Abstract
Objectives
Epidemiologic studies on eosinophilic esophagitis (EoE) are scarce and patient responders to proton pump inhibitor (PPI) therapy have usually been excluded. We aimed to evaluate population-based incidence rates, prevalence and trends in adult EoE over the past decade, including responders to PPI therapy.
Methods
We conducted an analysis of a prospectively established case registry in the health area of Cáceres, located in midwestern Spain. From the first EoE case diagnosed in 2007, endoscopy and pathology reports up to December 2016 were manually reviewed. A baseline diagnosis of EoE was confirmed upon symptoms of esophageal dysfunction (dysphagia/food impaction) and esophageal eosinophilia ≥ 15 eos/HPF. All patients were re-evaluated on PPI therapy during follow-up.
Results
A total of 137 patients were diagnosed with EoE during the study period, of whom 63 (46%) achieved clinicohistologic remission on PPI therapy. The prevalence of autoimmune disorders was low. Mean incidence rate was 8.09 new cases/100,000 inhabitants/year, increasing to 9.95 during the last lustrum and peaking in 2016 with 13.7. This trend coincided with late declining of esophageal biopsies rate. Overall prevalence in 2016 was 81.73 patients/100,000 inhabitants, with the highest prevalence in males between age 35 and 44 years (273 cases/100,000). No seasonal variation was observed in the diagnosis of EoE (53% during pollen season vs. 47%, p = 0.4).
Conclusions
In midwestern Spain, incidence (13.7 cases/100,000 inhabitants/year) and prevalence (81.73 patients/100,000 inhabitants) in 2016 have grown remarkably in just one decade, coming closer to those figures recently reported for Crohn’s disease in Spain.
Keywords: Eosinophilic esophagitis, incidence, prevalence
Introduction
Eosinophilic esophagitis (EoE) is a chronic, immune/antigen-mediated esophageal disease characterized clinically by symptoms related to esophageal dysfunction and histologically by eosinophil-predominant inflammation.1 Since the first descriptions of the disease in the early 1990s,2,3 EoE is presently the second cause of chronic esophageal inflammation after gastro-esophageal reflux disease (GERD) and the most common cause of dysphagia and food impaction among children and young adults. A recent systematic review with meta-analysis on population-based studies reported a pooled incidence of 3.7/100,000 inhabitants/year and prevalence of EoE of 22.7 cases/100,000.4 However, a remarkably high heterogeneity (I2 99.9%) was observed for both incidence and prevalence across individual studies, likely due to research bias and variations in awareness and diagnostic criteria.
With regards to this latter issue, available guidelines for EoE have systematically recommended ruling out a diagnosis of EoE in those patients with clinical, endoscopic and histological features characteristic of EoE who achieved remission on proton pump inhibitor (PPI) therapy.1,5,6 A prospective series published in 2011 on this new entity, formerly called PPI-responsive esophageal eosinophilia, first questioned these diagnostic criteria.7 Since then, evolving evidence has shown that EoE patients responders and non-responders to PPI therapy are genetically, mechanistically, and phenotypically indistinguishable, and radically different from those with conventional GERD.8–10 Additionally, PPI therapy can exert a similar reduction of Th2 inflammation, restoration of esophageal mucosal integrity, and reversal of the abnormal gene expression signature in responders to PPI therapy, similar to the effects of topical steroids in patients with typical EoE. Thus, a recent consensus international report11 and updated evidence-based guidelines12 have suggested considering patients responders to PPI therapy as true EoE patients. Based on the aforementioned data, there might be concerns that exclusion of this subset of patients in epidemiological studies on EoE might have led to an underestimation of the disease. This issue is crucial if we consider that a recent meta-analysis revealed that 50% of pediatric and adult patients with suspected EoE are eventually responders to PPI therapy.13
The primary goal of this study was to assess the incidence and prevalence of EoE over the past decade in Cáceres, Spain. Aside from reporting the first prospective series on EoE responders to PPI therapy,7 the peculiarity of this health area is that all EoE patients have been systematically re-evaluated after PPI therapy after the first EoE case responder to PPI in 2007.14 Secondary aims of the study include trends in epidemiological figures, age distribution of the disease, seasonal variation in the diagnosis of EoE and prevalence of autoimmune disorders in our adult cohort of patients.
Methods
Study setting
The study was conducted in the health area of a geographical area located in midwestern Spain, within the autonomous region of Extremadura, sharing a border with Portugal. The study health area covers a population of 196,363 inhabitants, of whom 168,912 (86%) are older than 16 years old and 51% live in rural areas (data from 2014).15 All patients with suspected EoE living in this area are referred to our gastroenterology clinic at Hospital Universitario San Pedro de Alcantara, which is the referral center for the entire population aforementioned. Noteworthy, our Department of Gastroenterology is the referral center for emergency endoscopy for food bolus impaction from 3 p.m. on weekdays and for the entire weekend. There are two private gastroenterology clinics in our health area, but both also refer most of their EoE patients after the initial diagnosis to our gastroenterology clinic for specific treatment. All patients included gave their consent for endoscopic procedures and scientific use of clinical data. This study was approved by the institutional review board at Hospital Universitario San Pedro de Alcantara.
EoE definition and case identification
All newly diagnosed adult (≥16 years old) EoE patients from our health area between January 1, 2007 and December 31, 2016, were consecutively included in a prospective registry if they had upper gastrointestinal symptoms suggestive of esophageal dysfunction (e.g. dysphagia, food impaction, heartburn, reflux, chest pain) and infiltration of esophageal biopsies by 15 or more eosinophils per high-powered field (eos/HPF).11 Other potential causes of esophageal eosinophilia, including eosinophilic gastroenteritis, Crohn’s disease, drug hypersensitivity, parasites, esophageal caustications, hypereosinophilic syndrome, vasculitis, pemphigoid, connective tissue disorder, and graft-versus-host disease were ruled out based on medical records. All patients fulfilling our EoE definition underwent an eight-week PPI therapy and were endoscopically re-evaluated with esophageal biopsies.
The year 2007 was chosen as the starting year for the registry since the first EoE patient was diagnosed in our clinic that very year.13 A patient search was carried out in the registry; in addition, all reports from all endoscopic procedures performed since 2007 were also revised, and they were also cross-checked with databases from our previous studies7,8,16–18 and with the electronic database of the Pathology Department, which was also used to estimate the annual esophageal biopsy rates. This rate was defined by the annual number of endoscopies in which esophageal biopsies were taken because of clinical and/or endoscopic suspicion of EoE. This figure was then divided by 10 (e.g. 9.4 represents 94 endoscopies with esophageal biopsies in one year)
Data extraction
Demographic, age at diagnosis, type and duration of symptoms before diagnosis, clinical, endoscopic and histological data were extracted from electronic medical records. Likewise, follow-up data were all obtained from electronic medical records. All endoscopic procedures in which esophageal biopsies were specifically taken for a clinical (dysphagia/food impaction) or endoscopic suspicion of EoE were recorded. The pollen season in Cáceres was defined from March to July, according to the local aerobiologic information provided by the Spanish Society of Allergy and Clinical Immunology.19
To calculate the incidence of EoE, the number of new patients was identified for each year of the study, and then divided by the total population of the health area of Cáceres for the corresponding year. To calculate the prevalence of EoE, the cumulative number of patients for each year of the study was divided by the total population of our health area for the corresponding year. Data from populations for each year, broken down by gender and age, were obtained from official databases from the Spanish National Institute of Statistics (Instituto Nacional de Estadistica, INE) for each year within the study period. Figures referred to groups of 100,000 inhabitants.
Statistical analysis
The SPSS (version 21.0; SPSS Inc, Chicago, IL, USA) statistical analysis package was used. Categorical variables were described with percentages, and continuous variables were described with mean standard deviation or median (range) as appropriate. Associations between categorical variables were tested with the chi2 test (with Fisher correction when necessary), and continuous data were assessed using the two-sample t test or the Mann–Whitney U test for parametric and nonparametric data, respectively. p values lower than 0.05 were considered statistically significant.
Results
Baseline characteristics
During the study period, 137 patients resident in our health area were diagnosed with EoE, combining symptoms of esophageal dysfunction and esophageal eosinophilia > 15 eos/HPF. Fifty-nine additional adult EoE patients included in our registry were excluded from the analysis since they belonged to different health areas than ours. Baseline characteristics of the included patients are summarized in Table 1. As expected, young age (mean 36), male predominance (73%), atopic comorbidities (69%) and dysphagia (92%) were notably common. With the exception of hypothyroidism (6.5%), there was no increased prevalence of autoimmune diseases, including celiac disease, inflammatory bowel disease or systemic sclerosis. As for the age distribution, cases steadily increased, peaking in the 35-year to 44-year age range, with a sharp decrease after the age of 45. Of note, 63 patients (46%) achieved clinical and histological remission on PPI therapy.
Table 1.
Baseline characteristics of adult EoE patients in our center from 2007 to 2016.
| Number of patients | 137 |
|---|---|
| Age, mean (range) | 36 (16–74) |
| Male gender, n (%) | 101 (73%) |
| Atopic comorbidities, n (%) | 95 (69%) |
| Rhinoconjunctivitis | 86 (62%) |
| Asthma | 71 (52%) |
| Food allergy | 32 (23%) |
| Autoimmune/Other disorders, n (%) | |
| Celiac disease | 2 (1.4%) |
| Crohn’s disease | 0 |
| Ulcerative colitis | 0 |
| Rheumatoid arthritis | 0 |
| IgA deficiency | 0 |
| Systemic sclerosis | 0 |
| Sarcoidosis | 1 (0.7%) |
| Hypothyroidism | 9 (6.5%) |
| Alopecia areata | 1 (0.7%) |
| Psoriasis | 1 (0.7%) |
| Hodgkin lymphoma | 2 (1.4%) |
| Asperger syndrome | 1 (0.7%) |
| Attention deficit hyperactivity disorder | 1 (0.7%) |
| Diagnostic delay (months) from initial symptoms, mean (range) | 56 (0–324) |
| Symptoms, n (%) | |
| Food bolus impaction requiring emergency endoscopy | 53 (38%) |
| Dysphagia | 126 (92%) |
| Heartburn | 74 (54%) |
| Chest pain | 14 (10%) |
| Endoscopic findings, n (%) | |
| Rings | 89 (65%) |
| Furrows | 94 (68%) |
| Exudates | 47 (34%) |
| Edema | 125 (91%) |
| Strictures | 14 (10%) |
| Reflux esophagitis | 17 (12%) |
| Histologic findings (peak eos/HPF) | |
| Distal esophagus, mean (range) | 53 (7–165) |
| Proximal esophagus, mean (range) | 41 (0–61) |
| Remission on PPI therapy, n (%) | 63 (46%) |
EoE: eosinophilic esophagitis; IgA: immunoglobulin A; eos: eosinophil; HPF: high-power field; PPI: proton pump inhibitor.
Incidence
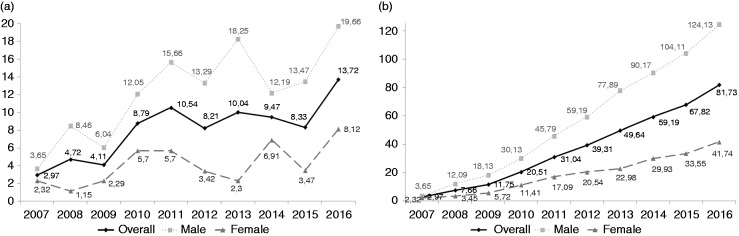
The incidence rates of EoE have rapidly increased in our health area over a 10-year period, since the first adult EoE case reported in 2007 (Table 2). Mean incidence was 8.09 new cases/100,000 inhabitants/year, but this figure increased to 9.95 cases/100,000/year during the last lustrum (2012–2016) and peaked in 2016 with 13.7 cases/100,000/year. Rising incidence of EoE was much more remarkable in males (mean 12.27/100,000/year, 15.37/100,000/year from 2012 to 2016) compared to that observed in females (mean 4.14/100,000/year, 4.87/100,000/year from 2012 to 2016). This temporal trend, broken down by gender, is illustrated in Figure 1.
Table 2.
Annual incidence of adult EoE in Caceres (Spain) between 2007 and 2016, broken down by gender and lustra
| Year | Population | Eosinophilic esophagitis cases | Overall incidence/100,000 inhabitants | Male incidence/100,000 inhabitants | Female incidence 100,000 inhabitants |
|---|---|---|---|---|---|
| 2007 | 168,508 | 5 | 2.97 | 3.65 | 2.32 |
| 2008 | 169,655 | 8 | 4.72 | 8.46 | 1.15 |
| 2009 | 170,184 | 7 | 4.11 | 6.04 | 2.29 |
| 2010 | 170,644 | 15 | 8.79 | 12.05 | 5.70 |
| 2011 | 170,755 | 18 | 10.54 | 15.66 | 5.70 |
| 2012 | 170,427 | 14 | 8.21 | 13.29 | 3.42 |
| 2013 | 169,206 | 17 | 10.04 | 18.25 | 2.30 |
| 2014 | 168,944 | 16 | 9.47 | 12.19 | 6.91 |
| 2015 | 168,085 | 14 | 8.33 | 13.47 | 3.47 |
| 2016 | 167,620 | 23 | 13.72 | 19.66 | 8.12 |
| Mean | 169,403 | 13.7 | 8.09 | 12.27 | 4.14 |
| Mean (2012–2016) | 168,856 | 16.8 | 9.95 | 15.37 | 4.84 |
Figure 1.
Incidence (A) and prevalence (B) rates of EoE by gender over the study period (2007–2016)
Prevalence
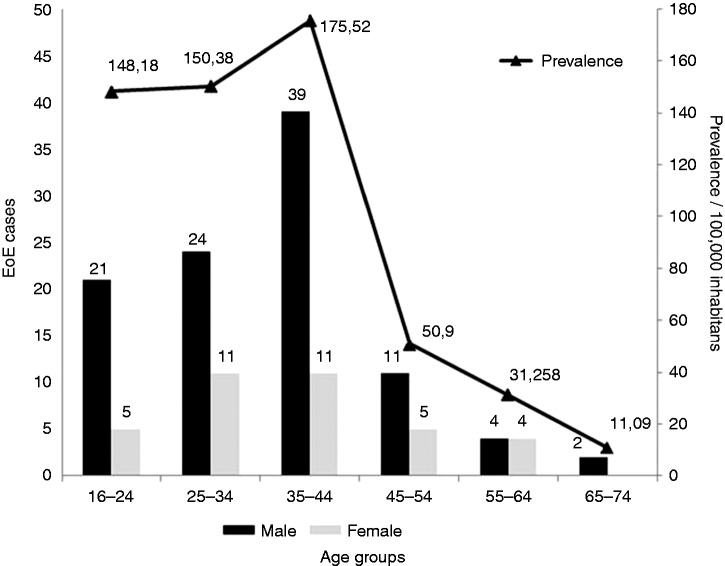
Consequently, the prevalence of EoE has dramatically risen in our health area during the study period. The figures, broken down by year and gender, are summarized in Table 3 and Figure 1. Mean prevalence was 37.16 cases/100,000, rising to 59.54 cases/100,000 over the last lustrum. Peak overall prevalence was reported in 2016, with 81.73 patients/100,000 inhabitants. As for age and gender groups, the highest prevalence rates in 2016 were reported in males between 16 and 24 (232 cases/100,000), 25 and 34 (199 cases/100,000) and especially between 35 and 44 (273 cases/100,000) (Figure 2).
Table 3.
Annual incidence of eosinophilic esophagitis in adult patients between 2007 and 2016 in Cáceres (Spain).
| Year | Overall prevalence/ 100,000 inhabitants | Male prevalence/ 100,000 inhabitants | Female prevalence/ 100,000 inhabitants | 16–24 years (M/F) | 25–34 years (M/F) | 35–44 years (M/F) | 45–54 years (M/F) | 55–64 years (M/F) | 65–74 years (M/F) |
|---|---|---|---|---|---|---|---|---|---|
| 2007 | 2.97 | 3.65 | 2.32 | 0 (0/0) | 3.52 (6.9/0) | 12.75 (12.6/12.8) | 0 (0/0) | 0 (0/0) | 0 (0/0) |
| 2008 | 7.66 | 12.09 | 3.45 | 4.64 (9.1/0) | 14.18 (20.8/7.3) | 22.29 (31.7 / 12.8) | 0 (0/0) | 4.93 (10/0) | 0 (0/0) |
| 2009 | 11.75 | 18.13 | 5.72 | 4.72 (9.2/0) | 25.11 (42.2/7.3) | 32.12 (44.8 / 19.3) | 0 (0/0) | 9.76 (9.8/9.7) | 0 (0/0) |
| 2010 | 20.51 | 30.13 | 11.41 | 19.19 (27.9/9.9) | 58.24 (85.3/29.8) | 42.30 (58.5 / 26.1) | 0 (0/0) | 9.71 (9.7/9.7) | 0 (0/0) |
| 2011 | 31.04 | 45.79 | 17.09 | 34.34 (57.1/10.1) | 81.65 (116.5/45.4) | 59.03 (84.8 / 33) | 6.51 (6.4/6.6) | 18.96 (18.9/19) | 0 (0/0) |
| 2012 | 39.31 | 59.19 | 20.54 | 66.01 (108.3/21) | 87.36 (119.5/54.1) | 69.74 (105.7 / 33.4) | 12.73 (12.6/12.9) | 23.05 (27.05/18.5) | 5.72 (12.4/0) |
| 2013 | 49.64 | 77.89 | 22.98 | 107.36 (167.3/44.1) | 89.43 (120.9/56.1) | 91.32 (148.4/33.9) | 22.04 (31.2/12.7) | 22.15 (26.3/17.9) | 11.44 (24.9/0) |
| 2014 | 59.19 | 90.17 | 29.93 | 118.88 (177.9/55.9) | 104.04 (132.4/74.1) | 123.60 (198.8/48.1) | 25.08 (37.3/12.6) | 25.76 (25.5/26) | 11.18 (24.1/0) |
| 2015 | 67.82 | 104.11 | 33.55 | 133.13 (203.5/57.5) | 112.40 (145.8/77.1) | 153.54 (243.1/63.1) | 34.67 (50.3/19) | 24.67 (24.4/24.9) | 10.97 (23.6/0) |
| 2016 | 81.73 | 124.13 | 41.74 | 148.18 (232/58.6) | 150.38 (199.7/97.7) | 175.52 (273.3/77.4) | 50.9 (69.9/31.9) | 31.28 (30.9/31.6) | 11.09 (23.8/0) |
| Mean | 37.16 | 56.53 | 18.87 | 63.65 | 72.63 | 78.22 | 15.19 | 17.03 | 5.04 |
| Mean (2012–2016) | 59.54 | 91.1 | 29.75 | 114.71 | 108.72 | 122.74 | 29.08 | 25.38 | 10.08 |
M: male; F: female.
Figure 2.
New cases and prevalence rates of EoE, stratified by age group and gender.
Impact of rates of esophageal biopsies
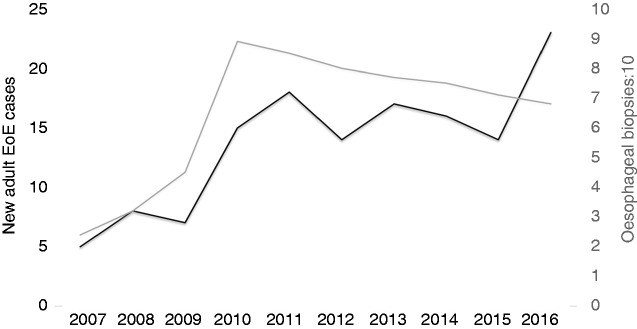
The reported increasing epidemiologic trends in EoE cases were initially associated with a parallel rising rate of esophageal biopsies, but peaking figures in 2015 and 2016 coincided with a further declining of esophageal biopsies rate (Figure 3).
Figure 3.
Yearly overall new EoE cases between 2007 and 2016, compared to the annual rate of esophageal biopsies (divided by ten) taken due to clinic or endoscopic suspicion of EoE.
Seasonal variation
We found no seasonal variation in the diagnosis of EoE in our study. A similar number of cases were diagnosed in and outside the pollen season (53% vs. 47%, p = 0.4).
Food impaction as onset of the disease and during follow-up
During the study period, 53 patients (39%) were diagnosed with food bolus impaction requiring emergency endoscopy. There were no differences in the proportion of patients diagnosed through emergency endoscopic removal between the first and second lustra of the study period (39% vs. 38%, p = 0.6). Thirty-six patients (26%) were lost to follow-up, of whom eight (30%) suffered further food bolus impaction requiring endoscopic removal. Three out of these eight patients were responders to PPI therapy.
Diagnostic delay compared between 2007–2011 and 2012–2016
There were no differences observed in the diagnostic delay (considered as the length from the onset of symptoms to the first diagnostic endoscopy), between the first and second lustra of the study period (54.2 months (0–240) vs. 52.28 months (0–324), p = 0.8).
Discussion
The present population-based study confirms an escalating epidemiologic trend of adult EoE, with similar or slightly higher incidence rates than those recently reported in the United States,20 Spain21 and Switzerland.22,23 Noteworthy, we herein report the highest prevalence for adult EoE ever reported so far.4 This finding is likely explained by systematic inclusion of responders to PPI therapy (46% in the present study). This subset of patients has been excluded from multiple previous studies, following the recommendations of 2007,5 20111 and 20136 guidelines. However, most recent consensus and evidence-based recommendations11,12 have underscored that no objective evidence can distinguish responders to PPI therapy from EoE patients, so these patients should likely be included within the EoE spectrum. As such, it is conceivable that most population-based studies might have previously underestimated the magnitude of EoE.
It is interesting to note that incidence rates for EoE reported in the present manuscript are similar to the most recent data for Crohn’s disease in Spain (incidence 8.9/100,000/year24 and 9.1/100,000/year25). Since Crohn’s disease was first described as a clinic and pathologic entity in 1932,26 its current prevalence from the most recent Spanish data (137.17/100,00025) is still far, but not that far, from the young EoE in Spain in 2016 (81.73/100,000). One can speculate whether EoE and Crohn’s disease are parallel emerging modern westernized diseases.27 As for EoE, large numbers of cases have been reported in westernized countries (North America, Western and Eastern Europe, and Australia), with fewer cases in South America, Asia and the Middle East.28 Cases from Northern Africa have recently been reported, and, as of yet, none in Sub-Saharan Africa or India.28 Thus, EoE is becoming a global disease affecting children and young adults who will suffer this chronic condition for several decades. Likewise, Crohn’s disease has been reported worldwide, but the incidence and prevalence also appear to be lower in Asia and the Middle East.29 Overall, we do believe that epidemiological figures of EoE will soon catch up with Crohn’s disease, with similar and even higher prevalence rates than that of Crohn’s disease likely expected for EoE in the next 10–20 years if the current epidemiologic trends persist.27
In line with previous findings by Dellon et al.,20 we corroborated that the prevalence of EoE steadily increases from adolescence and peaks in the age group between 34 and 45 years old, sharply decreasing after 45 years old. This is a counterintuitive finding, given the fact that EoE is a chronic and nonfatal disease, so the prevalence should theoretically continue to increase with age. Therefore, it is interesting to speculate whether EoE patients have been and are exposed to a triggering risk factor that was not present before the early 1990s.28
Similar to a recent study,30 we also demonstrate that increase in new EoE cases outpaced the use of endoscopic biopsy to rule out EoE. Contrary to another recent study,31 we could not find an increased prevalence of autoimmune disorders in our cohort of adult EoE patients. Only autoimmune hypothyroidism (6.5%) was more common that that expected in the general population and was almost universally present in women. Likewise, we could not observe a seasonal variation in the diagnosis of EoE. A first systematic review with meta-analysis found no significant variations in the seasonal distribution of either the diagnosis or clinical recrudescence of EoE throughout the year.32
Unfortunately, we could not prove enhanced rates of early detection of the disease or reduction of emergency endoscopies for food bolus impaction over time. Although lack of awareness on the importance of the disease may play a role, these findings likely reflect adult patient reluctance to consult for dysphagia or intermittent food impaction, since they are considered mild or unimportant symptoms.
The present study exhibits a number of strengths, including systematic PPI therapy for all patients with suspected EoE, prospective inclusion of all consecutively diagnosed patients in the supporting registry, and cross-checking with endoscopy and pathology databases, which allowed us to avoid fragmented medical record systems or resort to lack of validated disease definitions or coding definition. Limitations of the study might be our missing a small proportion of patients from private gastroenterology clinics not re-referred to our gastroenterology unit and the generalizability of our results. Unlike Cáceres (51% living in rural areas), only 13% of the Spanish population still live in rural areas. Accordingly, our findings may not necessarily be extrapolable to other populations within the same country, especially those living in urban areas.
In conclusion, the incidence and prevalence of EoE in Cáceres, located in midwestern Spain, have dramatically risen since the first case report in 2007. Currently, the incidence is similar to that recently reported in Spain for Crohn’s disease and the prevalence, despite a 70-year gap, is becoming steadily closer to that of Crohn’s disease. Indeed, the present study reports the highest prevalence for adult EoE ever reported so far. Our data likely underscore the real magnitude of an emergent relatively young disease. EoE is becoming a global disease affecting children and young adults who will suffer this chronic condition for several decades. This epidemiologic trend should lead to increased awareness campaigns, research about the early triggering factors of the disease and development of effective preventive strategies.
Acknowledgment
Author contributions are as follows: Javier Molina-Infante: study design, drafting of the article, data acquisition/interpretation and critical revision. Pedro Luis Gonzalez-Cordero, Hal Cliff Ferreira-Nossa, Pilar Mata-Romero: data acquisition/interpretation and critical revision. Alfredo J. Lucendo and Angel Arias: study design, extraction of population information, data interpretation and critical revision. All authors approved the final version of the manuscript.
Declaration of conflicting interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Ethics approval
This study was approved by the Institutional Review Board at Hospital Universitario San Pedro de Alcantara.
Informed consent
Informed consent, as approved by the Institution Review Board, was signed by all patients.
References
- 1.Liacouras CA, Furuta GT, Hirano I, et al. Eosinophilic esophagitis: Updated consensus recommendations for children and adults. J Allerg Clin Immunol 2011; 128: 3–10. [DOI] [PubMed] [Google Scholar]
- 2.Attwood SE, Smyrk TC, Demeester TR, et al. Esophageal eosinophilia with dysphagia. A distinct clinicopathologic syndrome. Dig Dis Sci 1993; 38: 109–116. [DOI] [PubMed] [Google Scholar]
- 3.Straumann A, Spichtin HP, Bernoulli R, et al. Idiopathic eosinophilic esophagitis: A frequently overlooked disease with typical clinical aspects and discrete endoscopic findings [article in German]. Schweiz Med Wochenschr 1994; 124: 1419–1429. [PubMed] [Google Scholar]
- 4.Arias Á, Pérez-Martínez I, Tenías JM, et al. Systematic review with meta-analysis: The incidence and prevalence of eosinophilic oesophagitis in children and adults in population-based studies. Aliment Pharmacol Ther 2016; 43: 3–15. [DOI] [PubMed] [Google Scholar]
- 5.Furuta GT, Liacouras CA, Collins MH, et al. Eosinophilic esophagitis in children and adults: A systematic review and consensus recommendations for diagnosis and treatment. Gastroenterology 2007; 133: 1342–1363. [DOI] [PubMed] [Google Scholar]
- 6.Dellon ES, Gonsalves N, Hirano I, et al. ACG Clinical Guideline: Evidence based approach to the diagnosis and management of esophageal eosinophilia and eosinophilic esophagitis. Am J Gastroenterol 2013; 108: 679–692. [DOI] [PubMed] [Google Scholar]
- 7.Molina-Infante J, Ferrando-Lamana L, Ripoll C, et al. Esophageal eosinophilic infiltration responds to proton pump inhibition in most adults. Clin Gastroenterol Hepatol 2011; 9: 110–117. [DOI] [PubMed] [Google Scholar]
- 8.Molina-Infante J, Rivas MD, Hernandez-Alonso M, et al. Remission in proton pump inhibitor-responsive esophageal eosinophilia correlates with downregulation of eotaxin-3 and TH2 cytokines, similarly to eosinophilic esophagitis after steroids. Aliment Pharmacol Ther 2014; 40: 955–965. [DOI] [PubMed] [Google Scholar]
- 9.van Rhijn BD, Weijenborg PW, Verheij J, et al. Proton pump inhibitors partially restore mucosal integrity in patients with proton pump inhibitor-responsive esophageal eosinophilia but not eosinophilic esophagitis. Clin Gastroenterol Hepatol 2014; 12: 1815–1823. [DOI] [PubMed] [Google Scholar]
- 10.Wen T, Dellon ES, Moawad FJ, et al. Transcriptome analysis of proton pump inhibitor-responsive esophageal eosinophilia reveals proton pump inhibitor-reversible allergic inflammation. J Allergy Clin Immunol 2015; 135: 187–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Molina-Infante J, Bredenoord AJ, Cheng E, et al. Proton pump inhibitor-responsive oesophageal eosinophilia: An entity challenging current diagnostic criteria for eosinophilic oesophagitis. Gut 2016; 65: 524–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lucendo AJ, Molina-Infante J, Arias A, et al. Guidelines on eosinophilic esophagitis: Evidence-based statements and recommendations for diagnosis and management in children and adults. United European Gastroenterol J. Epub ahead of print 23 January 2017. DOI: https://doi.org/10.1177/2050640616689525. [DOI] [PMC free article] [PubMed]
- 13.Lucendo AJ, Arias A, Molina-Infante J. Efficacy of proton pump inhibitor drugs for inducing clinical and histologic remission in patients with symptomatic esophageal eosinophilia: A systematic review and meta-analysis. Clin Gastroenterol Hepatol 2016; 14: 13–22. [DOI] [PubMed] [Google Scholar]
- 14.Molina-Infante J, Ferrando-Lamana L, Mateos-Rodríguez JM, et al. Overlap of reflux and eosinophilic esophagitis in two patients requiring different therapies: A review of the literature. World J Gastroenterol 2008; 14: 1463–1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Área de salud de Cáceres, servicio Extremeño de salud, http://www.areasaludcaceres.es/docs/files/2676-memoria-a769rea-salud-ca769ceres-2014.pdf (accessed 15 February 2017).
- 16.Molina-Infante J, Rodriguez-Sanchez J, Martinek J, et al. Long-term loss of response in proton pump inhibitor-responsive esophageal eosinophilia is uncommon and influenced by CYP2C19 genotype and rhinoconjunctivitis. Am J Gastroenterol 2015; 110: 1567–1575. [DOI] [PubMed] [Google Scholar]
- 17.Molina-Infante J, Arias A, Barrio J, et al. Four-food group elimination diet for adult eosinophilic esophagitis: A prospective multicenter study. J Allergy Clin Immunol 2014; 134: 1093–1099.e1. [DOI] [PubMed] [Google Scholar]
- 18.Molina-Infante J, Martin-Noguerol E, Alvarado-Arenas M, et al. Selective elimination diet based on skin testing has suboptimal efficacy for adult eosinophilic esophagitis. J Allergy Clin Immunol 2012; 130: 1200–1202. [DOI] [PubMed] [Google Scholar]
- 19.Sociedad española de alergología e immunología clínica. Niveles ambientales de pólenes, http://www.polenes.com/graficos/concentra?prov=C%E1ceres&codest=CAC (accessed 15 February 2017).
- 20.Dellon ES, Jensen ET, Martin CF, et al. Prevalence of eosinophilic esophagitis in the United States. Clin Gastroenterol Hepatol 2014; 12: 589–596.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arias A, Lucendo AJ. Prevalence of eosinophilic oesophagitis in adult patients in a central region of Spain. Eur J Gastroenterol Hepatol 2013; 25: 208–212. [DOI] [PubMed] [Google Scholar]
- 22.Hruz P, Straumann A, Bussmann C, et al. Escalating incidence of eosinophilic esophagitis: A 20-year prospective, population-based study in Olten County, Switzerland. J Allergy Clin Immunol 2011; 128: 1349–1350.e5. [DOI] [PubMed] [Google Scholar]
- 23.Giriens B, Yan P, Safroneeva E, et al. Escalating incidence of eosinophilic esophagitis in Canton of Vaud, Switzerland, 1993–2013: A population-based study. Allergy 2015; 70: 1633–1639. [DOI] [PubMed] [Google Scholar]
- 24.Lucendo AJ, Hervías D, Roncero Ó, et al. Epidemiology and temporal trends (2000–2012) of inflammatory bowel disease in adult patients in a central region of Spain. Eur J Gastroenterol Hepatol 2014; 26: 1399–1407. [DOI] [PubMed] [Google Scholar]
- 25.Fernández A, Hernández V, Martínez-Ares D, et al. Incidence and phenotype at diagnosis of inflammatory bowel disease. Results in Spain of the EpiCom study. Gastroenterol Hepatol 2015; 38: 534–540. [DOI] [PubMed] [Google Scholar]
- 26.Crohn BB, Ginzburg L, Oppeheimer GD. Regional ileitis: A pathologic and clinical entity. 1932. Mt Sinai J Med 2000; 67: 263–268. [PubMed] [Google Scholar]
- 27.Molina-Infante J, Schoepfer AM, Lucendo AJ, et al. Eosinophilic esophagitis: What can we learn from Crohn’s disease? United European Gastroenterol J. Epub ahead of print 29 September 2016. DOI: https://doi.org/10.1177/2050640616672953. [DOI] [PMC free article] [PubMed]
- 28.Dellon ES. Epidemiology of eosinophilic esophagitis. Gastroenterol Clin North Am 2014; 43: 201–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vegh Z, Burisch J, Pedersen N, et al. Incidence and initial disease course of inflammatory bowel diseases in 2011 in Europe and Australia: Results of the 2011 ECCO-EpiCom inception cohort. J Crohns Colitis 2014; 8: 1506–1515. [DOI] [PubMed] [Google Scholar]
- 30.Dellon ES, Erichsen R, Baron JA, et al. The increasing incidence and prevalence of eosinophilic oesophagitis outpaces changes in endoscopic and biopsy practice: National population-based estimates from Denmark. Aliment Pharmacol Ther 2015; 41: 662–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peterson K, Firszt R, Fang J, et al. Risk of autoimmunity in EoE and families: A population-based cohort study. Am J Gastroenterol 2016; 111: 926–932. [DOI] [PubMed] [Google Scholar]
- 32.Lucendo AJ, Arias Á, Redondo-González O, et al. Seasonal distribution of initial diagnosis and clinical recrudescence of eosinophilic esophagitis: A systematic review and meta-analysis. Allergy 2015; 70: 1640–1650. [DOI] [PubMed] [Google Scholar]