Abstract
Objective
The aim of the study is to determine the prevalence of gestational diabetes mellitus (GDM), its associated risk factors, foeto-maternal outcomes and prevalence of postnatal diabetes mellitus (DM).
Methods
This is a cross-sectional study using retrospective data from existing antenatal records of new antenatal women who registered at 72 public health clinics in Selangor in January 2014.
Results
A total of 745 antenatal records were reviewed. The prevalence of GDM women was 27.9% (n = 184). GDM risks were higher in women aged 35 years old and above and in those with maternal obesity. GDM women had a higher risk of having a non-spontaneous vaginal delivery compared to non-GDM women. The prevalence of postnatal DM among GDM mother was 12.1%. Working GDM mothers were at higher risk of developing postnatal DM.
Conclusion
The prevalence of GDM among newly registered women attending antenatal public health care in Selangor was higher than previous studies. Health care personnel need to be vigilant in screening women with risk factors.
Keywords: Gestational diabetes mellitus; antenatal clinic; selangor, prevalence; foeto-maternal outcome
Introduction
Gestational diabetes mellitus (GDM) is defined as any degree of glucose intolerance with onset or first recognition during pregnancy.1 Pregnancies affected by GDM imposes a risk for mother and child. The overall pregnancy outcomes among GDM mother were poorer with higher risks of spontaneous miscarriage, preterm labour and caesarean section.2 Risk of macrosomia, low Apgar score, need for intensive care unit admission, hypoglycaemia, congenital malformation and respiratory distress syndrome (RDS) were also higher among the infants of GDM mothers.2,3
The most commonly reported risk factors for GDM were older age, pre-pregnancy obesity, high parity, family history of diabetes (especially in first-degree relatives), previous history of GDM and previous obstetric outcome history (macrosomia infant, congenital malformation and recurrent abortions).4 Maternal weight and family history of GDM were also associated with maternal GDM.2,5
Women who have had GDM have a substantial risk of developing type 2 diabetes mellitus (T2DM), even though most women return to a normoglycaemia state shortly after delivery. Women with gestational diabetes were seven times more likely to develop type 2 diabetes compared with those who had a normoglycaemia pregnancy.6
Worldwide, it is estimated that GDM affects less than 1% to 28% of antenatal mothers.7 The combined prevalence of GDM in 15 multinational centres was 17.8%.8 Two previous studies showed the prevalence of (GDM) in Malaysia ranged from 18.3% and 24.9% respectively.9,10 However, not much recent data were available on GDM among antenatal mother attending the antenatal care in a public health clinic in Malaysia. The prevalence of DM has increased from 11.6% (2006) to 15.2% (2011) among adults in Malaysia.11,12 Hence it is expected that the prevalence of GDM would increase as well.
Public health clinics in Selangor screen for GDM selectively for patients with risk factors only. Screening is done using 75g oral glucose tolerance test (OGTT) at least once at or around 24 weeks gestation, unless there are indications for it to be done earlier.13 World Health Organization (WHO) guidelines also recommended to do diabetes screening among antenatal mothers at first antenatal visit and to be repeated at 24–28 weeks of gestation.14
Blood for glucose estimation is drawn after an overnight fast (baseline value) and 2 hours after the oral glucose load. The diagnosis of GDM is made if the patient satisfies the criteria for fasting blood glucose ≥ 5.6 or IGT with a 120-minute blood glucose value of ≥ 7.8 mmol/L.20
The risk factors of having an abnormal postpartum OGTT include insulin treatment during pregnancy, higher educational level, previous GDM, body mass index (BMI) more than 30 kg/m2 and women with more than two prior pregnancies are significantly associated with abnormal postpartum abnormal glucose tolerance test.15,16 Despite this, the rate of postpartum screening using OGTT remains low in clinical practice.17,18
The objectives of this paper are to determine the prevalence of GDM, it's associated risk factors and foeto-maternal outcome. The secondary objectives is to determine the prevalence of diabetes at 6-weeks postpartum and associated risk factors among antenatal women attending public health clinics in Selangor. The information can be applied to improve pregnancy care and further enhance pregnancy outcomes.
Method
A cross-sectional study was conducted among antenatal mothers who were registered at 72 public health clinics in Selangor in January 2014. Sample size was calculated using a formula for prevalence study (estimated prevalence of 25%, precision of 0.05, non-respondents of 20% and design effect of 2).19 The total sample size was 740 and allocated proportionately among all 10 districts in Selangor based on the total number of newly registered antenatal mothers in January 2014. The samples were then distributed evenly to all clinics in each district. For each clinic, samples were selected using a systematic random sampling technique. Antenatal women who were transferred out at any gestation and those with pre-existing Type 1 or Type 2 diabetes mellitus were excluded.
The data on demographic, risk factors, foeto-maternal outcome and postnatal OGTT were extracted from existing antenatal records. Respondents were diagnosed to have GDM when the fasting blood glucose was 5.6 mmol/L or more and/or 2-hours post prandial level was 7.8 mmol/L or more.20 The association between GDM and risk factors were analysed by chi-square test. As there might be cluster sampling effect in the public health clinic from the 10 districts, multivariate analysis using the generalized estimating equation (GEE) was used to predict the risk of GDM. All analysis were done using Social Package for the Social Sciences version 21.
Results
Socio-demographic characteristics of the respondents
A total of 745 antenatal records were reviewed. Only 659 (88.5%) had complete data were included in the analysis. The majority of the respondents had were Malaysian, aged less than 35 year, of Malay ethnicity, were working and had secondary education (Table 1).
Table 1.
Socio-demographic characteristics of respondents
| Socio-demography | n | % |
|---|---|---|
| Nationality | ||
| Malaysian | 598 | 90.7 |
| Non-Malaysian | 61 | 9.3 |
| Age group | ||
| Less than 35 years old | 561 | 85.1 |
| 35 years old and above | 98 | 14.9 |
| Ethnic group | ||
| Malays | 470 | 71.3 |
| Chinese | 42 | 6.4 |
| Indians | 71 | 10.8 |
| Others | 76 | 11.5 |
| Education level | ||
| No formal / primary education | 68 | 10.3 |
| Secondary education | 353 | 53.6 |
| Tertiary education | 234 | 35.5 |
| Occupation | ||
| Working | 363 | 55.6 |
| Housewife | 290 | 44.4 |
| Gravida | ||
| Primigravida | 193 | 29.3 |
| Multipara | 429 | 65.2 |
| Grand multipara | 36 | 5.5 |
| Trimester at booking | ||
| First trimester | 370 | 56.3 |
| Second trimester | 270 | 41.1 |
| Third trimester | 17 | 2.6 |
GDM and associated factors
The overall prevalence of GDM was 27.9%. Univariate analysis using chi-square (with Fisher's exact test when applicable) showed there were significant associations between GDM and age group, parity, maternal obesity, first-degree relative with DM, previous history of GDM, history of intra-uterine death and glycosuria of two or more occasions during pregnancy (Table 2).
Table 2.
Prevalence of GDM by socio-demography and risk factors
| Socio-demography | GDM n (%) | Non-GDM n (%) | p-Value (*with Fisher's exact test) |
|---|---|---|---|
| Overall | 184 (27.9) | 475 (72.1) | |
| Nationality | |||
| Malaysian | 164 (89.1) | 434 (91.4) | 0.374 |
| Non-Malaysian | 20 (10.9) | 41 (8.6) | |
| Age group | |||
| Less than 35 years old | 128 (69.6) | 433 (91.2) | <0.001 |
| 35 years old and above | 56 (30.4) | 42 (8.8) | |
| Ethnic group | |||
| Malays | 128 (69.6) | 342 (72.0) | 0.082 |
| Chinese | 17 (9.2) | 25 (5.3) | |
| Indians | 14 (7.6) | 57 (12.0) | |
| Others | 25(13.6) | 51 (10.7) | |
| Education level | |||
| No formal / primary education | 19 (10.3) | 49 (10.3) | 0.886 |
| Secondary education | 96 (52.2) | 257 (54.1) | |
| Tertiary education | 68 (37.5) | 166 (35.6) | |
| Occupation | |||
| Working | 95 (51.6) | 268 (56.4) | 0.238 |
| Housewife | 88 (48.4) | 202 (43.6) | |
| Gravida | |||
| Primigravida | 40 (21.7) | 153 (32.2) | 0.001 |
| Multipara | 126 (68.8) | 303 (63.8) | |
| Grand multipara | 18 (9.5) | 18 (4.0) | |
| Maternal obesity (BMI > 27.0 kg/m2 or maternal weight more than 80 kg) | |||
| Obese | 84 (45.7) | 101 (21.3) | <0.001 |
| Non-obese | 98 (54.3) | 371 (78.7) | |
| Weight gain more than 2 kg/week | |||
| Yes | 38 (20.7) | 84 (17.7) | 0.379 |
| No | 146 (79.3) | 391 (82.3) | |
| Previous macrosomia baby | |||
| Yes | 9 (4.9) | 10 (2.1) | 0.055 |
| No | 175 (95.1) | 465 (97.9) | |
| First-degree relative with DM | |||
| Yes | 61 (33.2) | 80 (16.8) | <0.001 |
| No | 123 (66.8) | 395 (83.2) | |
| History of GDM | |||
| Yes | 30 (16.3) | 28 (5.9) | <0.001 |
| No | 154 (83.7) | 447 (94.1) | |
| History of abortion : two times or more | |||
| Yes | 9 (4.9) | 18 (3.8) | 0.522 |
| No | 175 (95.1) | 457 (96.2) | |
| History of intrauterine death | |||
| Yes | 6 (3.3) | 5 (1.1) | 0.047 |
| No | 178 (96.7) | 470 (98.9) | |
| History of neonatal death | |||
| Yes | 3 (1.6) | 0.692* | |
| No | 181 (98.4) | 470 (98.9) | |
| History of foetal malformation | |||
| Yes | 1 (0.5) | 6 (1.3) | 0.680* |
| No | 183 (99.5) | 469 (98.7) | |
| Glycosuria two times or more | |||
| Yes | 14 (7.6) | 9 (4.9) | <0.001 |
| No | 170 (92.4) | 465 (95.1) | |
| Urinary tract infection/candidiasis | |||
| Yes | 17 (9.2) | 26 (5.5) | 0.079 |
| No | 167 (90.8) | 449 (94.5) | |
| History of polyhydramnios | |||
| Yes | 4 (2.2) | 2 (0.4) | 0.054* |
| No | 180 (97.8) | 473 (99.6) | |
p<0.05
GEEs showed significant association between GDM and age group, maternal obesity, first- degree relative with diabetes and previous history of pregnancy with GDM (Table 3).
Table 3.
Factors associated with GDM (using GEEs)
| Variable | Adjusted OR (95% confidence interval) | p-Value |
|---|---|---|
| Age group | ||
| Less than 35 years old | 1.00 | |
| 35 years old and above | 3.65 (2.21,6.01) | <0.001 |
| Gravida | ||
| Primigravida | 1.00 | |
| Multipara | 1.01(0.64,1.57) | 0.981 |
| Grand multipara | 1.50 (0.65,3.43) | 0.341 |
| Maternal obesity | ||
| Non-obese | 1.00 | |
| Obese | 2.37 (1.60,3.51) | <0.001 |
| First-degree relative with DM | ||
| Yes | 1.00 | |
| No | 1.88 (1.21,2.91) | 0.005 |
| History of GDM | ||
| Yes | 1.00 | |
| No | 2.36 (1.21,4.57) | 0.011 |
| History of intra-uterine death | ||
| Yes | 1.00 | 0.107 |
| No | 2.79 (0.80,9.68) | |
| Glycosuria two times or more | ||
| Yes | 1.00 | |
| No | 2.25 (0.77,6.58) | 0.140 |
In this study, the mean HbA1c was 5.5% with 21.2% (n=32) having a HbA1c level of 6.0% or greater. However, only 12.5% (n=23) were on insulin. There was a significant association between GDM and mode of delivery with a p-value of 0.001 (Figure 1). However, there was no association between GDM and foetal complications (birth weight, hypoglycaemia, obstructed labour, jaundice, intra-uterine death, stillbirth, foetal malformation, RDS and pre-term delivery).
Figure 1.
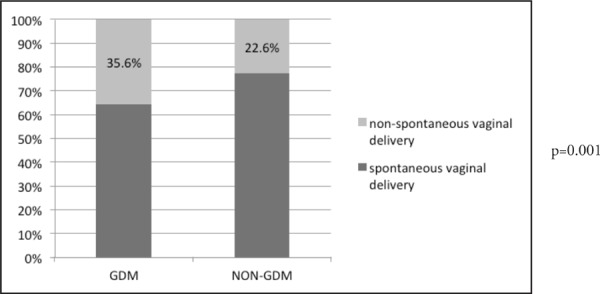
Comparison of mode of delivery between GDM and non-GDM
Postnatal DM
Only 99 (53.8%) of GDM women were screened with a postnatal OGTT of which, 12 (12.1%) had abnormal results. There was a significant association between abnormal postnatal OGTT with insulin management during the antenatal period (p<0.05), maternal obesity (p=0.005) and working mothers (p<0.05). However, no association was found for other risk factors such as maternal age, parity, family history of DM and previous history of GDM.
On further analysis, there were no differences in the sociodemographic characteristics between respondent who have done and not done OGTT (Table 4).
Table 4.
Sociodemographic characteristics between respondent who have done and not done OGTT.
| Socio-demography | OGTT Done n (%) | OGTT Not done n (%) | p- Value (##with Fisher's exact test) |
|---|---|---|---|
| Nationality | |||
| Malaysian | 90 (90.9) | 69 (88.5) | 0.593 |
| Non-Malaysian | 9 (9.1) | 9 (11.5) | |
| Age group | |||
| Less than 35 years old | 70 (68.6) | 51 (68.0) | 0.929 |
| 35 years old and above | 32 (31.4) | 24 (32.0) | |
| Ethnic group | |||
| Malays | 73 (71.6) | 53 (70.7) | 0.816 |
| Chinese | 10 (9.8) | 7 (9.3) | |
| Indians | 8 (7.8) | 4 (5.3) | |
| Others | 11 (10.8) | 11 (14.7) | |
| Education level | |||
| No formal / primary education | 12 (11.9) | 5 (6.7) | 0.317 |
| Secondary education | 52 (51.5) | 39 (52.0) | |
| Tertiary education | 37 (36.6) | 31 (41.3) | |
| Occupation | |||
| Working | 50 (49.5) | 43 (57.3) | 0.304 |
| Housewife | 51 (50.5) | 32 (42.7) | |
p<0.05
Discussion
The prevalence of GDM among newly registered antenatal women in the month of January 2014 in Selangor was 27.9%, which was higher than the 24.9% found in a study.10 This could be a reflection of the rise in the national prevalence of T2DM which from 11.6% in 2006 to 15.2% (in 2011) (NHMS 2006, NHMS 2011).11,12 The National Diabetes Registry report also showed that Selangor had the highest number of T2DM patients (n = 106,101; 16.2%).21 Another contributing reason could be the use of higher threshold levels for the diagnosis of GDM in previous studies higher cut-off point of fasting blood sugar more than 7 mmol/L and 2-hours postprandial of more than 7.8 mmol/L.10
Out of the 10 maternal risk factors identified, logistic regression showed that the significant associated variables were maternal age, obesity, previous history of GDM and first-degree family history of diabetes. Other risk factors were not found to be significant in this study.
GDM risks were higher in women aged 35 years old and above (p<0.001) with a fourfold increase. Other studies have also showed similar findings.5,9,10 Glucose tolerance is a function of insulin sensitivity and insulin secretion. Pancreatic β-cell function and insulin sensitivity fall with age. Women who were older more likely to have an inadequate β-cell response to stimulation and be more insulin-resistant than younger women, which, make gestational diabetes more likely.22
Family history of GDM is also one of the significant associated variables of GDM as shown in this study (p=0.005). Several studies also found that strong family history of diabetes, especially in first-degree relatives is one of the risk factors contributing to GDM.23,24,26 The familial association was most probably the product of minor alterations that occurred in infants of mothers with abnormal carbohydrate metabolism. This has been previously suggested by epidemiological studies in other populations.24,25
A meta-analysis done in 2007, noted that the risk of developing GDM is about four times higher among obese women.27 In this study, the odd ratio of developing GDM was 2.45 times higher among obese mothers. In obese women, the pathophysiology of GDM is primarily characterised by the pregnancy induced insulin resistance being amplified by the already elevated pre-pregnancy insulin resistance level.28 Maternal GDM and obesity are independently associated with adverse pregnancy outcomes.22,28 The hyperglycaemia and adverse pregnancy outcome study reported that a higher pre-pregnancy BMI and the BMI at 28 weeks were strongly correlated with increased insulin resistance at 28 weeks of gestation.29 Adipose tissue and placenta believed to produce a large amount of diabetogenic adipokines. Adipokine tumor necrosis factor alpha (TNF-α), is suspected to play an important role in insulin resistance pathways.30
Pregnancy complicated by GDM increase the risk of subsequent GDM.4 The magnitude of risk increases with the number of prior episodes of GDM. Women diagnosed with GDM in early pregnancy, before insulin resistance begins to rise, are likely to have a greater degree of hyperglycaemia, and therefore an increased likelihood of progression to abnormal glucose tolerance/diabetes.31
There was a significant difference in the mode of delivery of GDM women compared to non-GDM women (p=0.007). GDM women had a higher risk of having a non-spontaneous vaginal delivery such as operative deliveries (odds ratio [OR] of 1.9) compared to non-GDM women. This finding is similar with other maternal outcome studies. The rate of caesarean deliveries was higher in women with GDM.5 In a local study, the rate of caesarean section was found to be 10 times higher among diabetic women as compared to healthy women (28.5% vs 18.8%, OR 1.3).2
There was no significant difference in the foetal outcome of GDM women compared to non-GDM women in our study. Most of the GDM women in this study were well controlled (84.2% had HbAlc of ≤ 6%). Hence the finding of no significant adverse foetal outcome is expected. A high HbAlc level was shown to be associated with a higher incidence of macrosomia and RDS, as well as increased intensive care unit (ICU) admission.2 Other studies in the past have revealed that tight glycaemic control may significantly reduce poor perinatal outcome in women with diabetes.32 Treatment of diabetes in pregnancy, not only decreases perinatal morbidity but have also been found to augment the health-related quality of life of pregnant women.32
The prevalence of abnormal postpartum OGTT among GDM women in Selangor in this study was 12.1% which is lower compared to previous studies.33–35 It was reported that women with GDM have a 17% to 63% risk to develop postpartum diabetes within 5 to 16 years.36 In our study, the prevalence could be lower because of the shorter interval between delivery and screening which is approximately 6 weeks in this study. Therefore, prolonged periodical follow-up for this group of women may be needed to increase detection rate of abnormal postpartum OGTT. Abnormal postpartum glucose test was higher among GDM women who required insulin during pregnancy. This finding is consistent with other studies.37
Strength and limitations
This study was carried out at all public antenatal clinics in urban and rural areas within Selangor. These findings can potentially identify strategies to improve the management of future antenatal women. However, there was inadequate documentation as the data were collected retrospectively. There were also lack of results and defaulter tracing after postpartum glucose tolerance test resulting in a smaller sample size.
Conclusion
The prevalence of GDM among newly registered antenatal women in Selangor was 27.9% which was higher than previous studies. Factors associated with GDM were maternal age, obesity, history of first-degree relative with diabetes and previous history of GDM. Health care personnel may need to recognize and screen this group of women to detect and manage GDM early. Poor postpartum diabetes screening of GDM women demonstrates that strategies to improve their attendance are needed.
Recommendations
We need to ensure that all women at risk of GDM as in this study, should be screened for diabetes antenatally and postnatally. They also need to be on follow-up especially for those who were on insulin during pregnancy.
Acknowledgment
The authors would like to thank the Director General of Health Malaysia, the Selangor Health Director, Selangor District Health Officers for their kind permission and support to conduct and present this study. We would also like to thank the nursing staff for their invaluable assistance in data collection.
References
- 1.Metzger BE, Coustan DR. Proceedings of the fourth-international workshop conference on gestational diabetes mellitus. Diabetes Care. 1998;21(Suppl.2):B1–67. (eds.) [PubMed] [Google Scholar]
- 2.Nirmala K, Hanis A, Izzat H, et al. Outcome of pregnancy among Malaysian women with diabetes mellitus - a single centre experience. MJPHM Heath Medicine. 2013;13(2):1–10. [Google Scholar]
- 3.Zhao E, Zhang F, Zeng X, et al. Association between maternal diabetes mellitus and the risk of congenital malformations: a meta-analysis of cohort studies. Drug Discov & Ther. 2015;9(4):274–81. doi: 10.5582/ddt.2015.01044. [DOI] [PubMed] [Google Scholar]
- 4.Erem C, Kuzu UB, Deger O, et al. Prevalence of gestational diabetes mellitus and associated risk factors in Turkish women : the Trabzon GDM Study. Arch Med Sci. 2015;11(4):724–35. doi: 10.5114/aoms.2015.53291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Das V, Kamra S, Mishra A, et al. Screening for gestational diabetes mellitus and maternal and foetal outcome. J Obstet Gynecol Ind. 2004;54(5):449–51. [Google Scholar]
- 6.Bellamy L, Casas JP, Hingorani AD, et al. Type 2 diabetes mellitus after gestational diabetes: a systematic review and meta-analysis. Lancet. 2009;373(9677):1773–9. doi: 10.1016/S0140-6736(09)60731-5. [DOI] [PubMed] [Google Scholar]
- 7.Jiwani A, Marseille E, Lohse N, et al. Gestational diabetes mellitus: results from a survey of country prevalence and practices. J Matern Fetal Neonatal Med. 2012;25(6):600–10. doi: 10.3109/14767058.2011.587921. [DOI] [PubMed] [Google Scholar]
- 8.Sacks DA, Hadden DR, Maresh M, et al. Frequency of gestational diabetes mellitus at collaborating centers based on IADPSG consensus panel—recommended criteria: the hyperglycemia and adverse pregnancy outcome (HAPO) Study. Diabetes Care. 2012;35(3):526–28. doi: 10.2337/dc11-1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Idris N, Che Hatikah CH, Murizah MZ. et al. Universal versus selective screening for detection of gestational diabetes mellitus in a Malaysian population. Malays Fam Physician. 2009;4(2&3):83–7. [PMC free article] [PubMed] [Google Scholar]
- 10.Shamsuddin K, Mahdi ZA, Siti Rafiaah I, et al. Risk factor screening for abnormal glucose tolerance in pregnancy. Int J Gynaecol Obstet. 2001;75(1):27–32. doi: 10.1016/s0020-7292(01)00468-4. [DOI] [PubMed] [Google Scholar]
- 11.Letchuman GR, Wan Nazaimoon WM, Wan Mohamad WB. et al. Prevalence of diabetes in Malaysia National Health Morbidity Survey. Med J Malaysia. 2010;65(3):180–6. [PubMed] [Google Scholar]
- 12.Jan Mohamed HJ, Yap RW, Loy SL, et al. Prevalence and determinants of overweight, obesity, and type 2 diabetes mellitus in adults in Malaysia. Asia Pac J Public Health. 2014;18:123–35. doi: 10.1177/1010539514562447. [DOI] [PubMed] [Google Scholar]
- 13.Ministry of Health Malaysia, author. Clinical Practice Guidelines Management of Type 2 Diabetes Mellitus. 4th. Kuala Lumpur: Ministry of Health, Malaysia; 2009. edition. [Google Scholar]
- 14.World Health Organization, author. Diagnostic criteria and classification of hyperglycaemia first detected in pregnancy. 2013. [PubMed]
- 15.Lobner K, Knopff A, Baumgarten A, et al. Predictors of postpartum diabetes in women with gestational diabetes mellitus. Diabetes. 2006;55(3):792–97. doi: 10.2337/diabetes.55.03.06.db05-0746. [DOI] [PubMed] [Google Scholar]
- 16.Capula C, Chiefari E, Vero A, et al. Predictors of postpartum glucose tolerance testing in Italian women with gestational diabetes mellitus. Diabetes Res Clin Pract. 2014;105(2):223–30. doi: 10.1016/j.diabres.2014.05.008. [DOI] [PubMed] [Google Scholar]
- 17.Smirnakis KV, Chasan-Tabar L, Wolf M, et al. Postpartum diabetes screening in women with a history of gestational diabetes. Obstet Gynecol. 2005;106(6):1297–303. doi: 10.1097/01.AOG.0000189081.46925.90. [DOI] [PubMed] [Google Scholar]
- 18.Keely E, Clark H, Karovitch A, et al. Screening for type 2 diabetes following gestational diabetes. Canadian Fam Physician. 2010;56:558–63. [PMC free article] [PubMed] [Google Scholar]
- 19.Naing L, Winn T, Rusli BN. Practical issues in calculating the sample size for prevalence studies. Archives of Orofacial Sciences. 2006;1:9–14. [Google Scholar]
- 20.Ministry of Health Malaysia, author. Perinatal Care Manual. 3rd. Kuala Lumpur: Ministry of Health, Malaysia; 2013. edition. [Google Scholar]
- 21.Ministry of Health Malaysia, author. National Diabetes Registry Report. Disease Control Division (Non-Communicable Disease Section. Kuala Lumpur: Ministry of Health, Malaysia; 2009–2012. [Google Scholar]
- 22.Jolly M, Sebire N, Harris J, et al. The risks associated with pregnancy in women aged 35 years or older. Hum Reprod. 2000;15(11):2433–7. doi: 10.1093/humrep/15.11.2433. [DOI] [PubMed] [Google Scholar]
- 23.Nor Azlin MI, Norkhatijah MA, Zaleha AM, et al. Gestational diabetes mellitus in primigravidae: a mild disease. Acta Medica (Hradec Kralove) 2011;54(1):21–4. doi: 10.14712/18059694.2016.12. [DOI] [PubMed] [Google Scholar]
- 24.Mamta B, Ramesha KN, Sankara PS, et al. Determinants of gestational diabetes mellitus: a case control study in a district tertiary care hospital in South India. Int J Diabetes Dev Ctries. 2010;30(2):91–6. doi: 10.4103/0973-3930.62599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pettitt DJ, Aleck KA, Baird HR, et al. Congenital susceptibility to NIDDM. Role of intrauterine environment. Diabetes. 1988;37:622–8. doi: 10.2337/diab.37.5.622. [DOI] [PubMed] [Google Scholar]
- 26.Sreekanthan K, Belisita A, Rajendran K. et al. Prevalence of gestational diabetes mellitus in a medical college in South India: a pilot study. IJCP Practice. 2015;25(4):342–7. [Google Scholar]
- 27.Torloni MR, Betran AP, Horta BL, et al. Pre-pregnancy BMI and the risk of gestational diabetes: a systematic review of the literature with meta-analysis. Obes Rev. 2009;10(2):194–203. doi: 10.1111/j.1467-789X.2008.00541.x. [DOI] [PubMed] [Google Scholar]
- 28.Kampmann U, Madsen LR, Skajaa GO, et al. Gestational diabetes: a clinical update. World J Diabetes. 2015;6(8):1065–72. doi: 10.4239/wjd.v6.i8.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Catalano PM, Mclntyre HD, Cruickshank JK, et al. The hyperglycemia and adverse pregnancy outcome study associations of GDM and obesity with pregnancy outcomes. Diabetes Care. 2012;35:780–6. doi: 10.2337/dc11-1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kirwan JP, Haugel-De MS, Lepercq J, et al. TNF-alpha is a predictor of insulin resistance in human pregnancy. Diabetes. 2002;51:2207–13. doi: 10.2337/diabetes.51.7.2207. [DOI] [PubMed] [Google Scholar]
- 31.Noctor E, Fidelma PD. Type 2 diabetes after gestational diabetes: the influence of changing diagnostic criteria. World J Diabetes. 2015;6(2):234–44. doi: 10.4239/wjd.v6.i2.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Crowther CA, Hiller JE, Moss JR, et al. Effect of treatment of gestational diabetes mellitus on pregnancy outcomes. N Engl J Med. 2005;352:2477–86. doi: 10.1056/NEJMoa042973. [DOI] [PubMed] [Google Scholar]
- 33.Ko GTC, Chan JCN, Tsang LWW, et al. Glucose tolerance and other cardiovascular risk factors in Chinese women with a history of gestational diabetes. Aust NZ J Obstet Gynecol. 1999;39:478–83. doi: 10.1111/j.1479-828x.1999.tb03138.x. [DOI] [PubMed] [Google Scholar]
- 34.Jang HC, Yim CH, Han KO, et al. Gestational diabetes mellitus in Korea: prevalence and prediction of glucose intolerance at early postpartum. Diabetes Res Clin Pract. 2003;61:117–24. doi: 10.1016/s0168-8227(03)00110-4. [DOI] [PubMed] [Google Scholar]
- 35.Agarwal MM, Punnose J, Dhatt GS, et al. Gestational diabetes: implications of variation in post-partum follow-up criteria. Eur J Obstet Gynecol Reprod Sci. 2004;113:149–53. doi: 10.1016/j.ejogrb.2003.09.021. [DOI] [PubMed] [Google Scholar]
- 36.Yun S, Kabeer NH, Zhu BP, et al. Modifiable risk factors for developing diabetes among women with previous gestational diabetes. Prev Chronic Dis. 2007;4(1):1–8. [PMC free article] [PubMed] [Google Scholar]
- 37.Kitzmiller JL, Leona D, Taslimi M. et al. Gestational diabetes after delivery short-term management and long-term risks. Diabetes Care. 2007;30(Suppl 2):S225–35. doi: 10.2337/dc07-s221. [DOI] [PubMed] [Google Scholar]


