Abstract
Background
Periodontitis and edentulism are prevalent in patients with cirrhosis, but their clinical significance is largely unknown.
Objective
The objective of this article is to determine the association of severe periodontitis and edentulism with mortality in patients with cirrhosis.
Methods
A total of 184 cirrhosis patients underwent an oral examination. All-cause and cirrhosis-related mortality was recorded. The associations of periodontitis and edentulism with mortality were explored by Kaplan–Meier survival plots and Cox proportional hazards regression adjusted for age, gender, cirrhosis etiology, Child–Pugh score, Model for End-Stage Liver Disease score, smoker status, present alcohol use, comorbidity, and nutritional risk score.
Results
The total follow-up time was 74,197 days (203.14 years). At entry, 44% of the patients had severe periodontitis and 18% were edentulous. Forty-four percent of the patients died during follow-up. Severe periodontitis was associated with higher all-cause mortality in the crude analysis (HR 1.56, 95% CI 1.06–2.54), but not in the adjusted analysis (HR 1.45, 95% CI 0.79–2.45). Severe periodontitis was even more strongly associated with higher cirrhosis-related mortality (crude HR 2.19, 95% CI 1.07–4.50 and adjusted HR 2.29, 95% CI 1.04–4.99). No association was found between edentulism and mortality.
Conclusion
The presence of severe periodontitis predicted a more than double one-year cirrhosis mortality. These findings may motivate intervention trials on the effect of periodontitis treatment in patients with cirrhosis.
Keywords: All-cause mortality, cirrhosis, mortality, oral health, periodontitis
Key Summary
Poor oral health is prevalent in patients with cirrhosis and may lead to systemic infections, which is a common and well-known risk factor for morbidity and mortality.
The association between periodontitis and the prognosis of cirrhosis is unknown.
This prospective cohort study on cirrhosis patients showed that the presence of severe periodontitis predicted higher mortality in patients with cirrhosis.
These findings motivate further studies. The association, if confirmed, seems to necessitate closer attention to oral health in the clinic for patients with cirrhosis.
Introduction
Poor oral health is prevalent in patients with cirrhosis1–5 and may lead to malnutrition and systemic infections, which are common and well-known risk factors for morbidity and mortality.6–8 The problem is mainly caused by oral infections such as periodontitis, which is an inflammatory disease of the tooth-supporting tissue and untreated may lead to tooth loss.9,10 Our previous cross-sectional studies in patients with cirrhosis indicated an association between oral infections and a higher nutritional risk score, systemic inflammation activation, and increased frequency of cirrhosis-related complications.11,12 Likewise, a recent retrospective study suggested an association between oral infections and progression of the cirrhosis disease,2 and research on a variety of disease populations indicates an association between periodontitis, tooth loss, and mortality.13–15
Still, the association between periodontitis and the prognosis of cirrhosis is unknown although the issue may well be of particular relevance to this population whose course is fraught with infections and other inflammation-related adverse events.8 Therefore, the aim of this study was to prospectively determine the association of severe periodontitis and edentulism with all-cause and cirrhosis-related mortality in patients with cirrhosis.
Materials and methods
This prospective cohort study was conducted between March 2013 and November 2015 at Aarhus University Hospital, Denmark. Eligible patients were consecutively enrolled at the Department of Hepatology and Gastroenterology for a program on oral health and oral infections in patients with cirrhosis,11,12 and the present study focuses on the follow-up data.
Eligible patients were adult men and woman with an established diagnosis of cirrhosis regardless of etiology and severity. The diagnosis of cirrhosis was based on medical history, physical examination, liver biopsy and/or pertinent clinical-biochemical and ultrasonographic findings. Patients were excluded if they could not speak or understand Danish, had dementia or were cognitively impaired, had malignant diseases, or had acute critical conditions such as overt hepatic encephalopathy or variceal bleeding.
The study was performed according to the Declaration of Helsinki. The Ethical Committee of Central Denmark Region approved the study (journal No. 1-10-72-128-12, September 2012). Written, informed consent was obtained from all patients included in the study.
Oral examination
At study entry, a standardized oral examination was performed. The oral mucosa was examined for pathologic changes. In the dentate patients, the number of teeth was counted. Clinical probing depths (PD) of the gingival pockets were measured parallel to the longitudinal axis of the tooth from the free gingival margin to the bottom of the periodontal pocket, i.e. to the tip of the periodontal probe. Clinical attachment level (CAL) of the gingiva was defined as the distance from the cemento-enamel junction (CEJ) to the tip of the periodontal probe. The distance from the free gingival margin to the CEJ was measured and CAL was obtained by subtracting this value from the PD. Gingival recession (i.e. displacement of marginal tissue apical to the CEJ) was recorded as a negative value and thereby added to the PD. PD and CAL were registered at six sites per tooth, measured in mm, and recorded to the nearest mm.
Severe periodontitis was defined as two or more interdental sites with CAL of 6 or more mm on different teeth and one or more interdental sites with PD of 5 or more mm. The definition is given by the working group of the Centers for Disease Control and Prevention in collaboration with the American Academy of Periodontology.16
Three authorized dental hygienists undertook the oral examinations. Prior to study start, they were all trained by the same experienced clinical examiner in periodontitis, a dentist from the Department of Odontology, Aarhus University, Denmark. Seventeen patients (11%) had at least two quadrants of their periodontal measurement (i.e. probing depth and clinical attachment level) repeated by the same or another hygienist in order to assess intra- and inter-examiner variability. The reproducibility was estimated by Lin’s concordance correlation coefficient,17 and the degree of agreement according to the categories suggested by McBride. The lower one-sided 95% confidence level and the concordance correlation coefficient ranged from 0.90 to 0.96 and 0.91 to 0.96, respectively. Thus, the degree of agreement was moderate to substantial,18 which we considered satisfactory for the task at hand.
Clinical data
Demographics, clinical information, and laboratory results at the time of the oral examination were asked or collected from the patients’ medical charts, including age, gender, smoker status (current, former, never), present alcohol use (yes or no), cirrhosis etiology, and comorbidity. The comorbidities were classified and a total score calculated by the Charlson Comorbidity Index.19 To gauge the severity of the cirrhosis disease, the Child–Pugh score and the Model for End-Stage Liver Disease (MELD) score were calculated.
Patients’ nutritional risk was assessed using the standardized screening tool NRS-2002. Patients were scored in each of the three components: (1) undernutrition (estimated by body mass index, percentage recent weight loss, and change in food intake), (2) disease severity, and (3) age, giving a total score of 0–7. Patients with a total score of 3 or above were classified as nutritionally at risk with a need for targeted nutritional therapy.20
All patients were prospectively followed in the department until death or study end at January 5, 2016. No patient was lost to follow-up.
After follow-up, dates of death were obtained from the Civil Registration System in Denmark, an administrative registry that is continuously updated with vital status and dates of death for all Danish citizens.21 The cause of death up to and including 2014 was obtained from the Danish Register of Causes of Death, which contains information on all death certificates since 1943.22 The cause of death for patients who died in 2015 was retrieved from their medical charts. Based on these data, death was classified as cirrhosis related or not cirrhosis related. Cirrhosis-related deaths were those which had at least one of the following listed as the cause of death or as part of the events leading to death: cirrhosis (K70.3, K74.6) and liver failure (K.70.4, K72.–). All other deaths were classified as not cirrhosis related.
At study entry 12 (7%) patients were under evaluation for liver transplantation, but none was transplanted during the study period.
Statistical analyses
We used the Mann–Whitney test (for age, MELD score, and nutritional risk score) and the chi-square test (for other characteristics) to test whether the patients with or without severe periodontitis and the patients with or without teeth were identical at study entry.
To examine the association of oral health with mortality two sets of analyses were performed. First, a Kaplan–Meier analysis was performed to estimate survival probability in patients with severe periodontitis as opposed to dentate patients without severe periodontitis, and in edentulous patients as opposed to dentate patients. Second, a Cox proportional hazards regression analysis was used to estimate the association between presence or absence of severe periodontitis and presence and absence of teeth on all-cause and cirrhosis-related mortality. The association was expressed as the hazard ratio (HR) with 95% confidence interval (CI), and the association was adjusted for confounding by age, gender, alcoholic cirrhosis, Child–Pugh score, MELD score, smoker status, present alcohol use, comorbidity, and nutritional risk score. The cirrhosis-related and non-cirrhosis-related mortality was computed, taking into account that they were competing risks.23 The proportional hazards assumption was evaluated graphically using log-log plots of the survival curves. The continuous variables were included untransformed in the analyses. In all tests a p value of 0.05 or less was considered to be statistically significant. The data were analyzed using Stata version 12.0 (Stata Corp LP, College Station, TX, USA).
Results
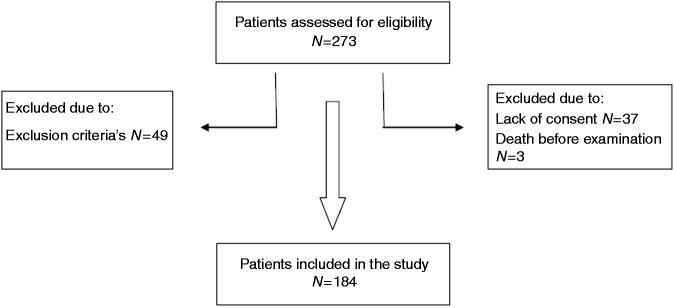
We screened 273 patients for eligibility, of whom 89 were excluded by the exclusion criteria, lack of consent, or death before the oral examination. Figure 1 gives the patient flow.
Figure 1.
Patient inclusion flowchart.
A total of 184 patients were included in the study. Their mean age at baseline was 61 years (range 21–87 years) and 120 (66%) were men. Their baseline characteristics are summarized in Table 1.
Table 1.
Characteristics of the patient cohort.
| Periodontitis |
Edentulism |
||||||
|---|---|---|---|---|---|---|---|
| All patients | Severe periodontitis | No severe periodontitis | p valuea | Edentulous | Dentate | p valueb | |
| Number of participants | 184 | 66 | 85 | 33 | 151 | ||
| Age, years median (range) | 62 (21–87) | 62 (42–84) | 60 (21–87) | 0.09 | 65 (40–85) | 61 (21–87) | 0.01 |
| Female/Male (%) | 34/66 | 29/71 | 42/58 | 0.09 | 24/76 | 37/63 | 0.17 |
| Cirrhosis etiology (%) | |||||||
| Alcohol | 73 | 85 | 67 | 0.01 | 64 | 75 | 0.18 |
| Cryptogenic | 12 | 12 | 11 | 0.78 | 15 | 11 | 0.55 |
| Autoimmune or cholestastic | 11 | 3 | 20 | 0.03 | 6 | 12 | 0.32 |
| Viral hepatitis B and/or C | 4 | 2 | 0.21 | 15 | 2 | 0.05 | |
| Cirrhosis severity | |||||||
| Child–Pugh class A/B/C (%) | 14/60/26 | 19/48/33 | 9/66/25 | 0.06 | 18/67/15 | 13/59/28 | 0.26 |
| MELD score median (range) | 11 (6–37) | 11 (6–33) | 11 (6–37) | 0.27 | 8 (5–20) | 11 (6–37) | 0.02 |
| Smoker habits (%) | |||||||
| Current/Former/Never | 16/45/39 | 20/51/29 | 12/37/51 | 0.03 | 18/52/30 | 15/44/41 | 0.53 |
| Daily alcohol use (%) | |||||||
| Yes/No | 38/62 | 43/57 | 39/61 | 0.58 | 27/73 | 41/59 | 0.14 |
| Charlson comorbidity index (%) | |||||||
| 0 | 59 | 56 | 67 | 0.19 | 48 | 62 | 0.06 |
| 1 | 24 | 29 | 22 | 22 | 24 | ||
| 2 | 13 | 15 | 6 | 21 | 11 | ||
| 3+ | 4 | 5 | 9 | 3 | |||
| Nutritional status median (range) | |||||||
| Nutritional risk score | 3 (1–7) | 3 (1–7) | 3 (1–6) | 0.13 | 3 (1–6) | 3 (1–7) | 0.58 |
A p value is given for the hypothesis that a characteristic is identical for patients with or without severe periodontitisa and patients with or without teethb.
MELD: Model of End-Stage Liver Disease.
The oral examination showed that 66 (44%) of the patients had severe periodontitis. Compared to patients without severe periodontitis more patients with severe periodontitis were smokers (20% vs. 12%) and had alcoholic cirrhosis (85% vs. 67%), but fewer had autoimmune or cholestatic cirrhosis (3% vs. 20%). There was no difference regarding age, gender, Child–Pugh score, MELD score, present alcohol use, comorbidities, and nutritional risk score between the patients with or without severe periodontitis.
Thirty-three (18%) patients were edentulous. Compared to dentate patients the edentulous patients were older (65 years vs. 60 years), more had viral hepatitis (15% vs. 2%), and they had a better MELD score (8 vs. 11), but there were no differences in gender, Child–Pugh score, smoking habits, present alcohol use, comorbidities, and nutritional risk score (Table 1).
The total follow-up time was 74,197 days (203.14 years) with a median per patient of 350 days (0.96 year).
Eighty-one patients (44%) died during follow-up. The presence of severe periodontitis was associated with higher all-cause mortality (crude HR 1.56, 95% CI 1.06–2.54). The confounder adjustments resulted in the same association with mortality, but its CI was wider (HR 1.45, 95% CI 0.79–2.45). No association was revealed between edentulism and mortality in any analysis (Table 2).
Table 2.
Oral health and risk of all-cause and cirrhosis-related mortality.
| All-cause mortality |
Cirrhosis-related mortality |
|||
|---|---|---|---|---|
| Crude HR (95% CI) | Adjusted HR (95% CI) | Crude HR (95% CI) | Adjusted HR (95% CI) | |
| Severe periodontitis vs. no severe periodontitis (N = 151) | 1.56 (1.06–2.54) | 1.45 (0.79–2.45) | 2.19 (1.07–4.50) | 2.29 (1.04–4.99) |
| Edentulous vs. dentate (N = 184) | 1.17 (0.63–2.21) | 1.46 (0.74–2.87) | 1.06 (0.45–2.54) | 1.24 (0.55–2.97) |
Associations are expressed as hazard ratios (HR) with confidence intervals (CI), and presented without adjustment and with adjustment for age, gender, alcoholic cirrhosis, Child–Pugh score, MELD score, smoker status, alcohol use, comorbidity, and nutritional risk score.
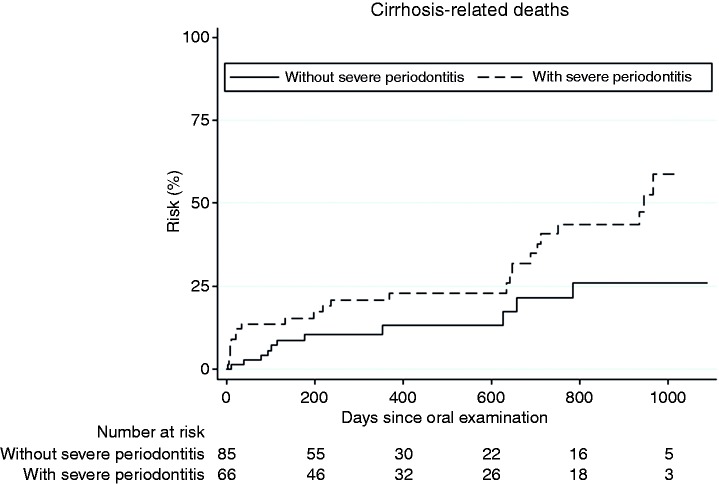
Of the 81 patients who died, 52 (64%) died from cirrhosis-related causes and 29 (36%) died from other causes. Severe periodontitis was even more strongly associated with cirrhosis-related mortality (crude HR 2.19, 95% CI 1.07–4.50 and adjusted HR 2.29, 95% CI 1.04–4.99) (Figure 2). Again, no association was observed between edentulism and cirrhosis-related mortality (Table 2).
Figure 2.
Cumulative risk of cirrhosis-related mortality caused by severe periodontitis. Number at risk indicates the number of patients at risk in each group at the corresponding time.
Discussion
This prospective cohort study on cirrhosis patients showed that the presence of severe periodontitis was associated with all-cause mortality in the crude analysis and cirrhosis-related mortality both in the crude and adjusted analyses. Edentulism showed no such association.
The most important strengths of our study are its prospective design, the systematic and detailed oral examination, and its complete follow-up to a statistically relevant number of deaths. The main limitation is its recruitment design, which implies that it cannot immediately be concluded that periodontitis was in fact the cause of the serious prognosis facing the patients, or whether the severe periodontitis resulted from more advanced cirrhosis. However, the lack of association at study entry between the Child–Pugh and MELD scores and the presence of periodontitis favors the first assumption. The relatively short follow-up period might be seen as a limitation but for the fact that nearly half of our participants died, in accordance with their known poor prognosis.24 Finally, our study comprised a broad spectrum of cirrhosis patients and may be seen as representative for an inpatient cirrhosis population, but still its single-center design may limit generalizability of the strength of the association to other cirrhosis populations. However, this limitation is probably not very important because the department both has a large local catchment population and also receives referred patients from other hospitals across Denmark. It may also be noted that the distribution of age, gender, and cirrhosis etiology of our patients corresponds to recent Danish nationwide studies.24 Confounding is always a worry in such association studies and we took care to control for obvious sources by statistically adjusting for the effects of a considerable number of demographic, individual, and disease characteristics. One obvious residual confounding factor might be unmeasured socioeconomic variables. This is likely not an important problem in this study as the Danish population is relatively homogenous with respect to income and disability status, but it may well be an important factor in other countries.25
The prevalence of severe periodontitis of 45% among our cirrhosis patients was very high compared with 10–15% in the general population.26 Likewise, edentulism in 18% was higher than the reported 7% in the general population,27 and most probably resulted from the frequent presence of destructive periodontal disease in line with other reports from cirrhosis cohorts.4,6
Alcohol is the most common etiology of cirrhosis in Denmark. In this study, more patients with alcoholic cirrhosis had severe periodontitis than patients with other cirrhosis etiologies. We found no association between self-reported present alcohol use and severe periodontitis; although studies have reported a life-time relationship.28 Possibly our patients followed advice to reduce their alcohol consumption while being former drinkers.
A large fraction of our patients were classified as having cryptogenic cirrhosis, which we now suspect may reflect former or present nonalcoholic steatohepatitis (NASH). Both obesity and diabetes are associated with periodontitis29 but we found no difference in the prevalence of obesity or diabetes between patients with cryptogenic cirrhosis and those with other cirrhosis etiologies (data not shown).
Periodontitis is reported to be associated with other diseases such as cardiovascular disease, chronic obstructive pulmonary disease, chronic kidney disease, diabetes mellitus, endocarditis, and rheumatoid arthritis.30 Periodontitis has also been shown to be associated with death from infection both in healthy and diseased populations.31 One motivation for conducting this study was the sparse information on the consequences of periodontitis for the course of cirrhosis, given the extensive knowledge about the importance of other infections. Our finding of a strong association between periodontitis and mortality is in line with the reports on other diseases,13 which may in a broad sense underline the importance of oral health.
We found that severe periodontitis was associated with increased all-cause mortality in the crude analysis. The magnitude of the association was the same in the adjusted analysis but its confidence limits included 1.0. This loss of association precision was probably due to the larger part of the all-cause mortality being in fact cirrhosis related, and the association to cirrhosis-related death was stronger as well as more precise after the confounder adjustment. This data structure expands and details the findings from other disease populations and may be taken to reflect a particularly marked importance of oral infections for the cirrhosis patients. This would be consistent with Åberg et al., who described a correlation between oral infections and accelerated liver disease measured by the change in MELD score in 38 patients awaiting liver transplantation.2 In accordance, a higher prevalence of P. gingivalis fimA genotype II, a gram-negative oral anaerobic bacterium strongly associated with periodontitis, was reported in a study of patients with cirrhosis versus patients with non-cirrhotic chronic hepatitis C.32 Our study corroborates and extends such reports by being larger, by having complete follow-up, and by adjusting for important confounders.
Infections were likely responsible for a large part of the more than doubled cirrhosis-related mortality that we found in the patients with severe periodontitis. Periodontitis is recognized as a persistent source of oral bacterial translocation to the bloodstream and as a factor causing systemic low-grade inflammation,33 and thus conceivably acts directly as a pathogenic link in the development of fatal cirrhosis complications, probably even aggravated by the immune-incompetence of cirrhosis. This is recognized in transplant hepatology, in which an oral examination of patients awaiting liver transplantation is routine to eliminate oral infection foci and prevent sepsis of oral origin. Our study suggests that all patients with cirrhosis would benefit from a systematic preventive oral care program and greater awareness of oral health. Ideally, such efforts should be evaluated in intervention trials, which have not been conducted in patients with cirrhosis. Further motivation lies in a small study of patients with chronic kidney diseases that showed a positive effect of treatment of periodontitis on all-cause mortality.34 Even in the general population periodontal treatment leads to a reduction in C-reactive protein.35
The lack of association between edentulism and all-cause mortality was unexpected as other studies indicate that lack of teeth may be related to higher mortality.15 The explanation in our study may be that patients without teeth cannot suffer from periodontitis and hence may be past the stage of oral neglect where oral bacterial translocation poses a threat to the patients. In support of this, the included edentulous patients at study entry had a more favorable MELD score.
In conclusion, the present study revealed a robust association between severe periodontitis and a higher all-cause and particularly cirrhosis-related mortality. These findings should be taken into account when defining the design of oral intervention studies in the future. In addition, pathophysiological studies to better understand the potential relationship between periodontitis and cirrhosis may be beneficial. The association, if confirmed, seems to necessitate closer attention to oral health in the clinical care of all patients with cirrhosis.
Acknowledgments
The authors thank dental hygienists Nanna Jensen, Natasja Nielsen and Susanne Hedegaard for performing the oral examinations.
Declaration of conflicting interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethics approval
The study was performed in accordance with the Declaration of Helsinki and was approved by the Ethical Committee of Central Denmark Region (journal No. 1-10-72-128-12).
Funding
This work was supported by grants from Aarhus University Hospital, Aase & Ejnar Danielsens Foundation, A.P. Møller Foundation, Central Denmark Region Foundation for Health Research, and Novo Nordisk Foundation.
Informed consent
All patients gave written informed consent before participation in the study.
References
- 1.Helenius-Hietala J, Meurman JH, Höckerstedt K, et al. Effect of the aetiology and severity of liver disease on oral health and dental treatment prior to transplantation. Transpl Int 2012; 25: 158–165. [DOI] [PubMed] [Google Scholar]
- 2.Åberg F, Helenius-Hietala J, Meurman J, et al. Association between dental infections and the clinical course of chronic liver disease. Hepatol Res 2014; 44: 349–353. [DOI] [PubMed] [Google Scholar]
- 3.Grønkjær LL, Vilstrup H. Oral health in patients with liver cirrhosis. Eur J Gastroenterol Hepatol 2015; 27: 834–839. [DOI] [PubMed] [Google Scholar]
- 4.Guggenheimer J, Eghtesad B, Close JM, et al. Dental health status of liver transplant candidates. Liver Transpl 2007; 13: 280–286. [DOI] [PubMed] [Google Scholar]
- 5.Lins L, Bittencourt PL, Evangelista MA, et al. Oral health profile of cirrhotic patients awaiting liver transplantation in the Brazilian northeast. Transplant Proc 2011; 43: 1319–1321. [DOI] [PubMed] [Google Scholar]
- 6.Singal AK, Salameh H, Kamath PS. Prevalence and in-hospital mortality trends of infections among patients with cirrhosis: A nationwide study of hospitalized patients in the United States. Aliment Pharmacol Ther 2014; 40: 105–122. [DOI] [PubMed] [Google Scholar]
- 7.Gunsar F, Raimondo ML, Jones S, et al. Nutritional status and prognosis in cirrhotic patients. Aliment Phamacol Ther 2006; 24: 563–572. [DOI] [PubMed] [Google Scholar]
- 8.Maharshi S, Sharma BC, Srivastava S. Malnutrition in cirrhosis increases morbidity and mortality. J Gastroenterol Hepatol 2015; 30: 1507–1513. [DOI] [PubMed] [Google Scholar]
- 9.Pihlstrom BL, Michalowicz BS, Johnson NW. Periodontal diseases. Lancet 2005; 366: 1809–1820. [DOI] [PubMed] [Google Scholar]
- 10.Ettinger RL, Qian F. Abutment tooth loss in patients with overdentures. J Am Dent Assoc 2004; 135: 739–746. [DOI] [PubMed] [Google Scholar]
- 11.Grønkjær LL, Holmstrup P, Schou S, et al. Periodontitis in cirrhosis. Submitted to BMC Gastroenterology, November 2016.
- 12.Grønkjær LL, Holmstrup P, Schou S, et al. Presence of tooth periapical radiolucency in patients with cirrhosis. Hepat Med 2016; 8: 97–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sharma P, Dietrich T, Ferro CJ, et al. Association between periodontitis and mortality in stage 3–5 chronic kidney disease: NHANES III and linked mortality study. J Clin Periodontol 2016; 43: 104–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Linden GJ, Linden K, Yarnell J, et al. All-cause mortality and periodontitis in 60-70-year-old men: A prospective cohort study. J Clin Periodontol 2012; 39: 940–946. [DOI] [PubMed] [Google Scholar]
- 15.Hu HY, Lee YL, Lin SY, et al. Association between tooth loss, body mass index, and all-cause mortality among elderly patients in Taiwan. Medicine (Baltimore) 2015; 94: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Page RC, Eke PI. Case definitions for use in population-based surveillance of periodontitis. J Periodontol 2007; 78: 1387–1399. [DOI] [PubMed] [Google Scholar]
- 17.Lin LI. A concordance correlation coefficient to evaluate reproducibility. Biometrics 1989; 45: 255–268. [PubMed] [Google Scholar]
- 18.McBride GB. A proposal for strength-of-agreement criteria for Lin’s Concordance Correlation Coefficient. 2005 NIWA Client Report: HAM2005-062.
- 19.Quan H, Li B, Couris CM, et al. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol 2011; 173: 676–682. [DOI] [PubMed] [Google Scholar]
- 20.Kondrup J, Allison SP, Elia M, et al. ESPEN guidelines for nutrition screening 2002. Clin Nutr 2003; 22: 415–421. [DOI] [PubMed] [Google Scholar]
- 21.Frank L. When an entire country is a cohort. Science 2000; 287: 2398–2399. [DOI] [PubMed] [Google Scholar]
- 22.Pedersen CB, Gøzche H, Møller J, et al. The Danish Civil Registration System. A cohort of eight million persons. Dan Med Bull 2006; 53: 441–449. [PubMed] [Google Scholar]
- 23.Marubini E, Valsecchi MG. Competing risks. In: Analysing survival data from clinical trials and observational studies, 1st ed Chichester, UK: John Wiley & Sons, 1995, pp. 331–363. [Google Scholar]
- 24.Jepsen P, Vilstrup H, Andersen PK, et al. Comorbidity and survival of Danish cirrhosis patients: A nationwide population-based cohort study. Hepatology 2008; 48: 214–220. [DOI] [PubMed] [Google Scholar]
- 25.Schmidt M, Schmidt SA, Sandegaard JL, et al. The Danish national patient registry: A review of content, data quality, and research potential. Clin Epidemiol 2015; 17: 449–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Petersen PE, Ogawa H. Strengthening the prevention of periodontal disease: The WHO approach. J Periodontol 2005; 76: 187–193. [DOI] [PubMed] [Google Scholar]
- 27.Petersen PE, Ekholm O, Jürgensen N. Surveillance of adult dental health status and dental visits in Denmark—the situation in 2005 and the development since 1987 [article in Danish]. Danish Dent J 2010; 114: 480–491. [Google Scholar]
- 28.Lages E, Costa FO, Lages EM, et al. Risk variables in the association between frequency of alcohol consumption and periodontitis. J Clin Periodontol 2012; 39: 115–122. [DOI] [PubMed] [Google Scholar]
- 29.Caldwell SH, Oelsner DH, Iezzoni JC, et al. Cryptogenic cirrhosis: Clinical characterization and risk factors for underlying disease. Hepatology 1999; 29: 664–669. [DOI] [PubMed] [Google Scholar]
- 30.Linden GJ, Lyons A, Scannapieco FA. Periodontal systemic associations: Review of the evidence. J Clin Periodontol 2013; 40(Suppl 14): 8–19. [DOI] [PubMed] [Google Scholar]
- 31.Hujoel PP, Drangsholt M, Spiekerman C, et al. An exploration of the periodontitis-cancer association. Ann Epidemiol 2003; 13: 312–316. [DOI] [PubMed] [Google Scholar]
- 32.Nagao Y, Kawahigashi Y, Sata M. Association of periodontal diseases and liver fibrosis in patients with HCV and/or HBV infection. Hepat Mon 2014; 14: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gendron R, Grenier D, Maheu-Robert L. The oral cavity as a reservoir of bacterial pathogens for focal infections. Microbes Infect 2000; 2: 897–906. [DOI] [PubMed] [Google Scholar]
- 34.de Souza CM, Braosi AP, Luczyszyn SM, et al. Association among oral health parameters, periodontitis, and its treatment and mortality in patients undergoing hemodialysis. J Periodontol 2014; 85: 169–178. [DOI] [PubMed] [Google Scholar]
- 35.Mattila K, Vesanen M, Valtenon V, et al. Effect of treating periodontitis on C-reactive protein levels: A pilot study. BMC Infect Dis 2000; 2: 30–32. [DOI] [PMC free article] [PubMed] [Google Scholar]