Chronic myelomonocytic leukemia (CMML) is an aggressive hematological malignancy characterized by sustained peripheral blood monocytosis and an inherent risk for leukemic blast transformation1,2. Patients with CMML have between 10–15 mutations per kilobase of coding DNA regions; with majority of these (>90%) involving epigenetic regulator genes (TET2 60%, ASXL1 40%), splicing machinery (SRSF2 40%), and cell signaling (oncogenic RAS pathway 30%)3–5. The polycomb group proteins play an important role in transcriptional repression by regulating chromatin modifications and consist of two canonical polycomb repressive complexes (PRC) PRC1 and PRC26. PRC2, comprises EED (embryonic ectoderm development protein), EZH2 (enhancer of zeste homolog 2), SUZ12 (suppressor of zeste 12 protein homolog), and RBBP4/7 (retinoblastoma binding protein), is recruited to chromatin and results in the trimethylation of lysine 27 of the histone 3 mark (H3K27me3), a repressive mark silencing gene transcription. In addition, ASXL1 (additional sex combs like 1) has been shown to associate with PRC2 and results in global reductions in H3K27me3, suggesting a regulatory role in the PRC27.
ASXL1 mutations (frameshift and nonsense) result in the truncation of the ASXL1 protein, are seen in ~40% of CMML patients, and independently and adversely impact overall survival (OS)4,8. EZH2 mutations (chromosome 7q36.1) in CMML, unlike in epithelial malignancies and lymphoproliferative disorders, are loss-of-function mutations and are uncommon (<5%), with an indeterminate prognostic impact4,9. Given that the co-occurrence of these mutations have been documented in myeloid neoplasms and that theoretically these could further impact the repressive role of the PRC2, we examined a large and informative CMML data set to assess the prognostic impact of ASXL1 and EZH2 co-mutations in CMML10.
Patients with 2016 WHO (World Health Organization)-defined CMML were identified from the institutional database1. Al.(BM) biopsies and cytogenetic studies performed at diagnosis. A 29 gene panel next-generation sequencing assay was carried out on BM DNA specimens on all 277 patients obtained at diagnosis for the following genes: TET2, DNMT3A, IDH1, IDH2, ASXL1, EZH2, SUZ12, SRSF2, SF3B1, ZRSR2, U2AF1, PTPN11, Tp53, SH2B3, RUNX1, CBL, NRAS, KRAS, JAK2, CSF3R, FLT3, KIT, CALR, MPL, NPM1, CEBPA, IKZF, ETNK1, and SETBP1, by previously described methods5,9. All statistical analyses considered parameters obtained at time of CMML diagnosis. Differences in the distribution of continuous variables between categories were analyzed by either Mann–Whitney or Kruskal–Wallis tests. Patient groups with nominal variables were compared by χ2 test. OS was calculated from the date of first referral to date of death or last contact. Leukemia-free survival (LFS) was calculated from the date of first referral to date of leukemic transformation or death/last contact. Overall and LFS curves were prepared by the Kaplan–Meier method and compared by the log-rank test. Cox proportional hazard regression model was used for multivariable analysis.
Two hundred and seventy-seven WHO-defined CMML patients were included in the study; median age 72 years (range, 18–92), 66% males. ASXL1 mutations were identified in 138 (50%) patients, whereas EZH2 mutations were identified in 7 (3%) patients; all 7 (100%) being co-mutated for ASXL1 (Table 1). EZH2 mutation types included; missense 3 (43%), nonsense 2 (28%), and 1 (14%) each for frameshift mutations and intronic mutations impacting splicing (splice site mutation). Four (57%) patients with EZH2 mutations had a proliferative CMML phenotype, while three (43%) patients had an abnormal karyotype, including trisomy 8, isochromosome 17q, and a balanced translocation—t(3;12;6)(p21;q21;q23), respectively. Mutational frequencies in the ASXL1/EZH2 co-mutated cohort included TET2 57%, RUNX1 43%, SRSF2 29%, and 14% each for JAK2V617F, FLT3-ITD, BCOR, CBL, NRAS, and SETBP1. Notably there were no mutations involving DNMT3A, IDH1, IDH2, SF3B1, U2AF1, ZRSR2, or TP53. In comparison to ASXL1mut/EZH2wt and ASXL1wt/EZH2wt, patients with ASXL1/EZH2 co-mutations were more likely to have additional mutations involving RUNX1 (43%, p = 0.001), BCOR (14%, p < 0.0001), FLT3-ITD (14%, p = 0.04) and less likely to have SF3B1 mutations (0%, p = 0.003). The ASXL1/EZH2 co-mutated patients were also more likely to have higher risk stratification by the ASXL1 integrated Mayo Molecular Model (p < 0.0001) and the GFM CMML prognostic model (p < 0.0001).
Table 1.
Clinical and laboratory characteristics of 277 CMML patients stratified by their ASXL1 and EZH2 mutational status
| Variables | All patients with CMML (n = 277) | ASXL1mt/EZH2mt CMML patients (n = 7, 3%) | ASXL1mt CMML patients (n = 131, 47%) | ASXL1wt CMML patients (n = 139, 50%) | P-value comparing ASXL1mt/EZH2mt patients with ASXL1mt and ASXL1wt |
|---|---|---|---|---|---|
| Age in years; median (range) | 72.3 (18–92) | 67 (65–79) | 72.5 (27–92) | 72.4 (18–92) | 0.7 |
| Males; n (%) | 183 (66) | 6 (86) | 89 (68) | 88 (63) | 0.4 |
| Hemoglobin, g/dL; median (range) | 10.7 (6.4–17) | 11.3 (7.2–15) | 10.7 (6.4–16.8) | 10.9 (7.1–17) | 0.7 |
| WBC × 109/L; median (range) | 12.3 (2–265) | 14.1 (6–51) | 14.3 (2–265) | 10 (2–186) | 0.01 |
| ANC × 109/L; median (range) | 6.2 (0.1–151 | 6.3 (3.2–32) | 7.6 (0.2–151) | 5.2 (0.1–143) | 0.04 |
| AMC × 109/L; median (range) | 2.5 (1–40) | 2.8 (1–8) | 3 (1–40) | 2 (1–30) | 0.007 |
| ALC × 109/L; median (range) | 1.7 (0–22) | 1.6 (1.2–6) | 1.9 (0.4–22) | 1.6 (0–11) | 0.13 |
| Platelets × 109/L; median (range) | 98 (10–840) | 211 (25–526) | 95 (10–726) | 100 (12–840) | 0.1 |
| Presence of circulating immature myeloid cells; n (%) | 156 (56) | 5 (71) | 81 (62) | 70 (50) | 0.09 |
| PB blast %; median (range) | 0 (0–19) | 0 (0–4) | 0 (0–19) | 0 (0–12) | 0.6 |
| BM blast %; median (range) | 3 (0–19) | 1 (0–10) | 4 (0–19) | 3 (0–18) | 0.08 |
| Lactate dehydrogenase levels IU/ml; median (range) | 226 (84–1296) | 271 (163–604) | 243 (84–1296) | 215 (131–719) | 0.1 |
| Cytogenetics | (n=267) | (n=124) | (n=136) | 0.7 | |
| abnormal; n (%) | 85 (32) | 3 (43) | 41 (33) | 41 (30) | |
| FAB CMML classification | (n=276) | (n=138) | 0.005 | ||
| Proliferative | 135 (49) | 4 (57) | 77 (59) | 54 (39) | |
| Dysplastic | 141 (51) | 3 (43) | 54 (41) | 84 (61) | |
| Therapy-related CMML; n (%) | 28 (10 | 1 (14) | 11 (8) | 16 (12) | 0.7 |
| Next-generation sequencing analysis; n (%) | |||||
| 1. Epigenetic regulators | |||||
| TET2 | 154 (56) | 4 (57) | 59 (45) | 91 (65) | 0.003 |
| DNMT3A | 16 (6) | 0 | 7 (5) | 9 (6) | 0.7 |
| IDH1 | 5 (2) | 0 | 3 (2) | 2 (1) | 0.8 |
| IDH2 | 16 (6) | 0 | 8 (6) | 8 (6) | 0.8 |
| 2. Transcription factors | |||||
| RUNX1 | 21 (8) | 3 (43) | 11 (8) | 7 (5) | 0.001 |
| BCOR | 1 (0.5) | 1 (14) | 0 | 0 | <0.0001 |
| 3. Spliceosome components | |||||
| SF3B1 | 15 (5) | 0 | 1 (1) | 14 (10) | 0.003 |
| SRSF2 | 129 (47) | 2 (29) | 63 (48) | 64 (46) | 0.6 |
| U2AF1 | 19 (7) | 0 | 12 (9) | 7 (5) | 0.3 |
| ZRSR2 | 8 (3) | 0 | 2 (2) | 6 (4) | 0.4 |
| 4. Cell signaling | |||||
| JAK2 V617F | 20 (7) | 1 (14) | 9 (7) | 10 (7) | 0.8 |
| MPL | 2 (1) | 0 | 0 | 2 (1) | 0.4 |
| SH2B3 | 1 (0.5) | 0 | 1 (1) | 0 | 0.6 |
| CBL | 37 (13) | 0 | 20 (15) | 17 (12) | 0.4 |
| NRAS | 44 (16) | 1 (14) | 25 (19) | 18 (13) | 0.4 |
| KRAS | 12 (4) | 1 (14) | 8 (6) | 3 (2) | 0.1 |
| PTPN11 | 8 (3) | 0 | 5 (4) | 3 (2) | 0.6 |
| CSF3R | 4 (1) | 0 | 2 (2) | 2 (1) | 0.9 |
| C-KIT | 8 (3) | 0 | 4 (3) | 4 (3) | 0.9 |
| FLT3 | 7 (3) | 1 (14) | 1 (1) | 5 (4) | 0.04 |
| NPM1 | 1 (0.5) | 0 | 0 | 1 (1) | 0.6 |
| CALR | 1 (0.5) | 0 | 0 | 1 (1) | 0.6 |
| 5. Tumor suppressor genes | |||||
| Tp53 | 7 (3) | 0 | 1 (1) | 6 (4) | 0.2 |
| 6. Others | |||||
| SETBP1 | 37 (13) | 1 (14) | 26 (20) | 10 (7) | 0.009 |
| CEBPA | 3 (1) | 0 | 1 (1) | 2 (1) | 0.8 |
| 2016 WHO morphological subtypes; n (%) | (n=275) | (n=129) | 0.9 | ||
| CMML-0 | 155 (56) | 5 (71) | 73 (57) | 77 (55) | |
| CMML-1 | 70 (25) | 1 (14) | 31 (24) | 38 (27) | |
| CMML-2 | 50 (18) | 1 (14) | 25 (19) | 24 (17) | |
| Spanish cytogenetic risk stratification; n (%) | (n=270) | (n=126) | (n=137) | 0.5 | |
| Low | 202 (75) | 4 (57) | 90 (71) | 108 (79) | |
| Intermediate | 42 (16) | 2 (29) | 22 (17) | 18 (13) | |
| High | 26 (10) | 1 (14) | 14 (11) | 11 (18) | |
| Mayo-French cytogenetic risk stratification; n (%) | (n=270) | (n=126) | (n=137) | 0.2 | |
| Low | 201 (74) | 4 (57) | 89 (71) | 108 (79) | |
| Intermediate | 55 (20) | 2 (29) | 32 (25) | 21 (15) | |
| High | 14 (5) | 1 (14) | 5 (4) | 8 (6) | |
| Mayo prognostic model; n (%) | (n=274) | (n=129) | (n=138) | 0.14 | |
| Low | 86 (31) | 0 | 35 (27) | 51 (37) | |
| Intermediate | 88 (32) | 4 (57) | 45 (35) | 39 (28) | |
| High | 100 (36) | 3 (43) | 49 (38) | 48 (35) | |
| Molecular Mayo model; n (%) | (n=260) | (n=126) | (n=127) | <0.0001 | |
| Low | 20 (8) | 0 | 0 | 20 (16) | |
| Intermediate-1 | 67 (26) | 0 | 19 (15) | 48 (38) | |
| Intermediate-2 | 83 (32) | 4 (57) | 37 (29) | 42 (33) | |
| High | 90 (35) | 3 (43) | 70 (56) | 17 (13) | |
| GFM CMML prognostic model; n (%) | (n=268) | (n=129) | (n=132) | <0.0001 | |
| Low | 117 (44) | 1 (14) | 29 (22) | 87 (66) | |
| Intermediate | 100 (37) | 4 (57) | 59 (46) | 37 (28) | |
| High | 51 (19) | 2 (29) | 41 (32) | 8 (6) | |
| Leukemic transformation; n (%) | 48 (17) | 0 | 27 (21) | 21 (15) | 0.23 |
| Deaths; n (%) | 169 (61) | 3 (43) | 87 (66) | 79 (57) | 0.2 |
| Follow-up in months; median (range) | 16 (0.03–194) | 6 (0.2–24)0.07- | 15 (0.07–183) | 19 (0.03–194) | 0.08 |
The bold values represent statistically significant p values, p < 0.05
mt mutant, wt wild type, WBC white blood cell count, ANC absolute neutrophil count, AMC absolute monocyte count, ALC absolute lymphocyte count, PB peripheral blood, BM bone marrow, WHO World Health Organization, GFMGroupe Francophone des Myélodysplasies, FABFrench–American–British, BT blast transformation
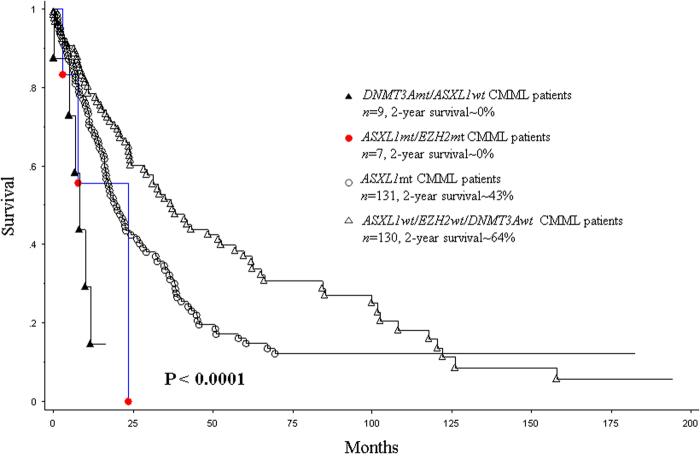
At last follow-up (median, 16 months), 169 (61%) deaths and 48 (17%) leukemic transformations were documented. Median survival for ASXL1/EZH2 co-mutated patients was 16 months, in comparison to 20 months for ASXL1mt/EZH2wt and 33 months for ASXL1wt/EZH2wt patients (p < 0.0001, Fig. 1). In a univariate analysis, survival (OS) was adversely impacted by male sex (p = 0.03), low hemoglobin (HB < 10 gm/dl, p = 0.001), high white blood cell count (WBC > 15 × 10(9)/L; p = 0.0003), high absolute monocyte count (AMC > 10 × 10(9)/L, p = 0.0002), high absolute lymphocyte count (p = 0.02), presence of circulating immature myeloid cells (IMC, p = 0.002), peripheral blood (p = 0.001) and BM (p = 0.045) blast %, abnormal karyotype (p = 0.0008), absence of TET2 mutations (p = 0.0003), presence of ASXL1 (p = 0.009), DNMT3A (p = 0.001), and Tp53 (p = 0.02) mutations. EZH2 mutations by themselves did not impact OS (p = 0.2); however, when analyzed in the context of ASXL1mt/EZH2mt status, in comparison to ASXL1mutations, the adverse impact of the co-mutations was significantly stronger (p = 0.04, HR 2.9, 95% CI 1.1–9.5). In a multivariable survival analysis that included the aforementioned significant variables, only high WBC > 15 × 10(9)/L (p = 0.005, HR 1.005, 95% CI 1.003–1.009), male sex (p = 0.002, HR 1.7, 95% CI 1.2–2.4), presence of IMC (p = 0.009, HR 1.5, 95% CI 1.1–2.4), presence of ASXL1/EZH2 co-mutations (p = 0.03, HR 2.1 95% CI 1.2–3.2), presence of DNMT3A mutations (p = 0.002, HR 2.8, 95% CI 1.4–5.4), and absence of TET2 mutations (p = 0.0006, HR 1.7, 95% CI 1.2–2.4) independently and adversely impacted OS. The prognostic relevance of ASXL1/EZH2 co-mutational status was lost when assessed in context of the Mayo Molecular Model (p = 0.2) and the GFM CMML model (p = 0.4). Neither did ASXL1 (p = 0.14) nor EZH2 (p = 0.3) mutations impact LFS.
Fig. 1. Overall survival of ASXL1, EZH2, and DNMT3A mutational status.
.
In summary, our study has demonstrated that EZH2 mutations are infrequent (<5%) in WHO-defined CMML, almost always co-occur with ASXL1 mutations, are not associated with DNMT3A or SF3B1 mutations, and are frequently associated with a “proliferative” CMML phenotype. While EZH2 mutations themselves did not impact either OS or LFS, ASXL1/EZH2 co-mutated patients had higher risk stratification by the ASXL1-integrated CMML prognostic models and had a shorter survival, in comparison to ASXL1mt patients alone. Mechanistic studies, including chromatin immunoprecipitation and sequencing (ChIP-seq) are needed to see if the ASXL1/EZH2 co-mutational status indeed synergistically impacts PRC2 activity and further depletes methylation of H3K27, resulting in unbridled transcription.
Acknowledgements
Current publication is supported in part by grants from the “The Gerstner Family Career Development Award” and the “Mayo Clinic Center for Individualized Medicine, Mayo Clinic, Rochester, MN, USA”. This publication was supported by CTSA Grant Number KL2 TR000136 from the National Center for Advancing Translational Science (NCATS). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Arber DA, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127:2391–2405. doi: 10.1182/blood-2016-03-643544. [DOI] [PubMed] [Google Scholar]
- 2.Patnaik MM, Tefferi A. Chronic myelomonocytic leukemia: 2016 update on diagnosis, risk stratification, and management. Am. J. Hematol. 2016;91:631–642. doi: 10.1002/ajh.24396. [DOI] [PubMed] [Google Scholar]
- 3.Merlevede J, et al. Mutation allele burden remains unchanged in chronic myelomonocytic leukaemia responding to hypomethylating agents. Nat. Commun. 2016;7:10767. doi: 10.1038/ncomms10767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patnaik MM, et al. ASXL1 and SETBP1 mutations and their prognostic contribution in chronic myelomonocytic leukemia: a two-center study of 466 patients. Leukemia. 2014;28:2206–2212. doi: 10.1038/leu.2014.125. [DOI] [PubMed] [Google Scholar]
- 5.Patnaik MM, et al. Prognostic interaction between ASXL1 and TET2 mutations in chronic myelomonocytic leukemia. Blood Cancer J. 2016;6:e385. doi: 10.1038/bcj.2015.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iwama A. Polycomb repressive complexes in hematological malignancies. Blood. 2017;130:23–29. doi: 10.1182/blood-2017-02-739490. [DOI] [PubMed] [Google Scholar]
- 7.Abdel-Wahab O, et al. ASXL1 mutations promote myeloid transformation through loss of PRC2-mediated gene repression. Cancer Cell. 2012;22:180–193. doi: 10.1016/j.ccr.2012.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Itzykson R, et al. Prognostic score including gene mutations in chronic myelomonocytic leukemia. J. Clin. Oncol. 2013;31:2428–2436. doi: 10.1200/JCO.2012.47.3314. [DOI] [PubMed] [Google Scholar]
- 9.Patnaik MM, et al. DNMT3A mutations are associated with inferior overall and leukemia-free survival in chronic myelomonocytic leukemia. Am. J. Hematol. 2017;92:56–61. doi: 10.1002/ajh.24581. [DOI] [PubMed] [Google Scholar]
- 10.Abdel-Wahab O, et al. Concomitant analysis of EZH2 and ASXL1 mutations in myelofibrosis, chronic myelomonocytic leukemia and blast-phase myeloproliferative neoplasms. Leukemia. 2011;25:1200–1202. doi: 10.1038/leu.2011.58. [DOI] [PMC free article] [PubMed] [Google Scholar]