Abstract
Extracellular signal-regulated kinase (ERK) signaling regulates hormone action in the reproductive axis, but specific mechanisms have yet to be completely elucidated. In the current study, ERK1 null and ERK2 floxed mice were combined with a gonadotropin-releasing hormone receptor (GnRHR)–internal ribosomal entry site-Cre (GRIC) driver. Female ERK double-knockout (ERKdko) animals were hypogonadotropic, resulting in anovulation and complete infertility. Transcript levels of four gonadotrope-specific genes (GnRHR and the three gonadotropin subunits) were reduced in pituitaries at estrus in ERKdko females, and the postcastration response to endogenous GnRH hyperstimulation was blunted. As females aged, they exhibited abnormal ovarian histology, as well as increased body weight. ERKdko males were initially less affected, showing moderate subfertility, up to 6 months of age. Male ERKdko mice also displayed a blunted response to endogenous GnRH following castration. By 12 months of age, ERKdko males had reduced testicular weights and sperm production. By 18 months of age, the ERKdko males displayed reduced testis and seminal vesicle weights, marked seminiferous tubule degeneration, and a 77% reduction in sperm production relative to controls. As the GRIC is also active in the male germ line, we examined the specific role of ERK loss in the testes using the stimulated by retinoic acid 8 (Stra8)-Cre driver. Whereas ERK loss in GRIC and Stra8 males resulted in comparable losses in sperm production, seminiferous tubule histological degeneration was only observed in the GRIC-ERKdko animals. Our data suggest that loss of ERK signaling and hypogonadotropism within the reproductive axis impacts fertility and gonadal aging.
We characterize the impact of deletion of ERKs within the pituitary gonadotrope on female and male fertility. We focus on the role of ERK signaling during gonadal aging.
The hypothalamic-pituitary-gonadal (HPG) axis regulates reproduction through multiple interconnected endocrine-feedback loops (1, 2). Neurons within the hypothalamus secrete gonadotropin-releasing hormone (GnRH) into the hypophyseal portal vasculature to act on gonadotrope cells in the anterior pituitary (3, 4). Gonadotrope cells are characterized by expression of the GnRH receptor (GnRHR), and comprise ∼7% to 10% of the anterior pituitary cell population (5). In response to GnRH stimulation, the gonadotrope produces and secretes two peptide hormones: luteinizing hormone (LH) and follicle-stimulating hormone (FSH) (6, 7). These glycoprotein hormones are comprised of two gonadotropin-specific subunits, LHβ and FSHβ, which combine with a common glycoprotein hormone α subunit (CGA) to create bioactive gonadotropins (8). In turn, these hormones are secreted into the systemic circulation and act upon the gonads to control steroidogenesis and gametogenesis (9–12).
The isolation of specific signaling pathways using gene-excision techniques within the HPG axis of the mouse clarifies the role and requirement for specific cell-signaling events on fertility. To this end, we focused on the role of extracellular signal-regulated kinase (ERK) 1 and 2 in pituitary gonadotropes. Whereas whole-body knockout (KO) of ERK2 results in embryonic lethality, ERK1 null (ERK1−/−) animals are viable and fertile (13–16). Therefore, the understanding of the role of ERK2 in reproductive tissues requires a cell-specific KO. Within the GnRH signaling network, ERKs control immediate-early gene expression and activation via reversible phosphorylation. Activation of factors, such as early growth response factor 1, c-Fos, activating transcription factor 3, and NR4A1, is crucial for appropriate response(s) to GnRH stimulation of gonadotropin subunit genes (among others) and is all ERK signaling dependent (17, 18). Thus, ERK1/2 signaling is an integral regulator of gonadotrope function and of other cells/tissues in the HPG axis (19–23).
Multiple laboratories, including our own, have used gonadotrope-specific gene deletion models to understand the role of genes or signaling intermediates in the neuroendocrine axis (24–26). Previously, we used a CGA promoter-Cre driver mouse strain to understand the role of ERK1/2 in pituitary gonadotrope behavior (25). Although useful, CGA-Cre is not specific to gonadotropes, as this Cre driver is expressed in the pituitary thyrotrope lineage, which also expresses CGA endogenously. The central caveat in our early studies was that of the ERK-deficient animals generated using CGA-Cre, only ∼50% survived to weaning. Of those animals surviving to adulthood, thyroid-stimulating hormoneβ expression levels were normal, suggesting a euthyroid state in this cohort. However, ∼50% of the CGA-Cre ERK-deficient animals failed to survive the neonatal period for unknown reasons, raising the possibility of the potential impact of loss of ERK signaling in the thyrotrope lineage as a potentially confounding component of the animals that did not survive. A novel gonadotrope-specific Cre driver, the GnRHR–internal ribosomal entry site–Cre (GRIC) mouse, has more recently been used to study ablation of genes, specifically in gonadotropes (5, 24, 27–32). In this model, Cre recombinase was “knocked in” downstream of the GnRHR coding region, along with an internal ribosomal entry site. These mice express a bicistronic mRNA from which the GnRHR and the Cre recombinase proteins are independently translated (5). GRIC-Cre recombinase activity has been detected in this model as early as embryonic day (e)12.75 in the pituitary and increases thereafter during development (27), markedly later than expression of CGA (e9.5 with robust expression at e12.5) (33). This difference in the timing of GnRHR and CGA (and hence, Cre) expression could potentially result in differences in the effects of ERK deletion between the two Cre models and warrants further investigation (25, 27).
Although the role of ERK signaling in reproduction has been well characterized in young adult animals, the effects of ERK loss, specifically in the gonadotrope of aging animals, have been largely ignored. Both males and females experience changes in the HPG axis as they age. Changes in gonadal function and in secretion of gonadotropins and steroid hormones have been documented in aging animals and humans. In rodent models, altered estrous cyclicity in aging female animals is associated with decreased LH and increased FSH secretion (34, 35), coincident with altered steroidogenesis and decreased serum progesterone (36). Interestingly, these effects can be mitigated by repeated pregnancy or progesterone substitution (37). Males also display similar alterations in gonadotropin levels with decreased quality and quantity of LH and increased FSH secretion, inversely correlating with sperm count (37–39).
Here, we investigated the effects of ERK1/2 loss in female and male mice as they age. We show that loss of ERK signaling impacts gonadotropin production and the timing of gonadal degeneration in males and females. In males, impaired spermatogenesis likely derives both from loss of gonadotropin stimulation and from GRIC-mediated ERK deletion in the germ line. Collectively, our studies provide insights into the effect(s) of hypogonadotropism on gonadal competence as mice age.
Materials and Methods
Animals
Animals were handled in compliance with protocols approved by the Cornell University Institutional Animal Care and Use Committee. ERK1−/−, ERK2 floxed (ERK2fl/fl), and GRIC mice have been described previously (5, 24, 25). ERK1/2 KO animals were designated ERK double KO (ERKdko; ERK1−/−, ERK2fl/fl, Cre+/−) and compared with control animals (ERK1−/−, ERK2fl/fl, Cre−/−) lacking the Cre allele. For breeding challenges, males of both genotypes were paired with one control and one ERKdko female. Females were checked daily for copulatory plugs and monitored for changes in body weight and signs of pregnancy and parturition. The reverse orientation splice acceptor (Rosa) 26 reporter and the stimulated by retinoic acid 8 (Stra8)-Cre driver mouse strains were obtained from The Jackson Laboratory (Bar Harbor, ME).
Genotyping
Genomic DNA was isolated from tail snips (3 mm) using an E-Z Tissue DNA Kit (Omega Biotek, Norcross, GA), per the manufacturer’s instructions. Routine polymerase chain reaction (PCR) genotyping was performed on animals, as previously described (40). PCR-based confirmation of ERK1−/− and ERK2fl/fl, Rosa26 reporter, Stra8-Cre, and GRIC alleles was performed with primers in Table 1.
Table 1.
PCR Primers Used in Genotype Analyses
| Primer | Primer Name | Sequence (5′ to 3′) |
|---|---|---|
| Rosa26 reporter | Rosa26 forward | TAA GCC TGC CCA GAA GAC TC |
| Rosa26 reverse | AAA GTC GCT CTG AGT TGT TAT | |
| Rosa26 common | TCC AGT TCA ACA TCA GCC GCT ACA | |
| ERK1 | ERK1 forward | AAG GTT AAC ATC CGG TCC AGC A |
| ERK1 reverse | AAG CAA GGC TAA GCC GTA CC | |
| ERK2 | ERK2 forward | AGC CAA CAA TCC CAA CCC TG |
| ERK2 reverse | GGC TGC AAC CAT CTC ACA AT | |
| GnRHR | GnRHR forward | GAA CTA CAG CTG AAT CAG TC |
| GnRHR reverse | CTC TAA CAA ACT CTG TAC A | |
| GnRHR homozygous | CGG AAT TCA TCG ATC ATA TCA GAT CC | |
| Stra8 | Stra8 forward | GTG CAA GCT GAA CAA CAG GA |
| Stra8 reverse | AGG GAC ACA GCA TTG GAG TC |
Histology and immunofluorescence staining
Tissues were fixed in 10% neutral-buffered formalin, paraffin-embedded, serially sectioned at 4 µm, and stained with hematoxylin and eosin using standard histological techniques. Sections were scanned and digitized using an Aperio Scanscope (Vista, CA) and analyzed using ImageScope (Leica Biosystems, Buffalo Grove, IL). For characterization of the ovarian luteal populations, every third section throughout the entire ovary was examined microscopically for identification of luteal tissue in both ERKdko and control females. For characterization of the testicular tissues, every third section was examined microscopically for histological evaluation. The largest sections were chosen, four slides total, and 20 seminiferous tubules were selected for analysis of tubule diameter from each section in both ERKdko and control males.
Fixed pituitary sections were stained with antibodies directed against LHβ (A. F. Parlow, National Hormone and Peptide Program, Torrance, CA) and ERK2 (Santa Cruz Biotechnology, Dallas, TX). Sections were deparaffinized in xylene and then rehydrated through graded ethanol washes. Antigen retrieval was carried out by boiling in a citrate buffer (10 mM sodium citrate, 0.005% Tween 20, pH 6.0), and sections were permeablized in phosphate-buffered saline (PBS) containing 0.2% Triton X-100 (PBST). Sections were blocked for 4 hours in blocking solution containing 22.5 mg/ml glycine, 10% normal goat serum, and 3% bovine serum albumin in PBST. Antibody directed against ERK2 (1:100) was applied first, overnight at room temperature; sections were washed in PBST and then exposed to secondary antibody (1:100) for 1 hour at room temperature. ERK2-stained sections were then washed in PBST and reblocked in blocking solution for 8 hours. LHβ antibody was then applied at a titer of 1:50 overnight at room temperature and washed and secondary antibody applied at 1:500 for 1 hour at room temperature. Sections were washed in PBST and coverslips applied. Image acquisition was performed using a Zeiss Imager Z1 microscope under 20, 40, or 60× magnifying objectives. Images were processed using ZEN 2 software (Carl Zeiss, Oberkochen, Germany).
Vaginal cytology
The vaginal vault was gently swabbed to make a cytological smear. The smear was stained with Wright’s Giemsa stain and examined with light microscopy. One hundred cells were counted daily for each animal with epithelial cells and leukocytes distinguished by morphology. An animal was deemed to be in estrus with >85% superficial epithelial cells. Animals in diestrus were identified by the predominant presence of leukocytes in the cytological smear.
Epididymal sperm count
After euthanasia, testes and epididymides from males of both genotypes were dissected free. Individual epididymi were placed in 1 ml of a solution containing 4% bovine serum albumin in PBS and tubules extracted. The preparation was incubated (32°F) for 20 minutes. Formalin (10%; 480 µL) was mixed with 20 µL of the sperm preparation and placed on a hemocytometer, and sperm were counted to determine sperm numbers.
Gonadectomy
Ovariectomies and castrations were performed at 5 to 6 months of age under Avertin (tribromoethanol; Sigma-Aldrich, St. Louis, MO) general anesthesia, with standard aseptic techniques. The castrations were performed with ventral midline incisions, and the ovariectomies were performed with flank incisions. Animals were given ketoprofen postoperatively for pain control. The animals were euthanized 5 days postoperatively, and blood and pituitaries were collected.
Serum hormone analyses
The blood was allowed to clot for 15 minutes and then centrifuged for 10 minutes at 2500 rpm. The serum was collected and frozen at −80°F. Pituitaries were snap frozen. Serum was analyzed at the University of Virginia Ligand Core through enzyme-linked immunosorbent assay (ELISA) multiplex, in duplicate or using in-house FSH and LH assays, as previously described (28). Specifically for the FSH assay, we used a Luminex Assay (Millipore, Burlington, MA), which results in ∼10-fold lower FSH concentrations compared with the ELISA multiplex assay used by the University of Virginia Ligand Core. Whereas this is a consistent result, the reasons for this difference are unclear, as the Luminex Assay contains proprietary reagents. As a consequence, we considered relative changes in FSH concentrations. Testosterone was assayed using a commercially available kit (IBL, Minneapolis, MN), per the manufacturer’s instructions.
RNA isolation and quantitative PCR
Tissues were collected, and Trizol (Thermo Fisher, Waltham, MA) extraction was performed per the manufacturer’s instructions to isolate total RNA. Reverse transcription in 1000 µg reactions was performed using the High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA), according to the manufacturer’s directions. Quantitative PCR (qPCR) was performed using SYBR Green (Thermo Fisher) and primers listed in Table 2. Amplifications were carried out using a CFX96 Touch Real-Time optical character recognition detection system (Bio-Rad Laboratories, Berkeley, CA). RNA levels were standardized using the internal control glyceraldehyde 3-phosphate dehydrogenase and an internal RNA pool and assessed using ΔΔ comparative threshold methodology, where the serum pool was set to a value of 1.0 for other comparisons (25).
Table 2.
PCR Primers Used in qPCR Analyses
| Primer | Primer Name | Sequence (5′ to 3′) |
|---|---|---|
| Glyceraldehyde-3-phosphate dehydrogenase | Gapdh forward | ATGTTTGTGATGGGTGTGAA |
| Gapdh reverse | ATGCCAAAGTTGTCATGGAT | |
| Gonadotropin-releasing hormone receptor | Gnrhr forward | TGCTCGGCCATCAACAACA |
| Gnrhr reverse | GGCAGTAGAGAGTAGGAAAAGGA | |
| Luteinizing hormone β-subunit | Lhb forward | CTGAGCCCAAGTGTGGTGTG |
| Lhb reverse | GACCATGCTAGGACAGTAGCC | |
| Follicle-stimulating hormone β-subunit | Fshb forward | GCCATAGCTGTGAATTGACCA |
| Fshb reverse | AGATCCCTAGTGTAGCAGTAGC | |
| Common α subunit | Cga forward | TCCAGGGCATATCCCACTCC |
| Cga reverse | CATTTCCCATTACTGTGGCCTTA |
Abbreviation: Gapdh, glyceraldehyde 3-phosphate dehydrogenase.
TUNEL staining
Terminal deoxynucleotidyl transferase deoxyuridine triphosphate nick end labeling (TUNEL) was carried out as previously described (41).
β-Galactosidase in vitro assay
For β-galactosidase in vitro assays, tissues were fixed in 4% paraformaldehyde/PBS for 1 hour at 4°C and then rinsed three times for 30 minutes each in a rinse buffer [100 mM sodium phosphate (pH 7.3), 2 mM MgCl2, 0.01% sodium deoxycholate, 0.02% volume-to-volume ratio (%) Nonidet P-40]. Tissues were stained overnight in staining buffer (rinse buffer with 5 mM potassium ferricyanide, 5 mM potassium ferrocyanide, 1 mg/ml 5-bromo-4-chloro-3-indolyl-d-galactoside), then fixed overnight in 10% formalin, and finally, washed with distilled water twice for 30 minutes. Tissues were dehydrated by sequential ethanol washes (70%, 95%, twice in 100%) and then washed in methyl salicylate until the tissue cleared.
Statistics
Pairwise comparisons were made by Student's t test. When appropriate, a two-way analysis of variance was used, with Tukey’s multiple comparisons test. All data are expressed as means ± standard error of the mean (SEM). P < 0.05 was considered statistically significant.
Results
ERKdko females are infertile, hypogonadotropic, and anovulatory
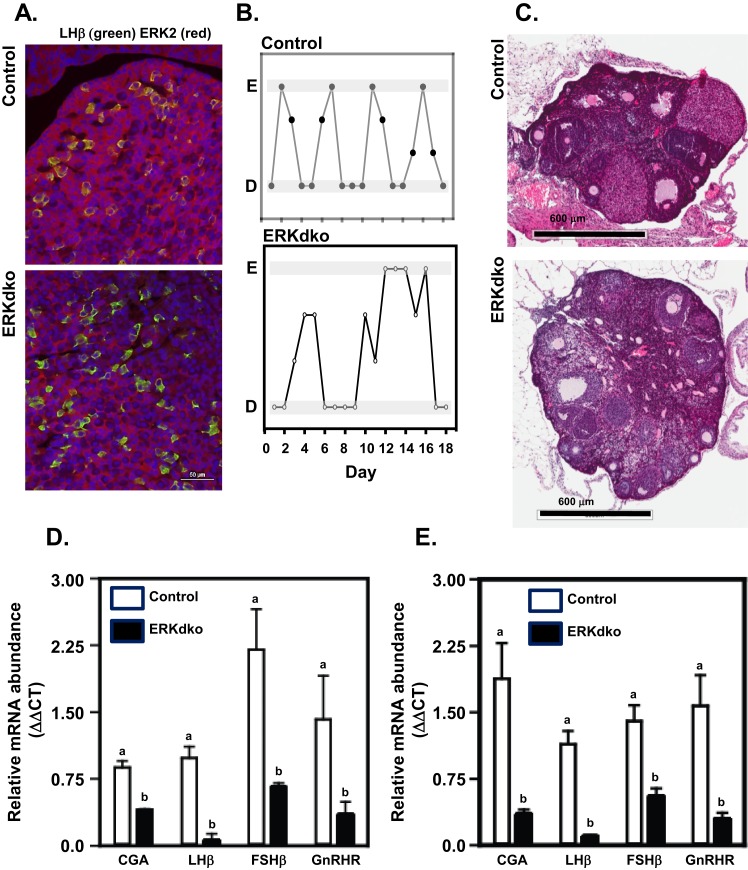
Validation of the GRIC ERKdko model was carried out using immunofluorescence studies, examining the overlapping expression of LHβ (to mark gonadotropes) and ERK2 in pituitary sections obtained from randomly cycling adult female mice (Fig. 1A). In control animals, all LHβ-expressing cells displayed overlapping expression of ERK2. In contrast, LHβ-expressing cells in the ERKdko females did not have an overlapping expression pattern with ERK2, consistent with gene excision in gonadotropes. Female control and ERKdko mice were paired with control males and monitored daily for copulatory plugs. Whereas 100% of control females exhibited plugs within the first 3 days of pairing, only 33% of ERKdko females exhibited plugs when paired with males for 30 days. All control females had litters at ∼20 days following copulation, whereas no ERKdko animals had litters (Table 3).
Figure 1.
GRIC ERKdko female mice display irregular estrous cycle-like vaginal cytology, hypogonadotropism, and anovulation. (A) Pituitary sections from randomly cycling adult female mice were stained for LHβ (green; to identify gonadotropes) and ERK2 (red). In control animals, overlapping expression of LHβ and ERK2 (yellow) occurred in all cells, whereas in the ERKdko animals, a similar level of overlapping expression was not observed. 4′,6-Diamidino-2-phenylindole nuclear staining is shown in blue. (B) ERKdko animals exhibit irregular periods of estrus and diestrus based on vaginal cytology compared with control animals. (C) Histological sections of ovaries stained with hematoxylin and eosin from control and ERKdko animals. Note the lack of corpora lutea (CL) present in the ovary of ERKdko females. (D) ERKdko animals had substantial reduction in all four gonadotrope-specific gene transcript levels (CGA, LHβ, FSHβ, GnRHR) at 6 months of age compared with control animals (P < 0.05). (E) Gonadotrope-specific transcript levels remained significantly lower in aged ERKdko female mice (12 months) when compared with aged control littermates (P < 0.05). a,bDifferent P < 0.05. D, vaginal cytology at diestrus; E, vaginal cytology at estrus; ΔΔCT, ΔΔ comparative threshold.
Table 3.
Reproductive Characteristics of Control and GRIC ERKdko Female Mice
| Control | GRIC ERKdko | |
|---|---|---|
| Estrous cycle length, days | 5.3 ± 0.3a (n = 5) | 9.2 ± 1.0b (n = 5) |
| Time in estrus, days | 1.4 ± 0.1a (n = 5) | 3.2 ± 0.5b (n = 5) |
| Time in diestrus, days | 1.9 ± 0.2a (n = 5) | 4.3 ± 0.5b (n = 5) |
| CL/ovary | 6.3 ± 1.3a (n = 6) | 0b (n = 6) |
| Mean litter size | 7.0 ± 0.6a (n = 3) | 0b (n = 6) |
Data are presented as means ± SEM and sample size (n) shown in parentheses.
Abbreviation: CL, corpora lutea.
Different P < 0.05.
We used vaginal cytology to assess dynamics of the estrous cycle. Control animals had a cycle length of 5.3 ± 0.26 days. ERKdko animals had a significantly longer interval (9.2 ±1.01 days) between estrus-like periods, as determined by cytology (Table 3). The ERKdko animals showed diestrus- and estrus-like cytology (Fig. 1A; Table 3); however, the duration of diestrus and estrus intervals was significantly prolonged, with a clear lack of normal periodicity seen in control females, consistent with previous observations (25). To understand better the relationship between estrous cycle behavior and ovarian activity, ovaries were collected at presumptive estrus and examined by histology. Whereas both control and ERKdko animals had ovarian follicles in various stages of maturation, control ovaries displayed an average of 6.3 corpora lutea (CL) per ovary compared with a conspicuous absence of CL in the ERKdko ovaries, indicating an anovulatory phenotype (Fig. 1B; Table 4). Body weights, uterine wet weights, and ovarian weights at estrus were not significantly different between control and ERKdko animals at 6 months of age (Table 4).
Table 4.
BW and Ovarian and Uterine WTs at 6 and 12 Months of Age in Control and GRIC ERKdko Female Mice
| Control | GRIC ERKdko | |
|---|---|---|
| BW, g (6 mo) | 24.3 ± 1.4a (n = 14) | 27.4 ± 1.3a (n = 21) |
| BW, g (12 mo) | 28.9 ± 0.6a (n = 23) | 36.3 ± 1.2b (n = 12) |
| Ovarian WT/BW, mg (6 mo) | 3.0 × 10−3 ± 0.1 × 10−3a (n = 4) | 2.2 × 10−3 ± 0.1 × 10−3a (n = 4) |
| Ovarian WT/BW, mg (12 mo) | 1.2 × 10−3 ± 0.09 × 10−3a (n = 13) | 0.8 × 10−3 ± 0.1 × 10−3b (n = 7) |
| Uterine WT/BW, g (6 mo) | 0.05 ± 0.1a (n = 4) | 0.03 ± 0.003a (n = 4) |
| Uterine WT/BW, g (12 mo) | 0.04 ± 0.004a (n = 13) | 0.02 ± 0.001b (n = 7) |
Data are presented as means ± SEM and sample size (n) shown in parentheses.
Abbreviations: BW, body weight; WT, weight.
Different P < 0.05.
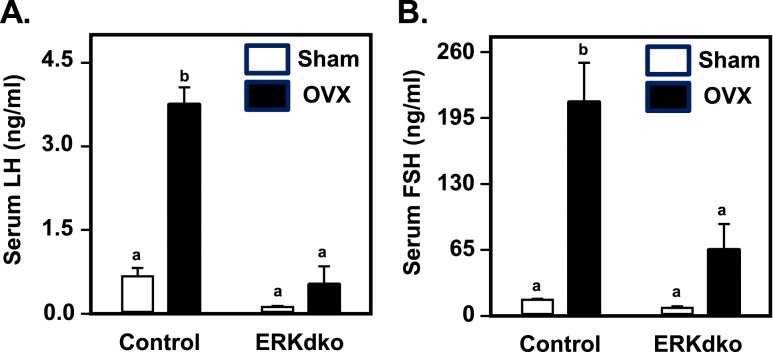
To assess the impact of ERK deletion on gonadotrope cell function in female mice, we used qPCR to determine the abundance of four genes known to define the gonadotrope cell lineage (Cga, Lhb, Fshb, and Gnrhr; Fig. 1C). The mRNA expression of all four gonadotrope genes at estrus was decreased in ERKdko females at 6 months of age, compared with age-matched control females. This was consistent with reduced levels of LH and FSH following ovariectomy in circulation of ERKdko relative to control females (Fig. 2A and 2B; sham-operated controls).
Figure 2.
ERKdko females have a blunted response to GnRH hyperstimulation following ovariectomy. (A) Control animals showed substantial increases in serum LH concentrations following ovariectomy (OVX) compared with sham-operated controls (Sham). In ERKdko animals, basal LH secretion was reduced in sham-operated animals, and response to OVX was blunted. (B) Control animals showed substantial increases in serum FSH concentrations following OVX compared with sham-operated controls (Sham). This effect was blunted in ERKdko female mice. LH and FSH assays were multiplex ELISAs (see Materials and Methods section). a,bDifferent P < 0.05.
To determine the response of ERKdko and control females to endogenous hyperstimulation by GnRH, animals were sham operated as a control or ovariectomized and then euthanized after 5 days to analyze changes in serum gonadotropins. We found a substantial increase in serum LH and FSH following ovariectomy in control animals, which was blunted significantly in ovariectomized ERKdko females (Fig. 2A and 2B). These findings are generally consistent with previously published data on conditional ERK deletion from our group using the CGA-Cre driver (25).
Female ERKdko animals show altered age-related changes in body weight and ovarian histology
Female control and ERKdko animals were maintained until ∼12 months of age to assess the impact of loss of ERK signaling on age-related changes within the reproductive axis. These animals received identical access to food and water and were not given the opportunity to reproduce. At the time of weaning through 6 months of age, there were no differences in body weight between genotypes. At ∼12 months of age, body weight was ∼25% higher in ERKdko compared with control females. Absolute ovarian and uterine weights were reduced in older ERKdko females compared with the control genotype, and this was amplified when correcting for differences in body weight (Table 4).
Aged ERKdko females (∼12 months) also showed signs of abnormal ovarian histology, perhaps reflecting premature gonadal degeneration. Consistent with the younger ERKdko females, ovarian histology revealed an absence of CL in the aged ERKdko females. Furthermore, aged females showed loss of normal ovarian architecture, with abnormal accumulations of extracellular matrix and regions of marked acellularity, particularly within the medullary region of the ovary. Ovarian histology in control animals appeared unremarkable, with multiple CL present and normal architecture throughout the ovary (Fig. 3A and 3B). Comparison of uterine histology between genotypes in the aged females was unremarkable (data not shown), suggesting that changes in ovarian architecture were specific within the reproductive axis.
Figure 3.
Aging reveals marked changed in ovarian histology in ERKdko animals. (A) ERKdko animals show abnormal accumulations of extracellular matrix and regions of marked acellularity in histological sections of ovary at 12 months of age. Original magnification, ×500 μm. (B) Higher magnification (×200 μm) of areas of acellularity and extracellular matrix accumulation within the ovaries is depicted.
To understand the changes in pituitary gonadotrope function, we performed qPCR on pituitaries from ∼12-month-old females. Transcript levels of the four gonadotrope genes were not significantly different between 6- and 12-month-old control animals. However, all of these transcript levels were significantly reduced in aged ERKdko females (Fig. 1D).
ERKdko males exhibit moderate subfertility
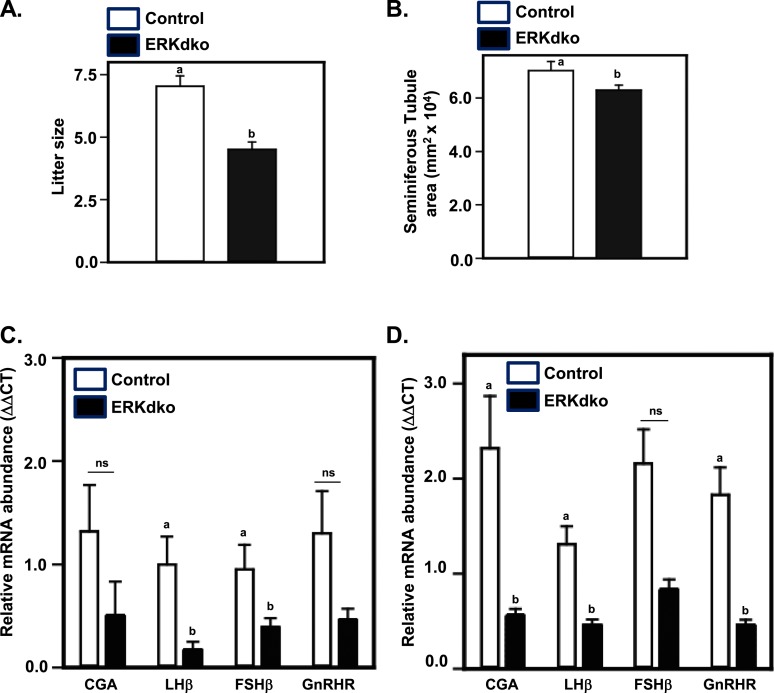
Males of both genotypes were capable of producing copulatory plugs. There was no difference between control and ERKdko males in days to first plug following pairing or in the number of copulatory plugs detected before pregnancy (data not shown). However, ERKdko males sired smaller litters compared with control males (Fig. 4A). There was no substantial difference in testis weights between control and ERKdko males at 6 months of age (Table 5). However, ERKdko males displayed a mild, but statistically significant reduction in epididymal sperm counts at 6 months of age (Table 3). This corresponded with a modest reduction in seminiferous tubule area (Fig. 4B). Sperm morphology was grossly normal for both genotypes (data not shown).
Figure 4.
ERKdko males display reduced fertility and are hypogonadotropic. (A) Six-month-old ERKdko males had a significantly reduced litter size compared with control animals. (B) ERKdko males have a significantly reduced seminiferous tubule area compared with control animals at 6 months of age. (C) Six-month-old ERKdko animals have significantly reduced transcript levels of LHβ and FSHβ compared with control animals. Steady-state levels of CGA and GnRHR were numerically smaller but not statistically different (ns) at 6 months of age. (D) Eighteen-month-old ERKdko males had a substantial reduction in CGA, LHβ, and FSHβ transcript levels compared with controls. Steady-state levels of FSHβ mRNA were numerically smaller but not statistically different (ns) at 18 months of age. a,bDifferent P < 0.05.
Table 5.
Testis and Seminal Vesicle Weights and Total Epididymal Sperm at 6, 12, and 18 Months of Age in Control, GRIC ERKdko, and Stra8 ERKdko Male Mice
| Control | GRIC ERKdko | STRA8 ERKdko | |
|---|---|---|---|
| BW, g (6 mo) | 29.0 ± 0.76a (n = 7) | 28.6 ± 0.93a (n = 6) | |
| BW, g (12 mo) | 30.9 ± 1.32a (n = 14) | 36.3 ± 1.78a (n = 12) | |
| BW, g (18 mo) | 30.3 ± 0.49a (n = 14) | 33.72 ± 2.78a (n = 12) | |
| Testis, mg/BW, g (6 mo) | 3.6 × 10−3 ± 0.1 × 10−3a (n = 7) | 3.4 × 10−3 ± 0.4 × 10−3a (n = 6) | |
| Testis, mg/BW, g (12 mo) | 3.8 × 10−3 ± 0.1 × 10−3a (n = 14) | 2.5 × 10−3 ± 0.1 × 10−3b (n = 12) | |
| Testis, mg/BW, g (18 mo) | 4.1 × 10−3 ± 0.1 × 10−3a (n = 14) | 2.8 × 10−3 ± 0.2 × 10−3b (n = 12) | 3.7 × 10−3 ± 0.1 × 10−3c (n = 6) |
| Seminal vesicle, mg (6 mo) | 288.2 ± 22.3a (n = 7) | 250.2 ± 16.6a (n = 6) | |
| Seminal vesicle, mg (12 mo) | 395.3 ± 54.3a (n = 14) | 258.4 ± 29.6a (n = 12) | |
| Seminal vesicle, mg (18 mo) | 362.7 ± 24.8a (n = 14) | 257.8 ± 8.2b (n = 12) | 429.3 ± 60.5c (n = 6) |
| Total epididymal sperm (6 mo) | 20.6 × 106 ± 0.8 × 106a (n = 7) | 17.0 × 106 ± 1.1 × 106b (n = 6) | |
| Total epididymal sperm (12 mo) | 14.7 × 106 ± 0.4 × 106a (n = 14) | 7.8 × 106 ± 0.2 × 106b (n = 12) | |
| Total epididymal sperm (18 mo) | 13.4 × 106 ± 0.6 × 106a (n = 14) | 3.8 × 106 ± 0.4 × 106b (n = 12) | 5.3 × 106 ± 0.3 × 106c (n = 6) |
Data are presented as means ± SEM and sample size (n) shown in parentheses.
Different P < 0.05.
To assess the effect(s) of ERK deletion on gonadotropin subunit and Gnrhr mRNA levels, qPCR was performed on pituitaries from control and ERKdko males at 6 months of age (Fig. 4C). Consistent with responses in young ERKdko females at estrus (Fig. 1C), ERKdko males displayed reduced expression of Lhb and Fshb mRNAs compared with control males. Although numerically lower, Cga and Gnrhr mRNAs were not statistically different between genotypes.
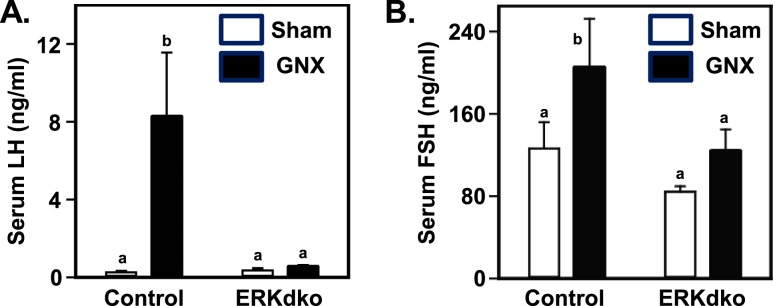
To assess the impact of hyperstimulation with endogenous GnRH, male control and ERKdko animals were castrated or underwent a sham surgery. Animals were euthanized after 5 days, and serum concentrations of LH and FSH were measured. Again, consistent with control females, castration resulted in a marked increase in LH and FSH secretion compared with sham-operated controls (Fig. 5). Strikingly, the postcastration rise in LH secretion was completely blocked in ERKdko males. FSH levels overall were lower in ERKdko males relative to controls, but here, as well, the increase postcastration was not statistically significant.
Figure 5.
ERKdko males have a blunted response to GnRH hyperstimulation following castration. (A) Control animals showed substantial increases in serum LH concentrations following castration (GNX) compared with sham-operated controls (Sham). In ERKdko animals, the response to GNX was abrogated. (B) Serum FSH response to GNX in control and ERKdko males was numerically higher but not statistically different compared with sham-operated controls (Sham). LH and FSH assays were multiplex ELISAs (see Materials and Methods section). a,bDifferent P < 0.05.
ERKdko males exhibit premature testicular abnormalities
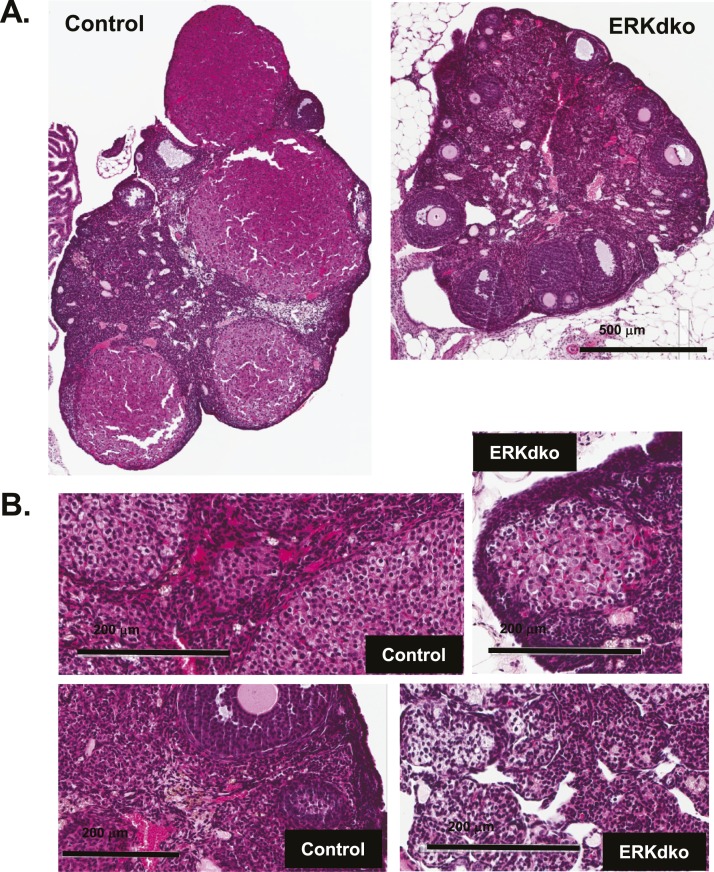
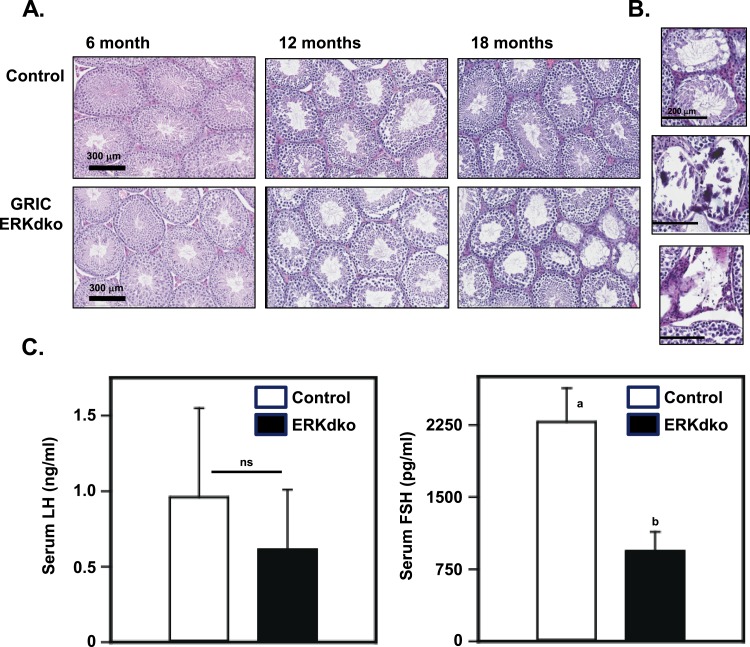
Control and ERKdko males were assessed at 6, 12, and 18 months of age for body weight, testis weights, epididymal sperm counts, and seminal vesicle weights (Table 5). By 12 and 18 months of age, ERKdko animals had substantially lower sperm counts and testis weights compared with control males. Seminal vesicle weights in ERKdko animals were reduced in the 18-month-old group compared with controls. Age-related abnormalities were also evident in histopathology (Fig. 6A and 6B), which revealed evidence of testicular abnormalities in ERKdko animals at 18 months but not in age-matched control testes. The testes showed areas of marked testicular degeneration, calcification, aspermatic tubules, and spermatid giant cells (Fig. 6B). TUNEL staining revealed a fourfold increase (1.03 ± 0.31 TUNEL+ cells/tubule in control vs 4.3 ± 0.47 TUNEL+ cells in ERKdko testis) in apoptotic cells in the ERKdko testis in aged animals. These TUNEL+ cells were localized to the spermatogonial epithelium of the tubule and did not specifically align with regions of marked degeneration (Fig. 6B).
Figure 6.
Aging reveals marked changed in testicular histology in ERKdko animals. (A) Testes of ERKdko animals showed grossly normal morphology until 18 months of age, where they display signs of testicular degeneration and dysplasia, including regions of marked calcification, aspermatic tubules, and giant spermatid cells. (B) Higher magnification (×200 μm) of regions showing age-related degeneration, calcification, and loss of tubules. (C) LH secretion is numerically lower but not statistically significant (ns) in ERKdko males at 18 months of age. Serum FSH concentrations are reduced (a,bdifferent P < 0.05) in ERKdko males at 18 months of age. LH and FSH assays were in-house assays (see Materials and Methods section).
Transcript levels for the four signature gonadotrope genes were assessed in pituitaries of males of 6, 12, and 18 months. Neither control nor ERKdko animals showed substantial changes in transcript levels within genotypes between 6 and 12 months of age. However, there was a substantial decrease between control and ERKdko animals at 18 months in Cga, Lhb, and Gnrhr (Fig. 4D). Interestingly, we found no substantial differences between genotypes or ages in circulating testosterone levels, indicating that despite low levels of LH, these were sufficient to maintain testosterone levels in ERKdko males (data not shown). Serum LH levels were not statistically different between genotypes at 18 months of age; however, ERKdko males did display numerically reduced serum LH (Fig. 6C). Serum FSH levels were not substantially different between genotypes or ages at 6 or 12 months. However, aged ERKdko males (18 months) had a substantial reduction in serum FSH compared with control animals of the same age (Fig. 6C).
Testicular degeneration of aging ERKdko males is likely a result of hypogonadotropism and not loss of germ-cell ERK signaling
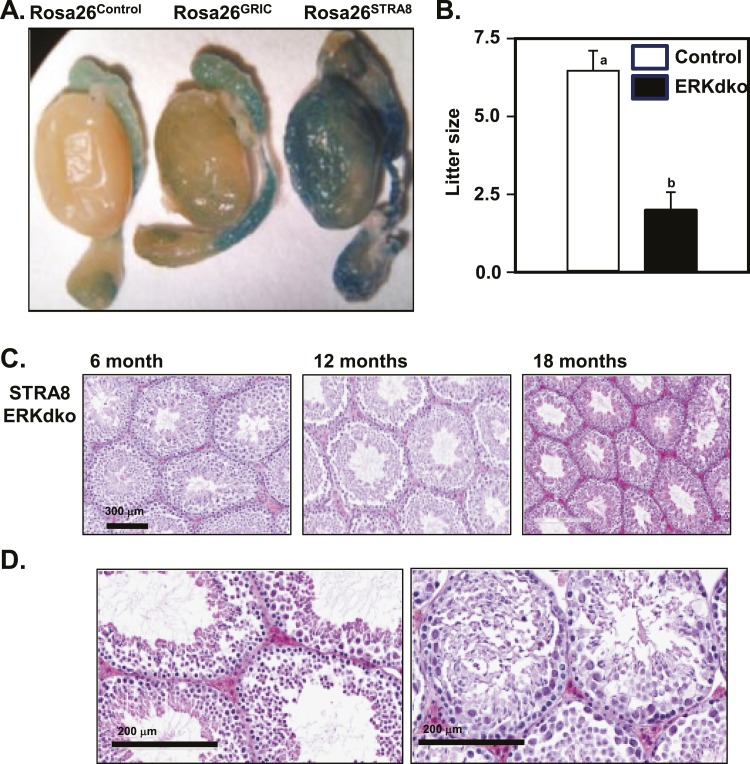
The GRIC-Cre driver has been reported to display Cre expression within the germ-cell lineage in the testes (24). We confirmed these observations using assessment of reporter gene activity in Rosa26-βgal mice harboring a GRIC allele (in situβ-galactosidase staining; Fig. 7A). To parse the relative roles of ERK deletion in gonadotropes and in germ cells, we characterized aged males from a Stra8-Cre line on the same ERK1−/−/ERK2fl/fl background. Consistent with the GRIC-Cre driver, the Stra8-Cre driver is expressed in the spermatogonia, as well as in later stages of spermatogenesis (42) but in contrast, is not expressed within the pituitary gland. Stra8 ERKdko males showed a moderate decrease in sperm count, a marked decrease in litter size, but interestingly, no substantial change in testicular size, testes-to-body weight ratio, body weight, or seminal vesicle weight compared with control animals (Table 5; Fig. 7B). Histological assessment of Stra8-Cre ERKdko testes revealed relatively minor degenerative changes at 18 months of age compared with control animals, consistent with the advanced age of the animals (Fig. 7D). By comparison, testicular degeneration with age was markedly more severe in the GRIC-Cre ERKdko animals (Fig. 6A and 6B), suggesting that ERK signaling within gonadotropes was essential for maintaining testicular architecture during aging.
Figure 7.
Direct deletion of ERKs in the spermatogonial lineage results in loss of sperm reproduction and fertility but not histological degeneration. (A) The GRIC- and testis-specific Stra8-Cre drivers were compared directly using the Rosa26 β-galactosidase reporter mouse strain. β-Galactosidase staining of whole testes revealed background staining in epididymides in all three genotypes and specific staining within the spermatogonial lineage in the GRIC- and Stra8-Cre strains, albeit in variable staining intensities. (B) Stra8-ERKdko males sire smaller litters compared with control males (a,bdifferent P < 0.05). (C and D) Histological section of testes at 6, 12, and 18 months of age in the Stra8-ERKdko males reveals age-related changes in testicular histopathology, inconsistent with degenerative changes observed in the GRIC ERKdko males at 18 month of age (Fig. 6).
Discussion
In the current studies, GRIC-mediated ERK excision in animals of both sexes resulted in a hypogonadotropic phenotype, with a reduced responsiveness to endogenous GnRH stimulation. Both sexes had reduced fertility at 6 months of age, with males being subfertile and females being infertile and anovulatory. We hypothesize that this difference between males and females is a result of the difference in LH and FSH requirements controlling the reproductive cycle. In males, LH and FSH subunit transcript levels and secretion in ERKdko animals appear to be sufficient to maintain fertility, albeit at a suboptimal level. Females, on the other hand, have a notably more complex requirement for gonadotropin production and secretion during the reproductive cycle, particularly with regard to LH biosynthesis, leading to the preovulatory LH surge and ovulation (25). Albeit reduced, FSH levels in this model appear to be sufficient to stimulate folliculogenesis to large antral follicle stages. ERKdko mice fail to ovulate, as reflected by the complete absence of CL in their ovaries. This could result from at least two possible mechanisms, which are not mutually exclusive. First, mice may be incapable of producing LH surges of sufficient amplitude to trigger ovulation because of reduced LH biosynthesis. Second, reduced LH secretion may blunt ovarian estradiol production to levels insufficient to induce a positive-feedback effect at the hypothalamic and/or pituitary levels. Given the present data, we are unable to parse these alternatives absolutely. Although ERK signaling has been shown to be the primary pathway for LH biosynthesis, other GnRH- and activin-dependent signaling cascades have been shown to contribute to LH and FSH production, which is likely how the ERKdko animals retain some gonadotropin production (43–45).
Our rationale for using the GRIC deleter strain in the present studies was our uncertainty regarding the specific effects of ERK deletion in gonadotropes vs thyrotropes using the CGA deleter strain in our previous studies (25). However, the phenotypes of the two models were remarkably similar, with some exceptions. The models converge to demonstrate definitively that ERK signaling in gonadotropes is essential for fertility in female mice. Although FSH production was blunted in both Cre models, females displayed folliculogenesis to the large antral follicle stage, suggesting that infertility derives principally from LH deficiency. There were apparent differences in the extent of disruption of estrous cyclicity between the two models, as assessed by vaginal cytology. The GRIC ERKdko females appear to proceed through all phases of the estrous cycle, with markedly variable intervals of time in estrus- and diestrus-like stages. In contrast, ERK deletion in the CGA model resulted in vaginal cytology more consistently reminiscent of persistent anestrus. Importantly, we never observed CL in ovaries of GRIC ERKdko females. Therefore, it appears that both models were anovulatory. These data suggest that measures of vaginal cytology alone are not sufficient to establish true estrous cyclicity. An additional difference between the two models was the extent of the FSH impairment, which was more extreme using the GRIC driver. However, consistent with the CGA model, FSH levels remained sufficient for folliculogenesis. Whereas the reasons behind these differences are not completely clear, this may be a result of the timing of the onset of Cre recombinase activity and/or Cre penetrance. CGA-Cre activity is detectable at e9.5 and increases markedly by e12.5 (33), whereas GRIC-Cre activity is first detectable at e12.75, with more marked activity as gestational age increases (25, 46). Multiple markers of pituitary and gonadotrope differentiation and function, such as fibroblast growth factor 8, bone morphogenetic protein 4 and 2, and the LIM homeodomain transcription factors, Isl1, Lhx3, and Lhx4, are expressed during an earlier developmental window, so loss of ERK in those cells could potentially have broad impacts on pituitary lineage specification and differentiation (47).
In the GRIC-Cre model, chronic hypogonadotropism occurs in parallel with a progressively degenerative gonadal phenotype in both sexes; however, the timing varied between females and males. Abnormal ovarian histology was initially detected at 12 months of age, with changes more obvious within the medullary region of the ovary. These types of degenerative changes could have developed at any time between the 6- and 12-month timeframe. By contrast, aged (18-month-old) ERKdko males displayed a more severe phenotype in the testes compared with younger time points measured, including marked loss of sperm production, reduced testicular and seminal vesicle weights, as well as areas of moderate to severe testicular degeneration. Reduced sperm production in the GRIC ERKdko model was entirely consistent with similar losses in sperm production with ERK deletion using the Stra8-Cre driver, suggesting that loss of ERK signaling in the germ line was detrimental to sperm production over time, independent of a hypogonadotropic phenotype. Interestingly, marked testicular histological degeneration was only observed in the GRIC ERKdko males, indicating that gonadotropin support from the pituitary is necessary to maintain normal testicular histology during aging. For reasons that are presently unclear, degeneration was localized to focal areas and not generalized throughout the testis. Interestingly, testis weights and sperm counts decreased between 6 and 12 months in males, but the decrease in seminal vesicle weights and the degenerative histological changes were only detected at 18 months of age. Seminal vesicle weight is typically correlated with testosterone, but we did not observe decreases in serum testosterone in the ERKdko mice. Two alternative possibilities may explain this finding: (1) our testosterone measures were a single point in time and may have missed differences attributable to the pulsatile nature of testosterone secretion, or (2) seminal vesicles have been shown to express LH receptors, and the seminal vesicle weight loss may have been a result of chronically low levels of LH in 18-month-old ERKdko males (48).
The results reported here are important for several reasons. First, the use of the GRIC-Cre driver confirms the importance of GnRH-induced ERK signaling within the gonadotrope, particularly regarding LH synthesis and secretion in female mice. ERKdko female mice display altered estrous cycle behavior and are infertile, consistent with our previous studies (25). Second, our data show, for the first time, that loss of ERK signaling in gonadotropes in female mice alters ovarian aging, with marked histological degeneration that is not apparent in age-matched control mice. Third, we discovered that the fertility phenotype of male ERKdko mice is progressive, with modest subfertility in young animals becoming more exaggerated with increasing age. This derived from at least two mechanisms: (1) the loss of local effects of ERK signaling within the germ line, as demonstrated using the Stra8-Cre driver, and (2) the effects of loss of ERK signaling within the gonadotrope, presumably via induction of a prolonged hypogonadal state. An indirect but critical finding of these studies is the identification of a role for ERK signaling in male germ cells. We speculate that the GRIC ERKdko mouse is a potentially interesting model to study the specific effects of chronic hypogonadotropism on the gonads during aging. This is not an area that has been well characterized at the level of cell signaling in model systems, such as the mouse, and may add substantially to our understanding of idiopathic hypogonadotropic–hypogonadal patients with genetic deficiencies in GnRH production and/or secretion (49, 50).
Appendix Table.
Antibodies
| Peptide/Protein Target | Antigen Sequence (if Known) | Name of Antibody | Manufacturer, Catalog No., and/or Name of Individual Providing the Antibody | Species Raised in; Monoclonal or Polyclonal | Dilution Used | RRID |
|---|---|---|---|---|---|---|
| LH/FSH | Not available | LH antibody | UVA Ligand Core Multiplex Assay/Millipore Multiplex Assay | Unknown/proprietary | AB_2716840 | |
| LH | Not available | LH antibody | National Hormone and Peptide Program/in-house ELISA/McGill University/IF | Rabbit; polyclonal | IF: 1:50 | Dr. Janet Roser, Department of Animal Science, University of California, Davis, Cat. no. 581B7, RRID:AB_2665514; A. F. Parlow, National Hormone and Peptide Program, Cat. no. rLH, RRID:AB_2665533 |
| FSH | Not available | FSH antibody | National Hormone and Peptide Program/in-house ELISA/McGill University | Rabbit; polyclonal | A. F. Parlow, National Hormone and Peptide Program, Cat. no. AFP-C0972881, RRID:AB_2687903 | |
| ERK2 | Not available | ERK2 antibody SC | Santa Cruz Biotechnology/IF | Mouse; monoclonal | IF:1:50 | Santa Cruz Biotechnology, Cat. no. sc-1647, RRID:AB_627547 |
Abbreviations: IF, immunofluorescence; RRID, Research Resource Identifier; UVA, University of Virginia.
Acknowledgments
We thank members of the M.S.R. laboratory for helpful discussions in the development of this manuscript.
Financial Support: These studies were supported by US National Institutes of Health/Eunice Kennedy Shriver National Institute of Child Health and Human Development Grants R01 HD034722 and R21 HD082780 (to M.S.R.) and Canadian Institutes of Health Research Grant MOP-123447 and Natural Sciences and Engineering Research Council of Canada Grant 2015-05178 (to D.J.B.).
Disclosure Summary: The authors have nothing to disclose.
Abbreviations:
- CGA
common glycoprotein hormone α subunit
- CL
corpora lutea
- e
embryonic day
- ELISA
enzyme-linked immunosorbent assay
- ERK
extracellular signal-regulated kinase
- ERK1−/−
extracellular signal-regulated kinase 1 null
- ERK2fl/fl
extracellular signal-regulated kinase 2 floxed
- ERKdko
extracellular signal-regulated kinase double-knockout
- FSH
follicle-stimulating hormone
- GnRHR
gonadotropin-releasing hormone receptor
- GRIC
gonadotropin-releasing hormone receptor–internal ribosomal entry site-Cre
- HPG
hypothalamic-pituitary-gonadal
- KO
knockout
- LH
luteinizing hormone
- mRNA
messenger RNA
- PBS
phosphate-buffered saline
- PBST
phosphate-buffered saline containing 0.2% Triton X-100
- PCR
polymerase chain reaction
- qPCR
quantitative polymerase chain reaction
- Rosa
reverse orientation splice acceptor
- SEM
standard error of the mean
- Stra8
stimulated by retinoic acid 8
- TUNEL
terminal deoxynucleotidyl transferase deoxyuridine triphosphate nick end labeling.
References
- 1. Bliss SP, Navratil AM, Xie J, Roberson MS. GnRH signaling, the gonadotrope and endocrine control of fertility. Front Neuroendocrinol. 2010;31(3):322–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Brown JL, Roberson M. Novel insights into gonadotropin-releasing hormone action in the pituitary gonadotrope. Semin Reprod Med. 2017;35(2):130–138. [DOI] [PubMed] [Google Scholar]
- 3. Clarke IJ, Cummins JT. The temporal relationship between gonadotropin releasing hormone (GnRH) and luteinizing hormone (LH) secretion in ovariectomized ewes. Endocrinology. 1982;111(5):1737–1739. [DOI] [PubMed] [Google Scholar]
- 4. Knobil E. The GnRH pulse generator. Am J Obstet Gynecol. 1990;163(5):1721–1727. [DOI] [PubMed] [Google Scholar]
- 5. Wen S, Schwarz JR, Niculescu D, Dinu C, Bauer CK, Hirdes W, Boehm U. Functional characterization of genetically labeled gonadotropes. Endocrinology. 2008;149(6):2701–2711. [DOI] [PubMed] [Google Scholar]
- 6. Wildt L, Häusler A, Marshall G, Hutchison JS, Plant TM, Belchetz PE, Knobil E. Frequency and amplitude of gonadotropin-releasing hormone stimulation and gonadotropin secretion in the rhesus monkey. Endocrinology. 1981;109(2):376–385. [DOI] [PubMed] [Google Scholar]
- 7. Coss D. Regulation of reproduction via tight control of gonadotropin hormone levels [published online ahead of print 22 March 2017]. Mol Cell Endocrinol. doi: 10.1016/j.mce.2017.03.022. [DOI] [PMC free article] [PubMed]
- 8. Gharib SD, Wierman ME, Shupnik MA, Chin WW. Molecular biology of the pituitary gonadotropins. Endocr Rev. 1990;11(1):177–199. [DOI] [PubMed] [Google Scholar]
- 9. Leung PC, Armstrong DT. Interactions of steroids and gonadotropins in the control of steroidogenesis in the ovarian follicle. Annu Rev Physiol. 1980;42(1):71–82. [DOI] [PubMed] [Google Scholar]
- 10. Moyle WR, Ramachandran J. Effect of LH on steroidogenesis and cyclic AMP accumulation in rat Leydig cell preparations and mouse tumor Leydig cells. Endocrinology. 1973;93(1):127–134. [DOI] [PubMed] [Google Scholar]
- 11. Richards JS, Pangas SA. New insights into ovarian function. Handb Exp Pharmacol. 2010;198:3–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Richards JS, Pangas SA. The ovary: basic biology and clinical implications. J Clin Invest. 2010;120(4):963–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hatano N, Mori Y, Oh-hora M, Kosugi A, Fujikawa T, Nakai N, Niwa H, Miyazaki J, Hamaoka T, Ogata M. Essential role for ERK2 mitogen-activated protein kinase in placental development. Genes Cells. 2003;8(11):847–856. [DOI] [PubMed] [Google Scholar]
- 14. Pàges G, Guérin S, Grall D, Bonino F, Smith A, Anjuere F, Auberger P, Pouysségur H. Defective thymocyte maturation in p44 MAP kinase (ERK 1) knockout mice. Science. 1999;286(5443):1374–1377. [DOI] [PubMed] [Google Scholar]
- 15. Saba-El-Leil MK, Vella FD, Vernay B, Voisin L, Chen L, Labrecque N, Ang SL, Meloche S. An essential function of the mitogen-activated protein kinase Erk2 in mouse trophoblast development. EMBO Rep. 2003;4(10):964–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Selcher JC, Nekrasova T, Paylor R, Landreth GE, Sweatt JD. Mice lacking the ERK1 isoform of MAP kinase are unimpaired in emotional learning. Learn Mem. 2001;8(1):11–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bliss SP, Navratil AM, Xie J, Miller A, Baccarini M, Roberson MS. ERK signaling, but not c-Raf, is required for gonadotropin-releasing hormone (GnRH)-induced regulation of Nur77 in pituitary gonadotropes. Endocrinology. 2012;153(2):700–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bliss SP, Navratil AM, Breed M, Skinner DC, Clay CM, Roberson MS. Signaling complexes associated with the type I gonadotropin-releasing hormone (GnRH) receptor: colocalization of extracellularly regulated kinase 2 and GnRH receptor within membrane rafts. Mol Endocrinol. 2007;21(2):538–549. [DOI] [PubMed] [Google Scholar]
- 19. de Lamirande E, Gagnon C. The extracellular signal-regulated kinase (ERK) pathway is involved in human sperm function and modulated by the superoxide anion. Mol Hum Reprod. 2002;8(2):124–135. [DOI] [PubMed] [Google Scholar]
- 20. Vantaggiato C, Formentini I, Bondanza A, Bonini C, Naldini L, Brambilla R. ERK1 and ERK2 mitogen-activated protein kinases affect Ras-dependent cell signaling differentially. J Biol. 2006;5(5):14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Peng J, Tang M, Zhang BP, Zhang P, Zhong T, Zong T, Yang B, Kuang HB. Kisspeptin stimulates progesterone secretion via the Erk1/2 mitogen-activated protein kinase signaling pathway in rat luteal cells. Fertil Steril. 2013;99(5):1436–1443.e1. [DOI] [PubMed] [Google Scholar]
- 22. Su YQ, Denegre JM, Wigglesworth K, Pendola FL, O’Brien MJ, Eppig JJ. Oocyte-dependent activation of mitogen-activated protein kinase (ERK1/2) in cumulus cells is required for the maturation of the mouse oocyte-cumulus cell complex. Dev Biol. 2003;263(1):126–138. [DOI] [PubMed] [Google Scholar]
- 23. Fan HY, Liu Z, Shimada M, Sterneck E, Johnson PF, Hedrick SM, Richards JS. MAPK3/1 (ERK1/2) in ovarian granulosa cells are essential for female fertility. Science. 2009;324(5929):938–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tran S, Zhou X, Lafleur C, Calderon MJ, Ellsworth BS, Kimmins S, Boehm U, Treier M, Boerboom D, Bernard DJ. Impaired fertility and FSH synthesis in gonadotrope-specific Foxl2 knockout mice. Mol Endocrinol. 2013;27(3):407–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bliss SP, Miller A, Navratil AM, Xie J, McDonough SP, Fisher PJ, Landreth GE, Roberson MS. ERK signaling in the pituitary is required for female but not male fertility. Mol Endocrinol. 2009;23(7):1092–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wang H, Hastings R, Miller WL, Kumar TR. Fshb-iCre mice are efficient and specific Cre deleters for the gonadotrope lineage. Mol Cell Endocrinol. 2016;419:124–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wen S, Ai W, Alim Z, Boehm U. Embryonic gonadotropin-releasing hormone signaling is necessary for maturation of the male reproductive axis. Proc Natl Acad Sci USA. 2010;107(37):16372–16377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Li Y, Schang G, Boehm U, Deng CX, Graff J, Bernard DJ. SMAD3 regulates follicle-stimulating hormone synthesis by pituitary gonadotrope cells in vivo. J Biol Chem. 2017;292(6):2301–2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Boerboom D, Kumar V, Boyer A, Wang Y, Lambrot R, Zhou X, Rico C, Boehm U, Paquet M, Celeste C, Kimmins S, Bernard DJ. β-Catenin stabilization in gonadotropes impairs follicle-stimulating hormone synthesis in male mice in vivo. Endocrinology. 2015;156(1):323–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fortin J, Boehm U, Deng CX, Treier M, Bernard DJ. Follicle-stimulating hormone synthesis and fertility depend on SMAD4 and FOXL2. FASEB J. 2014;28(8):3396–3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fortin J, Boehm U, Weinstein MB, Graff JM, Bernard DJ. Follicle-stimulating hormone synthesis and fertility are intact in mice lacking SMAD3 DNA binding activity and SMAD2 in gonadotrope cells. FASEB J. 2014;28(3):1474–1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fortin J, Kumar V, Zhou X, Wang Y, Auwerx J, Schoonjans K, Boehm U, Boerboom D, Bernard DJ. NR5A2 regulates Lhb and Fshb transcription in gonadotrope-like cells in vitro, but is dispensable for gonadotropin synthesis and fertility in vivo. PLoS One. 2013;8(3):e59058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cushman LJ, Burrows HL, Seasholtz AF, Lewandoski M, Muzyczka N, Camper SA. Cre-mediated recombination in the pituitary gland. Genesis. 2000;28(3-4):167–174. [DOI] [PubMed] [Google Scholar]
- 34. Broekmans FJ, Soules MR, Fauser BC. Ovarian aging: mechanisms and clinical consequences. Endocr Rev. 2009;30(5):465–493. [DOI] [PubMed] [Google Scholar]
- 35. Chan SW, Leathem JH. Aging and ovarian steroidogenesis in the rat. J Gerontol. 1977;32(4):395–401. [DOI] [PubMed] [Google Scholar]
- 36. Perry G, Roder H, Nunomura A, Takeda A, Friedlich AL, Zhu X, Raina AK, Holbrook N, Siedlak SL, Harris PL, Smith MA. Activation of neuronal extracellular receptor kinase (ERK) in Alzheimer disease links oxidative stress to abnormal phosphorylation. Neuroreport. 1999;10(11):2411–2415. [DOI] [PubMed] [Google Scholar]
- 37. Lu JK, LaPolt PS, Nass TE, Matt DW, Judd HL. Relation of circulating estradiol and progesterone to gonadotropin secretion and estrous cyclicity in aging female rats. Endocrinology. 1985;116(5):1953–1959. [DOI] [PubMed] [Google Scholar]
- 38. Neaves WB, Johnson L, Porter JC, Parker CR Jr, Petty CS. Leydig cell numbers, daily sperm production, and serum gonadotropin levels in aging men. J Clin Endocrinol Metab. 1984;59(4):756–763. [DOI] [PubMed] [Google Scholar]
- 39. Warner BA, Dufau ML, Santen RJ. Effects of aging and illness on the pituitary testicular axis in men: qualitative as well as quantitative changes in luteinizing hormone. J Clin Endocrinol Metab. 1985;60(2):263–268. [DOI] [PubMed] [Google Scholar]
- 40. Newbern J, Zhong J, Wickramasinghe RS, Li X, Wu Y, Samuels I, Cherosky N, Karlo JC, O’Loughlin B, Wikenheiser J, Gargesha M, Doughman YQ, Charron J, Ginty DD, Watanabe M, Saitta SC, Snider WD, Landreth GE. Mouse and human phenotypes indicate a critical conserved role for ERK2 signaling in neural crest development. Proc Natl Acad Sci USA. 2008;105(44):17115–17120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Clark PA, Brown JL, Li S, Woods AK, Han L, Sones JL, Preston RL, Southard TL, Davisson RL, Roberson MS. Distal-less 3 haploinsufficiency results in elevated placental oxidative stress and altered fetal growth kinetics in the mouse. Placenta. 2012;33(10):830–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bao J, Ma HY, Schuster A, Lin YM, Yan W. Incomplete cre-mediated excision leads to phenotypic differences between Stra8-iCre; Mov10l1(lox/lox) and Stra8-iCre; Mov10l1(lox/Δ) mice. Genesis. 2013;51(7):481–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Naor Z, Benard O, Seger R. Activation of MAPK cascades by G-protein-coupled receptors: the case of gonadotropin-releasing hormone receptor. Trends Endocrinol Metab. 2000;11(3):91–99. [DOI] [PubMed] [Google Scholar]
- 44. Navratil AM, Bliss SP, Berghorn KA, Haughian JM, Farmerie TA, Graham JK, Clay CM, Roberson MS. Constitutive localization of the gonadotropin-releasing hormone (GnRH) receptor to low density membrane microdomains is necessary for GnRH signaling to ERK. J Biol Chem. 2003;278(34):31593–31602. [DOI] [PubMed] [Google Scholar]
- 45. Benard O, Naor Z, Seger R. Role of dynamin, Src, and Ras in the protein kinase C-mediated activation of ERK by gonadotropin-releasing hormone [retracted article appears in J Biol Chem. 2017;292(21):8855]. J Biol Chem. 2001;276(7):4554–4563. [DOI] [PubMed] [Google Scholar]
- 46. Schang AL, Quérat B, Simon V, Garrel G, Bleux C, Counis R, Cohen-Tannoudji J, Laverrière JN. Mechanisms underlying the tissue-specific and regulated activity of the Gnrhr promoter in mammals. Front Endocrinol (Lausanne). 2012;3:162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ericson J, Norlin S, Jessell TM, Edlund T. Integrated FGF and BMP signaling controls the progression of progenitor cell differentiation and the emergence of pattern in the embryonic anterior pituitary. Development. 1998;125(6):1005–1015. [DOI] [PubMed] [Google Scholar]
- 48. Gonzales GF. Function of seminal vesicles and their role on male fertility. Asian J Androl. 2001;3(4):251–258. [PubMed] [Google Scholar]
- 49. Büchter D, Behre HM, Kliesch S, Nieschlag E. Pulsatile GnRH or human chorionic gonadotropin/human menopausal gonadotropin as effective treatment for men with hypogonadotropic hypogonadism: a review of 42 cases. Eur J Endocrinol. 1998;139(3):298–303. [DOI] [PubMed] [Google Scholar]
- 50. Kliesch S, Behre HM, Nieschlag E. High efficacy of gonadotropin or pulsatile gonadotropin-releasing hormone treatment in hypogonadotropic hypogonadal men. Eur J Endocrinol. 1994;131(4):347–354. [DOI] [PubMed] [Google Scholar]