Abstract
Trifluoromethylthiolated molecules are an important class of biologically active compounds and potential drug candidates. Because of the lack of efficient synthetic methods, catalytic enantioselective construction of these molecules is rare and remains a challenge. To expand this field, we herein disclose a bifunctional selenide-catalyzed approach for the synthesis of various chiral trifluoromethylthiolated tetrahydronaphthalenes bearing an all-carbon quaternary stereocenter with gem-diaryl-tethered alkenes and alkynes by merging desymmetrization and trifluoromethylthiolation strategy. The products are obtained in high yields with excellent enantio- and diastereo-selectivities. This method can be applied to the desymmetrization and sulfenylation of diols as well. Computational studies reveal that selenide can activate the electrophilic reagent better than sulfide, confirming the higher efficiency of selenide catalysis in these reactions. On the basis of the theoretical calculations, an acid-derived anion-binding interaction is suggested to exist in the whole pathway and accounts for the observed high selectivities.
Catalytic enantioselective synthesis of trifluoromethylthiolated molecules remains a challenge. Here, the authors report a bifunctional selenide-catalyzed approach for the synthesis of structurally complex chiral trifluoromethylthiolated tetrahydronaphthalenes by merging desymmetrization and trifluoromethylthiolation.
Introduction
In recent years, many efforts have been devoted to the incorporation of fluorine atoms or fluorine-containing groups such as trifluoromethyl (CF3), trifluoromethoxy (CF3O), and trifluoromethanesulfenyl (CF3S) ones into the parent molecules for various purposes because of the fluorine effect1–6. Among these endeavors, strategic synthesis of CF3S molecules has been paid special attention owing to the strong electron-withdrawing effect and extremely high lipophilicity value (πR = 1.44) of CF3S group5–12. However, little success has been achieved on enantioselective trifluoromethylthiolation until now, although stereogenic CF3S molecules warrant further studies considering the importance of chiral centers in medicine13–20. Thus, developing new methods to create versatile chiral CF3S molecules, especially those with an all-carbon quaternary stereocenter through a novel and enantioselective reaction mode, is highly desirable.
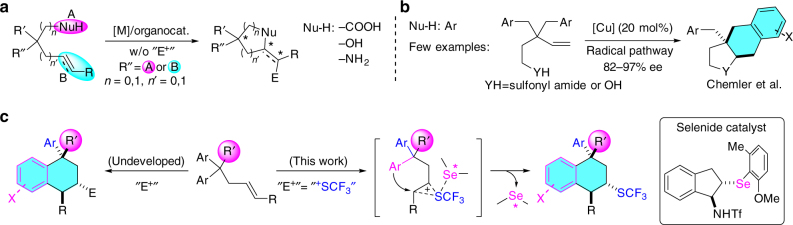
Catalytic enantioselective desymmetrization is an attractive strategy for the construction of chiral all-carbon quaternary stereocenters by the conversion of prochiral quaternary carbon centers21–23. Using this strategy, numerous valuable, potentially bioactive molecules having a chiral all-carbon quaternary center can be quickly accessed from different functionlized starting materials24–43. In particular, olefinic or alkynyl carboxylic acids33,34, alcohols35–39, and amines40–43 were frequently employed as the substrates to undergo enantioselective desymmetrization and cyclization to generate heterocycles by metal- or organocatalysis (Fig. 1a). In these transformations, the tethered nucleophile played an important role that it could bind a catalyst to guarantee an effective attack toward the multiple bond, which led to the formation of chiral products with high enantioselectivities. In contrast, enantioselective desymmetrization involving the attack of aryl group toward a multiple bond that results in the formation of multisubstituted tetrahydronaphthalene derivatives, an important class of bioactive compounds44–46, has been far less explored possibly because of the lack of the appropriate interaction between the aryl moiety and catalyst47–49. Only a few relevant examples have been reported by Chemler who utilized amine- or hydroxy-tethered alkenes for carboamination and etherification through a copper-catalyzed radical pathway (Fig. 1b) 50–53.
Fig. 1.
Enantioselective construction of all-carbon quaternary center-containing molecules via desymmetrization. a Known strategies for enantioselective desymmetrization. b Desymmetrization through copper-catalyzed radical pathway. c Enantioselective desymmetrization and trifluoromethylthiolation using aryl group as a nucleophile
Continuing our interest in Lewis basic selenium54–62-catalyzed trifluoromethylthiolation19,20,63–65, we intended to produce chiral CF3S molecules with an all-carbon quaternary stereocenter through an enantioselective, electrophilic desymmetrization, and trifluoromethylthiolation mode. We envisioned that when gem-diaryl-tethered alkenes were employed as the substrates, the aryl group on substrate could act as a nucleophile to attack chiral selenide-captured trifluoromethylthiiranium moiety to directly afford chiral CF3S tetrahydronaphthalenes (Fig. 1c). To cope with the main difficulty in this transformation, a proper chiral catalyst is essential that can control the attacking environment of the aryl group and thus induce the enantioselectivity of multiprochiral centers. Herein, we report our effort that gem-diaryl-tethered alkenes can undergo enantioselective desymmetrization and difunctionalization to efficiently afford CF3S-tetrahydronaphthalene derivatives with bifunctional selenide catalyst. The generated products contain one chiral quaternary carbon center and other two stereocenters. The developed method can be applied to enantioselective desymmetrization and sulfenylation of diols as well.
Results
Initial Attempts and Optimization of Reaction Conditions
We began our study of the electrophilic desymmetrization with 2,2-diphenyl olefinic benzamide 1a as the model substrate. It could be easily synthesized from diphenylacetonitrile, and possesses two phenyl groups as a nucleophile and an extra benzamide group. To test the desymmetrization of 1a, highly reactive electrophilic (PhSO2)2NSCF3 as the CF3S source and bifunctional catalyst C1 based on indane scaffold were utilized (Table 1). Based on our former observations20, selenide C1 with a triflic amide group was quite efficient for the trifluoromethylthiolation with the aid of acid. Pleasingly, at room temperature, the corresponding product 2a was smoothly formed rather than amination product from benzamide group in 94% nuclear magnetic resonance (NMR) yield with 89% ee and 5:1 dr using trimethylsilyl trifluoromethylsulfonate (TMSOTf) as the acid. Lowering the reaction temperature to −78 °C could quickly improve the enantioselectivity to 97% ee with unchanged diastereoselectivity (Table 1, entry 2). It is noted that sulfide catalyst C2 was not effective for this transformation at all under the similar conditions (Table 1, entry 3). To improve the diastereoselectivity of 2a, various aryl selenides based on C1 were tested for the reaction. While para-substituted phenyl group and meta-substituted phenyl group on the selenide had little influence, ortho-substituent on the phenyl ring largely enhanced the selectivity (Table 1, entries 5–8). To our delight, catalyst C7 bearing both ortho-methyl and methoxy groups was highly efficient to afford 2a in 99% yield with 99% ee and 50:1 dr. Using the mixed solvents of CH2Cl2 and (CH2Cl)2, the enantioselectivity of product 2a could be improved to > 99% (Table 1, entry 9). In addition, other acids including both Lewis acid or BrØnsted acid gave slightly lower enantioselectivity (Table 1, entries 10–12). It is noteworthy that the reaction could not go to completion and the corresponding product was formed in moderate selectivity under the optimal conditions when the substrate derived from 1a by further protecting nitrogen with methyl group was used (63% ee, see Supplementary Table 3 for details).
Table 1.
Screening of reaction conditions

|
Bz C6H5CO, Tf CF3SO2, TMSOTf Me3SiOSO2CF3, HPLC high-performance liquid chromatography, NMR nuclear magnetic resonance. Conditions: 1a (0.05 mmol), (PhSO2)2NSCF3 (1.5 equiv), catalyst (20 mol%), TMSOTf (1.0 equiv), CH2Cl2 (2.0 ml), 12 h. Yield refers to NMR yield using trifluoromethylbenzene as the internal standard. The ee value was determined by HPLC analysis on a chiral stationary phase. The dr value was determined by crude 19F NMR. *Mixed solvents of 1 ml CH2Cl2 and 1 ml (CH2Cl)2 were used. †BF3.OEt2 (1.0 equiv) as the acid. ‡TfOH (1.0 equiv) as the acid. §Tf2NH (1.0 equiv) as the acid
Desymmetrization and Trifluoromethylthiolation
With the optimal conditions in hand, we began to explore the substrate scope (Table 2). To ensure the full consumption of starting materials, 20 mol% of the catalyst loading was utilized for the transformations. Various aryl substituted olefins were first tested. All of them gave the corresponding products in good to excellent yields with excellent enantio- and diastereoselectivities (2a-h, 74–99% yields, 98–99% ees). Moreover, modified conditions were required for some substrates to give better yields or slightly better enantioselectivities. For example, the reactions could not go to completion under the optimal conditions for the formation of 2b–2d most likely because the weakly electron-withdrawing aryl group on the double bond eroded its reactivity toward CF3S cation. When the reaction temperature was raised to −60 °C, all these substrates were fully converted. Besides, low catalyst loading (10 mol%) and low concentration were appropriate for the generation of 2e and 2h to suppress the possible attack of the electron-rich aryl group of catalyst toward the iranium ion. It was worthy to mention that a substrate bearing ortho-methyl-substituted phenyl group still gave the desired product in excellent yield with excellent enantioselectivity in spite of the steric hindrance around the double bond (2f, 94% yield, >99% ee). Enantioselective desymmetrizaiton of alkyl-substituted olefins was carried out under the similar conditions. Substrates bearing methyl or phenylethyl group gave the corresponding products in good yields with excellent ees (2i, 97% ee; 2j, 97% ee). To our surprise, gem-dialkyl-substituted olefins could efficiently afford the products bearing another achiral quaternary carbon center with excellent enantioselectivities (2k, 92% ee; 2l, 97% ee), although large steric hindrance might affect the cyclization. Moreover, the developed method was also suitable for alkyne-derived compounds. Olefinic products were obtained in good yields. When phenyl-substituted substrate was utilized in the reaction, product 2m was formed with excellent ee (95% ee). The ethyl-substituted substrate gave 2n with a little lower ee (87%). These products contain a double bond, which can provide an opportunity for their further transformations. The absolute configuration of products was assigned to be 1R, 3S, 4S based on the X-ray crystallographic study of 2a.
Table 2.
Enantioselective desymmetrization and trifluoromethylthiolation of gem-diaryl tethered alkenes/alkynes

|
Bz C6H5CO, Ns 4-NO2C6H4SO2, Ac, CH3CO, Tf CF3SO2, TMSOTf Me3SiOSO2CF3, TIPSOTf iPr3SiOSO2CF3. Conditions: 1 (0.10 mmol), (PhSO2)2NSCF3 (1.5 equiv), TMSOTf (1.0 equiv), CH2Cl2 (2.0 ml) + (CH2Cl)2 (2.0 mL), −78 °C, 12 h. Yield is isolated yield. Ratio of ee was determined by HPLC analysis on a chiral stationary phase. Ratio of dr was determined by crude 19F NMR. Without note, diastereoselectivity is >99:1. *With 50:1 diastereoselectivity. †Reaction temperature: −60 °C. ‡CH2Cl2 (4.0 ml) + (CH2Cl)2 (4.0 ml) as the solvent; 10 mol% catalyst was used. §TMSOTf (2.0 equiv) was added. ∫With 8:1 diastereoselectivity. ǁTIPSOTf (1.0 equiv) instead of TMSOTf. ¶BF3.OEt2 (2.0 equiv) instead of TMSOTf
The effect of functional groups attached to the quaternary carbon center on substrates was investigated (Table 2). When substrate 1o with more acidic proton was used, the reaction proceeded efficiently to afford the carbocyclization product 2o. In contrast, when the nitrogen of 1o was protected by methyl group, the corresponding substrate 1o′ gave product 2o′ with lower enantioselectivity (85% ee). It was noted that when the phenyl group attached to the double bond on 1o was replaced by an alkyl group, CF3S-amination product was observed along with the formation of carbocyclization product. Free hydroxyl group on substrate had an impact on the enantioselectivity (2p, 81% ee). Compared to the reaction of 4-nitro-benzenesulfonamide (NsNH)-functionalized substrate, the decrease of enantioselectivity might attribute to OH-induced inappropriate H-bonding interaction between substrate and catalyst. When the hydroxyl group was protected by benzoyl or acyl group, the cyclization proceeded efficiently to produce the products with excellent ees (2q–s, 94–97% ees). It was noteworthy that the reaction of 1q was incomplete and afforded product 2q with 96% ee at −78 °C. Unexpectedly, when R′ group was hydrogen, the desired product 2t was still generated in 81% yield with 86% ee.
We then turned our attention to the desymmetrization with different gem-diaryl-tethered alkenes. Substrates with para- or ortho-substituted phenyl group at the quaternary carbon center gave the products in high yields with >99% ees under the similar conditions (2u, 2v, and 2y). When substrates with meta-substituted phenyl group at the quaternary carbon center were utilized, regioisomeric products were formed because of the site selectivity. The major isomer could be isolated with extremely high ees (2w, >99% ee; 2x, >99% ee). Fluorene-derived alkene underwent desymmetrization and cyclization to generate product 2z efficiently as well.
Practicability of the Developed System
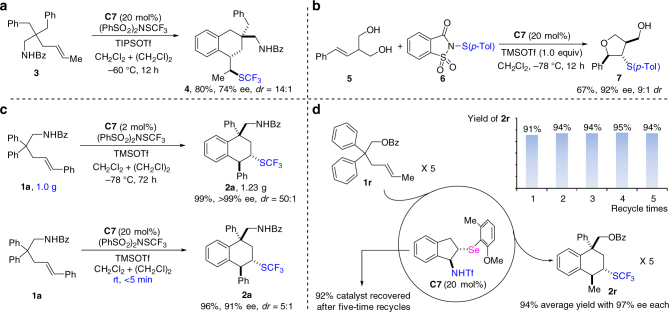
To test the generality of the developed method, alkene 3 with more flexible benzyl groups was examined under the similar conditions (Fig. 2a). Product 4 was formed in high yield with good enantioselectivity. When this method was applied to the desymmetrization and sulfenylation of 1a with sulfenylating reagents, no reaction occurred. This result was unexpected since the carbosulfenylation of alkenes has been realized by chiral selenophosphoramide catalysis66–68. Moreover, when olefinic diols were treated with sulfenylating reagent 6 in the presence of catalyst C7, thioproduct 7 was obtained in 67% with 92% ee and 9:1 dr via desymmetrization (Fig. 2b). The result shows that the developed reaction system has great potential for electrophilic functionalization of alkenes with different electrophilic reagents, and thus will trigger more explorations using the similar conditions.
Fig. 2.
Practicability of the developed system. a Transformation of substrate with flexible chain. b Desymmetrization and sulfenylation of diols. c Gram-scale reaction and reaction at room temperature. d Recycle of the catalyst
To further test the practical utility of the method, the reaction was scaled up with low catalyst loading. For example, desymmetrization of 1a (1.0 g) afforded product 2a (1.23 g) in 99% yield with excellent enantioselectivity (>99% ee) using 2 mol% C7 (Fig. 2c). This desymmetrization reaction could run at the room temperature, and was rapidly completed within 5 min using catalyst C7 to give product without much erosion of the selecticity. This result enhances the practicability of the method out of the lab. The recycle of the catalyst was also investigated. Alkene 1r was chosen as the substrate because of its easy separation from the catalyst (Fig. 2d). During the recycling, the product was obtained in high yield for each time, and its enantioselectivity remained unchanged. After being recycled five times, 92% catalyst was still recovered.
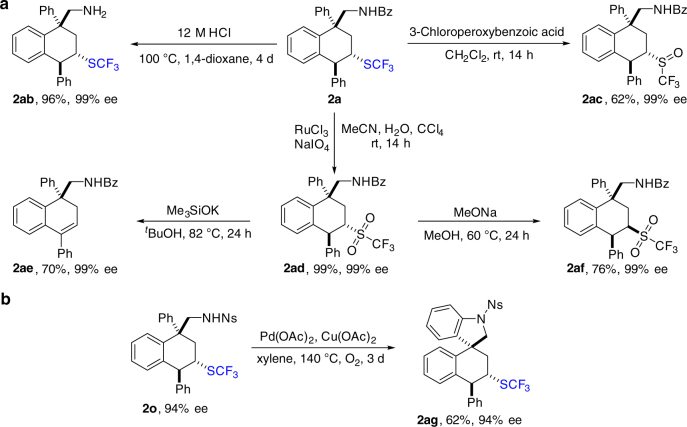
The functional groups on substrates not only helped to enhance the selectivity of the reaction, but also offered us a great opportunity to pursue further transformations of products. Some synthetic applications of 2a are depicted in Fig. 3 and all the derived compounds were isolated as single isomers. First, deprotection of benzoyl group on product 2a gave a free amine 2ab in 96% yield. The SCF3 group could be oxidized to both SOCF3 and SO2CF3 groups by the appropriate oxidative systems. Compounds with SO2CF3 group could be further converted69–71. The generated 2ad easily underwent the elimination of triflic group to form alkene 2ae with Me3SiOK. This provides a new route for the synthesis of valuable tetrahydronaphthalene derivatives, and shows a good potential of SCF3 group in synthetic utilities. Interestingly, 2af was formed as a diastereoisomer from 2ad when MeONa was used as the base. Furthermore, a spiroindoline derivative could be generated with 2o by an intramolecular Pd-catalyzed C–H amination. In the above-mentioned transformations, the erosion of enantioselectivity was not observed.
Fig. 3.
Further transformations of products. a Various transformations of 2a. b Intramolecular Pd-catalyzed C–H amination of 2o
Computational Studies
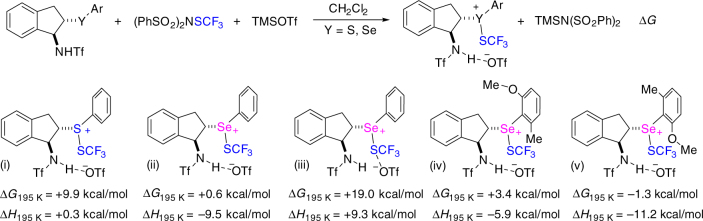
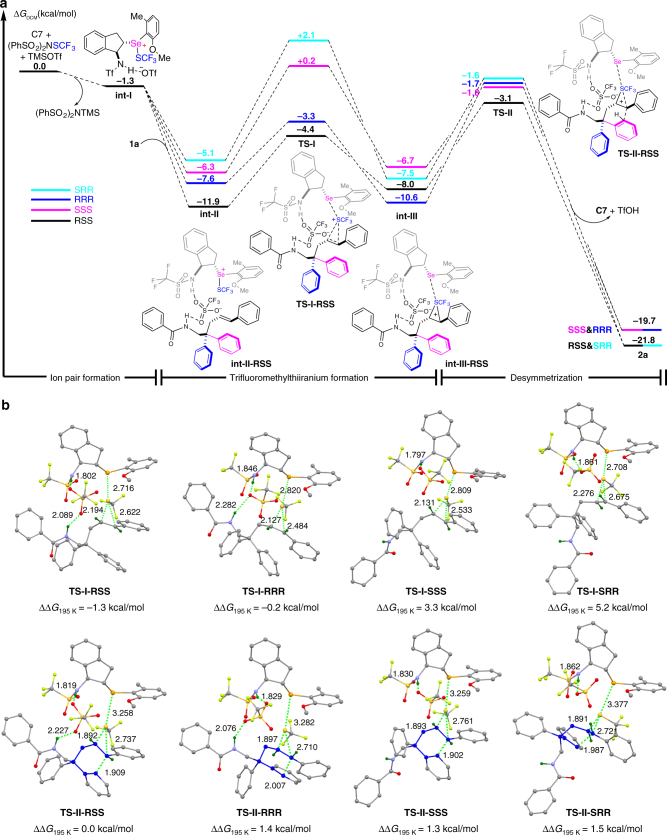
During the reaction for the formation of 2a, a complex containing a chalcogenide-captured CF3S cation was considered as the intermediate according to the work in which an active species was separated and could easily undergo the following step to afford the desired product for enantioselective sulfenofunctionalization of alkenes61. The formation of this intermediate is the commencement of the reaction and can be affected by the used chalcogenide catalysts. On the basis of the experimental results in Table 1 and our previous studies20, selenide catalysts are generally superior to the corresponding sulfide ones in promoting trifluoromethylthiolation, which reflects that selenides may activate CF3S-reagent easier to generate the ion pair intermediate than sulfides. To figure out the difference between sulfide and selenide catalysts, the impact of different catalysts on the formation of chalcogenide-captured CF3S cation was investigated. Five models with different binding interactions were proposed and the change of Gibbs free energy reflecting the difference between selenide and sulfide catalysts was calculated (Fig. 4). The results of ∆G clearly showed the huge difference caused by different catalysts. With the aid of the additive acid, the free energy for the activation of CF3S reagent by selenide is +0.6 kcal/mol in an exothermic process, but +9.9 kcal/mol is needed to promote such step using sulfide catalyst (Fig. 4i and 4ii). When TfO− anion binds to the acidic proton of the catalyst, the energy for the formation of cationic complex is largely lowered (Fig. 4ii vs. 4iii). Furthermore, when the optimal catalyst C7 is utilized, the activation energy of the step is lowest when the methyl and methoxy groups are at the appropriate positions (Fig. 4v). These computational results match experimental ones, and indicate that high-energy barrier is required for sulfide catalysis in the initial activation step and selenide is better than sulfide in the activation of the electrophilic reagent.
Fig. 4.
Computational studies. Change of Gibbs free energy based on computational studies
Proposed Mechanism
On the basis of the above results and DFT calculations, a possible reaction pathway is proposed (Fig. 5a). First, selenide catalyst activates CF3S reagent in the presence of Lewis acid to form intermediate int-I. Then, it reacts with substrate 1a to afford iranium ion int-III through transition state TS-I, after which the phenyl ring on the chain attacks the iranium ion to form the final product 2a. The reaction is spontaneous and exothermic according to calculating energies, which reasonably explains why the reaction is highly efficient under the optimal conditions. Considering the role of TfO− anion in the formation of int-I, an anion-binding interaction with a catalyst is proposed through the entire pathway. For substrate 1a with an NHBz group, an additional interaction between TfO− and the NHBz group is suggested to construct an anion bridge in the transformation based on DFT calculations. Interestingly, the proposed anion bridge can lower the energy of the intermediates. For example, when int-I directly binds to substrate 1a by hydrogen bonding, the formed intermediate has a higher energy of 2.3 kcal/mol in comparison to int-II (for details, see Supplementary Fig. 179). Moreover, it is noteworthy that the anion-binding interaction with the catalyst may provide a good chance for acids to participate in the construction of the chiral environment of reaction. Especially, the effect may be more evident when the substrates without H-bonding donor groups are utilized. Because of the anion-binding interaction with catalyst, the spatial hindrance of catalytic system is modified to further fix the absolute configuration of transition states. This can be the reason why products, e.g., 2q, 2r, and 2s, without H-bonding donor groups are generated in high enantioselectivities.
Fig. 5.
Proposed mechanism. a DFT calculations for reaction pathway at 195.15 K. b Calculated transition states related to TS-I and TS-II
When calculating the reaction pathway of 1a, it was found that the the highest energy appeared in different transition states for its four diastereomers. The highest energy is required for the attack of the phenyl ring toward the iranium ion to generate diastereomers (1R, 3S, 4S)-2a and (1R, 3R, 4R)-2a. For the formation of the other two diastereomers, the highest energy barrier lies in the step of the iranium ion formation (Fig. 5a). On the basis of the Curtin-Hammett Principle72, the formation of TS-I and TS-II involves in the enantiodetermination of chiral centers. The energy for the formation of their possible transition states is compared (Fig. 5b). A relative ∆∆G (5.2 kcal/mol) for TS-I-SRR is obtained to predict the enantioselectivity of the major product. The predicted value is 99.9%, which is close to the experimental result (2a, > 99% ee). The energy discrepancy in transition states mainly comes from the perturbance of interaction and the distortion of catalyst and substrate (see the distortion-interaction analysis in Supplementary Table 4). Such two factors affect the energy of TS-II-RRR (∆∆G = 1.4 kcal/mol) and TS-I-SSS (∆∆G = 3.3 kcal/mol) as well, which result in different diastereomers of reaction (drpredicted = 37:1). Furthermore, DFT calculations for the formation of 2q without H-bonding interaction between substrate and TfO− anion was also conducted based on the similar model. Similar results were obtained. By comparing the energy difference of the corresponding two transition states, TS-II’-RSS and TS-I’-SRR, the predicted enantioselectivity for the final product is 99.6% ee which is a little higher than the experimental result of 96% ee (∆∆G = 3.6 kcal/mol, see Supplementary Fig. 176 for details).
Discussion
In summary, we have developed an efficient approach for enantioselective desymmetrization and carbotrifluoromethylthiolation of gem-diaryl-tethered alkenes and alkynes to form chiral trifluoromethylthiolated tetrahydronaphthalenes by a bifunctional selenide catalyst. The desired products were obtained with excellent enantio- and diastereoselectivities. They could be further converted under mild conditions, which provided new pathways for the synthesis of various valuable tetrahydronaphthalene derivatives. The developed reaction could be scaled up to gram-scale and the catalytic system could also be used to the sulfenylation and desymmetrization of diols. These facts indicate that this method has great synthetic utility and practicality. Computational studies revealed the reason why selenide catalysis is more efficient than sulfide catalysis, and suggested an anion-binding interaction in the whole pathway. This work constitutes an additional strategy for the synthesis of chiral trifluoromethylthiolated molecules, highlights the efficiency of selenide catalysis, and is complementary to Lewis base catalysis.
Methods
Chiral Selenide-Catalyzed Desymmetrization
To a solution of olefin (0.1 mmol), (PhSO2)2N-SCF3 (59.8 mg, 0.15 mmol), and catalyst C7 (9.3 mg, 20 mol%) in solvent (CH2Cl2 2 ml, (CH2Cl)2 2 ml) at −78 °C was added TMSOTf (18.0 µl, 0.1 mmol). The resultant mixture was stirred at −78 °C for 12 h, and then quenched with MeOH (0.2 ml) and Et3N (0.2 ml), and concentrated in vacuo. The residue was purified by flash silica gel column chromatography to yield the corresponding CF3S product.
Chiral Selenide-Catalyzed Sulfenocyclization
To a solution of olefin 5 (17.8 mg, 0.1 mmol), saccharin-S(p-Tol) (36.6 mg, 0.12 mmol) and catalyst C7 (9.3 mg, 20 mol%) in solvent (CH2Cl2 4 ml) at −78 °C was added TMSOTf (18.0 µl, 0.1 mmol). The resultant mixture was stirred at −78 °C for 12 h, and then quenched by saturated NaHCO3 (1 ml) and then extracted with dichloromethane (8 ml ×4). The combined organic phases were concentrated in vacuo. The residue was purified by flash silica gel column chromatography to yield the corresponding thioproduct 7 (67%, 92% ee, 9:1 dr).
For nuclear magnetic resonance and high-performance liquid chromatography spectra, see Supplementary Figs 7–169.
Data Availability
The X-ray crystallographic coordinates for structures reported in this article have been deposited at the Cambridge Crystallographic Data Centre (CCDC), under deposition numbers CCDC 1523336, 1577179, 1532614, 1533403, and 1540104. The data can be obtained free of charge from The Cambridge Crystallographic Data Centre via http://www.ccdc.cam.ac.uk/data_request/cif. Any further relevant data are available from the authors upon reasonable request.
Electronic supplementary material
Acknowledgements
We thank Sun Yat-Sen University, the “One Thousand Youth Talents” Program of China and the Natural Science Foundation of Guangdong Province (Grant No. 2014A030312018) for financial support. We are grateful to our teammate, Dr. Jinji Wu, for single crystal structure analysis. We thank National Supercomputing Center in Shenzhen for providing computer service for our computational studies. We also thank Professor Vy Dong at UCI for the great suggestions about the manuscript.
Author contributions
J.L. started and performed the experiments and prepared Supplementary Information. Q.C. performed additional experiments with respect to substrate scope. X.C. performed the computational studies and revised the paper. X.Z. conceived and directed the project and wrote the manuscript.
Competing interests
The authors declare no competing financial interests.
Footnotes
Electronic supplementary material
Supplementary Information accompanies this paper at 10.1038/s41467-018-02955-0.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Xiaohui Cao, Email: caoxh5@mail.sysu.edu.cn.
Xiaodan Zhao, Email: zhaoxd3@mail.sysu.edu.cn.
References
- 1.Jeschke P. The unique role of fluorine in the design of active ingredients for modern crop protection. Chembiochem. 2004;5:570–589. doi: 10.1002/cbic.200300833. [DOI] [PubMed] [Google Scholar]
- 2.Liang T, Neumann CN, Ritter T. Introduction of fluorine and fluorine-containing functional groups. Angew. Chem. Int. Ed. 2013;52:8214–8264. doi: 10.1002/anie.201206566. [DOI] [PubMed] [Google Scholar]
- 3.Yang X, Wu T, Phipps RJ, Toste FD. Advances in catalytic enantioselective fluorination, mono‑,di‑, and trifluoromethylation, and trifluoromethylthiolation reactions. Chem. Rev. 2015;115:826–870. doi: 10.1021/cr500277b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Manteau B, et al. New trends in the chemistry of α-fluorinated ethers, thioethers, amines and phosphines. J. Fluor. Chem. 2010;131:140–158. doi: 10.1016/j.jfluchem.2009.09.009. [DOI] [Google Scholar]
- 5.Toulgoat F, Alazet S, Billard T. Direct trifluoromethylthiolation reactions: the “renaissance” of an old concept. Eur. J. Org. Chem. 2014;2014:2415–2428. doi: 10.1002/ejoc.201301857. [DOI] [Google Scholar]
- 6.Xu XH, Matsuzaki K, Shibata N. Synthetic methods for compounds having CF3-S units on carbon by trifluoromethylation, trifluoromethylthiolation, triflylation, and related reactions. Chem. Rev. 2015;115:731–764. doi: 10.1021/cr500193b. [DOI] [PubMed] [Google Scholar]
- 7.Ferry A, Billard T, Langlois BR, Bacqué E. Trifluoromethanesulfanylamides as easy-to-handle equivalents of the trifluoromethanesulfanyl cation (CF3S+): reaction with alkenes and alkynes. Angew. Chem. Int. Ed. 2009;48:8551–8555. doi: 10.1002/anie.200903387. [DOI] [PubMed] [Google Scholar]
- 8.Chen C, Chu L, Qing FL. Metal-free oxidative trifluoromethylthiolation of terminal alkynes with CF3SiMe3 and elemental sulfur. J. Am. Chem. Soc. 2012;134:12454–12457. doi: 10.1021/ja305801m. [DOI] [PubMed] [Google Scholar]
- 9.Yang YD, et al. Trifluoromethanesulfonyl hypervalent iodonium ylide for copper-catalyzed trifluoromethylthiolation of enamines, indoles, and β-keto esters. J. Am. Chem. Soc. 2013;135:8782–8785. doi: 10.1021/ja402455f. [DOI] [PubMed] [Google Scholar]
- 10.Danoun G, Bayarmagnai B, Gruenberg MF, Goossen LJ. Sandmeyer trifluoromethylthiolation of arenediazonium salts with sodium thiocyanate and Ruppert-Prakash reagent. Chem. Sci. 2014;5:1312–1316. doi: 10.1039/c3sc53076k. [DOI] [Google Scholar]
- 11.Guo S, Zhang X, Tang P. Silver-mediated oxidative aliphatic C-H trifluoromethylthiolation. Angew. Chem. Int. Ed. 2015;54:4065–4069. doi: 10.1002/anie.201411807. [DOI] [PubMed] [Google Scholar]
- 12.Mukherjee S, Maji B, Tlahuext-Aca A, Glorius F. Visible-light-promoted activation of unactivated C(sp3)–H bonds and their selective trifluoromethylthiolation. J. Am. Chem. Soc. 2016;138:16200–16203. doi: 10.1021/jacs.6b09970. [DOI] [PubMed] [Google Scholar]
- 13.Bootwicha T, et al. N-Trifluoromethylthiophthalimide: a stable electrophilic SCF3-reagent and its application in the catalytic asymmetric trifluoromethylsulfenylation. Angew. Chem. Int. Ed. 2013;52:12856–12859. doi: 10.1002/anie.201304957. [DOI] [PubMed] [Google Scholar]
- 14.Wang X, Yang T, Cheng X, Shen Q. Enantioselective electrophilic trifluoromethylthiolation of β-ketoesters: a case of reactivity and selectivity bias for organocatalysis. Angew. Chem. Int. Ed. 2013;52:12860–12864. doi: 10.1002/anie.201305075. [DOI] [PubMed] [Google Scholar]
- 15.Zhu XL, et al. In situ generation of electrophilic trifluoromethylthio reagents for enantioselective trifluoromethylthiolation of oxindoles. Org. Lett. 2014;16:2192–2195. doi: 10.1021/ol5006888. [DOI] [PubMed] [Google Scholar]
- 16.Deng QH, et al. Copper-boxmi complexes as highly enantioselective catalysts for electrophilic trifluoromethylthiolations. Chem. Eur. J. 2014;20:93–97. doi: 10.1002/chem.201303641. [DOI] [PubMed] [Google Scholar]
- 17.Zhao BL, Du DM. Enantioselective squaramide-catalyzed trifluoromethylthiolation-sulfur-Michael/Aldol cascade reaction: one-pot synthesis of CF3S-containing spiro cyclopentanone-thiochromanes. Org. Lett. 2017;19:1036–1039. doi: 10.1021/acs.orglett.6b03846. [DOI] [PubMed] [Google Scholar]
- 18.Zhang Z, et al. Catalytic asymmetric trifluoromethylthiolation via enantioselective [2,3]-sigmatropic rearrangement of sulfonium ylides. Nat. Chem. 2017;9:970–976. doi: 10.1038/nchem.2789. [DOI] [PubMed] [Google Scholar]
- 19.Liu X, et al. Enantioselective trifluoromethylthiolating lactonization catalyzed by an indane-based chiral sulfide. Angew. Chem. Int. Ed. 2016;55:5846–5850. doi: 10.1002/anie.201601713. [DOI] [PubMed] [Google Scholar]
- 20.Luo J, Liu Y, Zhao X. Chiral selenide-catalyzed enantioselective construction of saturated trifluoromethylthiolated azaheterocycles. Org. Lett. 2017;19:3434–3437. doi: 10.1021/acs.orglett.7b01392. [DOI] [PubMed] [Google Scholar]
- 21.Enríquez-Garcíawand Aacute, Kündig EP. Desymmetrisation of meso-diols mediated by non-enzymatic acyl transfer catalysts. Chem. Soc. Rev. 2012;41:7803–7831. doi: 10.1039/c2cs35049a. [DOI] [PubMed] [Google Scholar]
- 22.Petersen KS. Nonenzymatic enantioselective synthesis of all-carbon quaternary centers through desymmetrization. Tetrahedron Lett. 2015;56:6523–6535. doi: 10.1016/j.tetlet.2015.09.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zeng XP, et al. Catalytic enantioselective desymmetrization reactions to all-carbon quaternary stereocenters. Chem. Rev. 2016;116:7330–7396. doi: 10.1021/acs.chemrev.6b00094. [DOI] [PubMed] [Google Scholar]
- 24.Lee JY, You YS, Kang SH. Asymmetric synthesis of all-carbon quaternary stereocenters via desymmetrization of 2,2-disubstituted 1,3-propanediols. J. Am. Chem. Soc. 2011;133:1772. doi: 10.1021/ja1103102. [DOI] [PubMed] [Google Scholar]
- 25.Aikawa K, Okamoto T, Mikami K. Copper(I)-catalyzed asymmetric desymmetrization: Synthesis of five-membered-ring compounds containing all-carbon quaternary stereocenters. J. Am. Chem. Soc. 2012;134:10329–10332. doi: 10.1021/ja3032345. [DOI] [PubMed] [Google Scholar]
- 26.Saget T, Cramer N. Enantioselective C-H arylation strategy for functionalized dibenzazepinones with quaternary stereocenters. Angew. Chem. Int. Ed. 2013;52:7865–7868. doi: 10.1002/anie.201303816. [DOI] [PubMed] [Google Scholar]
- 27.Zhao Y, Jiang X, Yeung YY. Catalytic, enantioselective, and highly chemoselective bromocyclization of olefinic dicarbonyl compounds. Angew. Chem. Int. Ed. 2013;52:8597–8601. doi: 10.1002/anie.201304107. [DOI] [PubMed] [Google Scholar]
- 28.Cheng XF, et al. Pd(II)-catalyzed enantioselective C-H activation/C-O bond formation: synthesis of chiral benzofuranones. J. Am. Chem. Soc. 2013;135:1236–1239. doi: 10.1021/ja311259x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meng SS, et al. Chiral phosphoric acid catalyzed highly enantioselective desymmetrization of 2-substituted and 2,2-disubstituted 1,3-diols via oxidative cleavage of benzylidene acetals. J. Am. Chem. Soc. 2014;136:12249–12252. doi: 10.1021/ja507332x. [DOI] [PubMed] [Google Scholar]
- 30.Partridge BM, González JS, Lam HW. Iridium-catalyzed arylative cyclization of alkynones by 1,4-iridium migration. Angew. Chem. Int. Ed. 2014;53:6523–6527. doi: 10.1002/anie.201403271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang W, Liu Y, Zhang S, Cai Q. Copper-catalyzed intramolecular desymmetric aryl C-O coupling for the enantioselective construction of chiral dihydrobenzofurans and dihydrobenzopyrans. Angew. Chem. Int. Ed. 2015;54:8805–8808. doi: 10.1002/anie.201503882. [DOI] [PubMed] [Google Scholar]
- 32.Wang Z, et al. Catalytic enantioselective intermolecular desymmetrization of azetidines. J. Am. Chem. Soc. 2015;137:5895–5898. doi: 10.1021/jacs.5b03083. [DOI] [PubMed] [Google Scholar]
- 33.Wilking M, Mück-Lichtenfeld C, Daniliuc CG, Hennecke U. Enantioselective, desymmetrizing bromolactonization of alkynes. J. Am. Chem. Soc. 2013;135:8133–8136. doi: 10.1021/ja402910d. [DOI] [PubMed] [Google Scholar]
- 34.Wilking M, Daniliuc CG, Hennecke U. Monomeric cinchona alkaloid-based catalysts for highly enantioselective bromolactonisation of alkynes. Chem. Eur. J. 2016;22:18601–18607. doi: 10.1002/chem.201604003. [DOI] [PubMed] [Google Scholar]
- 35.Arai MA, Kuraishi M, Arai T, Sasai H. A new asymmetric Wacker-type cyclization and tandem cyclization promoted by Pd(II)-spiro bis(isoxazoline) catalyst. J. Am. Chem. Soc. 2001;123:2907–2908. doi: 10.1021/ja005920w. [DOI] [PubMed] [Google Scholar]
- 36.Seiser T, Cramer N. Enantioselective C-C bond activation of allenyl cyclobutanes: access to cyclohexenones with quaternary stereogenic centers. Angew. Chem. Int. Ed. 2008;47:9294–9297. doi: 10.1002/anie.200804281. [DOI] [PubMed] [Google Scholar]
- 37.Tay DW, Leung GYC, Yeung YY. Desymmetrization of diolefinic diols by enantioselective aminothiocarbamate-catalyzed bromoetherification: synthesis of chiral spirocycles. Angew. Chem. Int. Ed. 2014;53:5161–5164. doi: 10.1002/anie.201310136. [DOI] [PubMed] [Google Scholar]
- 38.Ke Z, Tan C, Chen K, Yeung YY. Catalytic asymmetric bromoetherification and desymmetrization of olefinic 1,3-diols with C2-symmetric sulfides. J. Am. Chem. Soc. 2014;136:5627–5630. doi: 10.1021/ja5029155. [DOI] [PubMed] [Google Scholar]
- 39.Zi W, Toste FD. Gold(I)-catalyzed enantioselective desymmetrization of 1,3-diols through intramolecular hydroalkoxylation of allenes. Angew. Chem. Int. Ed. 2015;54:14447–14451. doi: 10.1002/anie.201508331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gu Q, You SL. Desymmetrization of cyclohexadienonesviacinchonine derived thiourea-catalyzed enantioselective aza-Michael reaction and total synthesis of (-)-Mesembrine. Chem. Sci. 2011;2:1519–1522. doi: 10.1039/c1sc00083g. [DOI] [Google Scholar]
- 41.Mourad AK, Leutzow J, Czekelius C. Anion-induced enantioselective cyclization of diynamides to pyrrolidines catalyzed by cationic gold complexes. Angew. Chem. Int. Ed. 2012;51:11149–11152. doi: 10.1002/anie.201205416. [DOI] [PubMed] [Google Scholar]
- 42.Babij NR, Wolfe JP. Desymmetrization of meso-2,5-diallylpyrrolidinyl oreas through asymmetric palladium-catalyzed carboamination: stereocontrolled synthesis of bicyclic ureas. Angew. Chem. Int. Ed. 2013;52:9247–9250. doi: 10.1002/anie.201302720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Manna K, Eedugurala N, Sadow AD. Zirconium-catalyzed desymmetrization of aminodialkenes and aminodialkynes through enantioselective hydroamination. J. Am. Chem. Soc. 2015;137:425–435. doi: 10.1021/ja511250m. [DOI] [PubMed] [Google Scholar]
- 44.Ward RS. Lignans, neolignans and related compounds. Nat. Prod. Rep. 1999;16:75–96. doi: 10.1039/a705992b. [DOI] [Google Scholar]
- 45.Razzakov NA, Vakhabov A, Aripova SF. Quaternary derivatives of α- and β-scopodonnines and their pharmacologic activity. Chem. Nat. Compd. 2003;39:215–217. doi: 10.1023/A:1024830417848. [DOI] [Google Scholar]
- 46.Gordaliza M, et al. Podophyllotoxin: distribution, sources, applications and new cytotoxic derivatives. Toxicon. 2004;44:441–459. doi: 10.1016/j.toxicon.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 47.Miller B, Shi X. Cyclization and rearrangement processes resulting from bromination of 3-benzylcycloalkenes. J. Org. Chem. 1993;58:2907–2909. doi: 10.1021/jo00062a043. [DOI] [Google Scholar]
- 48.Haro T, Nevado C. Flexible gold-catalyzed regioselective oxidative difunctionalization of unactivated alkenes. Angew. Chem. Int. Ed. 2011;50:906–910. doi: 10.1002/anie.201005763. [DOI] [PubMed] [Google Scholar]
- 49.Cacciuttolo B. Access to polycyclic derivatives by triflate-catalyzed intramolecular hydroarylation. Eur. J. Org. Chem. 2014;2014:7458–7468. doi: 10.1002/ejoc.201402972. [DOI] [Google Scholar]
- 50.Zeng W, Chemler SR. Copper(II)-catalyzed enantioselective intramolecular carboamination of alkenes. J. Am. Chem. Soc. 2007;129:12948–12949. doi: 10.1021/ja0762240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Miao L, et al. Diastereo- and enantioselective copper-catalyzed intramolecular carboamination of alkenes for the synthesis of hexahydro-1H-benz[f]indoles. Org. Lett. 2010;12:4739–4741. doi: 10.1021/ol102233g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Miller Y, Miao L, Hosseini AS, Chemler SR. Copper-catalyzed intramolecular alkene carboetherification: synthesis of fused-ring and bridged-ring tetrahydrofurans. J. Am. Chem. Soc. 2012;134:12149–12156. doi: 10.1021/ja3034075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bovino MT, et al. Enantioselective copper-catalyzed carboetherification of unactivated alkenes. Angew. Chem. Int. Ed. 2014;53:6383–6387. doi: 10.1002/anie.201402462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vedejs E, Denmark SE, editors. Lewis Base Catalysis in Organic Synthesis. Weinheim: Wiley-VCH; 2016. [Google Scholar]
- 55.Braga AL, Lüdtke DS, Vargas F, Braga RC. Catalytic applications of chiral organoselenium compounds in asymmetric synthesis. Synlett. 2006;37:1453–1466. doi: 10.1055/s-2006-941592. [DOI] [Google Scholar]
- 56.Braga AL, Lüdtke DS, Vargas F. Enantioselective synthesis mediated by catalytic chiral organoselenium compounds. Curr. Org. Chem. 2006;10:1921–1938. doi: 10.2174/138527206778521204. [DOI] [Google Scholar]
- 57.Denmark SE, Collins WR. Lewis base activation of lewis acids: development of a Lewis base catalyzed selenolactonization. Org. Lett. 2007;9:3801–3804. doi: 10.1021/ol701617d. [DOI] [PubMed] [Google Scholar]
- 58.Denmark SE, Beutner GL. Lewis base catalysis in organic synthesis. Angew. Chem. Int. Ed. 2008;47:1560–1638. doi: 10.1002/anie.200604943. [DOI] [PubMed] [Google Scholar]
- 59.Denmark SE, Kalyani D, Collins WR. Preparative and mechanistic studies toward the rational development of catalytic, enantioselective selenoetherification reactions. J. Am. Chem. Soc. 2010;132:15752–15765. doi: 10.1021/ja106837b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Denmark SE, Chi HM. Lewis base catalyzed, enantioselective, intramolecular sulfenoamination of olefins. J. Am. Chem. Soc. 2014;136:8915–8918. doi: 10.1021/ja5046296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Denmark SE, Hartmann E, Kornfilt DJP, Wang H. Mechanistic, crystallographic, and computational studies on the catalytic, enantioselective sulfenofunctionalization of alkenes. Nat. Chem. 2014;6:1056–1064. doi: 10.1038/nchem.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen F, Tan CK, Yeung YY. C2-symmetric cyclic selenium-catalyzed enantioselective bromoaminocyclization. J. Am. Chem. Soc. 2013;135:1232–1235. doi: 10.1021/ja311202e. [DOI] [PubMed] [Google Scholar]
- 63.Luo J, Zhu Z, Liu Y, Zhao X. Diaryl selenide catalyzed vicinal trifluoromethylthioamination of alkenes. Org. Lett. 2015;17:3620–3623. doi: 10.1021/acs.orglett.5b01727. [DOI] [PubMed] [Google Scholar]
- 64.Wu JJ, Xu J, Zhao X. Selenide-catalyzed stereoselective construction of tetrasubstituted trifluoromethylthiolated alkenes with alkynes. Chem. Eur. J. 2016;22:15265–15269. doi: 10.1002/chem.201603975. [DOI] [PubMed] [Google Scholar]
- 65.Luo J, Liu X, Zhao X. Development of chalcogenide catalysts towards trifluoromethylthiolation. Synlett. 2017;28:397–401. doi: 10.1055/s-0036-1588568. [DOI] [Google Scholar]
- 66.Denmark SE, Jaunet A. Catalytic, enantioselective, intramolecular carbosulfenylation of olefins. J. Am. Chem. Soc. 2013;135:6419–6422. doi: 10.1021/ja401867b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Denmark SE, Chi HM. Catalytic, enantioselective, intramolecular carbosulfenylation of olefins. Mechanistic aspects: a remarkable case of negative catalysis. J. Am. Chem. Soc. 2014;136:3655–3663. doi: 10.1021/ja413270h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Denmark SE, Jaunet A. Catalytic, enantioselective, intramolecular carbosulfenylation of olefins. Preparative and stereochemical aspects. J. Org. Chem. 2014;79:140–171. doi: 10.1021/jo4023765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hendrickson JB, Gigs A, Wareing J. Triflones (CF3SO2C). A survey of reactivity and synthetic utility. J. Am. Chem. Soc. 1974;96:2275–2276. doi: 10.1021/ja00814a061. [DOI] [Google Scholar]
- 70.Hendrickson JB, Bair KW, Keehn PM. Conversion of triflones to ketones. J. Org. Chem. 1977;42:2935–2936. doi: 10.1021/jo00437a036. [DOI] [Google Scholar]
- 71.Hellmann G, et al. Chiral fluorinateda-sulfonyl carbanions: Enantioselective synthesis and electrophilic capture, racemization dynamics, and structure. Chem. Eur. J. 2013;19:3869–3897. doi: 10.1002/chem.201204014. [DOI] [PubMed] [Google Scholar]
- 72.Seeman JI. The Curtin-Hammett priciple and the Winstein-Holness equation: new definition and recent extensions to classical concepts. J. Chem. Ed. 1986;63:42–48. doi: 10.1021/ed063p42. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The X-ray crystallographic coordinates for structures reported in this article have been deposited at the Cambridge Crystallographic Data Centre (CCDC), under deposition numbers CCDC 1523336, 1577179, 1532614, 1533403, and 1540104. The data can be obtained free of charge from The Cambridge Crystallographic Data Centre via http://www.ccdc.cam.ac.uk/data_request/cif. Any further relevant data are available from the authors upon reasonable request.