Abstract
Attention can be oriented externally to the environment or internally to the mind, and can be derailed by interference from irrelevant information originating from either external or internal sources. However, few studies have explored the nature and underlying mechanisms of the interaction between different attentional orientations and different sources of interference. We investigated how externally- and internally-directed attention was impacted by external distraction, how this modulated internal distraction, and whether these interactions were affected by healthy aging. Healthy younger and older adults performed both an externally-oriented visual detection task and an internally-oriented mental rotation task, performed with and without auditory sound delivered through headphones. We found that the addition of auditory sound induced a significant decrease in task performance in both younger and older adults on the visual discrimination task, and this was accompanied by a shift in the type of distractions reported (from internal to external). On the internally-oriented task, auditory sound only affected performance in older adults. These results suggest that the impact of external distractions differentially impacts performance on tasks with internal, as opposed to external, attentional orientations. Further, internal distractibility is affected by the presence of external sound and increased suppression of internal distraction.
Introduction
Our ability to manage the barrage of sensory inputs that we encounter in the world is what allows us to make appropriate decisions and engage in complex, goal-directed behavior1. Allocation of attentional resources is essential for such goal-directed behaviors, and attention can be divided into external and internal subsystems2,3. Behaviors that are “externally-oriented” are those that depend on the presence of external stimuli (e.g., paying attention to external visual or auditory stimuli, reading, or memory encoding); behaviors that are internally-oriented are those that occur in the absence of any external stimuli (e.g., planning, memory retrieval, calculation, interoception/awareness of internal states and mental imagery). An obstacle to achieving high-level performance on attention-demanding tasks is interference by both external and internal distraction. Just as goal-directed activities can be derailed by interference from irrelevant stimuli in the external environment, interference can also arise from the internal milieu in the form of intrusive thoughts, emotions, and urges4–8, or as a complex interaction between these two sources.
Impact of External Interference
The vast majority of studies have focused on the impact of external interference on performance of a primary task, likely due to the convenience of being able to manipulate external factors in a laboratory environment. Externally-presented visual and auditory distractions have been shown to impair performance on numerous externally-oriented tasks9, including episodic memory retrieval10–12, categorization performance13, and attention14,15, and also on internally-oriented tasks such as working memory16,17. Further, we have shown that early engagement of top-down control mechanisms can impact stimulus processing and minimize distraction costs associated with external interference17,18. Further, resistance to the negative impact of distraction on working memory involves maintaining functional connectivity between the prefrontal cortex (PFC) and visual cortical regions19–21.
Studies of cognitive aging have shown that healthy older adults (OA) are particularly susceptible to interference, likely contributing to deficits in cognitive control and attention regulation22,23. Specifically, OA show impairments in both focusing and distributing attention in space24, time25, and toward important object features26–29. Such failures of attentional regulation likely contribute to an array of age-related cognitive impairments. Of particular note, we have shown that OA experience deficits in the suppression of externally presented distracting information that in turn has a negative impact on task performance18,23,30–36.
Impact of Internal Interference
Prior studies of internal interference have focused on what is referred to as internal distractions or intrusions, which have been conceptualized as ‘mind-wandering’8,37, ‘stimulus-independent thought’38, ‘self-generated thought’39, ‘task-unrelated thought’40, ‘spontaneous cognition’41, ‘introspectively oriented thought’40, and more indirectly as ‘spontaneous fluctuations of attention’42. Semantic differences aside, the fundamental characteristic of internal interference is that attention is derailed from an original task or goal and instead becomes focused on internal thoughts. This shift in attentional focus may be either intentional (i.e., diversions) or unintentional (i.e., intrusions) and can occur with or without awareness. Studies of the frequency of ‘mind-wandering’ show that between 30–50% of our waking thoughts are ‘stimulus-independent’ or not related to the primary task or goal at hand43–45. These off-task thoughts occur during almost every type of behavior and task that has been monitored43,44, and they result in demonstrable costs in task performance46.
Given the well-documented age-related declines in cognitive control and in external distractor suppression33,47,48, one might predict an increased susceptibility of OA to internal distractions. Surprisingly, several studies have shown that OA report significantly fewer internal distractions on a variety of tasks49–51. One interpretation of these findings is that OA have insufficient resources to maintain both task-relevant and irrelevant thoughts; this notion is consistent with the view that mind-wandering results from failures of cognitive control.
Interactions between Internal and External Attention and Interference
Surprisingly, relatively few studies have explored the nature and underlying mechanisms of interference in the context of differing attentional orientation (i.e., internally-focused or externally-oriented). Studies from our lab have shown that external distraction interacts with both externally-oriented tasks, such as memory encoding and attention52,53; as well as internally-oriented tasks, such as memory recall10,11. A few studies have attempted to link internal distractions to externally-directed attention by examining correlations between the propensity for mind-wandering and performance on attention demanding tasks54. To our knowledge, however, no studies have evaluated how attention orientation interacts with both external distraction and the regulation of internal distractions in a common experimental framework. For example, it is unknown whether it is more difficult to regulate internal distracting thoughts when one is engaged in an internal monitoring task vs. an external monitoring task and how this is modulated by external noise. The goal of this experiment was to study internal and external distraction during both internally- and externally-oriented tasks to understand how different attentional orientations impact distraction regulation and whether these processes change during the course of healthy aging.
Materials and Methods
Participants
The participants for the study were 25 healthy younger adults (YA) between the ages of 18 and 30 years (mean = 23.1 +/− 2.1; 13F, 12M) and 25 healthy older adults (OA) between the ages of 60 and 72 years (mean = 64.5 +/− 5.3; 14F, 11M). Data were excluded for 1 YA and 2 OA due to technical or procedural problems (it was determined after the end of the experiment that one OA had removed the headphones for half of the trials and the headphones malfunctioned for the other two excluded participants). All participants were fluent English speakers and had normal or corrected-to-normal vision (screened using a Snellen chart). Our exclusion criteria were: history of neurological or psychiatric disease, use of psychoactive medications, substance misuse, and presence of serious medical conditions, including history of heart disease, diabetes, and untreated hypertension. Those participants whose hypertension was controlled by prescription medication were admitted into the study. All participants gave informed consent and received monetary compensation for their participation. All methods were carried out in accordance with relevant guidelines and regulations approved by the UCSF Committee on Human Research’s Institutional Review Board.
To be included in this experiment, we required that all older adults be deemed cognitively normal (within 1.5 SDs) relative to normative values for age-matched controls based on a neuropsychological assessment that occurred within two years of being recruited to this study. The neuropsychological evaluation included the following tests: Mini Mental State Exam (MMSE), geriatric depression scale (GDS), California Verbal Learning Test—Second Edition (CVLT-II) working memory and verbal learning, Wechsler Adult Intelligence Scale-Revised (WAIS-R) incidental recall, Stroop interference test, and the Trail-Making Test (A and B) of visual-motor sequencing, semantic fluency, and phonemic fluency measures from the Delis–Kaplan Executive Function System (D-KEFS).
Stimuli and cognitive tasks
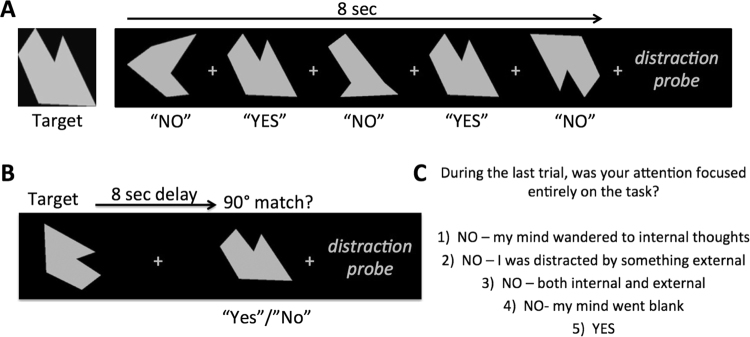
For the experimental paradigm, all participants performed two cognitive tasks: an externally-oriented (EXT) visual target detection task and an internally-oriented (INT) mental rotation task (Fig. 1). In order to attempt to equate the cognitive demands of the two tasks, identical stimuli were used for both tasks and a thresholding run was conducted in each participant for each task prior to the experimental runs. The stimuli consisted of abstract gray shapes presented on a black background. All stimuli were presented using PsychoPy v1.76 software and were displayed at 768 × 1024 pixel resolution on an LCD computer monitor positioned approximately 60 cm away from the participant. Auditory stimuli were used in a previous study of auditory distraction on long-term memory10, and consisted of 30 segments of recordings made at several public locations (i.e., a busy cafe, a children’s playground, a church mass, and an outdoor sporting event). No sound clips contained discernable words, just ambient sounds from the environment where they were recorded. All of the 8 sec sound clips were band-pass filtered at 1 kHz and their average root-mean-square power spectral densities were normalized in Matlab. Auditory stimuli were presented using Sennheiser HD202 noise-cancelling headphones. In an attempt to subjectively equate volume levels of auditory stimuli across participants, the auditory volume was initially set at a specified volume and then titrated to the point where the participants were able to comprehend several discernable words that were played among ambient background sounds during a pre-experiment test. This procedure is one that we have used successfully in the past10 to ensure that all stimuli were presented well above threshold.
Figure 1.
Experimental tasks used to assess (A) externally-oriented attention and (B) internally-oriented attention; (C) distraction probe presented to participants after every 8-sec trial to assess the presence and nature of any distractions that occurred during the previous trial.
Procedure
The experiment consisted of a thresholding run for each task followed by four blocks of both tasks (INT and EXT), with each block consisting of 30 trials. Two blocks of each task were completed with the addition of background auditory sound and two without sound; participants wore the noise-canceling headphones on all blocks, regardless of the presence or absence of auditory sound. The order of tasks was randomized for each participant.
Perceptual Thresholding
The purpose of the thresholding runs was to attempt to equate performance across tasks without sound and individuals as well as across groups of younger and older adults. For the EXT thresholding run, each trial began with participants seeing a target object on the screen and a brief reminder of the instructions. Participants were allowed to view the target item for as long as necessary. Then participants were shown probe items that were either: 1) identical matches to the target, 2) target objects that were rotated by a certain number of degrees, or 3) novel objects. Each probe item appeared for 1000 ms. Participants were instructed to respond “yes” only if a probe matched the target identically, including orientation of rotation. For the INT thresholding run, each trial began by seeing a target object that was presented for 1000 ms and participants were told to mentally rotate the object 90 degrees clockwise. After a 2000 ms delay, participants were shown target objects that were either correctly rotated 90 degrees clockwise or that were rotated by some other number of degrees. If the target matched the 90° rotation, participants were told to respond “yes” and “no” if the rotation deviated from 90°. For both conditions, an adaptive staircase algorithm then adjusted the degree of rotation based on performance: following a correct rejection of a rotated target, the degree of rotation was decreased by 1° on the subsequent trial (thus making it closer to the original target and increasing difficulty); following a false positive response to a rotated target, the degree of rotation was increased by 2° on the subsequent trial. These increments were chosen in order to find a perceptual threshold for each participant where they could perform the task at approximately 70–75% correct. Participants completed a total of 100 thresholding trials each of the EXT and INT tasks. Each participant’s thresholding level was defined as the average degree of rotation for the last 10 trials of each condition, resulting in unique thresholds that were used to set the difficultly levels independently for EXT and INT.
External Task
Each EXT trial began with a reminder of the target item (1000 ms), followed by a fixation cross (500 ms), and then presentation of five probes (1000 ms each), interleaved with fixation during an ISI (500 ms), resulting in 8 sec trials (Fig. 1A). For each stimulus, participants responded “yes” if the probe was an identical match to the target and “no” otherwise. Probes consisted of object that were: 1) identical to the target (33% of probes; “yes” response), 2) targets that were rotated the number of degrees determined by EXT thresholding (33%; “no” response), or 3) novel abstract shapes (33%; “no” response). Immediately after each sequence of five probes, participants were given a self-paced distraction probe (see description below), followed by a 1000 ms inter-trial interval.
Internal Task
Each 8 sec INT trial began with the presentation of one of 30 abstract shapes (2000 ms), followed by a delay (5000 ms) during which only a fixation cross appeared on a black screen (Fig. 1B). During the delay, participants were told to mentally rotate the target object 90° clockwise. Following the delay, a probe item was presented (1000 ms) and participants responded “yes” if the probe was a correctly rotated target (50% of trials) or “no” if the rotation deviated from 90° (50% of trials). For non-match trials, targets were rotated the number of degrees determined by INT thresholding. Following each trial, participants were given a self-paced distraction probe (see description below), followed by a 1000 ms inter-trial interval.
Distraction Probes
After each trial in the two tasks above, participants were presented a distraction probe designed to provide a subjective measure of internal and external distractions during each of the above tasks. Our probe was based on well-validated, experience sampling techniques adapted from previous studies of mind-wandering5,7,37,44,55,56. Such indices have been shown to correlate with other laboratory measures and real-world assays of mind-wandering44–46. In our experiment, after each trial, participants were asked to report via button press whether their attention was focused entirely on the task during the entire trial. If not, participants were asked to indicate if they were distracted by 1) internal thoughts, 2) something external, 3) both internal and external things, or 4) if their “mind just went blank.” The exact language used in the distraction probe prompt is provide in Fig. 1C.
Results
INT and EXT Task Performance
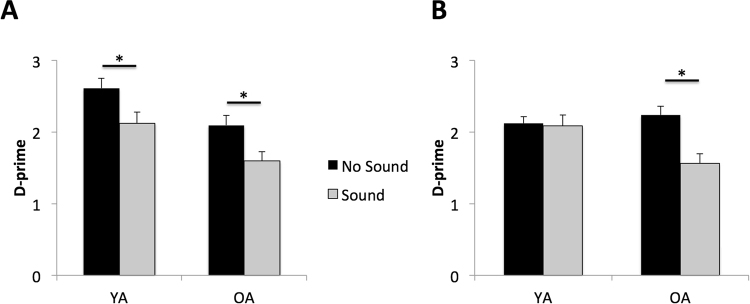
Task performance was indexed using the discrimination metric d′57, which helped to control for potential differences in false alarms or bias between the two tasks. To assess the impact of age and external auditory sound on task performance between the INT and EXT tasks, we used a 3-way mixed repeated measures multivariate general linear model (GLM) with auditory sound (present or absent) and task orientation (INT or EXT) as the within-subject factors and age group (YA vs OA) as the between subject factor (Fig. 2). In order to help account for minor differences between YA and OA in baseline task performance, we also included each participant’s INT and EXT thresholding level result as continuous covariates. This model revealed a significant 3-way interaction of sound X orientation X age (F1,43 = 14.8, p = 0.0004, partial eta2 = 0.18). In order to determine whether a particular task orientation was driving the 3-way interaction, additional post-hoc 2-way repeated measures multivariate GLMs were performed separately for d′ scores on the EXT and INT. For performance on the EXT task, we did not find a significant age by sound interaction (F1,45 = 0.03, p = 0.95, partial eta2 = 0.002). We found significant main effects of auditory sound on task performance (F1,45 = 24.0, p < 0.001, partial eta2 = 0.35), such that both YA and OA showed diminished performance on trials in which the auditory sound was present, and a main effect of age (F1,45 = 9.4, p = 0.004, partial eta2 = 0.17), such that performance was lower in OA overall, as compared to YA. For YA, d′ on the EXT task without auditory sound was 2.6 (SEM = 0.14) and with auditory sound it was 2.1 (SEM = 0.13). For OA, d′ on the task without auditory sound was 2.1 (SEM = .15) and with auditory sound it was 1.6 (SEM = 0.13). For INT, a significant interaction of age by condition (F1,45 = 9.7, p < 0.003, partial eta2 = 0.18) revealed that performance in OA was more negatively affected by the presence of auditory sound than was performance in YA (Fig. 2B). Within-subject comparisons confirmed that for YA d′ on INT did not differ between the no-sound (mean = 2.12 ± 0.1) and sound (mean = 2.08 ± 0.12) conditions (t23 = 0.24, p = 0.81); whereas OA showed a significant decrease in d′ on the sound (mean = 2.24 ± 0.15) as compared to the no-sound (mean = 1.56 ± 0.13) conditions (t22 = 4.4, p = 0.0002).
Figure 2.
Performance (d′) on the (A) external task (EXT), where both groups showed a significant decrease in d′ with auditory sound, and on the (B) internal task (INT), where only OA showed a significant decrease in d′ with auditory sound (*p < 0.02; error bars = SEM).
Impact of Auditory Sound on Distraction Reports
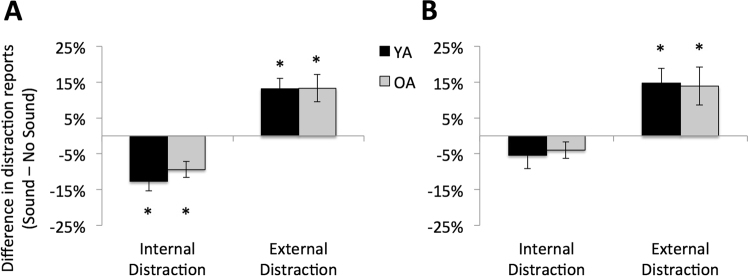
With respect to the participant’s distraction reports, our main question of interest was the impact of auditory sound on the relative frequency of self-reported internal and external distractions. We defined internal distractions as a response of “internal” or “both” to the distraction probe and external distraction as responses of “external” or “both”; the frequency of “zone-out” trials was quite low overall (less that 5% of responses in all participants), so this category of distraction was omitted from analysis. Raw frequencies of distraction reports for each category are presented in Table 1. To streamline analyses tailored to our primary hypothesis of interest, we generated difference scores for internal and external distraction reports by subtracting the percentage of trials with each type of distraction in the sound condition from the percentage in the no-sound condition. As a result, we used one-sample t-tests for a difference from zero to assess a significant difference in distraction reports between conditions. Due to the number of comparisons, we report both raw and Bonferroni corrected (indicated by “pcorr”) statistics. On the EXT task, the presence of external auditory sound led to a decrease in the number of internal distractions and an increase in the number of external distractions in both groups (Fig. 3A). Specifically, in YA the presence of auditory sound led to a 12.6% (SEM = 3.0%; t22 = −4.7, p = 0.0001, pcorr = 0.0008) reduction of internal distractions and a 13.2% (SEM = 2.9%; t22 = 4.4, p = 0.0002, pcorr = 0.002) increase of external distractions; in OA, the presence of auditory sound led to a 9% (SEM = 2.2%; t22 = −4.3, p = 0.0003, pcorr = 0.002) reduction of internal distractions and a 13.3% (SEM = 3.8%; t22 = 3.5, p = 0.002, pcorr = 0.016) increase of external distractions. On the INT task, the presence of external auditory sound led to an increase in the number of external distractions in both groups (Fig. 3B), but neither group showed a significant decrease in internal distractions. In YA, the presence of auditory sound led to a 5% (SEM = 3.3%; t22 = −1.6, p = 0.12, pcorr = 0.96) reduction of internal distractions and a 13% (SEM = 2.9%; t22 = 4.4, p = 0.0002, pcorr = 0.002) increase of external distractions; in OA, the presence of auditory sound led to a 4% (SEM = 2.3%; t22 = −2.0, p = 0.06, pcorr = 0.48) increase in internal distractions and a 14% (SEM = 5.3%; t22 = 3.5, p = 0.003, pcorr = 0.024) increase of external distractions.
Table 1.
Distraction Frequencies.
| Distraction: | Young Adults | Older Adults | |||
|---|---|---|---|---|---|
| Internal | External | Internal | External | ||
| EXT | No Sound | 14 | 2 | 11 | 1 |
| Sound | 6 | 10 | 6 | 9 | |
| INT | No Sound | 11 | 1 | 8 | 1 |
| Sound | 8 | 9 | 3 | 10 | |
Mean number of internal vs external distractions reported by young and older adults in response to the distraction probes (60 probes total per condition) for the externally-oriented (EXT) and internally-oriented (INT) task performed with or without presentation of auditory sounds.
Figure 3.
Effect of auditory sound on distraction reports for the (A) external task and (B) internal task (*one-sample t-test, pcorr < 0.02; error bars = SEM).
Task Performance and Suppression of Internal Distractions
To determine the extent to which susceptibility to internal distractions impacted task performance, we correlated the difference scores for reports of internal distractions on the sound vs. no-sound conditions with performance on the task conditions that contained external auditory sounds. We did not find a significant correlation between performance on the INT task and change scores for either internal (r46 = 0.14, p = 0.35; pcorr = 1.4) or external (r46 = −0.24, p = 0.11; pcorr = 0.44) distractions. For EXT, however, we found a significant negative correlation between the percent reduction of internal distractions between sound and no-sound conditions and task performance (r = −0.41, p = 0.005, pcorr = 0.02), such that in greater suppression of internal distractions in the presence of auditory sound was associated with better task performance (Fig. 4). We did not find a significant correlation between change in external distractions and task performance on EXT (r46 = 0.21, p = 0.16; pcorr = 0.64).
Figure 4.
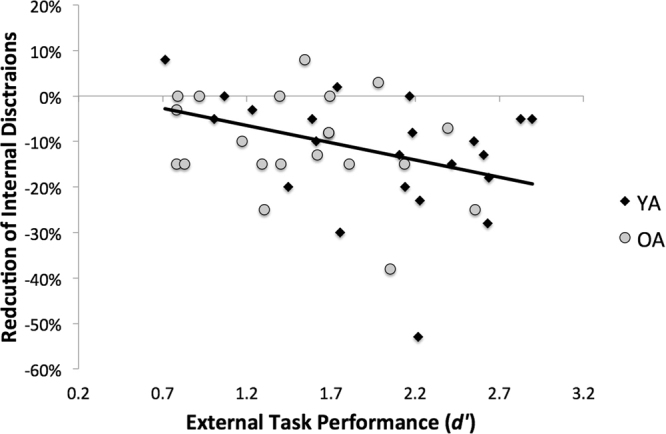
Task Performance and Suppression of Internal Distractions. Reduction of internal distractions between the sound and no-sound conditions predicted task performance on the external task in the presence of auditory sound for both YA (black diamonds) and OA (gray circles).
Discussion
In the present study, we sought to determine whether external interference had a differential impact on task performance depending on whether attention was directed internally or externally, and we examined the extent to which internal distractibility, or mind-wandering was affected by the presence of external distractions. We were also interested in understanding how modulation of internal distractibility in the presence of external distraction relates to task performance, and whether these dynamics are affected by healthy aging. Healthy YA and OA performed two tasks—one that was externally-oriented and one with an internally-oriented focus of attention—with and without auditory sound. In terms of performance on the two tasks, we found that auditory sound induced a significant decrease in performance in both young and older adults on the EXT task, and this was accompanied by a shift in the type of distractions reported such that the presence of external auditory sound led to a suppression of internal distractions and increased external distraction. We also found that the degree of suppression of internal distractions predicted successful performance on the EXT task. On the internal task, sound only affected performance in OA and performance did not correlate with changes in internal distraction reports.
Impact of external sound on task performance and distractibility
Internal and external distractions each can lead to performance decrements on a variety of tasks15,18,23,30–36,58,59 but few studies have examined both types of distractions in a single experiment60,61. Those studies that have done so report a fairly even split in the frequency of these two types of distractions62. Subsequent research has led to the emergence of two distinct views of internal versus external distractions. On the one hand, some researchers have proposed that internal distractions (e.g., mind-wandering) are distinguishable from external distractions both neurally and behaviorally60,62,63. Often referred to as the decoupling hypothesis, this line of evidence suggests that the process of being internally distracted is fundamentally different from the process of being externally distracted, and the process of turning one’s attention inward may actually decrease ones propensity to be distracted by external stimuli, by decoupling or disengaging with the external environment. More recently, Unsworth and McMillan (2014) used latent variable analysis to show that, while highly correlated, external and internal distractions (in the form of task-unrelated thoughts) are in fact distinct entities, but also found evidence that when a person is in a state of mind-wandering, they are less likely to be distracted by external stimuli.
While a few studies have sought to characterize the relationship between internal and external distractions, no other studies have, to our knowledge, sampled the frequency of these two types of distractions while experimentally manipulating the amount of external sound present during tasks with differing loci of attentional focus. Rather, most studies of mind-wandering have relied on naturally occurring background sounds as sources of external distraction. In the present study, we manipulated the auditory environment that participants experienced during task engagement, such that it was either quiet or there was auditory sound presented via headphones. In doing so, our study revealed a trade off, or push-pull, relationship between internal and external distractions in both YA & OA in the setting of sound presented during a task. However, this effect was most pronounced when the task had an external orientation (visual target discrimination), where there was an increase in external distractions and a suppression of internal distractions in the presence of external auditory sound. When the orientation of the task was internal, we still saw an increase in the frequency of external distractions in the presence of auditory sound, but without an accompanying suppression of internal distractions. Thus, while much prior research has focused on how the act of focusing ones attention internally can impact susceptibility to external distractions60,62,63, here we add to this literature by showing that, when the external sound is added, attention can be pulled significantly away from internal distractions as it is shifted to the external source of distractions. An interesting question is whether there is a critical auditory threshold at which point the background sound begins to have an impact on internal distractibility. The use of a single, standardized, decibel level in the present study precludes further insight into such questions.
Critically, we found that the suppression of internal distractions predicted performance on the externally-oriented task, such that participants who reduced their internal distractions most in the sound condition had the highest task performance. Indeed, we found no clear link between an increase in external distractions and task performance; rather, suppression of internal distractions under the more distracting sound condition appears to have a more prominent association with performance. This finding could provide a possible explanation for why some people actually seek out noisy environments (e.g., coffee shops) to engage in attention-demanding task (e.g., studying or writing). Namely, the presence of external distractions could aid in the suppression of internal distractions, in turn facilitating task performance. Our results would also predict that such behavior would have a differential benefit, depending on the task demands, and performance under externally noisy conditions would likely be associated with improved performance on tasks with an external focus of attention (e.g., using a laptop or mobile device or reading a book) more than tasks with an internal focus (e.g., introspection or mental planning). At the same time, not all individuals feel they benefit from studying in a noisy environment. Unfortunately the present sample size doesn’t allow a thorough examination of the degree to which individual differences might factor into our findings.
Interestingly, internal distractions have been linked to increased activity in the default mode network (DMN)5,8,62,64, and activity in this network tends to be negatively correlated with activation of attention networks and with performance on attention-demanding tasks41,65–67. A future investigation of brain activity during a cognitive paradigm similar to that employed here would shed light on the nature of the neural underpinnings of internal and external distractions.
Impact of age on susceptibility to internal and external distractions
Studies in our lab have consistently shown that OA experience deficits in the suppression of externally-presented distracting information31, that these deficits occur at early visual processing stages18,32,36,52 and that this is mediated by a failure to maintain functional connectivity between prefrontal and visual cortices31. This susceptibility to external distractions in OA leads to disruption of performance in numerous domains, including working memory18,32, attention68, and long-term memory10,11. In contrast to this deficit in external distraction suppression, OA have been shown to exhibit fewer internal distractions during certain tasks compared to young adults49,50,69,70. This potential discrepancy between deficits in internal and external distractor suppression in OA clearly warrants exploration. Others have proposed that the apparent decrease in mind wandering frequency in OA actually stems from diminished cognitive control51.
Indeed, there is evidence that ‘mind-wandering’ reflects both state-dependent changes in cognitive status, varying inversely with both task difficulty and arousal37,55, and trait-level individual differences in executive function44. In general, the frequency of reporting internal distractions is inversely correlated with executive processes. Mind-wandering increases as tasks become well-practiced58; it does not affect performance on easy, mundane tasks, but negatively impacts tasks that require cognitive control38. Further, the frequency of internal distractions correlates negatively with WM capacity7,8,44. A growing body of research implicates inefficient regulation of cognitive control processes as a core mechanism underlying many of the cognitive deficits associated with healthy aging47,71–75. Taken together, these findings have led to the notion that OA may have insufficient cognitive resources to maintain both task-relevant and irrelevant thoughts during task performance. Such a view would be consistent with the idea that mind-wandering results from cognitive control failures regardless of age. According to the reduced cognitive resources theory of aging, OA may have fewer overall cognitive resources than YA. When engaged in an attention-demanding task, OA thus invest more of their available resources into performing the task, and have less ability to simultaneously engage in mind-wandering or internal thoughts that are not task-relevant59.
Our findings add to this literature on the nature of internal distractions in healthy aging. On the one hand, we found no difference in the impact of external sound on the relative frequencies of internal versus external distraction reports between YA and OA. Both groups showed a shift from more internal distractions under relatively quiet task conditions to increased external distractions (and fewer internal distractions) when faced with externally-presented auditory sound. Interestingly, on the EXT task, both groups exhibited a performance cost associated with the auditory sound and external distractions, whereas on the internally-oriented task, only OA showed a cost related to external sound. This finding raises the intriguing possibility that there may be differential effects of age on the interaction between distraction and the ability to perform attentionally-demanding tasks depending on the focus of the task at hand.
To our knowledge, no other studies have examined the impact of age on two separate tasks with differing (internal vs external) orientations, with and without the presence of auditory distractions. From this parametric examination, we were able to determine that OA appear to be more susceptible to distractions, regardless of task orientation, whereas YA seem to be more resilient to distractions on a task with an internal orientation. Previous research in our lab has shown that when OA are faced with a need to multitask, they tend to be more impaired on a concurrent working memory task (i.e., an internally-oriented task), when compared to YA76. Further, while YA are quickly able to reengage functional connectivity between the networks associated with the working memory trace, OA appear less able to reengage and the network remains uncoupled following distraction. The present findings may shed new light on the question of whether there are separable, even dissociable, neurocognitive systems underlying internal and external attention2,3. A possible explanation for our current findings is that performing a task with an internal orientation while listening to distracting background noise constitutes a form of task-switching, and, consistent with previous reports, OA are more impaired on such tasks16,76,77. In contrast, performing an externally oriented task may require less switching between systems, but may place an undue burden on the limited resources available for processing external stimuli, thus leading to decrements in performance in both YA and OA. Future studies with neuroimaging measures would help to clarify the nature of the neural systems that are at play during performance of tasks with internal vs external orientations.
It should be noted that the present study has some limitations. The sample size was relatively small and, while many of the magnitudes of behavioral effects were large, others were only modest, and thus warrant additional exploration and replication in future studies employing larger samples. In addition, it should be noted that our participants did not undergo formal screening for hearing acuity/impairment. While we did attempt to subjectively equate volume levels of auditory stimuli across participants, ensuring that all stimuli were presented well above threshold, we cannot rule out the possibility that differences in hearing might have impacted our results. Future studies of auditory distraction in older adults should include a more formal test of hearing abilities.
Acknowledgements
We thank A.J. Simon, Hyein Cho, Andrew Garcia, Sophia Corona, Minsu Kim, Jordie Martin, and Julia Kang for help with data collection and analysis. We also thank Joaquin Anguera and Peter Wais for helpful comments on the original draft of the manuscript and for helpful discussions during the revision process. This research was supported by NIH grants R21-AG021525 and R01- AG049424.
Author Contributions
D.A.Z. and A.G. conceived and designed the study. J.J. and D.A.Z. designed the experiments, collected data, and conducted the analyses. D.A.Z. and A.G. wrote the manuscript. All authors reviewed and approved the paper.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
David A. Ziegler, Email: david.ziegler@ucsf.edu
Adam Gazzaley, Email: adam.gazzaley@ucsf.edu.
References
- 1.Gazzaley A, Nobre AC. Top-down modulation: bridging selective attention and working memory. Trends Cogn Sci. 2012;16:129–135. doi: 10.1016/j.tics.2011.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chun MM, Golomb JD, Turk-Browne NB. A taxonomy of external and internal attention. Annu Rev Psychol. 2011;62:73–101. doi: 10.1146/annurev.psych.093008.100427. [DOI] [PubMed] [Google Scholar]
- 3.Dixon ML, Fox KC, Christoff K. A framework for understanding the relationship between externally and internally directed cognition. Neuropsychologia. 2014;62:321–330. doi: 10.1016/j.neuropsychologia.2014.05.024. [DOI] [PubMed] [Google Scholar]
- 4.Beauregard M, Levesque J, Bourgouin P. Neural correlates of conscious self-regulation of emotion. J Neurosci. 2001;21:RC165. doi: 10.1523/JNEUROSCI.21-18-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dam-Larsen S, et al. Final results of a long-term, clinical follow-up in fatty liver patients. Scandinavian journal of gastroenterology. 2009;44:1236–1243. doi: 10.1080/00365520903171284. [DOI] [PubMed] [Google Scholar]
- 6.Dolcos F, McCarthy G. Brain systems mediating cognitive interference by emotional distraction. J Neurosci. 2006;26:2072–2079. doi: 10.1523/JNEUROSCI.5042-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kane MJ, et al. For whom the mind wanders, and when: an experience-sampling study of working memory and executive control in daily life. Psychol Sci. 2007;18:614–621. doi: 10.1111/j.1467-9280.2007.01948.x. [DOI] [PubMed] [Google Scholar]
- 8.Mason MF, et al. Wandering minds: the default network and stimulus-independent thought. Science. 2007;315:393–395. doi: 10.1126/science.1131295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berti S, Schroger E. A comparison of auditory and visual distraction effects: behavioral and event-related indices. Brain Res Cogn Brain Res. 2001;10:265–273. doi: 10.1016/S0926-6410(00)00044-6. [DOI] [PubMed] [Google Scholar]
- 10.Wais PE, Gazzaley A. The impact of auditory distraction on retrieval of visual memories. Psychon Bull Rev. 2011;18:1090–1097. doi: 10.3758/s13423-011-0169-7. [DOI] [PubMed] [Google Scholar]
- 11.Wais PE, Martin GM, Gazzaley A. The impact of visual distraction on episodic retrieval in older adults. Brain Res. 2012;1430:78–85. doi: 10.1016/j.brainres.2011.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zeamer C, Fox Tree JE. The process of auditory distraction: disrupted attention and impaired recall in a simulated lecture environment. J Exp Psychol Learn Mem Cogn. 2013;39:1463–1472. doi: 10.1037/a0032190. [DOI] [PubMed] [Google Scholar]
- 13.Wais PE, Gazzaley A. External distraction impairs categorization performance in older adults. Psychol Aging. 2014;29:666–671. doi: 10.1037/a0037617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wetzel N, Schroger E. On the development of auditory distraction: A review. Psych J. 2014;3:72–91. doi: 10.1002/pchj.49. [DOI] [PubMed] [Google Scholar]
- 15.Forster S, Lavie N. Entirely irrelevant distractors can capture and captivate attention. Psychon Bull Rev. 2011;18:1064–1070. doi: 10.3758/s13423-011-0172-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.West R. Visual distraction, working memory, and aging. Mem Cognit. 1999;27:1064–1072. doi: 10.3758/BF03201235. [DOI] [PubMed] [Google Scholar]
- 17.Rutman AM, Clapp WC, Chadick JZ, Gazzaley A. Early top-down control of visual processing predicts working memory performance. J Cogn Neurosci. 2010;22:1224–1234. doi: 10.1162/jocn.2009.21257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gazzaley A, Cooney JW, Rissman J, D’Esposito M. Top-down suppression deficit underlies working memory impairment in normal aging. Nature Neuroscience. 2005;8:1298–1300. doi: 10.1038/nn1543. [DOI] [PubMed] [Google Scholar]
- 19.Gazzaley A, Rissman J, Desposito M. Functional connectivity during working memory maintenance. Cogn Affect Behav Neurosci. 2004;4:580–599. doi: 10.3758/CABN.4.4.580. [DOI] [PubMed] [Google Scholar]
- 20.Gazzaley A, et al. Functional interactions between prefrontal and visual association cortex contribute to top-down modulation of visual processing. Cereb Cortex. 2007;17(Suppl 1):i125–135. doi: 10.1093/cercor/bhm113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rissman J, Gazzaley A, D’Esposito M. Measuring functional connectivity during distinct stages of a cognitive task. Neuroimage. 2004;23:752–763. doi: 10.1016/j.neuroimage.2004.06.035. [DOI] [PubMed] [Google Scholar]
- 22.Mishra J, Anguera JA, Ziegler DA, Gazzaley A. A cognitive framework for understanding and improving interference resolution in the brain. Prog Brain Res. 2013;207:351–77. doi: 10.1016/B978-0-444-63327-9.00013-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clapp WC, Gazzaley A. Distinct mechanisms for the impact of distraction and interruption on working memory in aging. Neurobiology of Aging. 2012;33(1):134–48. doi: 10.1016/j.neurobiolaging.2010.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Madden DJ, et al. Aging and attentional guidance during visual search: functional neuroanatomy by positron emission tomography. Psychology and aging. 2002;17:24–43. doi: 10.1037/0882-7974.17.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zanto TP, et al. Age-related changes in orienting attention in time. J Neurosci. 2011;31:12461–12470. doi: 10.1523/JNEUROSCI.1149-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schiavetto A, Kohler S, Grady CL, Winocur G, Moscovitch M. Neural correlates of memory for object identity and object location: effects of aging. Neuropsychologia. 2002;40:1428–1442. doi: 10.1016/S0028-3932(01)00206-8. [DOI] [PubMed] [Google Scholar]
- 27.Grady CL, et al. Age-related changes in cortical blood flow activation during visual processing of faces and location. J Neurosci. 1994;14:1450–1462. doi: 10.1523/JNEUROSCI.14-03-01450.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bollinger J, Rubens MT, Masangkay E, Kalkstein J, Gazzaley A. An expectation-based memory deficit in aging. Neuropsychologia. 2011;49:1466–1475. doi: 10.1016/j.neuropsychologia.2010.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Quigley C, Muller MM. Feature-selective attention in healthy old age: a selective decline in selective attention? J Neurosci. 2014;34:2471–2476. doi: 10.1523/JNEUROSCI.2718-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Berry AS, et al. The influence of perceptual training on working memory in older adults. PLoS ONE. 2010;5:e11537. doi: 10.1371/journal.pone.0011537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Clapp WC, Rubens MT, Sabharwal J, Gazzaley A. A deficit in switching between functional brain networks underlies the impact of multitasking on working memory in older adults. Proc Natl Acad Sci USA. 2011;108:7212–7217. doi: 10.1073/pnas.1015297108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gazzaley A, et al. Age-related top-down suppression deficit in the early stages of cortical visual memory processing. Proc Natl Acad Sci USA. 2008;105:13122–13126. doi: 10.1073/pnas.0806074105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gazzaley A, D’Esposito M. Top-down modulation and normal aging. Ann N Y Acad Sci. 2007;1097:67–83. doi: 10.1196/annals.1379.010. [DOI] [PubMed] [Google Scholar]
- 34.Gazzaley A, Sheridan MA, Cooney JW, D’Esposito M. Age-related deficits in component processes of working memory. Neuropsychology. 2007;21:532–539. doi: 10.1037/0894-4105.21.5.532. [DOI] [PubMed] [Google Scholar]
- 35.Zanto TP, Hennigan K, Ostberg M, Clapp WC, Gazzaley A. Predictive knowledge of stimulus relevance does not influence top-down suppression of irrelevant information in older adults. Cortex. 2010;46:564–574. doi: 10.1016/j.cortex.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zanto TP, Toy B, Gazzaley A. Delays in neural processing during working memory encoding in normal aging. Neuropsychologia. 2010;48:13–25. doi: 10.1016/j.neuropsychologia.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smallwood J, Schooler JW. The restless mind. Psychol Bull. 2006;132:946–958. doi: 10.1037/0033-2909.132.6.946. [DOI] [PubMed] [Google Scholar]
- 38.Teasdale JD, et al. Stimulus-independent thought depends on central executive resources. Mem Cognit. 1995;23:551–559. doi: 10.3758/BF03197257. [DOI] [PubMed] [Google Scholar]
- 39.Callard F, Smallwood J, Margulies DS. Default Positions: How Neuroscience’s Historical Legacy has Hampered Investigation of the Resting Mind. Front Psychol. 2012;3:321. doi: 10.3389/fpsyg.2012.00321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fransson P. How default is the default mode of brain function? Further evidence from intrinsic BOLD signal fluctuations. Neuropsychologia. 2006;44:2836–2845. doi: 10.1016/j.neuropsychologia.2006.06.017. [DOI] [PubMed] [Google Scholar]
- 41.Andrews-Hanna JR, Reidler JS, Huang C, Buckner RL. Evidence for the default network’s role in spontaneous cognition. Journal of neurophysiology. 2010;104:322–335. doi: 10.1152/jn.00830.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cohen MR, Maunsell JH. When attention wanders: how uncontrolled fluctuations in attention affect performance. J Neurosci. 2011;31:15802–15806. doi: 10.1523/JNEUROSCI.3063-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Killingsworth MA, Gilbert DT. A wandering mind is an unhappy mind. Science. 2010;330:932. doi: 10.1126/science.1192439. [DOI] [PubMed] [Google Scholar]
- 44.McVay JC, Kane MJ, Kwapil TR. Tracking the train of thought from the laboratory into everyday life: an experience-sampling study of mind wandering across controlled and ecological contexts. Psychon Bull Rev. 2009;16:857–863. doi: 10.3758/PBR.16.5.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smilek D, Carriere JS, Cheyne JA. Failures of sustained attention in life, lab, and brain: ecological validity of the SART. Neuropsychologia. 2010;48:2564–2570. doi: 10.1016/j.neuropsychologia.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 46.Smallwood J, Fishman DJ, Schooler JW. Counting the cost of an absent mind: mind wandering as an underrecognized influence on educational performance. Psychon Bull Rev. 2007;14:230–236. doi: 10.3758/BF03194057. [DOI] [PubMed] [Google Scholar]
- 47.Braver TS, Barch DM. A theory of cognitive control, aging cognition, and neuromodulation. Neurosci Biobehav Rev. 2002;26:809–817. doi: 10.1016/S0149-7634(02)00067-2. [DOI] [PubMed] [Google Scholar]
- 48.Ziegler DA, et al. Cognition in healthy aging is related to regional white matter integrity, but not cortical thickness. Neurobiol Aging. 2010;31:1912–1926. doi: 10.1016/j.neurobiolaging.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Giambra LM. Task-unrelated-thought frequency as a function of age: a laboratory study. Psychol Aging. 1989;4:136–143. doi: 10.1037/0882-7974.4.2.136. [DOI] [PubMed] [Google Scholar]
- 50.Jackson JD, Balota DA. Mind-Wandering in Younger and Older Adults: Converging Evidence From the Sustained Attention to Response Task and Reading for Comprehension. Psychology and aging. 2012;27:106–119. doi: 10.1037/a0023933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McVay JC, Meier ME, Touron DR, Kane MJ. Aging ebbs the flow of thought: Adult age differences in mind wandering, executive control, and self-evaluation. Acta Psychologica. 2013;142:136–147. doi: 10.1016/j.actpsy.2012.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Clapp WC, Gazzaley A. Distinct mechanisms for the impact of distraction and interruption on working memory in aging. Neurobiol Aging. 2012;33:134–148. doi: 10.1016/j.neurobiolaging.2010.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zanto TP, Gazzaley A. Neural suppression of irrelevant information underlies optimal working memory performance. J Neurosci. 2009;29:3059–3066. doi: 10.1523/JNEUROSCI.4621-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brefczynski-Lewis JA, Lutz A, Schaefer HS, Levinson DB, Davidson RJ. Neural correlates of attentional expertise in long-term meditation practitioners. Proc Natl Acad Sci USA. 2007;104:11483–11488. doi: 10.1073/pnas.0606552104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Braboszcz C, Delorme A. Lost in thoughts: neural markers of low alertness during mind wandering. Neuroimage. 2010;54:3040–3047. doi: 10.1016/j.neuroimage.2010.10.008. [DOI] [PubMed] [Google Scholar]
- 56.Davidson RJ. Empirical explorations of mindfulness: conceptual and methodological conundrums. Emotion (Washington, D.C.) 2010;10:8–11. doi: 10.1037/a0018480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Macmillan, N. A. & Creelman, C. D. Detection theory: A user’s guide (2nd ed.). Mahwah, New Jersey: Lawrence Erlbaum Associates. (2005).
- 58.Smallwood J, et al. Subjective experience and the attentional lapse: task engagement and disengagement during sustained attention. Consciousness and cognition. 2004;13:657–690. doi: 10.1016/j.concog.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 59.Maillet D, Schacter DL. From mind wandering to involuntary retrieval: Age-related differences in spontaneous cognitive processes. Neuropsychologia. 2016;80:142–156. doi: 10.1016/j.neuropsychologia.2015.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Unsworth N, McMillan BD. Similarities and differences between mind-wandering and external distraction: a latent variable analysis of lapses of attention and their relation to cognitive abilities. Acta Psychol (Amst) 2014;150:14–25. doi: 10.1016/j.actpsy.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 61.Handy TC, Kam JW. Mind wandering and selective attention to the external world. Can J Exp Psychol. 2015;69:183–189. doi: 10.1037/cep0000051. [DOI] [PubMed] [Google Scholar]
- 62.Stawarczyk D, Majerus S, Maj M, Van der Linden M, D’Argembeau A. Mind-wandering: phenomenology and function as assessed with a novel experience sampling method. Acta Psychol (Amst) 2011;136:370–381. doi: 10.1016/j.actpsy.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 63.Barron E, Riby LM, Greer J, Smallwood J. Absorbed in thought: the effect of mind wandering on the processing of relevant and irrelevant events. Psychol Sci. 2011;22:596–601. doi: 10.1177/0956797611404083. [DOI] [PubMed] [Google Scholar]
- 64.Gruberger M, Ben-Simon E, Levkovitz Y, Zangen A, Hendler T. Towards a neuroscience of mind-wandering. Frontiers in human neuroscience. 2011;5:56. doi: 10.3389/fnhum.2011.00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Andrews-Hanna JR, Reidler JS, Sepulcre J, Poulin R, Buckner RL. Functional-anatomic fractionation of the brain’s default network. Neuron. 2010;65:550–562. doi: 10.1016/j.neuron.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- 67.Fox KC, Spreng RN, Ellamil M, Andrews-Hanna JR, Christoff K. The wandering brain: meta-analysis of functional neuroimaging studies of mind-wandering and related spontaneous thought processes. Neuroimage. 2015;111:611–621. doi: 10.1016/j.neuroimage.2015.02.039. [DOI] [PubMed] [Google Scholar]
- 68.Cashdollar N, et al. Prolonged disengagement from attentional capture in normal aging. Psychology and aging. 2013;28:77–86. doi: 10.1037/a0029899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zavagnin M, Borella E, De Beni R. When the mind wanders: Age-related differences between young and older adults. Acta Psychologica. 2014;145:54–64. doi: 10.1016/j.actpsy.2013.10.016. [DOI] [PubMed] [Google Scholar]
- 70.Frank DJ, Nara B, Zavagnin M, Touron DR, Kane MJ. Validating older adults’ reports of less mind-wandering: An examination of eye movements and dispositional influences. Psychol Aging. 2015;30:266–278. doi: 10.1037/pag0000031. [DOI] [PubMed] [Google Scholar]
- 71.Miyake A, et al. The unity and diversity of executive functions and their contributions to complex “Frontal Lobe” tasks: a latent variable analysis. Cognitive psychology. 2000;41:49–100. doi: 10.1006/cogp.1999.0734. [DOI] [PubMed] [Google Scholar]
- 72.Fletcher PC, Henson RN. Frontal lobes and human memory: insights from functional neuroimaging. Brain. 2001;124:849–881. doi: 10.1093/brain/124.5.849. [DOI] [PubMed] [Google Scholar]
- 73.Braver, T. S. & Ruge, H. Functional Neuroimaging of Executive Functions. in Handbook of Functional Neuroimaging of Cognition, 2nd Edition (eds. Cabeza, R. and Kingstone, A.) 307–347 (MIT Press, Cambridge, MA, 2006).
- 74.Salthouse TA, Atkinson TM, Berish DE. Executive functioning as a potential mediator of age-related cognitive decline in normal adults. Journal of experimental psychology. 2003;132:566–594. doi: 10.1037/0096-3445.132.4.566. [DOI] [PubMed] [Google Scholar]
- 75.Glisky, E. L. Changes in Cognitive Function in Human Aging. In Brain Aging: Models, Methods, and Mechanisms (ed. Riddle, D.R.) Chapter 1. (CRC Press, Boca Raton (FL), 2007). [PubMed]
- 76.Clapp WC, Rubens MT, Sabharwal J, Gazzaley A. Deficit in switching between functional brain networks underlies the impact of multitasking on working memory in older adults. Proc Natl Acad Sci USA. 2011;108:7212–7217. doi: 10.1073/pnas.1015297108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Neider MB, et al. Walking and talking: dual-task effects on street crossing behavior in older adults. Psychology and aging. 2011;26:260–268. doi: 10.1037/a0021566. [DOI] [PMC free article] [PubMed] [Google Scholar]