Abstract
Elevated mammographic density (MD) is an established breast cancer risk factor. Studies examining relationships between MD and breast cancer risk factors are limited in China, where established breast cancer risk factors are less prevalent but dense breasts are more prevalent than Western countries. This study included 11,478 women (45-69 years; 36% premenopausal) participating in an ongoing national cancer screening program in 11 urban provinces in China and predicted as having high-risk for breast cancer. Polytomous logistic regression was performed to assess associations between MD and risk factors by comparing each higher Breast Imaging Reporting and Data System (BI-RADS) category (2, 3, or 4) to the lowest category (BI-RADS, 1). We found associations of increasing age, body mass index, weight, postmenopausal status, and parity with lower MD. Higher levels of education, increasing height, and later first birth were associated with higher MD. These associations did not vary by menopausal status. Additionally, the association between longer period of breastfeeding and lower MD was seen among postmenopausal women only (Pinteraction = 0.003). Having first-degree relatives with breast cancer diagnosed before 50 years was associated with lower MD only among premenopausal women (Pinteraction = 0.061). We found effects of established breast cancer risk factors on MD showed similar directions in Chinese and Western women, supporting the hypothesis that MD represents cumulative exposure to breast cancer risk factors over the life course. Our findings help to understand the biological basis of the association of MD with breast cancer risk and have implications for breast cancer prevention research in China.
Epidemiology: Breast cancer risk factors similar in urban China and Western countries
Demographic variables linked to dense breast tissue — a strong risk factor for cancer — are similar among women living in China and the West. Xiaohong Yang from the US National Cancer Institute in Bethesda, Maryland, USA, and colleagues looked for predictors of elevated breast density, as measured on a mammogram, among participants of an ongoing cancer screening program in 11 urban provinces in China. In their analysis of 11,478 women aged 45 to 69 deemed at high-risk for breast cancer, the researchers identified many variables — incliuding age, height, weight, menopausal status, educational level and history of pregnancies — that were all related to mammographic density in some way. Similar associations were previously found among women in Western countries, suggesting that many of these risk factors are consistent across populations.
Introduction
The estimated age-standardized incidence rate of breast cancer in China (22.1 per 100,000 person-years) is currently lower compared with Western countries (e.g., 92 per 100,000 person-years in Northern America).1 However, incidence rates have increased rapidly during the past decades and breast cancer is now the most frequently diagnosed female cancer in Chinese and Asian women.2,3 Previous studies have suggested that rising breast cancer incidence in Asian women is largely attributable to changes in reproductive and lifestyle risk factors such as declining fertility rates, delayed age at first birth, reduced breastfeeding, and increasing body mass index (BMI).4–7
Mammographic density (MD) reflects the proportion of radiologically dense epithelial and stromal breast tissues that are visible on a mammogram. The association between higher MD and breast cancer risk has been reported in diverse populations.8–16 However, in China, where the mammogram screening program is available only in selected areas and populations,17 the evaluation of MD in relation to breast cancer risk is very limited. Several breast cancer risk factors including age, reproductive factors, body size, menopausal hormonal therapy, and dietary factors are associated with variations in MD.18–21 This research suggests these risk factors can influence changes in breast tissue, manifested as variations in MD. Well-known risk factors for breast cancer explain 20–30% of the variance in percent mammographic density (PMD) in Western populations,19,22 suggesting that additional factors influencing MD variation remain to be identified. Knowledge of the genetic basis of MD is limited, but twin studies suggest that MD is a highly heritable trait and the majority (60–70%) of the residual MD variance can be explained by genetic factors after accounting for known MD influencing factors.23 Several studies show ethnic variations in MD and indicate that ethnicity is one of the factors that influences MD and may influence some of the associations between MD and breast cancer risk factors.24–28
Accordingly, the expansion of MD studies to populations with distinct genomic architecture and environmental and lifestyle exposures may improve our understanding of factors that influence MD and have important public health implications. Compared to Western populations, Asians are known to have higher PMD but lower prevalence of well-established breast cancer risk factors associated with Western lifestyle. Therefore, MD-influencing factors might not have exactly same effects in Asians as in Western populations. In addition, it is unknown how exposures with higher prevalence among Asian women such as tea drinking would influence MD.
Prior studies evaluating MD influencing factors among Asian women were limited to immigrant Asians29,30 or more developed Asian countries, such as Singapore,31–34 Japan,35,36 and Korea.37–40 Except for one large population-based study,41 MD studies conducted in China have been small and examined only a limited number of risk factors.42 Because density decreases with menopause,43,44 it is important to evaluate risk factor associations separately for premenopausal and postmenopausal women. However, none of these studies evaluated factors that influence MD by menopausal status.41,42 Therefore, we comprehensively examined the associations of MD with reproductive and lifestyle factors considering menopausal status among Chinese women who participated in a large multi-city screening program in China and had high predicted risk of developing breast cancer.
Results
The distributions of selected characteristics are shown in Table 1. The mean age was 54.4 years (range, 45-69) and mean BMI was 24.1 kg/m2 (range, 15.8-36.4). Thirty percent of participants had first-degree relatives with breast cancer diagnosed before age 50. Most women were postmenopausal (64%), parous (94%), and reported that they had breastfed (83%), and had never smoked (87%) or drank alcohol (74%). The distributions of MD within Breast Imaging Reporting and Data System (BI-RADS) categories are shown for different age groups in Fig. 1. The overall distribution was as follows: 11% (1, almost entirely fat), 37% (2, scattered fibroglandular densities), 50% (3, heterogeneously dense), and 3% (4, extremely dense). Heterogeneously dense was the most common MD category for both premenopausal (65%) and postmenopausal (42%) women. As expected, MD decreased with increasing age (PMantel–Haenszel Chi-Square < 1.0E-30). MD also varied by family history, education, BMI, weight, age at menarche, parity, age at first full-term birth, breastfeeding, menopausal status, age at menopause, smoking, alcohol, and tea drinking (PChi-square or ANOVA < 0.05, Table 1).
Table 1.
Demographic characteristics, anthropometric measures, reproductive and lifestyle factors among participants of the Chinese Breast Cancer Screening Program (2013–2014)
| All (n = 11478) | BI-RADS = 1 (n = 1206) | BI-RADS = 2 (n = 4216) | BI-RADS = 3 (n = 5736) | BI-RADS = 4 (n = 320) | P a | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | n | % | |||
| Age | ||||||||||||
| Mean (SD) | 54.4 | 6.3 | 59.0 | 5.96 | 55.9 | 6.20 | 52.5 | 5.70 | 51.1 | 5.22 | <1.0E-30 | |
| 45-49 | 3016 | 26.3 | 84 | 7.0 | 729 | 17.3 | 2066 | 36.0 | 137 | 42.8 | <1.0E-30 | |
| 50–59 | 5760 | 50.2 | 534 | 44.3 | 2191 | 52.0 | 2881 | 50.2 | 154 | 48.1 | ||
| 60–69 | 2702 | 23.5 | 588 | 48.8 | 1296 | 30.7 | 789 | 13.8 | 29 | 9.1 | ||
| Family history of breast and/or ovarian cancers | ||||||||||||
| No | 5797 | 50.5 | 581 | 48.2 | 2109 | 50.0 | 2939 | 51.3 | 168 | 52.7 | 0.05 | |
| 1st or 2nd degree relativesb | 2205 | 19.2 | 226 | 18.7 | 814 | 19.3 | 1092 | 19.0 | 73 | 22.9 | ||
| 1st degree relatives with breast cancer <50 years | 3472 | 30.3 | 399 | 33.1 | 1292 | 30.7 | 1703 | 29.7 | 78 | 24.5 | ||
| Missing | 4 | 1 | 2 | 1 | ||||||||
| Education | ||||||||||||
| None-elementary | 1369 | 11.9 | 325 | 26.9 | 564 | 13.4 | 460 | 8.0 | 20 | 6.3 | <1.0E-30 | |
| Middle school | 3405 | 29.7 | 402 | 33.3 | 1321 | 31.3 | 1615 | 28.2 | 67 | 20.9 | ||
| High school | 4054 | 35.3 | 336 | 27.9 | 1510 | 35.8 | 2083 | 36.3 | 125 | 39.1 | ||
| College+ | 2650 | 23.1 | 143 | 11.9 | 821 | 19.5 | 1578 | 27.5 | 108 | 33.8 | ||
| Body mass index (kg/m2) | ||||||||||||
| Mean (SD) | 24.1 | 3.00 | 25.7 | 3.11 | 24.4 | 2.97 | 23.7 | 2.85 | 22.3 | 2.86 | <1.0E-30 | |
| <23 | 4188 | 38.1 | 231 | 20.3 | 1371 | 34.1 | 2392 | 43.3 | 194 | 62.6 | <1.0E-30 | |
| 23–24.9 | 2927 | 26.6 | 261 | 23.0 | 1073 | 26.7 | 1529 | 27.7 | 64 | 20.6 | ||
| 25+ | 3876 | 35.3 | 644 | 56.7 | 1582 | 39.3 | 1598 | 29.0 | 52 | 16.8 | ||
| Missing | 487 | 70 | 190 | 217 | 10 | |||||||
| Weight (kg) | ||||||||||||
| Mean (SD) | 61.6 | 8.10 | 64.8 | 8.15 | 62.2 | 8.06 | 60.7 | 7.86 | 56.7 | 7.46 | <1.0E-30 | |
| Missing | 281 | 55 | 109 | 111 | 6 | |||||||
| Height (cm) | ||||||||||||
| Mean (SD) | 159.8 | 4.52 | 159.2 | 4.72 | 159.8 | 4.54 | 160.0 | 4.44 | 159.5 | 4.57 | 6.1E-09 | |
| Missing | 229 | 19 | 89 | 116 | 5 | |||||||
| Age at menarche | ||||||||||||
| <13 | 1334 | 11.7 | 112 | 9.3 | 438 | 10.4 | 727 | 12.7 | 57 | 17.9 | 1.4E-20 | |
| 13–14 | 5245 | 45.8 | 469 | 39.1 | 1862 | 44.3 | 2749 | 48.0 | 165 | 51.7 | ||
| 15+ | 4874 | 42.6 | 620 | 51.6 | 1907 | 45.3 | 2250 | 39.3 | 97 | 30.4 | ||
| Missing | 25 | 5 | 9 | 10 | 1 | |||||||
| Parity | ||||||||||||
| Never | 683 | 6.0 | 37 | 3.1 | 229 | 5.4 | 380 | 6.6 | 37 | 11.6 | 3.0E-09 | |
| Ever | 10795 | 94.1 | 1169 | 96.9 | 3987 | 94.6 | 5356 | 93.4 | 283 | 88.4 | ||
| Age at first full term birthc | ||||||||||||
| <25 | 3500 | 32.5 | 457 | 39.3 | 1276 | 32.1 | 1681 | 31.5 | 86 | 30.4 | 2.5E-04 | |
| 25–26 | 3433 | 31.9 | 344 | 29.6 | 1277 | 32.1 | 1720 | 32.2 | 92 | 32.5 | ||
| 27–28 | 2136 | 19.8 | 217 | 18.6 | 796 | 20.0 | 1068 | 20.0 | 55 | 19.4 | ||
| 29+ | 1695 | 15.8 | 146 | 12.5 | 626 | 15.7 | 873 | 16.3 | 50 | 17.7 | ||
| Missing | 31 | 5 | 12 | 14 | 0 | |||||||
| Breastfeedingc | ||||||||||||
| No breastfeeding | 1870 | 17.3 | 174 | 14.9 | 645 | 16.2 | 982 | 18.3 | 69 | 24.4 | 2.5E-27 | |
| 1–6 months | 1866 | 17.3 | 156 | 13.3 | 699 | 17.5 | 952 | 17.8 | 59 | 20.8 | ||
| 7–12 months | 4276 | 39.6 | 396 | 33.9 | 1564 | 39.2 | 2200 | 41.1 | 116 | 41.0 | ||
| 13 months+ | 2783 | 25.8 | 443 | 37.9 | 1079 | 27.1 | 1222 | 22.8 | 39 | 13.8 | ||
| Menopausal status | ||||||||||||
| Premenopausal | 4100 | 35.7 | 154 | 12.8 | 1099 | 26.1 | 2665 | 46.5 | 182 | 56.9 | <1.0E-30 | |
| Postmenopausal | 7378 | 64.3 | 1052 | 87.2 | 3117 | 73.9 | 3071 | 53.5 | 138 | 43.1 | ||
| Age at menopause among postmenopausal women | ||||||||||||
| <49 | 2766 | 37.5 | 396 | 37.7 | 1134 | 36.4 | 1172 | 38.2 | 64 | 46.4 | <1.0E-30 | |
| 49–50 | 2613 | 35.4 | 347 | 33.0 | 1107 | 35.5 | 1116 | 36.4 | 43 | 31.2 | ||
| 51+ | 1997 | 27.1 | 308 | 29.3 | 876 | 28.1 | 782 | 25.5 | 31 | 22.5 | ||
| Missing | 2 | 1 | 1 | |||||||||
| Smoking | ||||||||||||
| Never | 10008 | 87.2 | 1075 | 89.1 | 3695 | 87.6 | 4957 | 86.4 | 281 | 87.8 | 0.01 | |
| Current | 1264 | 11.0 | 105 | 8.7 | 435 | 10.3 | 689 | 12.0 | 35 | 10.9 | ||
| Former | 206 | 1.8 | 26 | 2.2 | 86 | 2.0 | 90 | 1.6 | 4 | 1.3 | ||
| Alcohol consumption | ||||||||||||
| Never | 8456 | 73.7 | 920 | 76.3 | 3140 | 74.5 | 4154 | 72.4 | 242 | 75.6 | 0.03 | |
| Current | 2640 | 23.0 | 257 | 21.3 | 935 | 22.2 | 1383 | 24.1 | 65 | 20.3 | ||
| Former | 381 | 3.3 | 28 | 2.3 | 141 | 3.3 | 199 | 3.5 | 13 | 4.1 | ||
| Missing | 1 | 1 | ||||||||||
| Regular tea drinking | ||||||||||||
| No | 6087 | 57.9 | 568 | 51.5 | 2185 | 56.4 | 3137 | 59.9 | 197 | 68.4 | 1.9E-09 | |
| Yes | 4419 | 42.1 | 534 | 48.5 | 1692 | 43.6 | 2102 | 40.1 | 91 | 31.6 | ||
| Missing | 972 | 104 | 339 | 497 | 32 | |||||||
aResults from one-way analysis of variance (ANOVA) for continuous variables and χ2 test for categorical variables
bExcluding women with first-degree relatives with breast cancer <50 years
cParous women only
Fig. 1.
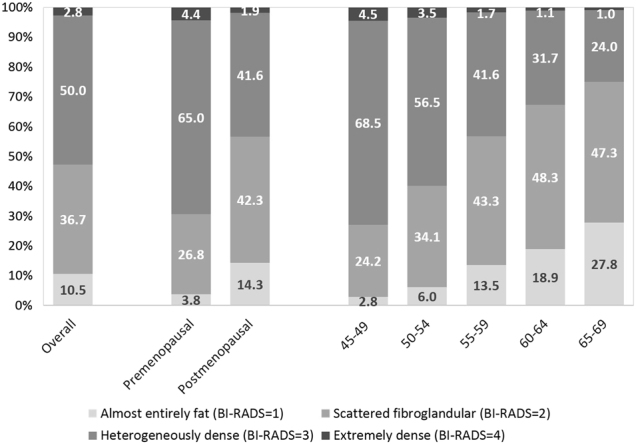
Distribution of mammographic density according to BI-RADS density categories by age and menopausal status
Table 2 shows the overall associations between examined risk factors and MD in the multivariable polytomous regression model. Older age (ORtrend = 0.80, 95% CI = 0.79-0.82; per 2-year increase), postmenopausal status (ORtrend = 0.73, 95% CI = 0.66-0.81), higher BMI (ORtrend = 0.78, 95% CI = 0.76-0.80; per 2 kg/m2), having first-degree relatives with breast cancer diagnosed before age 50 (OR = 0.87, 95% CI = 0.80-0.96), and being parous (ORtrend = 0.74, 95% CI = 0.62-0.88) were associated with lower MD. In contrast, higher education level was associated with higher MD. Later first full term-birth was associated with higher MD (ORtrend = 1.38, 95% CI = 1.21–1.57, P = 1.2E-06; 29+ vs. <25 years). Women who had breastfed for longer than a year were likely to have lower MD (ORtrend = 0.84, 95% CI = 0.74-0.96). When weight and height were analyzed separately to replace BMI in the model, height was positively associated with MD (ORtrend = 1.10, 95% CI = 1.08-1.12; per 2 cm increase) and weight was inversely associated with MD (ORtrend = 0.91, 95% CI = 0.90-0.92; per 2 kg increase). Tea drinking was associated with lower MD in the multivariable model without the adjustment of province (ORtrend = 0.72, 95% CI = 0.67–0.78; yes vs. no), however, the association became non-significant (ORtrend = 0.98, 95% CI = 0.90-1.07) with the adjustment due to the significant heterogeneity by province.
Table 2.
Associations between selected characteristics and mammographic density based on polytomous logistic regression in Chinese Breast Cancer Screening Program
| BI-RADS 2 vs. 1 | BI-RADS 3 vs. 1 | BI-RADS 4 vs. 1 | ORtrend (95% CI)b | P trend b | |
|---|---|---|---|---|---|
| OR (95% CI)a | OR (95% CI)a | OR (95% CI)a | |||
| Age | |||||
| Per 2 years | 0.84 (0.82–0.87) | 0.70 (0.68–0.72) | 0.64 (0.60–0.68) | 0.80 (0.79–0.82) | <1.0E-30 |
| Family history of breast and ovarian cancers | |||||
| 1st or 2nd degree relativesc | 1.06 (0.87–1.29) | 1.07 (0.87–1.32) | 1.28 (0.89–1.84) | 1.05 (0.94–1.16) | 0.02 |
| 1st degree relatives with breast cancer <50 years | 0.85 (0.71–1.00) | 0.80 (0.67–0.96) | 0.61 (0.43–0.87) | 0.87 (0.80–0.96) | 4.6E-04 |
| Education | |||||
| Middle school vs. none-elementary | 1.62 (1.33–1.98) | 2.13 (1.71–2.67) | 2.08 (1.18–3.66) | 1.61 (1.41–1.83) | 1.4E-12 |
| High school vs. none-elementary | 2.00 (1.62–2.47) | 2.85 (2.26–3.59) | 3.72 (2.15–6.42) | 1.84 (1.61–2.10) | 1.1E-19 |
| College +vs. none-elementary | 2.40 (1.86–3.11) | 4.44 (3.36–5.86) | 6.41 (3.59–11.45) | 2.34 (2.03–2.71) | <1.0E-30 |
| Body mass index | |||||
| Per 2 kg/m2 | 0.78 (0.74–0.81) | 0.66 (0.63–0.69) | 0.44 (0.40–0.49) | 0.78 (0.76–0.80) | <1.0E-30 |
| Heightd | |||||
| Per 2 cm | 1.13 (1.09–1.17) | 1.19 (1.15–1.24) | 1.33 (1.24–1.43) | 1.10 (1.08–1.12) | 1.1E-23 |
| Weightd | |||||
| Per 2 kg | 0.91 (0.89–0.93) | 0.85 (0.83–0.87) | 0.73 (0.70–0.76) | 0.91 (0.90–0.92) | <1.0E-30 |
| Age at menarche | |||||
| 13-14 vs. <13 | 1.14 (0.88–1.48) | 1.07 (0.82–1.40) | 0.91 (0.60–1.38) | 0.96 (0.85–1.10) | 0.56 |
| 15 + vs. <13 | 1.07 (0.83–1.39) | 0.95 (0.73–1.25) | 0.74 (0.47–1.16) | 0.90 (0.79–1.03) | 0.13 |
| Parity | |||||
| Parous vs. nulliparous | 0.67 (0.46–0.98) | 0.58 (0.39–0.86) | 0.34 (0.20–0.59) | 0.74 (0.62–0.88) | 4.9E-04 |
| Age at first full term birthe | |||||
| 25–26 vs. <25 | 1.37 (1.14–1.66) | 1.45(1.19–1.78) | 1.47 (1.02–2.12) | 1.17 (1.06–1.30) | 2.2E-03 |
| 27–28 vs. <25 | 1.32 (1.06–1.64) | 1.50 (1.19–1.89) | 1.49 (0.96–2.30) | 1.22 (1.08–1.37) | 9.3E-04 |
| 29+ vs. <25 | 1.55 (1.21–1.99) | 1.89 (1.46–2.45) | 2.09 (1.31–3.33) | 1.38 (1.21–1.57) | 1.2E-06 |
| Breastfeedinge | |||||
| 1–6 months vs. no breastfeeding | 1.42 (1.08–1.85) | 1.40 (1.06–1.85) | 1.26 (0.80–1.99) | 1.07 (0.94–1.23) | 0.31 |
| 7–12 months vs. no breastfeeding | 1.30 (1.04–1.64) | 1.38 (1.08–1.74) | 1.21 (0.81–1.81) | 1.12 (0.99–1.25) | 0.07 |
| 13 months+ vs. no breastfeeding | 0.99 (0.78–1.26) | 0.87 (0.68–1.13) | 0.45 (0.27–0.75) | 0.84 (0.74–0.96) | 0.01 |
| Menopausal status | |||||
| Postmenopausal vs. Premenopausal | 0.73 (0.58–0.92) | 0.55 (0.44–0.70) | 0.40 (0.28–0.59) | 0.73 (0.66–0.81) | 3.5E-09 |
aPolytomous logistic regression was used to estimate ORs and 95% CIs comparing each of the higher BI-RADS categories (2, 3, 4) to BI-RADS = 1, adjusted for province, age, family history, education, BMI, age at menarche, parity, and menopausal status
bCumulative logistic regression (CLOGIT function) and Wald χ2 test were used to estimate ORtrend and Ptrend, with MD modeled as an ordinal variable comparing higher to lower BI-RADS categories (with BI-RADS = 1 as the reference). Model was adjusted for the same factors as above
cExcluding women with first-degree relatieves with breast cancer <50 years
dBMI was replaced with height and weight in the model
eParous women only. Model was adjusted for province, age, family history, education, BMI, age at menarche, age at first full term birth, breastfeeding, and menopausal status
The distributions of risk factors for dense and non-dense groups are shown in Supplementary Table 1. Overall, associations for most risk factors examined were similar in premenopausal and postmenopausal women except for family history, parity and breastfeeding duration (Table 3). The association of family history (first-degree relatives with breast cancer before age 50) was associated with MD only among premenopausal women (OR = 0.75, 95% CI = 0.62–0.91) but not among postmenopausal women (OR = 1.01, 95% CI = 0.89–1.15). In contrast, statistically significant associations with parity and breastfeeding were only observed among postmenopausal women with the interaction term significant only for breastfeeding. In addition, among postmenopausal women, later age at menopause was associated with higher MD (ORtrend = 1.12, 95% CI = 1.05–1.20).
Table 3.
Associations between selected characteristics and mammographic density (BI-RADS 3–4 versus BI-RADS 1–2) by menopausal status
| Premenopausal (n = 4100) | Postmenopausal (n = 7378) | |||
|---|---|---|---|---|
| OR (95% CI)a,b | P | OR (95% CI)a,c | P | |
| Age | ||||
| Per 2 years | 0.81 (0.78–0.84) | 3.1E-27 | 0.80 (0.78–0.82) | <1.0E-30 |
| Family history of breast and ovarian cancers | ||||
| 1st or 2nd degree relativesd | 0.94 (0.76–1.17) | 0.42 | 1.07 (0.93–1.24) | 0.34 |
| 1st degree relatives with breast cancer <50 years | 0.75 (0.62–0.91) | 0.01 | 1.01 (0.89–1.15) | 0.72 |
| Education | ||||
| Middle school vs. none-elementary | 1.25 (0.90–1.72) | 0.18 | 1.51 (1.26–1.80) | 5.7E-06 |
| High school vs. none-elementary | 1.27 (0.92–1.76) | 0.14 | 1.76 (1.47–2.10) | 4.6E-10 |
| College +vs. none-elementary | 1.73 (1.24–2.41) | 0.001 | 2.29 (1.88–2.80) | 3.0E-16 |
| ORtrend (95 CI)1; Ptrend | 1.18 (1.08–1.29) | 0.000 | 1.28 (1.21–1.36) | 2.5E-16 |
| Body mass index | ||||
| Per 2 kg/m2 | 0.78 (0.74–0.82) | 1.2E-19 | 0.82 (0.79–0.85) | 2.1E-26 |
| Heighte | ||||
| Per 2 cm | 1.09 (1.05–1.14) | 4.7E-06 | 1.07 (1.05–1.10) | 1.2E-07 |
| Weighte | ||||
| Per 2 kg | 0.91 (0.89–0.93) | 2.4E-19 | 0.92 (0.91–0.94) | 7.9E-26 |
| Age at menarche | ||||
| 13–14 vs. <13 | 1.01 (0.80–1.26) | 0.97 | 0.90 (0.74–1.08) | 0.26 |
| 15+ vs. <13 | 0.85 (0.67–1.08) | 0.18 | 0.88 (0.73–1.07) | 0.20 |
| ORtrend (95 CI)a; Ptrend | 0.90 (0.81–1.01) | 0.08 | 0.95 (0.88–1.04) | 0.28 |
| Parity | ||||
| Ever vs. never | 0.87 (0.62–1.21) | 0.40 | 0.77 (0.61–0.97) | 0.02 |
| Age at first full term birthe,f | ||||
| 25–26 vs. <25 | 1.04 (0.86–1.26) | 0.69 | 1.13 (0.98–1.30) | 0.09 |
| 27–28 vs. <25 | 1.09 (0.86–1.38) | 0.48 | 1.21 (1.03–1.42) | 0.02 |
| 29+ vs. <25 | 1.29 (1.00–1.66) | 0.05 | 1.29 (1.08–1.54) | 0.01 |
| ORtrend (95 CI)a; Ptrend | 1.08 (1.00–1.17) | 0.06 | 1.09 (1.03–1.15) | 2.6E-03 |
| Breastfeedinge,f | ||||
| 1-6 months vs. no breastfeeding | 0.86 (0.67–1.11) | 0.24 | 1.13 (0.93–1.36) | 0.21 |
| 7-12 months vs. no breastfeeding | 1.28 (1.03–1.60) | 0.03 | 0.98 (0.84–1.16) | 0.85 |
| 13 months +vs. no breastfeeding | 0.98 (0.75–1.26) | 0.86 | 0.79 (0.66–0.95) | 0.01 |
| ORtrend (95 CI)a; Ptrend | 1.05 (0.97–1.14) | 0.26 | 0.92 (0.87–0.97) | 4.0E-03 |
| Age at menopause among postmenopausal women | ||||
| 49–50 vs. <49 | – | – | 1.12 (0.99–1.27) | 0.94 |
| 51+ vs. <49 | – | – | 1.26 (1.10–1.44) | 5.2E-03 |
| ORtrend (95 CI)a; Ptrend | – | – | 1.12 (1.05–1.20) | 1.1E-03 |
aLogistic regression was used to estimate ORs and 95% CIs comparing the dense (BI-RADS = 3, 4) to non-dense breast group (BI-RADS = 1, 2). ORtrend and Ptrend were obtained by treating categorical variables as ordinal variables
bAdjusted for province, age, family history, education, BMI, age at menarche, and parity
cAdjusted for province, age, education, BMI, age at menarche, parity, and age at menopause
dExcluding women with first-degree relatives with breast cancer <50 years
eBMI was replaced with height and weight in the model
fParous women only. Adjusted for province, age, menopausal status, education, BMI, age at menarche, age at first full term birth, and breastfeeding
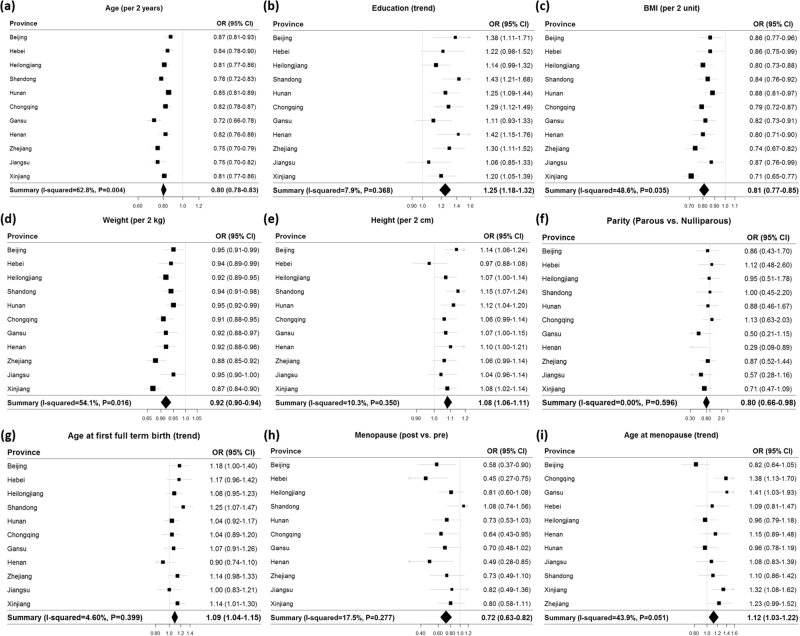
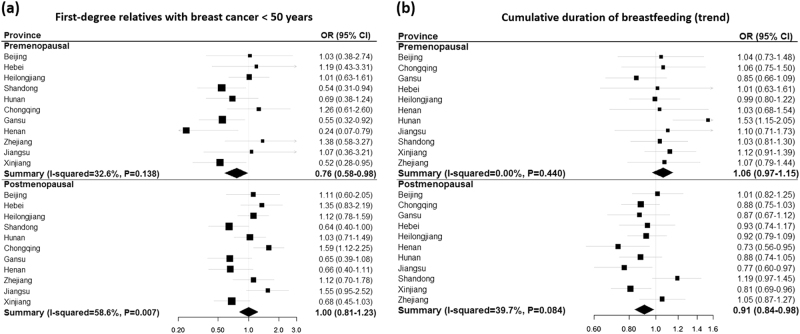
Figure 2 shows province-specific associations from the meta-analysis. Among all risk factors examined, the associations for MD with age, BMI and weight differed in magnitude, but not direction by province (Pheterogeneity < 0.05). Figure 3 shows province-specific associations for family history and breastfeeding and MD by menopausal status. The associations between MD and having a first-degree relative with breast cancer before age 50 showed heterogeneity by province (Fig. 3a, Pheterogeneity = 0.14 and 0.007 for premenopausal and postmenopausal women, respectively), whereas the associations with breastfeeding did not show significant variation across provinces in either premenopausal or postmenopausal women (Fig. 3b).
Fig. 2.
Associations of selected risk factors with mammographic density by province: a age, b education, c BMI, d weight, e height, f parity, g age at first full-term birth, h menopausal status, and i age at menopause. Province-specific ORtrend and Ptrend for having dense (BI-RADS, 3–4) compared to non-dense breast (BI-RADS, 1–2) were estimated using multivariable logistic regression adjusted for age (per 2-years), education (none to elementary, middle school, high school, college + ), BMI (per 2 kg/m2), age at menarche (<13, 13-14, 15+years old), parity (parous vs. nulliparous), and menopausal status (premenopausal and postmenopausal). Age at full-term birth (<25, 25–26, 27–28, 29+) was estimated only for parous women with additional adjustment for breastfeeding duration (no breastfeeding, 1–6 months, 7–12 months, 13 months+). OR for age at menopause (<49, 49-50, 51+) was estimated only for postmenopausal women. For categorical variables (education, age at first full term birth, and age at menopause), linear trend in association (Ptrend) was tested by treating each factor as an ordinal variable. Meta-analysis was performed based on the random-effects model
Fig. 3.
Associations of a having a first-degree relative with breast cancer before age 50 and b breastfeeding duration with mammographic density by province and menopausal status. Province-specific OR (95% CI) for having dense breast (BI-RADS, 3–4) compared with non-dense breast (BI-RADS, 1–2) was estimated by multivariable logistic regression. Meta-analysis was performed based on the random-effect model. a Group without family history of either breast or ovarian cancer was used as the reference. Model was adjusted for age (per 2 years), education (none to elementary, middle school, high school, college+), BMI (per 2 kg/m2), age at menarche (<13, 13-14, 15+years old), and parity (parous vs. nulliparous). Model for postmenopausal women was additionally adjusted for age at menopause (<49, 49-50, 51+). b Breastfeeding duration (no breastfeeding, 1–6 months, 7–12 months, 13 months+) was treated as an ordinal variable among parous women with the adjustment for age (per 2 years), family history (no history, first- and/or second-degree relatives with breast and/or ovarian cancers except for first-degree with breast cancer before age 50 years, and first-degree relative with breast cancer before age 50 years; premenopausal women only), education (none to elementary, middle school, high school, college+), BMI (per 2 kg/m2), age at menarche (<13, 13–14, 15+years old), age at full-term birth (<25, 25–26, 27–28, 29+), and age at menopause (<49, 49–50, 51+; postmenopausal women only)
Discussion
The goal of this study was to examine associations of MD with known breast cancer risk factors using data collected from a population-based multi-center screening program in China. Our study is one of the largest MD evaluations conducted among Asian women, which permitted stratification by menopausal status. Our results are consistent with the only other large study conducted among 28,388 Chinese, showing that MD was related to age, menopause, parity, and age at first full-term birth.41 However, the previous study did not collect information on several important risk factors (such as breastfeeding and age at menopause, etc.) and did not investigate the potential modification by menopausal status. Using a more comprehensive multivariable model, in addition to those expected associations, we observed that increasing height, higher education level, and later age at menopause were associated with higher MD, whereas having first-degree relatives with breast cancer diagnosed before age 50 was associated with lower MD. Further, we found that longer duration of breastfeeding was associated with lower MD but only among postmenopausal women. Taken together, these findings support the hypothesis that some established breast cancer risk factors may be mediated through MD, an intermediate phenotype for breast cancer risk, among Chinese women.
Similar associations of MD with age, weight, BMI, and parity observed in Asian and Western countries suggest that these factors have a similar impact on MD in different populations.29–42,45,46 However, it remains unclear whether the effect size for these MD-influencing factors differs by race/ethnicity. A longitudinal analysis showed that the age-related decline of PMD was slower among Japanese women compared to Caucasians,47 suggesting possible racial/ethnic heterogeneity in MD variations in relation to age. Similarly, although some researchers observed a strong inverse correlation between BMI (or weight) and PMD across diverse populations,22,40,48–50 the associations between absolute areas (dense or non-dense) and BMI varied significantly by ethnicity,22,30,32,40,50,51 which may be due to the substantial difference in body size and breast composition by ethnicity.29 Standardized quantitative MD measurements in diverse populations are needed to address this question.
The inverse associations of age and BMI with MD have been consistently found and was also confirmed in our study. The seemingly paradoxical age association was explained previously by the concept of ‘breast tissue aging (exposure)’ proposed by Pike.46 Specifically, in Pike’s model, the decline of PMD with age parallels that of the rate of ‘breast tissue aging’, which refers to the cumulative exposure of breast tissue to hormones, growth factors, and other carcinogens and was suggested as a more relevant measure in relation to MD compared with chronological age.45,46 In this model, both the rates of breast tissue aging and PMD are greatest at younger ages and decline with increasing (chronological) age, and both show decreases during pregnancy and menopause. The inverse association between BMI and MD likely reflects the correlation between body fat and fatty tissue within breasts. Previous studies have shown that the effects of weight and MD on breast cancer risk both became stronger after the adjustment for each other, suggesting that BMI and MD are negative confounders of each other in their associations with the risk of breast cancer and likely to operate via independent pathways.50
A recent meta-analysis estimated that breast cancer risk increased by 17% with every 10 cm increase in height52 and endogenous factor such as IGF-1 have been linked to underlying height, MD and breast cancer risk.53,54 However, prior evaluations of MD and height have led conflicting results; positive22,55 and null associations48,50 have been reported in both Asian and Western populations. More studies are needed to confirm the height–MD association.
The genetic basis of MD remains largely unknown. Previous studies showed that women with higher MD were more likely to have first-degree relatives with breast cancer56 and odds increased with the number of affected relatives.57,58 A recent genome-wide association study evaluating common genetic variants also identified shared genetic loci associated with both breast cancer risk and MD.59 Therefore, the unexpected association of lower density with positive family history of breast cancer observed in our study requires cautious interpretation. It is noteworthy that a recent Spanish study showed MD was significantly lower among BRCA2 mutation carriers compared to non-carriers in women from high-risk families.60 Therefore, it is possible that high-risk rare mutations and low-risk common variants may have different effect on MD. This notion is consistent with our observation of the significant association only among premenopausal women and women with first-degree earlier-onset family history, i.e., women more likely to carry high-risk alleles. In addition, our study participants were selected to be enriched for positive family history of breast and/or ovarian cancers, and therefore, the MD-family history association found in our study may not generalize to the general population.
Consistent with several studies,61,62 we found that higher levels of education were independently associated with higher MD, suggesting that the MD association with education could not be fully accounted for by measured variables such as BMI and reproductive factors. Other unmeasured lifestyle factors including dietary factors, physical activity, and socioeconomic status-related stress may also contribute and further investigation of these relationships is warranted in future studies.
We found that menopausal status was independently associated with MD after accounting for age and other confounders. This finding is consistent with previous evidence showing that age-related declines in MD are non-linear and that the rate of change may be faster among younger than older women.63 In our study, we also found that earlier age at menarche and later age at menopause were associated with higher MD, which supports the hypothesis that the cumulative exposure to endogenous hormones has a similar impact on MD and breast cancer risk.46 Likewise, the associations of nulliparity and older age at first full-term birth with higher MD also suggest that hormone-related risk factors may potentially affect breast cancer risk through MD.
The literature regarding breastfeeding and MD is inconsistent,32,35,38,42,64,65 which may be due to limited sample sizes in these studies, as well as a lack of information on breastfeeding duration. We observed that longer breastfeeding was associated with lower MD among postmenopausal women. The reason for difference by menopausal status is not clear, but it may partly involve the long-term reduction in prolactin levels among parous women,66 as circulating prolactin levels have been positively associated with MD among postmenopausal women in other studies.67,68 On the other hand, the lack of significant association among premenopausal women may be due to the relatively lower proportion of women with longer breastfeeding duration (≥1years, 21.1% among premenopausal vs. 28.4% among postmenopausal women). Additionally, we cannot exclude the possibility that the association with breastfeeding is mediated through parity since older Chinese women would have been more likely to have more children than younger women because of the one-child policy in China that started in the late 1970’s. This is an area for future exploration.
Previous studies reported associations of MD with lifestyle factors such as alcohol consumption,69,70 smoking,19,69 and tea,71 however, these factors were not associated with MD in our study. We found that tea drinkers were less likely to have dense breast, which is a potentially interesting finding since tea drinking has been shown to be protective for breast cancer by previous studies and the prevalence of tea drinking is high in China. However, the association was only seen in a few provinces (Chongqing and Gansu) and there was significant heterogeneity across provinces resulting in an overall lack of association after adjusting for province. In addition, detailed tea drinking behaviors (frequency, amount, duration, etc.) were not collected in this study. Future studies with detailed exposure measures are warranted to clarify the relationship between tea consumption and MD in Asian populations.
Major strengths of our study include the large number of study participants in an understudied Asian population, a comprehensive collection and analysis of multiple exposures of interest, centralized data collection, management, quality control, and quality assurance. All participating sites are first class tertiary hospitals in each province and all radiologists participating in the screening program have radiology certificate from the Department of Health and are required to have at least 5 years of clinical experiences in breast imaging. In addition, all participating radiologists received an intensive training before the screening program started followed with on-site training sessions during the screening program to enforce uniform standards for mammographic procedures and diagnosis and density reporting. Additionally, multi-regional participation has provided a distinct opportunity to assess regional heterogeneity in MD influencing factors. Several limitations of our study should be noted. First, MD was assessed using BI-RADS, which is subjective and known to vary across radiologists.72,73 Despite the centralized and on-site training to enforce the uniform MD measurement, measurement variability across different radiologists and screening sites is likely to exist. This subjectivity and variability may cause potential misclassification of MD levels and confound MD-risk factor associations. In addition, the MD associations observed in our study may also be confounded by other unmeasured or unknown factors such as lifestyle, social, and environmental factors and these factors are likely to vary across provinces (urbanization, air pollution, occupational exposure, etc.), which may partly explain the observed heterogeneity by province. Nevertheless, the associations between most factors examined and MD were consistent across different regions, suggesting that the observed associations were internally valid. Our study was also limited by the categorical BI-RADS measurement and the two-dimensional view. A quantitative measurement that assesses different phenotypes of MD (e.g., PMD, absolute dense area, non-dense area) accompanied by three-dimensional views or a volumetric approach may be more useful in establishing relationships between breast cancer risk factors and MD. Additionally, mammography was only performed on urban Chinese women who were predicted at being high-risk for breast cancer, and thus they are not representative of the general population. This design may account for the slightly higher proportion of dense breasts (BI-RADS 3 and 4; 54%) compared with the previous report (49.2%) of Chinese women within the same age groups.41
In summary, we found that later age at first full-term birth, later age at menopause, height, and higher education level were positively associated with MD, and age, BMI, family history of breast cancer, parity, and longer durations of breastfeeding were negatively related to MD among Chinese women at elevated breast cancer risk. Except for age, BMI, and family history of breast cancer, other established breast cancer risk factors showed the same direction of effect on MD, suggesting that the effect of these risk factors may be mediated through their influence on MD, an intermediate phenotype for breast cancer. Most associations were similar for premenopausal and postmenopausal women, except for breastfeeding duration, which showed stronger association among postmenopausal women. Further studies using standardized MD measures in diverse populations are warranted and may provide useful information to identify high-risk women particularly in low-resource countries where mammographic screening is not readily available to the general population.
Methods
Study population
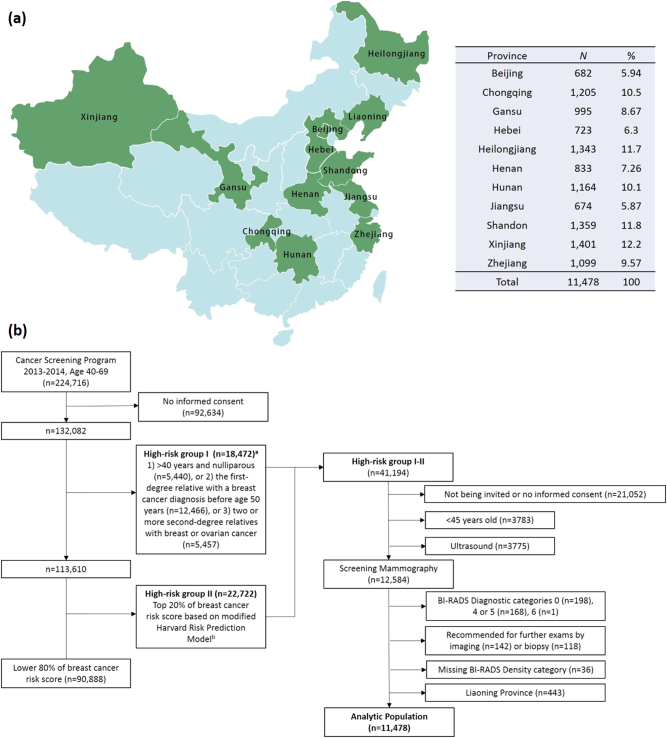
Study subjects were a subset of participants aged 40–69 in an ongoing cancer screening program in urban China led by the National Cancer Center/Cancer Hospital, Chinese Academy of Medical Sciences (CHCAMS). The goal of this program is to screen for the five most common cancers in China, including lung, breast, colorectal, upper digestive tract, and liver cancers. The program was initiated in 2012 and covered 12 provinces (Fig. 4a) in China between 2013 and 2014. Participants were interviewed to obtain risk factor data for the five targeted cancers using a standardized questionnaire. A risk prediction strategy was used to estimate risk for each cancer site. Individuals considered to be at high-risk for one of these five cancers were invited for further screening.
Fig. 4.
Schematic of the process of selecting high-risk women from the Chinese Breast Cancer Screening Program (2013-2014) for mammogram screening and inclusion in the density evaluation: a 12 participating provinces and number of participants by province b flowchart, aNot mutually exclusive, bRisk factors included in the prediction model were: body mass index (BMI), long-term mental depression, age at menarche, menstrual irregularity, age at menopause, age at first marriage, breastfeeding duration, history of benign breast disease, previous surgeries of the reproductive system, and family history of breast cancer. Note: Liaoning (n = 443) was not included in the final analysis
For breast cancer, two sequential methods were used to define high-risk groups. The first used information on family history of breast or ovarian cancer and parity, and the second using risk prediction software (Remote Data Collection System and Cancer Risk Assessment System) modified from the Harvard Risk Prediction Model74 based on prevalence of risk factors and relative risks in the Chinese population (Fig. 4b). A subset of women determined to be at high-risk for breast cancer were invited, on a first-come first-served basis, to participate in the breast cancer screening by ultrasound and/or mammographic imaging (only for women ≥ 45 years).
This study included women who participated in the screening program in 2013–2014 and had mammogram data available (Fig. 4b). Our eligible sample included 12,584 women from 17 cities located in one of 12 provinces (or municipalities): Beijing, Chongqing, Gansu, Hebei, Heilongjiang, Henan, Hunan, Jiangsu, Liaoning, Shandong, Xinjiang, and Zhejiang. We restricted study subjects to women with negative imaging findings (i.e., Breast Imaging Reporting and Data System (BI-RADS) diagnostic categories, 1, 2 or 3). We excluded women if they had incomplete diagnostic classification (BI-RADS diagnostic category = 0) or suspected/confirmed malignant tumors (BI-RADS diagnostic category = 4, 5, 6), were recommended for further exams by imaging, or had missing density data. We additionally excluded participants from Liaoning province (n = 443) since most women (96%) were classified as having heterogeneously dense breasts (BI-RADS density score = 3) and we suspected potential MD misclassification. Our final analytic sample included a total of 11,478 women. The distribution of number of participant, age, and menopausal status by province was shown in Supplementary Table 2. The mean age ranged from 52.8 in Xinjiang to 55.5 in Chongqing, and the proportion of postmenopausal women varied from 58% in Xinjiang to 71% in Chongqing. The study was approved by the Institutional Review Board of CHCAMS and informed consent was obtained from all participants in the study. The research was conducted following all relevant guidelines and procedures. This study was exempted from review by the Office of Human Subject Research Protections at the National Institutes of Health since NIH investigators do not have the access to the personal identifying information (Exempt Number: 13328).
Mammographic density assessment
Full-field digital mammograms were obtained in each participating hospital. A total of four films capturing craniocaudal and mediolateral oblique views of both breasts were obtained for each woman. Imaging results were read by trained radiologists in each designated hospital and categorized using the BI-RADS guidelines (BI-RADS diagnostic category (0–6) and BI-RADS density scoring system (1–4)) recommended by the American College of Radiology (4th edition).75 MD was categorized into four levels using the BI-RADS density scoring system: almost entirely fat (1), scattered fibroglandular densities (2), heterogeneously dense (3), and extremely dense (4). Maximal values for BI-RADS diagnostic and density categories were used across the two breasts.
Selected risk factors
Data on breast cancer risk factors were extracted from the baseline questionnaire, including age, family history of breast and/or ovarian cancers, education level, lifestyle factors (smoking, alcohol consumption, and regular tea drinking), and reproductive history (age at menarche, parity, age at first full-term birth, cumulative months of breastfeeding for all live-born children, menopausal status, and age at menopause). Height (cm) and weight (kg) were measured and BMI was calculated from weight and height as kg/m2.
Statistical analysis
Differences in the distribution of each risk factor by BI-RADS density categories were assessed using one-way analysis of variance (ANOVA) or χ2 test. To assess associations between MD and risk factors, we used polytomous logistic regression to compare each higher MD category (BI-RADS 2, 3, and 4) to BI-RADS = 1 (almost entirely fat, the referent group). Odds ratios (ORs) and corresponding 95% confidence intervals (CIs) were estimated using the PROC LOGISTIC procedure with the GLOGIT option. ORtrend (95% CI, Ptrend) across BI-RADS categories were also obtained using cumulative logistic regression with treating BI-RADS categories as an ordinal variable.
The base model included age, BMI, and province as covariates. For the multivariable analysis, we included all variables that had P < 0.05 in the base model. The final multivariable model included province, age (per 2 years), family history of breast and/or ovarian cancers (no history, first-degree relative with breast cancer before age 50 years, and other history), menopausal status (pre- or postmenopausal), education (none to elementary, middle school, high school, or college+), BMI (per 2 kg/m2), age at menarche ( < 13, 13–14, or 15+ years old), and parity (parous or nulliparous). Among parous women, age at first full-term birth (<25, 25–26, 27–28, or 29+ years), and breastfeeding duration (no breastfeeding, 1–6 months, 7–12 months, or 13 months+) were also included in the model. We also ran models in which we replaced BMI with height (per 2 cm) or weight (per 2 kg) to examine height and weight separately. Variables such as smoking (never, former, or current), alcohol consumption (never, former, or current), and regular tea drinking (yes or no) were not associated with MD in base model and therefore, were excluded from the final multivariable model. In the sensitivity analysis, the additional adjustment for these factors did not change the magnitude of the associations with other variables (data not shown).
Because density is known to decrease with menopause,43 we therefore stratified our analysis by menopausal status. In stratified analyses, BI-RADS categories were collapsed into dense (BI-RADS, 3 and 4) and non-dense (BI-RADS, 1 and 2, reference) groups because of small number in some extreme categories. Models for postmenopausal women were additionally adjusted for age at menopause (<49, 49-50, or 51+). The potential effect modification by menopausal status was tested by including an interaction term between each explanatory variable and menopausal status in the analysis. Since family history was associated with MD only among premenopausal women, it was only included in the analysis for premenopausal women.
To assess the potential heterogeneity across different provinces, we also stratified analyses by province and provided summary statistics using meta-analysis based on a random-effects model. Tests for heterogeneity across provinces were assessed by I2 statistics.
All statistical tests were two-sided. Meta-analysis was conducted using Stata/SE (version 11.2; StataCorp LP, College Station, TX) and all other analyses were conducted using SAS (version 9.3; SAS Institute Inc., Cary, NC).
Data and code availability statement
The data sets and code used and analyzed for the current study will be available from the corresponding author upon reasonable request without undue qualifications.
Electronic supplementary material
Acknowledgements
This research was funded by the Intramural Research Program of the Division of Cancer Epidemiology and Genetics in the National Cancer Institute at the National Institutes of Health; National Key Public Health Service Project, Cancer Screening Program in Urban China (China); and Research Special Fund for Public Welfare Industry of Health (201402003) (China).
Author contributions
All authors of this research paper have directly participated in the planning, execution, or analysis of the study. Specifically, J.R., J.L., N.H., M.D., X.R.Y., and J.H. contributed to study conception and design, and acquisition of data. H.S. performed statistical analyses and wrote the manuscript. J.R., G.L.G., and X.R.Y. made substantial contributions to the interpretation of data and critical revision of the manuscript. RP provided statistical guidance and contributed to data interpretation. Y.W., J.L.G., Y.F., J.S., K.Z., L.N., S.W., and L.W. helped with data acquisition and management.
Competing interests
The authors declare no competing financial interests.
Footnotes
Hyuna Sung, Jiansong Ren and Jing Li contributed equally to this work.
Min Dai, Xiaohong R. Yang and Jie He jointly supervised this work.
Electronic supplementary material
Supplementary information accompanies the paper on the npj Breast Cancer website (10.1038/s41523-018-0055-9).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ferlay J. et al. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC Cancer Base No. 11 [Internet]. International Agency for Research on Cancer, Lyon. http://globocan.iarc.fr (2013).
- 2.Fan L, et al. Breast cancer in China. Lancet Oncol. 2014;15:e279–289. doi: 10.1016/S1470-2045(13)70567-9. [DOI] [PubMed] [Google Scholar]
- 3.Shin HR, et al. Recent trends and patterns in breast cancer incidence among Eastern and Southeastern Asian women. Cancer Cause Control. 2010;21:1777–1785. doi: 10.1007/s10552-010-9604-8. [DOI] [PubMed] [Google Scholar]
- 4.Minami Y, et al. The increase of female breast cancer incidence in Japan: emergence of birth cohort effect. Int. J. Cancer. 2004;108:901–906. doi: 10.1002/ijc.11661. [DOI] [PubMed] [Google Scholar]
- 5.Leung GM, et al. Trends in breast cancer incidence in Hong Kong between 1973 and 1999: an age-period-cohort analysis. Brit. J. Cancer. 2003;88:2006. doi: 10.1038/sj.bjc.6601019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chia KS, et al. Profound changes in breast cancer incidence may reflect changes into a Westernized lifestyle: a comparative population-based study in Singapore and Sweden. Int. J. Cancer. 2005;113:302–306. doi: 10.1002/ijc.20561. [DOI] [PubMed] [Google Scholar]
- 7.Sim, X. L. et al. Ethnic differences in the time trend of female breast cancer incidence: Singapore. BMC cancer6,1968-2002 (2006). [DOI] [PMC free article] [PubMed]
- 8.Wolfe JN. Risk for breast cancer development determined by mammographic parenchymal pattern. Cancer. 1976;37:2486–2492. doi: 10.1002/1097-0142(197605)37:5<2486::AID-CNCR2820370542>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 9.Boyd NF, et al. Mammographic density and the risk and detection of breast cancer. N. Eng. J. Med. 2007;356:227–236. doi: 10.1056/NEJMoa062790. [DOI] [PubMed] [Google Scholar]
- 10.McCormack VA, Silva IDS. Breast density and parenchymal patterns as markers of breast cancer risk: A meta-analysis. Cancer Epidem Biomar. 2006;15:1159–1169. doi: 10.1158/1055-9965.EPI-06-0034. [DOI] [PubMed] [Google Scholar]
- 11.Jakes RW, Duffy SW, Ng FC, Gao F, Ng EH. Mammographic parenchymal patterns and risk of breast cancer at and after a prevalence screen in Singaporean women. Int. J. Epidemiol. 2000;29:11–19. doi: 10.1093/ije/29.1.11. [DOI] [PubMed] [Google Scholar]
- 12.Pettersson, A. et al. Mammographic density phenotypes and risk of breast cancer: a meta-analysis. J. Natl. Cancer106, 10.1093/jnci/dju078 (2014). [DOI] [PMC free article] [PubMed]
- 13.Kotsuma Y, et al. Quantitative assessment of mammographic density and breast cancer risk for Japanese women. Breast. 2008;17:27–35. doi: 10.1016/j.breast.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 14.Maskarinec G, Meng L. A case-control study of mammographic densities in Hawaii. Breast Cancer Res. Treat. 2000;63:153–161. doi: 10.1023/A:1006486319848. [DOI] [PubMed] [Google Scholar]
- 15.Maskarinec G, Pagano I, Lurie G, Wilkens LR, Kolonel LN. Mammographic density and breast cancer risk: the multiethnic cohort study. Am. J. Epidemiol. 2005;162:743–752. doi: 10.1093/aje/kwi270. [DOI] [PubMed] [Google Scholar]
- 16.Maskarinec G, et al. Mammographic density and breast cancer risk by family history in women of white and Asian ancestry. Cancer Causes Control. 2015;26:621–626. doi: 10.1007/s10552-015-0551-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goss PE, et al. Challenges to effective cancer control in China, India, and Russia. Lancet Oncol. 2014;15:489–538. doi: 10.1016/S1470-2045(14)70029-4. [DOI] [PubMed] [Google Scholar]
- 18.Li T, et al. The association of measured breast tissue characteristics with mammographic density and other risk factors for breast cancer. Cancer Epidemiol. Biomark. Prev. 2005;14:343–349. doi: 10.1158/1055-9965.EPI-04-0490. [DOI] [PubMed] [Google Scholar]
- 19.Vachon CM, Kuni CC, Anderson K, Anderson VE, Sellers TA. Association of mammographically defined percent breast density with epidemiologic risk factors for breast cancer (United States) Cancer Causes Control. 2000;11:653–662. doi: 10.1023/A:1008926607428. [DOI] [PubMed] [Google Scholar]
- 20.Martin, L. J. & Boyd, N. F. Mammographic density—potential mechanisms of breast cancer risk associated with mammographic density: hypotheses based on epidemiological evidence. Breast Cancer Res.10, 10.1186/bcr1831 (2008). [DOI] [PMC free article] [PubMed]
- 21.Masala G, et al. Dietary and lifestyle determinants of mammographic breast density. A longitudinal study in a Mediterranean population. Int. J. Cancer. 2006;118:1782–1789. doi: 10.1002/ijc.21558. [DOI] [PubMed] [Google Scholar]
- 22.Boyd NF, et al. The relationship of anthropometric measures to radiological features of the breast in premenopausal women. Br. J. Cancer. 1998;78:1233–1238. doi: 10.1038/bjc.1998.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boyd NF, et al. Heritability of mammographic density, a risk factor for breast cancer. New Engl. J. Med. 2002;347:886–894. doi: 10.1056/NEJMoa013390. [DOI] [PubMed] [Google Scholar]
- 24.Chen Z, et al. Does mammographic density reflect ethnic differences in breast cancer incidence rates? Am. J. Epidemiol. 2004;159:140–147. doi: 10.1093/aje/kwh028. [DOI] [PubMed] [Google Scholar]
- 25.Maskarinec G, Pagano I, Chen Z, Nagata C, Gram IT. Ethnic and geographic differences in mammographic density and their association with breast cancer incidence. Breast Cancer Res. Treat. 2007;104:47–56. doi: 10.1007/s10549-006-9387-5. [DOI] [PubMed] [Google Scholar]
- 26.Maskarinec G, Meng L, Ursin G. Ethnic differences in mammographic densities. Int. J. Epidemiol. 2001;30:959–965. doi: 10.1093/ije/30.5.959. [DOI] [PubMed] [Google Scholar]
- 27.Maskarinec G, Nagata C, Shimizu H, Kashiki Y. Comparison of mammographic densities and their determinants in women from Japan and Hawaii. Int. J. Cancer. 2002;102:29–33. doi: 10.1002/ijc.10673. [DOI] [PubMed] [Google Scholar]
- 28.Mariapun S, Li J, Yip CH, Taib NA, Teo SH. Ethnic differences in mammographic densities: an Asian cross-sectional study. PLoS One. 2015;10:e0117568. doi: 10.1371/journal.pone.0117568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tseng M, Byrne C. Adiposity, adult weight gain and mammographic breast density in US Chinese women. Int. J. Cancer. 2011;128:418–425. doi: 10.1002/ijc.25338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Habel LA, et al. Mammographic density in a multiethnic cohort. Menopause. 2007;14:891–899. doi: 10.1097/gme.0b013e318032569c. [DOI] [PubMed] [Google Scholar]
- 31.Heng D, et al. Risk factors for breast cancer associated with mammographic features in Singaporean Chinese women. Cancer Epidemiol. Biomark. Prev. 2004;13:1751–1758. [PubMed] [Google Scholar]
- 32.Ursin G, et al. Associations between soy, diet, reproductive factors, and mammographic density in Singapore Chinese Women. Nutr. Cancer. 2006;56:128–135. doi: 10.1207/s15327914nc5602_2. [DOI] [PubMed] [Google Scholar]
- 33.Wu AH, et al. Green tea, soy, and mammographic density in Singapore Chinese women. Cancer Epidem Biomar. 2008;17:3358–3365. doi: 10.1158/1055-9965.EPI-08-0132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee E, et al. Genetic variation in peroxisome proliferator-activated receptor gamma, soy, and mammographic density in Singapore Chinese women. Cancer Epidemiol. Biomark. Prev. 2012;21:635–644. doi: 10.1158/1055-9965.EPI-11-1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ishihara S, et al. Association between mammographic breast density and lifestyle in Japanese women. Acta Med. Okayama. 2013;67:145–151. doi: 10.18926/AMO/50407. [DOI] [PubMed] [Google Scholar]
- 36.Kawahara M, et al. Factors influencing breast density in Japanese women aged 40-49 in breast cancer screening mammography. Acta Med. Okayama. 2013;67:213–217. doi: 10.18926/AMO/51065. [DOI] [PubMed] [Google Scholar]
- 37.Sung J, et al. Genetic influences on mammographic density in Korean twin and family: the Healthy Twin study. Breast Cancer Res. Treat. 2010;124:467–474. doi: 10.1007/s10549-010-0852-9. [DOI] [PubMed] [Google Scholar]
- 38.Sung J, Song YM, Stone J, Lee K, Lee D. Reproductive factors associated with mammographic density: a Korean co-twin control study. Breast Cancer Res. Treat. 2011;128:567–572. doi: 10.1007/s10549-011-1469-3. [DOI] [PubMed] [Google Scholar]
- 39.Sung J, Song YM, Stone J, Lee K. The relationship between bone mineral density and mammographic density in Korean women: the healthy twin study. Breast Cancer Res. Treat. 2011;129:583–591. doi: 10.1007/s10549-011-1523-1. [DOI] [PubMed] [Google Scholar]
- 40.Sung J, Song YM, Stone J, Lee K, Kim SY. Association of body size measurements and mammographic density in Korean women: the healthy twin study. Cancer Epidemiol. Prev. Biomark. 2010;19:1523–1531. doi: 10.1158/1055-9965.EPI-09-1005. [DOI] [PubMed] [Google Scholar]
- 41.Dai H, et al. Distribution of mammographic density and its influential factors among Chinese women. Int. J. Epidemiol. 2014;43:1240–1251. doi: 10.1093/ije/dyu042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang YP, et al. Influence of factors on mammographic density in premenopausal Chinese women. Eur. J. Cancer Prev. 2016;25:306–311. doi: 10.1097/CEJ.0000000000000177. [DOI] [PubMed] [Google Scholar]
- 43.Boyd NF, Martin LJ, Yaffe MJ, Minkin S. Mammographic density: a hormonally responsive risk factor for breast cancer. J. Br. Menopause Soc. 2006;12:186–193. doi: 10.1258/136218006779160436. [DOI] [PubMed] [Google Scholar]
- 44.Boyd N, et al. A longitudinal study of the effects of menopause on mammographic features. Cancer Epidemiol. Prev. Biomark. 2002;11:1048–1053. [PubMed] [Google Scholar]
- 45.Boyd NF, et al. Mammographic breast density as an intermediate phenotype for breast cancer. Lancet Oncol. 2005;6:798–808. doi: 10.1016/S1470-2045(05)70390-9. [DOI] [PubMed] [Google Scholar]
- 46.Pike MC, Krailo MD, Henderson BE, Casagrande JT, Hoel DG. Hormonal risk-factors, breast-tissue age and the age-incidence of breast-cancer. Nature. 1983;303:767–770. doi: 10.1038/303767a0. [DOI] [PubMed] [Google Scholar]
- 47.Maskarinec G, Pagano I, Lurie G, Kolonel LN. A longitudinal investigation of mammographic density: the multiethnic cohort. Cancer Epidemiol. Prev. Biomark. 2006;15:732–739. doi: 10.1158/1055-9965.EPI-05-0798. [DOI] [PubMed] [Google Scholar]
- 48.Brisson J, et al. Height and weight, mammographic features of breast tissue, and breast cancer risk. Am. J. Epidemiol. 1984;119:371–381. doi: 10.1093/oxfordjournals.aje.a113755. [DOI] [PubMed] [Google Scholar]
- 49.Sala E, et al. High-risk mammographic parenchymal patterns and anthropometric measures: a case-control study. Br. J. Cancer. 1999;81:1257–1261. doi: 10.1038/sj.bjc.6690838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Boyd NF, et al. Body size, mammographic density, and breast cancer risk. Cancer Epidemiol. Prev. Biomark. 2006;15:2086–2092. doi: 10.1158/1055-9965.EPI-06-0345. [DOI] [PubMed] [Google Scholar]
- 51.Stone J, Warren RML, Pinney E, Warwick J, Cuzick J. Determinants of percentage and area measures of mammographic density. Am. J. Epidemiol. 2009;170:1571–1578. doi: 10.1093/aje/kwp313. [DOI] [PubMed] [Google Scholar]
- 52.Green J, et al. Height and cancer incidence in the Million Women Study: prospective cohort, and meta-analysis of prospective studies of height and total cancer risk. Lancet Oncol. 2011;12:785–794. doi: 10.1016/S1470-2045(11)70154-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Endogenous H, et al. Insulin-like growth factor 1 (IGF1), IGF binding protein 3 (IGFBP3), and breast cancer risk: pooled individual data analysis of 17 prospective studies. Lancet Oncol. 2010;11:530–542. doi: 10.1016/S1470-2045(10)70095-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Martin LJ, Boyd NF. Mammographic density. Potential mechanisms of breast cancer risk associated with mammographic density: hypotheses based on epidemiological evidence. Breast Cancer Res. 2008;10:201. doi: 10.1186/bcr1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dite GS, et al. Predictors of mammographic density: insights gained from a novel regression analysis of a twin study. Cancer Epidem Biomar. 2008;17:3474–3481. doi: 10.1158/1055-9965.EPI-07-2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ziv E, Shepherd J, Smith-Bindinan R, Kerlikowske K. Mammographic breast density and family history of breast cancer. J. Natl. Cancer Inst. 2003;95:556–558. doi: 10.1093/jnci/95.7.556. [DOI] [PubMed] [Google Scholar]
- 57.Crest AB, Aiello EJ, Anderson ML, Buist DSM. Varying levels of family history of breast cancer in relation to mammographic breast density (United States) Cancer Cause Control. 2006;17:843–850. doi: 10.1007/s10552-006-0026-6. [DOI] [PubMed] [Google Scholar]
- 58.Martin LJ, et al. Family history, mammographic density, and risk of breast cancer. Cancer Epidemiol. Biomark. Prev. 2010;19:456–463. doi: 10.1158/1055-9965.EPI-09-0881. [DOI] [PubMed] [Google Scholar]
- 59.Stone J, et al. Novel associations between common breast cancer susceptibility variants and risk-predicting mammographic density measures. Cancer Res. 2015;75:2457–2467. doi: 10.1158/0008-5472.CAN-14-2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cajal, T. R. Y. et al. Mammographic density and breast cancer in women from high risk families. Breast Cancer Res.17, 10.1186/s13058-015-0604-1 (2015). [DOI] [PMC free article] [PubMed]
- 61.Aitken, Z. Mammographic density and markers of socioeconomic status: a cross-sectional study. BMC Cancer10, 10.1186/1471-2407-10-35 (2010). [DOI] [PMC free article] [PubMed]
- 62.Sung J, Song YM. The relationship between education level and mammographic density. Eur. J. Cancer Prev. 2015;24:491–496. doi: 10.1097/CEJ.0000000000000120. [DOI] [PubMed] [Google Scholar]
- 63.McCormack VA, Perry NM, Vinnicombe SI, Silva ID. Changes and tracking of mammographic density in relation to Pike’s model of breast tissue aging: a UK longitudinal study. Int. J. Cancer. 2010;127:452–461. doi: 10.1002/ijc.25252. [DOI] [PubMed] [Google Scholar]
- 64.Riza E, et al. Correlates of high-density mammographic parenchymal patterns by menopausal status in a rural population in Northern Greece. Eur. J. Cancer. 2005;41:590–600. doi: 10.1016/j.ejca.2004.12.014. [DOI] [PubMed] [Google Scholar]
- 65.Leinster SJ, Walsh PV, Whitehouse GH, al-Sumidaie AM. Factors associated with mammographic parenchymal patterns. Clin. Radiol. 1988;39:252–256. doi: 10.1016/S0009-9260(88)80519-1. [DOI] [PubMed] [Google Scholar]
- 66.Wang DY, et al. The permanent effect of reproductive events on blood prolactin levels and its relation to breast cancer risk—a population study of postmenopausal women. Eur. J. Cancer Clin. 1988;24:1225–1231. doi: 10.1016/0277-5379(88)90132-0. [DOI] [PubMed] [Google Scholar]
- 67.Boyd NF, et al. The association of breast mitogens with mammographic densities. Brit J. Cancer. 2002;87:876–882. doi: 10.1038/sj.bjc.6600537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Greendale GA, et al. Serum prolactin levels are positively associated with mammographic density in postmenopausal women. Breast Cancer Res. Treat. 2007;105:337–346. doi: 10.1007/s10549-006-9454-y. [DOI] [PubMed] [Google Scholar]
- 69.Brand JS, et al. Influence of lifestyle factors on mammographic density in postmenopausal women. PLoS One. 2013;8:e81876. doi: 10.1371/journal.pone.0081876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Quandt Z, et al. The association of alcohol consumption with mammographic density in a multiethnic urban population. BMC Cancer. 2015;15:1094. doi: 10.1186/s12885-015-1094-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wu AH, et al. Green tea, soy, and mammographic density in Singapore Chinese women. Cancer Epidemiol. Biomark. Prev. 2008;17:3358–3365. doi: 10.1158/1055-9965.EPI-08-0132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ciatto S, et al. Categorizing breast mammographic density: intra- and interobserver reproducibility of BI-RADS density categories. Breast. 2005;14:269–275. doi: 10.1016/j.breast.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 73.Kerlikowske K, et al. Variability and accuracy in mammographic interpretation using the American College of Radiology Breast Imaging Reporting and Data System. J. Natl. Cancer Inst. 1998;90:1801–1809. doi: 10.1093/jnci/90.23.1801. [DOI] [PubMed] [Google Scholar]
- 74.Colditz GA, et al. Harvard report on cancer prevention volume 4: Harvard Cancer Risk Index. Risk Index Working Group, Harvard Center for Cancer Prevention. Cancer Causes Control. 2000;11:477–488. doi: 10.1023/A:1008984432272. [DOI] [PubMed] [Google Scholar]
- 75.D’Orsi C. J., Sickles E. A., Mendelson E. B., Morris E. A. et al. ACR BI-RADS® Atlas, Breast Imaging Reporting and Data System. (American College of Radiology, Reston, VA, 2013).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data sets and code used and analyzed for the current study will be available from the corresponding author upon reasonable request without undue qualifications.