Figure 1). Age, gender, disease duration, colonic extension of disease, medical maintenance treatment and prevalence of primary sclerosing cholangitis among the study population are presented in Table 1. According to the total Mayo score, among the patients with active disease, 4% (n = 6) had severe disease, 27% (n = 42) moderate disease and 39% (n = 61) mild disease. Among the patients classified as having active disease, 29% (n = 46) were in clinical remission according to the Mayo score, but did not meet the study criteria for deep remission due to mild endoscopic inflammation (endoscopic subscore = 1 (n = 30)), blood in the stool (bleeding subscore = 1 (n = 2)) or a relapse within 3 months prior to study visit (n = 14).
Table 1.
Comparison of demographic data and disease characteristics in patients with ulcerative colitis (UC) with high fatigue and no/mild fatigue.
| UC high fatigue (N = 116) | UC no/mild fatigue (N = 172) | p | |
|---|---|---|---|
| Years of age, mean (min–max) | 39 (19–69) | 44 (18–73) | <.01 |
| Female gender, n (%) | 61 (52) | 55 (32) | <.01 |
| Mean disease duration (SD), years | 11 (9) | 14 (12) | .02 |
| Proctitis/left sided/ extensive, % | 18/27/55 | 18/31/51 | .74 |
| Primary sclerosing cholangitis, n (%) | 1 (1) | 7 (4) | .15 |
| 5-ASA, oral, n (%)a | 92 (79) | 123 (72) | .17 |
| 5-ASA, topical, n (%)a | 7 (6) | 3 (2) | .10 |
| Thiopurines, n (%)a | 23 (20) | 30 (17) | .64 |
| Anti-TNF-α, n (%)a | 9 (8) | 7 (4) | .20 |
| Corticosteroids, oral, n (%)a | 0 (0) | 1 (0) | 1.00 |
| Corticosteroids, topical, n (%)a | 3 (3) | 0 (0) | .06 |
Ongoing treatment prior to study visit.
Anaemia and iron deficiency
In total, 5% (n = 13) fulfilled the criteria for anaemia, and iron deficiency was identified in 20% (n = 51) of the patients (Figure 2). Due to missing data, it was not possible to classify 29 patients as being iron deficient or not.
Fatigue
The levels of fatigue in the full study population measured by the MFI domains were (mean (SD)) General fatigue: 11.1 (4.3); Physical fatigue: 9.9 (4.5); Reduced activity: 9.5 (4.1); Reduced motivation: 8.3 (3.7) and Mental fatigue: 8.8 (3.7). The overall prevalence of high fatigue (MFI General fatigue ≥ 13) among the patients was 40% (n = 116).
Comparison between patients with and without high fatigue
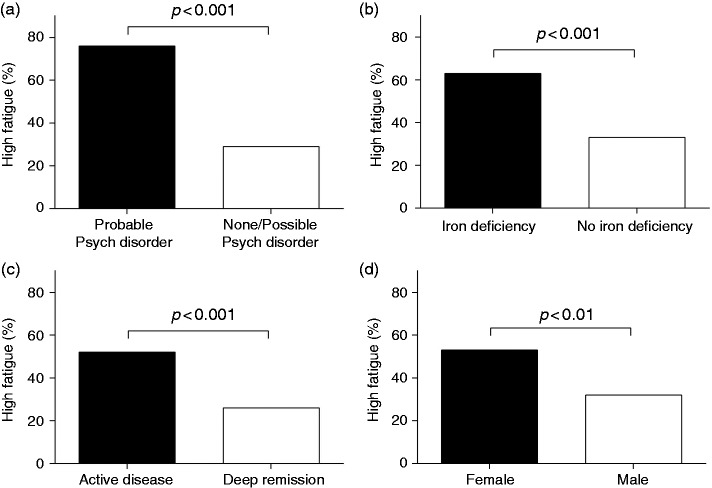
Comparisons between the groups of patients with high fatigue and no/mild fatigue concerning demographic data, disease characteristics and medical treatment are shown in Table 1. The patients with high fatigue had a lower mean age and a shorter duration of disease, and female gender was more prevalent compared with the patients with no/mild fatigue. However, no differences in current medical treatment were observed between the two groups. The clinical disease activity score was higher and iron deficiency, but not anaemia, was more prevalent among patients with high fatigue. Moreover, these patients reported poorer psychological well-being and reduced quality of life compared with patients with no/mild fatigue (Table 2). The markers for increased immune activity, faecal calprotectin and serum IL-17A, were significantly higher among the patients reporting high fatigue. However, hs-CRP and the other cytokines measured in serum samples did not differ between the two groups (Table 3). The variables that differed significantly between the two groups in the initial comparisons were included in a multivariate logistic regression analysis. Representing the variables reflecting inflammatory activity in the initial comparisons (Mayo score, faecal calprotectin and IL-17A), the study population was dichotomized into those with active disease and those in deep remission (Figure 1), and this variable was included in the analysis. The population was dichotomized into clinically applicable psychiatric distress (probable psychiatric disorder (HAD total ≥ 13) or no/possible psychiatric disorder (HAD total < 13)). Age, female gender and iron deficiency were also included in the analysis, but quality of life and disease duration were not, since they were not considered to be possible causes of fatigue. The multivariate logistic regression analysis identified the following independent risk factors for high fatigue (odds ratio (95% confidence interval)): probable psychiatric disorder, 6.2 (3.1–12.2); iron deficiency, 2.5 (1.2–5.1); active disease, 2.2 (1.2–3.9) and female gender, 2.1 (1.1–3.7), R2 = 0.32. Age was not an independent risk factor for high fatigue: 0.98 (0.96–1.01). The differences in prevalence of high fatigue among the patients with and without these risk factors are shown in Figure 3. Further comparisons showed higher levels of fatigue in all of the MFI domains in patients with the identified risk factors for high fatigue (probable psychiatric disorder, iron deficiency, active disease and female gender (p < .05)) except for mental fatigue and reduced motivation and activity when comparing females and males. Since it is difficult to determine a cut-off for iron deficiency in patients with IBD,14 a post-hoc analysis was performed to compare patients with low levels of ferritin ( < 30 µg/L = iron deficiency) and high levels of ferritin ( > 100 µg/L = iron sufficient) regardless of disease activity and systemic inflammatory activity (levels of CRP). This analysis showed that patients with low levels of ferritin reported higher levels of fatigue on all MFI domains (p < .01). Further, high fatigue was more prevalent among patients with ferritin < 30 µg/L compared with patients with ferritin > 100 µg/L (66% vs. 31%, p < .001).
Table 2.
Comparison of clinical disease activity, anaemia, iron deficiency, psychological distress and quality of life in patients with ulcerative colitis (UC) with high fatigue and no/mild fatigue.
| UC high fatigue (N = 116) | UC no/ mild fatigue (N = 172) | p | |
|---|---|---|---|
| Mayo scorea | 3.4 (3.1) | 1.7 (2.5) | <.001 |
| Anaemia, n (%) | 7 (6) | 6 (3) | .39 |
| Iron deficiency, n (%)b | 32 (32) | 12 (19) | <.001 |
| Anxiety and depression (HAD)a,c | 12.2 (6.9) | 4.9 (4.5) | <.001 |
| Quality of life (IBDQ)a,d | 156 (31) | 196 (21) | <.001 |
Mean (SD).
Missing data: high fatigue n = 16, no/mild fatigue n = 13.
Hospital Anxiety and Depression scale, total score.
Inflammatory Bowel Disease Questionnaire.
Table 3.
Comparison of inflammatory markers between patients with ulcerative colitis (UC) with high and no/mild fatigue.
| UC high fatigue (N = 116) | UC no/mild fatigue (N = 172) | p | |
|---|---|---|---|
| Calprotectin (µg/g)a | 112 (27–663) | 53 (17–190) | <.01 |
| hs-CRP (mg/L)b | 1.40 (0.39–4.03) | 1.00 (0.40–3.00) | .35 |
| IL-17A (pg/mL)c | 1.79 (0.80–4.01) | 0.96 (0.58–1.80) | <.01 |
| IL-6 (pg/mL)c | 0.84 (0.41–2.16) | 0.88 (0.44–2.11) | .66 |
| IL-1β (pg/mL)c | 0.02 (0.00–0.18) | 0.03 (0.00–0.30) | .55 |
| IL-10 (pg/mL)c | 0.46 (0.17–0.97) | 0.41 (0.21–1.06) | .52 |
| IL-8 (pg/mL)c | 53 (18–1310) | 73 (20–1451) | .62 |
| TNF-α (pg/mL)c | 1.85 (1.54–2.89) | 2.16 (1.62–2.96) | .07 |
| IL-13 (pg/mL)c | 2.69 (0.28–12.03) | 2.68 (0.32–13.15) | .60 |
| IFN- (pg/mL)c | 6.10 (3.68–12.84) | 6.04 (3.51–9.91) | .50 |
| IL-4 (pg/mL)c | 0.03 (0.00–0.17) | 0.02 (0.00–0.09) | .11 |
| IL-12p70 (pg/mL)c | 0.20 (0.07–0.39) | 0.15 (0.07–0.43) | .46 |
All values expressed as median (IQR). Missing data: an = 18, bn = 29, cn = 40.
Figure 3.
Differences in the prevalence of high fatigue among patients with and without the independent risk factors for high fatigue: (a) probable psychiatric disorder, (b) iron deficiency, (c) active disease and (d) female gender.
Comparison between patients in deep remission with and without high fatigue
The prevalence of high fatigue among the patients with UC in deep remission (n = 35, 26%) was further investigated. As observed in the total study population, female gender was more common, the degree of psychological distress was higher, quality of life was reduced and iron deficiency tended to be more prevalent among the patients in deep remission with high fatigue than in patients with no/mild fatigue. Patients in deep remission with high fatigue also reported more severe gastrointestinal symptoms compared with patients in deep remission with no/mild fatigue. Further, the proportion of patients who fulfilled the Rome III criteria for IBS tended to be larger among patients with high fatigue (Table 4). To explore the role of low-grade immune activity, the levels of mucosal and systemic inflammatory markers were also compared between the UC patients in deep remission with high fatigue and no/mild fatigue. However, the patients in deep remission with high fatigue did not have higher levels of inflammatory markers (calprotectin, hs-CRP and systemic cytokines) compared with the patients with no/mild fatigue (p = NS for all comparisons).
Table 4.
Comparison between UC patients in deep remission (UCR) with high and no/mild fatigue.
| UCR high fatigue (N = 35) | UCR no/mild fatigue (N = 98) | p | |
|---|---|---|---|
| Years of age, mean (min–max) | 40 (19–66) | 43 (18–73) | .29 |
| Female gender, n (%) | 20 (57) | 31 (32) | .01 |
| GI symptoms (GSRS)a | 2.1 (0.8) | 1.7 (0.6) | <.01 |
| IBS-like symptoms, n (%) | 10 (29) | 14 (14) | .07 |
| Anxiety and depression (HAD)b | 11.3 (6.3) | 4.4 (3.9) | <.001 |
| Quality of life (IBDQ)c | 178 (22) | 204 (15) | <.001 |
| Anaemia, n (%) | 0 (0) | 2 (2) | .54 |
| Iron deficiency, n (%)d | 6 (19) | 7 (8) | .10 |
Gastrointestinal symptoms (Gastrointestinal Symptom Rating Scale), mean (SD).
Hospital Anxiety and Depression scale, total score, mean (SD).
Inflammatory Bowel Disease Questionnaire, mean (SD).
Missing data: UCR high fatigue n = 3, no/mild fatigue n = 6.
Discussion
In this large cross-sectional study, we confirmed that fatigue is a common problem in patients with UC. In line with previous reports, we found psychological distress, disease activity and female gender to be important factors for fatigue,1,2,4–6,9,12 but also suggest iron deficiency as an independent risk factor for high fatigue in patients with UC. Furthermore, we demonstrated a high prevalence of fatigue in UC patients during deep remission and that low-grade immune activity does not seem to be the cause of fatigue among these patients.
Fatigue is a complex and multidimensional symptom with both physical and mental components. Persistent fatigue might reflect underlying somatic and/or psychiatric disease or might even be a side effect of medical treatment.10 In this study, the prevalence of high fatigue, defined as an MFI General fatigue score ≥ 13,3 was 40% in the total study population. Moreover, 52% among the patients with active UC and 26% in the group of patients who fulfilled the criteria for deep remission suffered from high fatigue. These results are in line with a previous Nordic study.6 Even higher prevalence of high fatigue among IBD patients with active disease has been reported (70–75%).1,5 This discrepancy might be explained by the fact that in our study, a large proportion of the patients classified with active disease had mild disease activity. The prevalence of high fatigue among patients with UC in deep remission in our study is lower compared with previous reports on patients with quiescent IBD.3–5 A possible explanation is the strict criteria for deep remission used in our study.
Psychosocial factors have been associated with fatigue among patients with IBD,1,4,5,8 which was confirmed in the present study, where high fatigue in UC patients was associated with higher levels of anxiety and depression and reduced health-related quality of life. Probable psychiatric disease was identified as an independent risk factor for high fatigue in the regression analysis, and the patients with a probable psychiatric disorder had higher scores on all MFI domains, further supporting the importance of evaluating psychological distress among UC patients suffering from fatigue. This observation was, however, expected, since symptoms included in the criteria for depressive disorders, such as perceived fatigue, loss of energy, diminished interest in activity, psychomotor retardation and reduced ability to concentrate, also are central symptoms in the assessment of fatigue by the MFI.38
The patients with high fatigue had higher inflammatory disease activity, measured by the total Mayo score and faecal calprotectin, compared with the patients with no/mild fatigue, which is in agreement with previous studies.1,2,5,6,11,13 Systemic immunological activity, measured by IL-17A, was also elevated among the patients with high fatigue. The elevated serum levels of IL-17A might reflect the colonic disease activity. Previous work from our group demonstrated a correlation between levels of IL-17A in serum and clinical disease activity in UC patients.39 The level of fatigue among patients with IBD has been reported to be similar to that in patients with other chronic inflammatory diseases, such as rheumatic diseases and cancer,3 and systemic inflammatory activity (IL-1, IL-6, IL-8 and TNF-α) has been suggested to be a common link between fatigue, pain and psychological distress.16 Our patients with high fatigue had increased levels of IL-17A, but no other serum cytokines were elevated. This result indicates that systemic immune activity might contribute to generation of fatigue among patients with UC. However, the increased colonic inflammation observed among the patients might also affect the central nervous system directly through the autonomous nerve system, that is, the brain–gut axis, which has been suggested to be a possible pathway for fatigue.16
Anaemia and iron deficiency are possible contributing factors to fatigue. Iron is essential for the production of red blood cells,14 but is also important for normal function of the cells of the brain.40 Therefore, iron deficiency might affect both cognitive and emotional factors.40 Anaemia has been correlated with fatigue in patients with IBD in some studies,2,4 while others found no correlation.3,5,6,13 We observed no difference comparing anaemia among UC patients with high fatigue and no/mild fatigue, but these data must be interpreted with caution, since the prevalence of anaemia was very low (5%). Further, the cut-off for anaemia is the lower limit of the normal range, which means that a proportion of the patients might have had reduced haemoglobin levels compared with their normal level without fulfilling the criteria for anaemia. However, the present study shows that patients with UC with concomitant iron deficiency have higher levels of fatigue compared with patients without iron deficiency. Iron deficiency was also an independent risk factor for high fatigue. In contrast, three previous studies found no correlation between iron deficiency and fatigue in IBD patients.6,12,13 A possible explanation for these conflicting data might be different definitions of iron deficiency and the use of different serological markers for iron deficiency. In the present study, the patients were thoroughly examined to identify active disease (faecal calprotectin and endoscopy), and soluble transferrin receptor and transferrin saturation were used in combination with levels of ferritin to classify patients as iron deficient or not in accordance with ECCO guidelines.14 Moreover, in this study, patients with ferritin > 100 µg/L had lower levels of fatigue compared with those who had ferritin < 30 µg/L, further supporting the idea that iron deficiency seems to be of importance for the generation of fatigue. However, from the results of this cross-sectional study, it is not possible to conclude that fatigue among these patients would be improved by iron supplement treatment.
The majority of previous studies report higher levels of fatigue among female IBD patients.1,4, 6,11 In this study, female gender was more prevalent among the UC patients with high fatigue and was also identified as an independent risk factor for high fatigue. This result is also in line with studies on chronic fatigue syndrome, which is more prevalent among women.41 Possible explanations for the gender differences are hormonal and biopsychosocial factors (including stress-induced immunological alterations).41
Although several independent factors associated with high fatigue were identified by our logistic regression analysis, only about a third of the variance in fatigue could be explained by factors evaluated in this study, which points to the complexity of evaluating fatigue.
Our study demonstrates that patients with psychological distress, active disease and iron deficiency report higher levels of fatigue in all MFI domains. Similar observations have been reported in IBD patients with active disease.4–6 Additionally, in the study by Goldenberg et al.,13 IBD patients with iron deficiency tended to have higher levels of mental fatigue, but no difference was observed in the other domains of fatigue. The observation that iron deficiency might affect cognitive components is interesting, since a recent meta-analysis on non-anaemic iron-deficient patients reported that iron supplementation can improve fatigue, prostration and concentration but not exercise tolerance.42
The prevalence of high fatigue (26%) among the patients with UC in deep remission is, however, evidently high, since the definition of high fatigue (MFI General fatigue ≥ 13) is based on the 95th percentile of healthy controls in a previous Dutch study.3 To understand why a large proportion of the UC patients without apparent disease activity experienced severe fatigue, we further analysed these patients and found similar results as in the total study population concerning psychological distress, gender and iron deficiency. Additionally, the patients in deep remission with high fatigue reported more gastrointestinal symptoms, and IBS-like symptoms tended to be more prevalent among the patients with high fatigue compared with patients with no/mild fatigue. Our results are in line with the results from a Norwegian study that reported higher levels of fatigue among UC patients in clinical remission with IBS-like symptoms compared with patients in remission without IBS-like symptoms.23 These results could be interpreted as a sign of low-grade inflammatory disease activity, but this could not be confirmed, since the patients in deep remission with and without high fatigue had similar levels of colonic inflammation and systemic immune activity.
Strengths of the present study are the large cohort of UC patients and the thorough investigation with endoscopy and inflammatory markers in stool and blood samples in order to evaluate on-going inflammatory activity. We decided not to include patients with Crohn’s disease, as it is more difficult to verify that these patients are in deep remission compared with patients with UC. Another advantage is that we evaluated the potential relationship between fatigue and systemic immune activity. Further strengths are the careful evaluation of the patients’ iron status with multiple serological markers combined with the cautious investigation of inflammatory activity in accordance with recent ECCO guidelines.14 A limitation of the study is the cross-sectional design, which makes it impossible to evaluate the causes and consequences of high fatigue in a longitudinal perspective. Moreover, the study did not include healthy controls.
To conclude, high fatigue is common among UC patients with active disease as well as among patients in deep remission. Iron deficiency seems to be of importance for fatigue, and we confirm that psychological distress, disease activity and female gender are associated with high fatigue. However, low-grade inflammatory activity does not seem to be the cause of fatigue among UC patients in deep remission. Prospective and comparative studies are needed to further evaluate the mechanisms behind fatigue in patients with UC and to determine appropriate treatments for this category of patients in order to improve their quality of life.
Acknowledgements
We would like to thank our colleagues at Sahlgrenska University Hospital/Sahlgrenska (Dr Antal Bajor, Dr Per Hedenström and Dr Björn Lindkvist), Södra Älvsborg Hospital/Borås (Dr Anders Lasson) and Norra Älvsborg Hospital/Tollhättan (Dr Dietrich Ahlhausen and Dr Eszter Benyei) for participating in the recruitment of study patients.
Funding
This work was supported by the Swedish Medical Research Council (grants 13409, 21691 and 21692), the Health & Medical Care Committee of the Regional Executive Board Region in Västra Götaland (119011), the Göteborg Medical Society and foundation of Elin and Carl Linder (GLS-406621), the Swedish Society of Medicine (SLS-329111), and the Faculty of Medicine, University of Gothenburg.
References
- 1.Graff LA, Clara I, Walker JR, et al. Changes in fatigue over 2 years are associated with activity of inflammatory bowel disease and psychological factors. Clin Gastroenterol Hepatol 2013; 11: 1140–1146. [DOI] [PubMed] [Google Scholar]
- 2.Jelsness-Jorgensen LP, Bernklev T, Henriksen M, et al. Chronic fatigue is more prevalent in patients with inflammatory bowel disease than in healthy controls. Inflamm Bowel Dis 2011; 17: 1564–1572. [DOI] [PubMed] [Google Scholar]
- 3.Minderhoud IM, Oldenburg B, van Dam PS, et al. High prevalence of fatigue in quiescent inflammatory bowel disease is not related to adrenocortical insufficiency. Am J Gastroenterol 2003; 98: 1088–1093. [DOI] [PubMed] [Google Scholar]
- 4.Romberg-Camps MJ, Bol Y, Dagnelie PC, et al. Fatigue and health-related quality of life in inflammatory bowel disease: Results from a population-based study in the Netherlands: The IBD-South Limburg cohort. Inflamm Bowel Dis 2010; 16: 2137–2147. [DOI] [PubMed] [Google Scholar]
- 5.Graff LA, Vincent N, Walker JR, et al. A population-based study of fatigue and sleep difficulties in inflammatory bowel disease. Inflamm Bowel Dis 2011; 17: 1882–1889. [DOI] [PubMed] [Google Scholar]
- 6.Bager P, Befrits R, Wikman O, et al. Fatigue in out-patients with inflammatory bowel disease is common and multifactorial. Aliment Pharmacol Ther 2012; 35: 133–141. [DOI] [PubMed] [Google Scholar]
- 7.Czuber-Dochan W, Ream E, Norton C. Review article: Description and management of fatigue in inflammatory bowel disease. Aliment Pharmacol Ther 2013; 37: 505–516. [DOI] [PubMed] [Google Scholar]
- 8.Jelsness-Jorgensen LP, Bernklev T, Henriksen M, et al. Chronic fatigue is associated with impaired health-related quality of life in inflammatory bowel disease. Aliment Pharmacol Ther 2011; 33: 106–114. [DOI] [PubMed] [Google Scholar]
- 9.Cohen BL, Zoega H, Shah SA, et al. Fatigue is highly associated with poor health-related quality of life, disability and depression in newly-diagnosed patients with inflammatory bowel disease, independent of disease activity. Aliment Pharmacol Ther 2014; 39: 811–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smets EM, Garssen B, Bonke B, et al. The Multidimensional Fatigue Inventory (MFI) psychometric qualities of an instrument to assess fatigue. J Psychosom Res 1995; 39: 315–325. [DOI] [PubMed] [Google Scholar]
- 11.Opheim R, Fagermoen MS, Bernklev T, et al. Fatigue interference with daily living among patients with inflammatory bowel disease. Qual Life Res 2014; 23: 707–717. [DOI] [PubMed] [Google Scholar]
- 12.Grimstad T, Norheim KB, Isaksen K, et al. Fatigue in newly diagnosed inflammatory bowel disease. J Crohns Colitis 2015; 9: 725–730. [DOI] [PubMed] [Google Scholar]
- 13.Goldenberg BA, Graff LA, Clara I, et al. Is iron deficiency in the absence of anemia associated with fatigue in inflammatory bowel disease? Am J Gastroenterol 2013; 108: 1392–1397. [DOI] [PubMed] [Google Scholar]
- 14.Dignass AU, Gasche C, Bettenworth D, et al. European consensus on the diagnosis and management of iron deficiency and anaemia in inflammatory bowel diseases. J Crohns Colitis 2015; 9: 211–222. [DOI] [PubMed] [Google Scholar]
- 15.Lopez A, Cacoub P, Macdougall IC, et al. Iron deficiency anaemia. Lancet 2016; 387: 907–916. [DOI] [PubMed] [Google Scholar]
- 16.Louati K, Berenbaum F. Fatigue in chronic inflammation - a link to pain pathways. Arthritis Res Ther 2015; 17: 254–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haney E, Smith ME, McDonagh M, et al. Diagnostic methods for myalgic encephalomyelitis/chronic fatigue syndrome: A systematic review for a National Institutes of Health Pathways to Prevention Workshop. Ann Intern Med 2015; 162: 834–840. [DOI] [PubMed] [Google Scholar]
- 18.Whitehead WE, Palsson O, Jones KR. Systematic review of the comorbidity of irritable bowel syndrome with other disorders: What are the causes and implications? Gastroenterology 2002; 122: 1140–1156. [DOI] [PubMed] [Google Scholar]
- 19.Ohman L, Simren M. Pathogenesis of IBS: Role of inflammation, immunity and neuroimmune interactions. Nat Rev Gastroenterol Hepatol 2010; 7: 163–173. [DOI] [PubMed] [Google Scholar]
- 20.Frandemark A, Jakobsson Ung E, Tornblom H, et al. Fatigue: A distressing symptom for patients with irritable bowel syndrome. Neurogastroenterol Motil. Epub ahead of print 11 July 2016. DOI: 10.1111/nmo.12898. [DOI] [PubMed] [Google Scholar]
- 21.Jonefjall B, Strid H, Ohman L, et al. Characterization of IBS-like symptoms in patients with ulcerative colitis in clinical remission. Neurogastroenterol Motil 2013; 25: 756–e578. [DOI] [PubMed] [Google Scholar]
- 22.Simren M, Axelsson J, Gillberg R, et al. Quality of life in inflammatory bowel disease in remission: The impact of IBS-like symptoms and associated psychological factors. Am J Gastroenterol 2002; 97: 389–396. [DOI] [PubMed] [Google Scholar]
- 23.Jelsness-Jorgensen LP, Bernklev T, Moum B. Fatigue and disease-related worries among inflammatory bowel disease patients in remission; is it a reflection of coexisting IBS-like symptoms? A short report. J Psychosom Res 2012; 73: 469–472. [DOI] [PubMed] [Google Scholar]
- 24.Halpin SJ, Ford AC. Prevalence of symptoms meeting criteria for irritable bowel syndrome in inflammatory bowel disease: Systematic review and meta-analysis. Am J Gastroenterol 2012; 107: 1474–1482. [DOI] [PubMed] [Google Scholar]
- 25.Lennard-Jones JE. Classification of inflammatory bowel disease. Scand J Gastroenterol Suppl 1989; 170: 2–6. discussion 16–19. [DOI] [PubMed] [Google Scholar]
- 26.Schroeder KW, Tremaine WJ, Ilstrup DM. Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. A randomized study. N Engl J Med 1987; 317: 1625–1629. [DOI] [PubMed] [Google Scholar]
- 27.Rogler G, Vavricka S, Schoepfer A, et al. Mucosal healing and deep remission: What does it mean? World J Gastroenterol 2013; 19: 7552–7560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Silverberg MS, Satsangi J, Ahmad T, et al. Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: Report of a Working Party of the 2005 Montreal World Congress of Gastroenterology. Can J Gastroenterol 200519 Suppl A): 5A–36A. [DOI] [PubMed] [Google Scholar]
- 29.Roseth AG, Fagerhol MK, Aadland E, et al. Assessment of the neutrophil dominating protein calprotectin in feces. A methodologic study. Scand J Gastroenterol 1992; 27: 793–798. [DOI] [PubMed] [Google Scholar]
- 30.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand 1983; 67: 361–370. [DOI] [PubMed] [Google Scholar]
- 31.Spinhoven P, Ormel J, Sloekers PP, et al. A validation study of the Hospital Anxiety and Depression Scale (HADS) in different groups of Dutch subjects. Psychol Med 1997; 27: 363–370. [DOI] [PubMed] [Google Scholar]
- 32.Stjernman H, Granno C, Bodemar G, et al. Evaluation of the Inflammatory Bowel Disease Questionnaire in Swedish patients with Crohn’s disease. Scand J Gastroenterol 2006; 41: 934–943. [DOI] [PubMed] [Google Scholar]
- 33.Guyatt G, Mitchell A, Irvine EJ, et al. A new measure of health status for clinical trials in inflammatory bowel disease. Gastroenterology 1989; 96: 804–810. [PubMed] [Google Scholar]
- 34.Dimenas E, Glise H, Hallerback B, et al. Quality of life in patients with upper gastrointestinal symptoms. An improved evaluation of treatment regimens? Scand J Gastroenterol 1993; 28: 681–687. [DOI] [PubMed] [Google Scholar]
- 35.Svedlund J, Sjodin I, Dotevall G. GSRS–a clinical rating scale for gastrointestinal symptoms in patients with irritable bowel syndrome and peptic ulcer disease. Dig Dis Sci 1988; 33: 129–134. [DOI] [PubMed] [Google Scholar]
- 36.Whitehead W. Validation working team, Rome questionnaire committee. Development and validation of the Rome III diagnostic questionnaire. In: Drossman DA, Corazziari E, Delvaux M, et al. (eds) Rome III. The Functional Gastrointestinal Disorders. 3rd ed. McLean, Virginia: Degnon Associates, Inc; 2006, pp. 835–853.
- 37.Longstreth GF, Thompson WG, Chey WD, et al. Functional bowel disorders. Gastroenterology 2006; 130: 1480–1491. [DOI] [PubMed] [Google Scholar]
- 38.Diagnostic and statistical manual of mental disorders. 4th text revision ed. Washington, DC: American Psychiatric Association, 2000.
- 39.Ohman L, Dahlen R, Isaksson S, et al. Serum IL-17A in newly diagnosed treatment-naive patients with ulcerative colitis reflects clinical disease severity and predicts the course of disease. Inflamm Bowel Dis 2013; 19: 2433–2439. [DOI] [PubMed] [Google Scholar]
- 40.Kim J, Wessling-Resnick M. Iron and mechanisms of emotional behavior. J Nutr Biochem 2014; 25: 1101–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jason LA, Richman JA, Rademaker AW, et al. A community-based study of chronic fatigue syndrome. Arch Intern Med 1999; 159: 2129–2137. [DOI] [PubMed] [Google Scholar]
- 42.Pratt JJ, Khan KS. Non-anaemic iron deficiency - a disease looking for recognition of diagnosis: A systematic review. Eur J Haematol 2016; 96: 618–628. [DOI] [PubMed] [Google Scholar]