Abstract
Soybean cyst nematode (SCN) Heterodera glycines is an obligate parasite that relies on the secretion of effector proteins to manipulate host cellular processes that favor the formation of a feeding site within host roots to ensure its survival. The sequence complexity and co-evolutionary forces acting upon these effectors remain unknown. Here we generated a de novo transcriptome assembly representing the early life stages of SCN in both a compatible and an incompatible host interaction to facilitate global effector mining efforts in the absence of an available annotated SCN genome. We then employed a dual effector prediction strategy coupling a newly developed nematode effector prediction tool, N-Preffector, with a traditional secreted protein prediction pipeline to uncover a suite of novel effector candidates. Our analysis distinguished between effectors that co-evolve with the host genotype and those conserved by the pathogen to maintain a core function in parasitism and demonstrated that alternative splicing is one mechanism used to diversify the effector pool. In addition, we confirmed the presence of viral and microbial inhabitants with molecular sequence information. This transcriptome represents the most comprehensive whole-nematode sequence currently available for SCN and can be used as a tool for annotation of expected genome assemblies.
Introduction
The soybean cyst nematode (SCN) Heterodera glycines is the most economically important pathogen of soybean, causing over one billion dollars in yield loss annually1. This microscopic roundworm begins its life cycle as an egg in the soil, undergoing one molt before hatching as a second-stage juvenile (J2). Once the nematode has hatched, it migrates through the soil towards a host plant where it invades the root tissue and migrates towards the vasculature, selecting a single cell to establish a feeding site called a syncytium. At this point, the nematode penetrates the cell wall using its stylet and releases a set of secretions into the host cell, including effector proteins. Stylet-secreted effector proteins identified to date share many characteristics including the presence of a signal peptide, lack of a transmembrane domain, and expression in the esophageal gland cells2. These effector proteins manipulate the host cell by modulating a variety of cellular processes to make it more suitable for the nematode, including suppression of host defense and stress responses and causing significant transcriptional re-programming in the host cell nucleus3. Effectors harboring nuclear localization signals are recognized by host cellular machinery for targeting to the nucleus where they modulate host nuclear functions4. Similar to effectors delivered by the stylet of piercing/sucking insects, the type III secretion system of bacteria or the haustorium of pathogenic fungi and parasitic plants, these effectors represent an interface between the nematode pathogen and host5,6. Once the feeding site is established, the nematode becomes sedentary and relies entirely on the host for nutrition for the remainder of its life cycle. The nematode slowly swells up as it undergoes a series of molts and differentiates into either a male or a female. Females protrude from the roots while the males regain mobility and exit the root to fertilize females, following which the males die. The females eventually die after fertilization, their bodies hardening into a protective casing for the eggs called a cyst that breaks off into the soil and begins the process anew. The early stages of the nematode infection cycle represent a key point in determining the fate of a cyst nematode. Whether or not the nematode will survive long enough to complete its life cycle depends on the ability of the nematode to survive and circumvent the hostile environment presented by the plant host.
In recent years, next generation sequencing technologies have been applied to several plant-parasitic nematode species, resulting in the assembly of complete genomes for Meloidogyne hapla, M. incognita, Globodera ellingtonia, G.pallida, G. rostochiensis, Ditylenchus destructor, and Bursaphelenchus xylophilus7–12. Despite the enormous economic importance of H. glycines, no finished and comprehensively annotated genome is currently available. In the absence of a sequenced genome, several other plant-parasitic nematode systems have turned to de novo transcriptome-level studies instead13–16. These studies were able to identify key features of the interaction of plant host and nematode pathogen, including the discovery of new effectors.
In the SCN system, studies have primarily focused on identifying and characterizing stylet-secreted effectors produced in the esophageal gland cells, which has resulted in the identification of 72 SCN effectors17–19. These studies based their identification of SCN effectors on the presence of a signal peptide as well as expression in the esophageal gland cells confirmed by in situ hybridization. Multiple functional studies have since been performed using these effectors, identifying host targets and characterizing their role in cyst nematode parasitism reviewed in20. Though the approach focused on the gland cells has been highly successful in identifying stylet-secreted effector proteins, low abundance transcripts, those harboring non-canonical secretion signals, and those encoding secreted proteins originating in other structures of the nematode, such as amphids21, are lacking. A global analysis allows for a comprehensive assessment of effectors, enabling studies to assess effector variation within and across populations to identify highly variable effectors potentially correlated with virulence, as well as those effectors highly conserved across the population that may be key components of the SCN infection process. Effector variation has been shown to be important in other plant pathogens such as bacteria and fungi as a tactic for evading host recognition and resistance22,23.
To provide comprehensive biological insight and a tool for comparative analyses between different nematode species and populations of H. glycines in the absence of a reference genome, a de novo transcriptome assembly of early life stages was generated. An analysis of the transcriptome confirmed previous reports of microorganisms present within the nematode with molecular details and identified new parallels to other plant-parasitic nematode species. We then performed multiple analyses focused on effectors; both predicting novel effectors using a newly developed bioinformatics tool called N-Preffector that is not reliant on the presence of a signal peptide and investigating variation of previously identified stylet-secreted effector protein sequences. This allowed for the identification of an additional suite of novel effectors that may play a pivotal role in SCN infection and could serve as potential targets for future development of novel SCN control strategies.
Results
Transcriptome sequencing and assembly
To gain global insights into the transcriptomic response associated with the establishment of SCN infection, mRNA sequencing of pre-parasitic second-stage juvenile (ppJ2) and parasitic second-stage juvenile (pJ2) life stages infecting a resistant and susceptible host was conducted, yielding a total of 603.6 million paired 100 base reads. Following initial filtering steps and removal of reads mapping to the soybean genome, the final input for transcriptome assembly was 430 million reads. Trinity de novo transcriptome assembly resulted in a final assembly of 147,910 transcripts with a total assembly length of 46.7 Mb and estimated 23-fold transcriptome coverage. The average length of these transcripts was 658 base pairs (bp) with a N50 of 1,085 bp (Table 1). When translated, 78,625 resulting proteins were predicted. This transcriptome assembly was then assessed using BUSCO (Benchmarking Universal Single-Copy Orthologs)24. Based on the 429 single copy orthologs for eukaryotes, the SCN assembly is 68% complete, with an additional 14% of the orthologs represented in fragmented transcripts and the remaining 18% missing from the transcriptome.
Table 1.
de novo transcriptome assembly statistics for the SCN early life stage assembly.
| Number of transcripts | 147,910 |
| Total assembly length (Mb) | 46.7 |
| Number of trinity ‘genes' | 71,093 |
| N50 (bp) | 1,085 |
| Maximum contig size (bp) | 11,112 bp |
| Minimum contig size (bp) | 201 bp |
| Average contig length (bp) | 658 bp |
| Predicted proteins | 78,625 |
| BUSCO score | C: 68%, F: 14%, M: 18% |
The assembly was generated from H. glycines pre-parasitic second-stage juvenile samples as well as parasitic second-stage juvenile samples from susceptible and resistant host interactions using the Trinity de novo transcriptome assembly tool. The transcriptome was assessed for completeness using the tool BUSCO (benchmarking universal single-copy orthologs) to identify complete (C), fragmented (F), and missing (M) sequences representing conserved orthologs found in all eukaryotes.
Annotation of transcripts
Transcripts from the H. glycines transcriptome were annotated following the Trinotate pipeline25. Transcripts were first compared to GenBank, Swissprot, and TrEMBL databases using BLASTX, resulting in a total of 66,601 (45.03%) out of the 147,910 transcripts annotated using an e-value cutoff of 1e–5 (S1 Table). When examining the species distribution of these significant hits, most transcripts hit to prior H. glycines database entries followed by animal-parasitic nematode species such as Ascaris suum and Strongyloides ratti (Fig. 1). In total, 1315 species are represented in the BLASTX results representing a broad variety of genera. Other species of note in the annotated transcripts include Cardinium endosymbionts of Encarsia pergandiella and Bemesia tabaci as well as several soybean cyst nematode associated viruses26–31. The virus sequences from the H. glycines PA3 population sequenced in this study are described by Ruark et al.32 and the endosymbiont-associated transcripts were characterized in more detail as described below.
Figure 1.
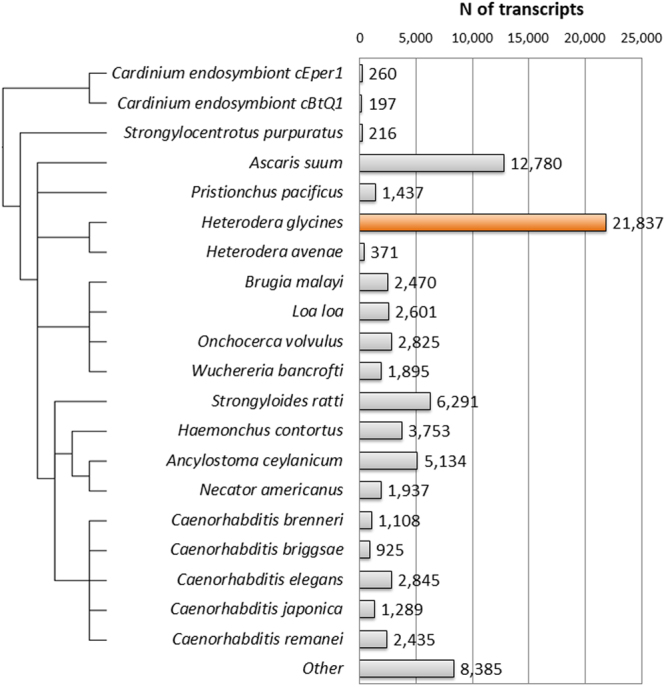
Species distribution of predicted homologues to H. glycines. Homologues were predicted using a BLASTX search against several protein databases at an e-value cutoff of 1e–5. The top 20 species with the most homologues are shown here. The resulting species evolutionary relationship was obtained from NCBI Taxonomy Browser83 and visualized using IcyTree84.
Transcripts were further compared to several nematode species with sequenced and annotated genomes representing free-living, animal-parasitic, and plant-parasitic trophic groups to identify potential overlap and genes that are uniquely shared between SCN and one other nematode species. The H. glycines transcriptome uniquely shares 76 potential homologs with Bursaphelenchus xylophilus, 313 homologs with Meloidogyne hapla, 200 with M. incognita, and 7,721 with Globodera pallida. In addition, the transcriptome shares 11 homologs with the free-living nematode Pristionchus pacificus, 28 with the free-living nematode Caenorhabditis elegans, and 84 homologs with the animal-parasitic nematode A. suum (Fig. 2; S2 Table).
Figure 2.
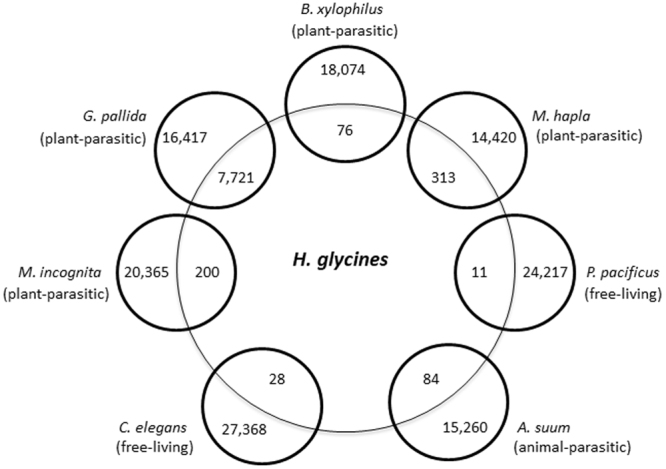
H. glycines orthologs in proteomes from sequenced nematodes with diverse feeding behaviors. The interior numbers represent predicted H. glycines proteins that only have orthologs identified in one of the seven other nematode species examined. Exterior numbers represent sequenced nematode proteins with no unique orthologs in the early parasitic H. glycines transcriptome.
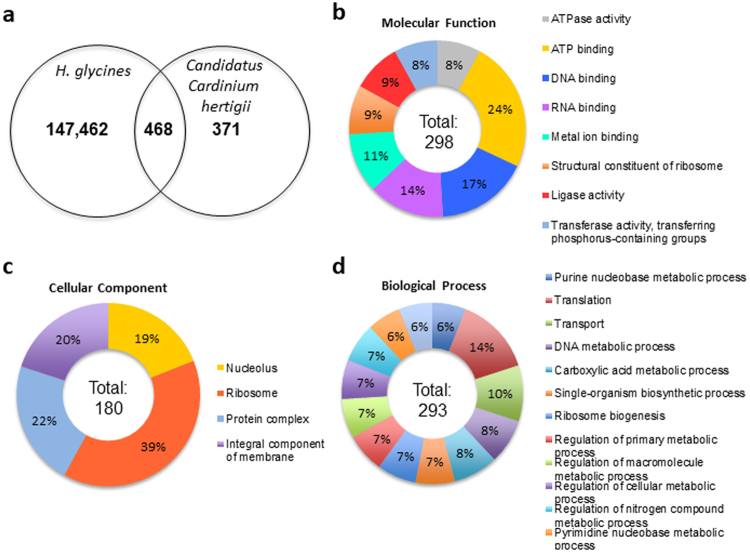
Identification and GO annotation of endosymbiont-associated transcripts from the H. glycines transcriptome
Prior microscopic analysis of SCN indicated the presence of a bacterial endosymbiont28,31. Within the early parasitic SCN transcriptome we identified 468 transcripts annotated as endosymbiont-associated transcripts, all of which were confirmed by BLASTX mapping to the Cardinium hertigii proteome (Fig. 3a; S3 Table). To further examine the potential functional significance of this inhabitant on SCN biology, GO terms were assigned to the 468 endosymbiont-associated transcripts using BLAST2GO, resulting in GO annotation of 328 of the 468 transcripts (S3 Table). Of those sequences with GO annotation within molecular function, the majority were involved in ATP binding, with 24% of the annotated transcripts falling into this category, followed by DNA (17%) and RNA (14%) binding (Fig. 3b). The cellular compartment represented by the greatest number of transcripts was the ribosome (39%) (Fig. 3c). The most significant biological processes represented among annotated transcripts were translation (14%) and transport (10%) (Fig. 3d).
Figure 3.
Identification and characterization of ‘Candidatus Cardinium hertigii’-associated transcripts within the H. glycines early life stage transcriptome. Transcripts from the H. glycines transcriptome were extracted and mapped against the proteome for Candidatus Cardinium hertigii to identify potential endosymbiont-associated transcripts, resulting in the identification of 468 of the 839 described proteins for this endosymbiont within the SCN early life stage transcriptome (a). Available gene ontology annotation was added to the endosymbiont-associated transcripts by BLAST2GO and grouped by the parent terms molecular function (b), cellular component (c), and biological process (d).
SCN stylet-secreted effector protein analysis
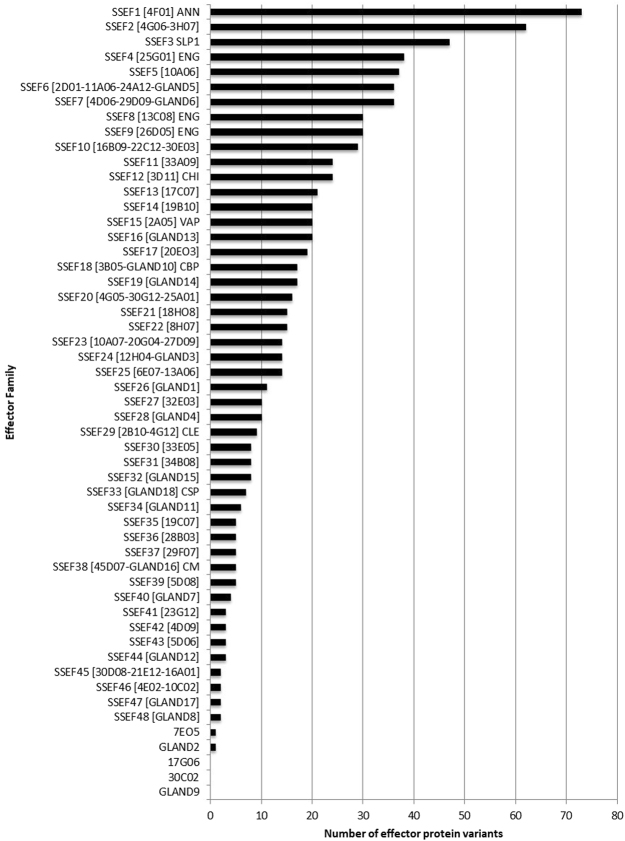
Effector proteins originating in the esophageal gland cells and secreted through the stylet play critical roles in the SCN infection process. Therefore, we first examined the 72 previously identified stylet-secreted H. glycines effectors17–19 within the transcriptome. Of these, transcripts corresponding to each effector were identified using a BLASTN search, indicating that the transcriptome contained sufficient depth to detect expression of the known gland cell effector repertoire of SCN. An analysis of effector variation within the population was then performed. We first grouped the known effectors into stylet-secreted effector families (SSEFs) with greater than 70% sequence identity. To assess the level of variation of these known effectors within the sequenced H. glycines population, the predicted peptide sequences were mined for protein variants using BLASTP at a 1e–5 cutoff. Protein variants were identified for 69 of the 72 known effectors (Fig. 4). The remaining three (17G06, 30C02, and GLAND9) were found to have single nucleotide insertions and/or deletions leading to a frame shift in the predicted peptide, resulting in a completely different peptide compared to the reference sequence, and consequently were not examined for sequence variation. A wide scope of variation was identified, with some effectors having over 70 predicted protein variants across the population (e.g., annexin 4F01), while others were limited to a single, highly conserved protein sequence (e.g., 7E05, protein unknown function).
Figure 4.
Variation of known effectors in the H. glycines early life stage transcriptome. Protein variants of previously published H. glycines effectors17–19 were identified using a BLASTP search at a 1e-5 cutoff and counted. Known effector sequences with >70% amino acid identity were grouped into stylet-secreted effector families (SSEF) to facilitate the analysis. Available functional annotation for effector families is indicated as follows: ANN = annexin-like; SLP1 = SNARE-like protein 1; ENG = endoglucanase; CHI = chitinase; VAP = venom allergen-like protein; CBP = cellulose-binding protein; CLE = CLAVATA3/EMBRYO SURROUNDING REGION (CLE)-like; CSP = circumsporozoite protein; CM = chorismate mutase.
We then examined the expression of known SCN effectors during a compatible and an incompatible interaction to determine if the host environment influences the expression of any of these effectors. The effectors were split into two different groups (upregulated or downregulated) based on their expression pattern from the pre-parasitic second-stage juvenile (J2) stage to the parasitic J2 life stage and then compared across the two conditions (S4 Table). Most of the known SCN effectors followed the same pattern of expression across both comparisons, but the level of expression change was slightly reduced in the incompatible interaction. However, a subset of effectors exhibited an opposite trend of increased expression in the incompatible interaction, including members of SSEFs 1 [4F01], 9 [26D05], 17 [20E03], 22 [8H07], 45 [30D08, 21E12, 16A01], 39 [5D08], and 11 [33A09].
Effector alternative splicing analysis
To analyze alternative splicing (AS) as a potential mechanism of effector variation, we used the 72 previously identified stylet-secreted H. glycines effector candidates17–19. Similar to the protein analysis of known SCN effectors, transcripts corresponding to each effector were identified using a BLASTN search in order to determine AS relationships. The major differences from the protein analysis were the use of a higher sequence similarity threshold (>85% identity) and the use of a gap penalty of 0. These two constraints were implemented to reduce false positives and improve true positives since gaps are expected to occur and should have a higher percent identity if AS occurs. In total, 395 AS transcripts were identified for the 72 previously known SCN effectors (Table 2), with the number of AS variants per each effector ranging from 1 to 38. Using these 395 AS transcripts, differential expression analysis was conducted to determine statistically significant AS transcripts for comparison between the ppJ2 and pJ2 life stages as well as between two different host interactions in the pJ2 life stage, an incompatible and compatible interaction. In total, 129 AS transcripts representing 44 known SCN effectors were determined to be statistically significant with respect to host interaction groups and 276 AS transcripts representing 58 known SCN effectors were statistically significant with respect to life stages, with 127 overlapping transcripts (98.4%) between stages (Table 2).
Table 2.
Summary statistics for alternative splicing analysis of known SCN effectors.
| Known effectors | AS transcripts | |
|---|---|---|
| Total | 72 | 395 |
| Significant for host interaction (compatible vs incompatible) | 44 | 129 |
| Significant for life stage (ppJ2 vs pJ2) | 58 | 276 |
Alternative splicing analysis was performed on the previously published SCN effectors using the de novo transcriptome assembly. Splice variants were identified for known effectors and then analyzed for differential expression based on host interaction and nematode life stage.
To explore the effect that AS may have on protein function, functional domain analysis was conducted on the 395 AS transcripts. For this, we determined the changes in the functional domain architectures between specific AS isoforms. Since AS often alters the reading frame, all six reading frames were analyzed. Of the 72 effectors, 7 did not have any identified functional protein domains. In total, 513 protein functional domains for the remaining 65 effectors (7.9 domains per an isoform, on average) were identified using InterPro33. For the 395 AS transcripts, 910 protein functional domains were identified (2.3 domain, on average), with 108 transcripts with no functional domains identified. When considering each effector and their AS transcripts, 37 out of 65 effectors (57%) had AS events that altered the predicted domain architecture. The 395 transcripts included 198 architectures with no change, 247 with at least one added functional domain, and 78 with one or more functional domains deleted. We note that the numbers of domain architectures do not add up to 395 because in some cases a transcript belonging to one effector was identified as the AS transcript from a different effector.
To analyze the functional changes in more detail, case studies of two effectors, GLAND13 and HgCLE (Heterodera glycines CLAVATA3/EMBRYO SURROUNDING REGION-like), were considered together with their AS transcripts. GLAND13 was chosen to demonstrate a simple example of a clear association between a protein function and AS variation due to the differentially spliced isoforms. On the other hand, HgCLE was chosen to demonstrate the structural and functional complexity that could be invoked through alternatively spliced isoforms. The architecture of the GLAND13 protein was predicted to have two functional domains that corresponded almost exactly to the two exons (Fig. 5a). These two protein domains were associated with glycosyl hydrolase, a five-blade beta propeller domain, and concanavalin A-like lectin/glucanase protein domain (InterPro IDs: IPR023296 and IPR013320, respectively). Both of the functional domains are known to associate with metabolism. Our de novo AS analysis determined two different transcripts associated with GLAND13. The primary transcript included both protein domains, while the secondary transcript had exon 1 spliced out. It is possible for the reading frame to be altered if an AS event modifies the beginning of the gene. However, in our case the reading frame was preserved, which caused a removal of the glycosyl hydrolase domain, while leaving intact the glucanase domain. The functional implications of this removal are yet to be experimentally characterized. However, it was clear from the analysis that the primary transcript was important for life stage and was upregulated in the parasitic stage (p-value is 9.07E-11). Additionally, the secondary transcript was important for both life stage and host interaction (p-value 1.29E-5, Fig. 5b). This transcript was upregulated in the parasitic stage, but to a greater extent in nematodes infecting a resistant host plant.
Figure 5.
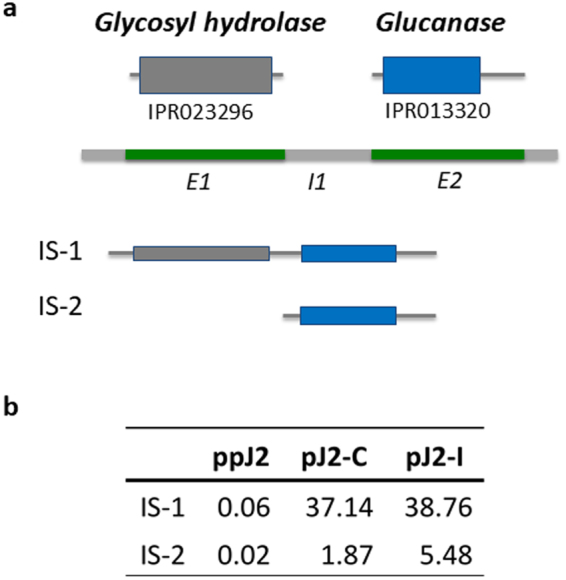
Gene structure, protein functional domain architecture, and isoform protein products for GLAND13. Domain architecture and the retained protein domains in each of two isoforms, IS-1 and IS-2 (a). Expression of each isoform (transcripts per million) in pre-parasitic second-stage juveniles (ppJ2) and parasitic J2 (pJ2) life stages during a compatible (c) or incompatible (I) host interaction (b). The first isoform was significant for life stage change (p-value is 9.07E-11), while the second isoform was significant for both life stage and host interaction changes (p-value 1.29E-5).
HgCLE genomic architecture includes four exons that were consistent with four functional subunits: signal peptide, variable domain I, variable domain II, and the CLE domain34. The N-terminal signal peptide is important for secretion of the peptide out of the nematode esophageal gland cell while variable domain I has been shown to function in targeting of the effector within the host plant cell35. The HgCLE effector family [2B10-4G12] contains two known members with high levels of sequence conservation at the amino acid level, the only differences existing within the variable domains. The CLE domain is processed to release a small peptide that functions within the host plant as a ligand mimic34. Based on the AS analysis, there were 8 transcripts associated with HgCLE. To improve the AS analysis, the corresponding HgCLE2 genomic DNA sequence was retrieved from NCBI GenBank (GenBank ID: FJ503005.1) and compared with these 8 transcripts (Fig. 6a). While the genomic sequence was obtained from a nematode population that was different from the one used in this study, it was expected that there would be a significant sequence similarity between the gene sequence and the AS isoforms if there were AS events associated with intron retention. Transcript 1 corresponded to the full sequence of HgCLE2 retaining all four exons. Transcript 2 included exon 1–3, but retained intron 3 and lacked exon 4, which was associated with the CLE domain. Transcript 3 contained exon 1 and 2, but retained a modified version of intron 1. Transcript 4 was similar to transcript 3 except intron 3 was retained. Transcript 5 included just exon 1 and 2. Transcript 6 included modified versions of intron 1 and exon 2. Transcript 7 included a modified version of exon 2. Transcript 8 included exon 1. With respect to the differential expression analysis, transcript 1, 3, 5 and 7 were statistically significant (p-values ranging from 5.50E-04 to 9.638E-05) for life stage and host interaction, transcripts 2 and 4 were statistically significant (p-value 5.27E-07 and 4.96E-10) only in regards to the life stage, and transcripts 6 and 8 were not differentially expressed between any group (Fig. 6b).
Figure 6.
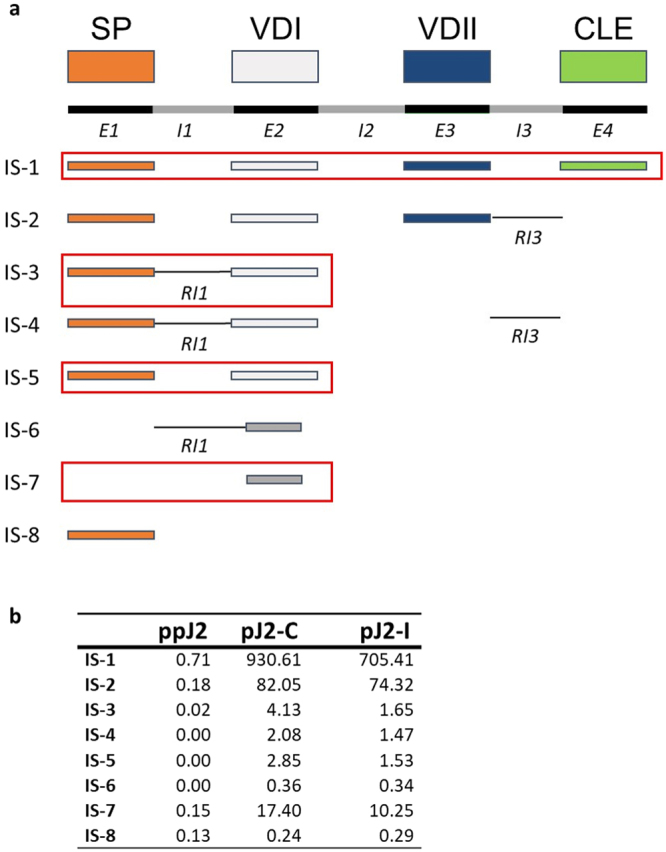
Gene structure, protein functional domain architecture, and isoform protein products for HgCLE2. Domain architecture and the retained protein domains in each of eight isoforms, IS-1 and IS-8 (a). Shown in red are the retained introns. Each retained intron was modified as a result of AS. Dark grey boxes correspond to a modified VD1 domain due to AS. Expression of each isoform (transcripts per million) in pre-parasitic second-stage juveniles (ppJ2) and parasitic J2 (pJ2) life stages during a compatible (c) or incompatible (I) host interaction (b). Red boxes highlight transcripts that were statistically different for both life stage and host interaction groups.
Novel effector prediction
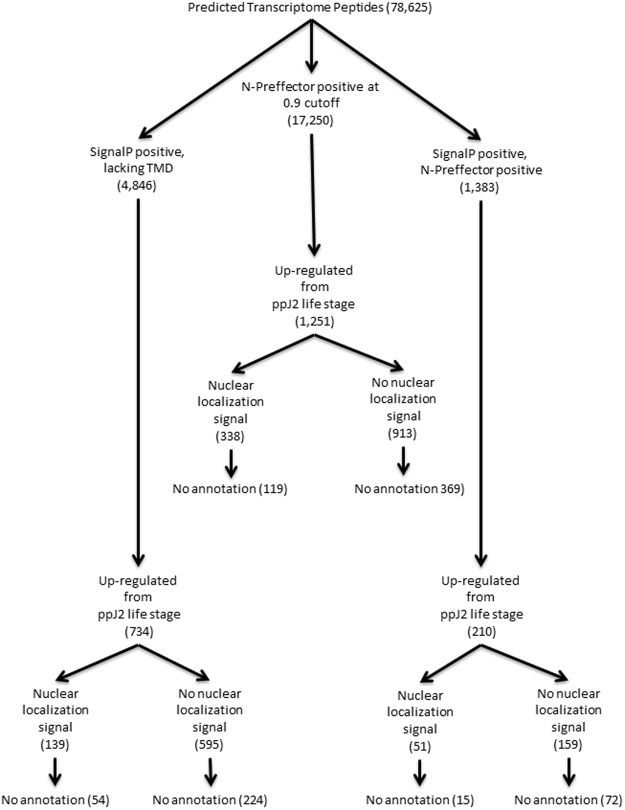
We then performed a comprehensive effector analysis on the SCN transcriptome. Effectors were predicted using two separate pipelines, and then the results were compared to determine the overlap of each pipeline (Fig. 7; S5 Table). The first of these pipelines relies on the presence of a signal peptide and follows the method used in previous studies for the prediction of putative stylet-secreted effectors18,19. This pipeline predicted 4,846 putative effectors. To identify putative new effectors with higher confidence, we focused on genes upregulated from the pre-parasitic J2 to parasitic J2 life stage and analyzed the sequences for the presence of a nuclear localization signal (NLS). A NLS combined with an N-terminal signal peptide is a strong indicator for localization of these effectors into host cell nuclei where they can play a variety of functions including regulation of plant defense responses20,36. Following these filtering steps, this pipeline predicted 734 effector candidates, including 139 nuclear localization signal (NLS)-positive effector candidates up-regulated from the pre-parasitic J2 to the parasitic J2 life stage (Fig. 7; S5 Table). The 72 known SCN effector proteins, known to contain signal peptides, were re-discovered at a rate of 74% using this pipeline. This pipeline is reliant upon the presence of a N-terminal signal peptide, which may not be present if the N-terminus is absent from the transcript. This is reflected in the fact that several known SCN effectors were not recovered despite their nucleotide sequences being present within the transcriptome. A second pipeline independent of the presence of a signal peptide was performed using N-Preffector, a machine learning algorithm trained on known nematode and bacterial effectors. The N-Preffector-based pipeline predicted 1,251 putative effectors, including 338 NLS positive effector candidates up-regulated from the pre-parasitic J2 to the parasitic J2 life stage (Fig. 7; S5 Table). In this pipeline, 67% of the known SCN effectors were re-discovered. When the two pipelines were compared, 210 effector candidates were found to be overlapping, including 51 NLS positive candidates (Fig. 7; S5 Table). Many of these sequences have little or no annotation available. Among those sequences with available annotation are many homologs of effectors from other plant-parasitic nematodes that were not previously identified or characterized in H. glycines (S6 Table). These include effectors such as glutathione synthetase37 and members of the SPRYSEC family38.
Figure 7.
Secreted effector protein prediction in the early life stage transcriptome of H. glycines. Predicted peptides from the transcriptome were put through two separate pipelines to identify candidate effectors. One pipeline utilized prediction of a signal peptide and lack of a predicted transmembrane domain (TMD) while the other utilized N-Preffector, a machine learning algorithm. Numbers shown here are predicted peptides remaining after each step in the pipeline.
Discussion
In this study, we sequenced the transcriptome of the early life stages of the plant-parasitic nematode Heterodera glycines, including the infective (pre-parasitic) second-stage juvenile (J2) life stage and the parasitic J2 life stage in two different host conditions, resistant and susceptible. We then carried out a de novo transcriptome assembly with an emphasis on assessing the level of variation of known effectors within a single population and identifying novel secreted effectors within H. glycines that may play important roles in establishing a parasitic interaction with its host, soybean. The resulting transcriptome from these samples consisted of nearly 150,000 transcripts encoding 78,625 predicted proteins. There are several possible explanations for the large number of transcripts identified. First, to generate the transcriptome a large population of nematodes was sequenced. The inherent genotypic heterogeneity present within the population may lead to many variants of the same gene being represented within the transcriptome. In addition, following transcriptome assembly no expression threshold was applied. This was done to capture any rare or lowly expressed transcripts within the population. Of the 147,910 transcripts contained within the H. glycines transcriptome, 66,601 (48%) were annotated based on BLAST homology. Many of these potential homologues existed in other nematode species, including plant- and animal-parasitic nematodes. In addition, some transcripts showed homology to a bacterial endosymbiont from the genus Cardinium, of Encarsia pergandiella, a parasitic wasp, and Bemesia tabaci, a whitefly. Previous work identified this endosymbiont and characterized it as Candidatus Paenicardinium endonii, later renamed to Candidatus Cardinium hertigii28,39. However, little is known about the function of this endosymbiont and what role it may play, if any, in plant parasitism. Related endosymbionts found in insects and arachnids have been shown to have prominent impacts on their hosts, leading to changes in host reproductive capacity and also modulating host immunity40. To better understand the function of a putative endosymbiont in H. glycines all transcripts associated with this endosymbiont were identified and extracted from the transcriptome, representing a majority of the characterized sequences for this endosymbiont. Those transcripts identified were primarily associated with metabolic processes, which may contribute to both nematode and endosymbiont metabolism. Further studies into the function of this endosymbiont and any effect on parasitism removing the endosymbiont has will be vital in elucidating what role it plays inside the nematode. In addition to a bacterial endosymbiont, several putative homologs from viruses were also identified. Previously, researchers found representative viruses from the Bornaviridae, Rhabdoviridae, and Bunyaviridae families contained within H. glycines26,32. Thus, there appears to be a significant microbial community active within H. glycines that has until now remained largely unexplored. Further examination of these organisms could reveal vital connections that can be exploited for improving resistance against SCN.
Stylet-secreted effectors represent a key component of the plant-nematode interaction, serving a wide variety of functions required for successful invasion and establishment of the nematode feeding site. Previous studies have identified a suite of these effectors using microaspiration techniques to isolate the contents of the esophageal gland cells where these genes are expressed18,19. These studies then prioritized potential effectors based on those sequences possessing a signal peptide and lacking a transmembrane domain. Despite previous knowledge about the effector repertoire of SCN, very little is known about the structure of these sequences within a population, specifically how these sequences vary from one individual to another. To address this question, we undertook an effector variation analysis within the transcriptome, identifying putative variants of known effectors and examining their level of variation within the population. Within the SCN transcriptome, predicted sequence variants of known effectors ranged from over 70 (effector 4F01) down to one (effectors 7E05 and GLAND2). This effector variation may be a result of different alleles being present in the population and/or reflect variation in the copy numbers of genes encoding related effectors. The level of variation of these effectors is likely related to the function of the effector in question. For example, a highly variable effector such as 4F01 may be under constant selection pressure to avoid host recognition, resulting in a wide level of variation across the gene pool. A prior study demonstrated that 4F01 might function as a mimic of host plant annexins to promote successful plant infection41. By contrast, effectors with limited variation across the population are likely constrained by their function. It would be interesting to see how a highly virulent population or a field population compares to the highly inbred population used here for sequencing. Certain effectors may be expanded or reduced depending on the population and host selection pressure. Effectors with a very low number of variants across populations may represent key elements of infection that could be targeted for further study in the attempt at identifying a novel source of broad spectrum SCN resistance.
One potential mechanism of gene regulation that can introduce variation into genetic sequences is alternative splicing. Previous work has identified alternative splicing in stylet-secreted effectors from SCN on an individual basis and demonstrated that expression of these variants was impacted by the life stage of the nematode42,43. As sequence data become available for plant-parasitic nematodes, these types of analysis can be expanded to larger scales. For example, a comprehensive analysis of alternative splicing events conducted across the effector complement of the potato cyst nematode G. pallida using the sequenced genome found that 38% of these genes undergo alternative splicing and that certain families of effectors show increased occurrence of splicing relative to others44. With the early parasitic transcriptome generated in this study we were able to perform a large-scale alternative splicing analysis on the known effectors of SCN and identified significant changes in the expression of alternatively spliced transcripts for a majority of the effectors between the ppJ2 and pJ2 life stages as well as between compatible and incompatible host interactions. Changes in effector splicing across life stages as the nematode begins infection may be important for altering the protein function or activity to facilitate migration and establishment of the nematode feeding site. We then examined alternative splicing of effectors between a compatible and an incompatible host interaction, identifying a smaller subset of effectors with significant expression changes between these two conditions. These splice variants may be useful once again for altering function and activity of the effectors, potentially after being triggered by perception of host resistance by the nematode. By expressing an alternate version of the effector sequence, the nematode may avoid direct recognition of the host or recognition of the function that effector performs. Once additional populations of SCN have been sequenced it will be interesting to see whether these splice variants are involved in virulence on other sources of SCN resistance and if these can be targeted to improve overall resistance to this pathogen.
We also mined the early parasitic transcriptome to identify additional effectors expressed within H. glycines using the SignalP predictive tool, as well as a novel pipeline called N-Preffector. The use of N-Preffector allowed for the identification of an entirely new class of effectors not necessarily containing a signal peptide. Examples of secreted effectors lacking a signal peptide have been shown in other plant-parasitic nematode species such as G. rostochiensis, where they have been shown to play a role in disrupting host reactive oxygen species production45,46. These effectors may contain a previously unknown secretion signal or utilize a novel secretion pathway in order to be secreted. Between the two pathways utilized for effector discovery, 86% of known SCN effectors were re-discovered within the early parasitic SCN transcriptome. The remaining 14% were not re-discovered either due to truncated sequences relative to the reference sequence or a change in the predicted protein sequence between the transcriptome and reference sequence. It is interesting to note that 47% of these effectors were identified by both pipelines, but included different effectors. This illustrates the potential advantage of using both pipelines to accurately detect all possible effectors including those that one pipeline may not identify. The signal peptide-dependent method is excellent at predicting putative effectors, but misses out on transcripts that may be truncated or simply lack the signal peptide, which can be complemented using the N-Preffector pipeline. It should also be noted that in this study an expression change between life-stages was used as a parameter for effector prediction and to limit the overall number of false positives. For this reason, the possibility exists that some putative effectors with very low expression levels may have eluded discovery. One example is HgCLEB, which is expressed at low levels and therefore was not discovered in the effector pipeline, but later identified using a targeted search of the transcriptome47.
The novel effector candidates identified by these two pipelines represent a set of genes for downstream expression and functional analysis to investigate the interaction between SCN and soybean. Many of these sequences have little or no annotation available, much like the original gland isolated effector sequences obtained for H. glycines17–19. These novel effector sequences may play pivotal roles in nematode parasitism and will require more in depth functional studies to determine their function. Among those sequences with available annotation are many homologs of effectors from other plant-parasitic nematodes that were not identified or characterized in H. glycines previously. Included in this category are genes such as the glutathione synthetase family, the novel G. rostochiensis effector E9, and candidates showing homology to the SPRYSEC family of effectors from G. rostochiensis. Glutathione synthetases have many potential roles in the interaction between the nematode and host plant. In the interaction of the root-knot nematode M. incognita it was found that glutathione is needed for successful infection of the host plant Medicago truncatula37. In addition, glutathione synthetase genes were found to be greatly expanded in the genome of the potato cyst nematode G. pallida, where these genes are theorized to be involved in protection of the nematode from antioxidant proteins as well as potentially in nematode nutrition8. Several transcripts annotated as glutathione synthetase also contained a secretion signal, something that differentiates them from glutathione synthetases found in animal parasites that may function within the nematode. The putative effector E9 has been identified in both G. rostochiensis and G. pallida and was confirmed to be expressed in gland cells via in situ hybridization44,48. Thus far little is known about the function of the E9 effector, other than it being expressed in the gland cells of Globodera species. The SPRYSEC effectors on the other hand have been heavily investigated in the Globodera-tomato pathosystem, with demonstrated roles in the suppression of plant immune responses38,48. To date, SPRYSEC effectors have not been identified in the genome sequence of root-knot nematodes8 however, entries in non-redundant sequence databases suggest they may be present in other cyst nematodes and lesion nematodes49. Thus, these could be very interesting candidates for comparative analysis across virulent populations of SCN to determine whether or not they play the same role as in Globodera spp. Another effector candidate of note is a putative secreted calreticulin. A calreticulin secreted by M. incognita is necessary for successful infection and may play a role in suppression of plant defenses; functions that may be retained in H. glycines50. Another nematode effector homolog group identified in the transcriptome involved in suppression of host defenses are the C-type lectins (CTLs) from Rotylenchus reniformus. These effectors were identified in the R. reniformus transcriptome and subsequently shown to be expressed in the hypodermis of parasitic stages of the nematode51. It is hypothesized that these effectors are involved in protecting the nematode from environmental stress. While these homologs are all predicted to have the same function in H. glycines as their originating species, further functional characterization is necessary to confirm this.
Interestingly, we identified several effector candidates with sequence similarity to proteins originating in plants and other organisms. These included multiple effector candidates with homology to members of the plant RING/U-box superfamily of proteins. These proteins are typically involved in protein modification and regulation of plant pathways, including defense responses and regulation of cell death52. Nematode mimics of these proteins may be involved in manipulation or suppression of host defense pathways in order to allow successful establishment of the feeding site. Among the identified effector candidates are also several homologs related to plant metabolism and cell wall degradation. These included arabinosidase, fructosidase, glycoside hydrolase, and expansin. These cell wall modifying proteins have been shown to aid in the loosening and degradation of polysaccharides present in the plant cell wall53–55 and have been identified from other plant-parasitic nematodes where they play a crucial function in migration and establishment of the nematode feeding site56,57. Therefore, these plant mimics all represent avenues of study to be pursued in order to better understand the interplay between SCN and its plant host, soybean.
In conclusion, a de novo transcriptome of the pre-parasitic and parasitic second-stage juvenile life stages of H. glycines has been generated, annotated, and comprehensively mined for putative effector sequences. Within this transcriptome novel effector candidates were identified utilizing a new prediction tool not reliant on sequences possessing a signal peptide, N-Preffector. In addition, the level of variation of previously identified H. glycines effectors was examined for the first time at the population level and identified highly conserved and highly variable effectors. Finally, this transcriptome provides a useful genetic resource that will aid in annotation of the SCN genome. Combining these data will provide insights into the biology of SCN with the hopes of discovering new ways to combat this pathogen.
Materials and Methods
Nematode cultivation and isolation
The SCN inbred population PA3 (HG Type 0) was propagated under greenhouse conditions on susceptible soybean Williams 82 or EXF63. Freshly hatched pre-parasitic second-stage juveniles (ppJ2) were inoculated onto 10-day old seedlings of the susceptible host or the resistant host (cv. Forrest) and the inoculated plants were placed in the greenhouse. The remaining ppJ2 nematodes were pelleted by centrifugation and flash-frozen in liquid nitrogen and stored at −80 °C prior to RNA isolation. Five days post-inoculation, parasitic second-stage juveniles (pJ2) nematodes were isolated from the roots by blending the roots for 30 s in a kitchen blender. Following this, the root homogenate was poured over a nested stack of sieves with pore sizes of 850 µm, 250 µm, and 25 µm before purifying the nematodes from the sample using sucrose centrifugal flotation58. Samples were frozen in liquid nitrogen and stored at −80 °C prior to RNA isolation.
RNA isolation and sequencing
RNA was isolated from frozen nematode pellets using the PerfectPure Fibrous Tissue Kit (5Prime) and a modified version of the manufacturer’s extraction protocol. Tissue was homogenized in 30 second intervals in the provided lysis solution containing 0.5 µM TCEP using a bead beater and 1.0 mm zirconia beads, followed by a 30 second incubation on ice. This was repeated three times. The sample was centrifuged briefly at room temperature before transferring the supernatant to a fresh tube. Following lysis and homogenization, 10 µl of the provided Proteinase K was added and the sample was allowed to incubate on ice for 10 minutes, after which the manufacturer’s protocol for RNA purification was followed. RNA quality was determined using a Fragment Analyzer (Advanced Analytical) and quantified using a Qubit Fluorometer prior to library preparation. RNA-seq libraries (ppJ2, pJ2 infecting susceptible host, pJ2 infecting resistant host) were constructed using the TruSeq mRNA Stranded Library Prep Kit (Illumina) and sequenced on the Illumina HiSeq. 2500 platform in a paired-end manner (2 × 100 for ppJ2 and pJ2-Compatible samples and 2 × 50 for pJ2-Incompatible sample). Library preparation and high-throughput sequencing services were performed at the University of Missouri DNA Core Facility. Three biological replicates of each sample were sequenced.
De novo transcriptome assembly
Prior to assembly, raw reads from these libraries were filtered using Trimmomatic59 to remove low quality reads. The remaining reads were paired and orphan reads discarded. High quality paired-end reads were used as input for transcriptome assembly. De novo transcriptome assembly was completed using the de Bruijn graph-based tool Trinity60. As part of the assembly process, an in silico read normalization step was performed. Assembly quality was then assessed by mapping raw reads back to transcripts using Bowtie261 at default parameters.
Transcriptome annotation and quantification
The transcriptome was annotated following the established Trinotate pipeline60. Homology searches were performed against the protein sequences contained in Genbank62 and UniProt63 databases using BLASTX at an evalue cutoff of 1e-564. Transcripts were translated into protein using TransDecoder, a component of Trinity60. HMMER and Pfam databases were used to predict protein domains contained within each transcript65,66. Presence of a signal peptide was determined using SignalP version 4.0 and TMHMM version 2.0 was utilized to identify predicted transmembrane domains67,68. The resulting annotation information was then combined and pooled into a SQLite database. In addition, sequenced nematode genomes were leveraged to identify potential homologs within the transcriptome. For this, predicted protein datasets from the genomes of Bursaphalenchus xylophilus, Meloidogyne hapla, Meloidogyne incognita, Globodera pallida, Pristionchus pacificus, Ascaris suum, and Caenorhabditis elegans were downloaded from WormBase (http://ws204.wormbase.org/) and used69. BLASTP hits from the H. glycines transcriptome with e-values less than 1e–5 were considered potential homologs. Lists of potential homologs from each of the seven species examined were then compared and contrasted to determine uniquely shared homologs between the sequenced nematode and H. glycines.
For quantification and differential expression analysis, reads from the libraries used for assembly were mapped and quantified using RSEM70 to determine transcript abundance. RSEM was utilized as it has been shown to correlate well with RT-qPCR measurements and produce expression values with high accuracy71. Following quantification, differential expression analysis was conducted using edgeR72, identifying all genes with a minimum 4-fold expression difference and under a p-value cutoff of 0.001 between any of the samples.
Identification of endosymbiont sequences within the H. glycines transcriptome
The entire de novo early parasitic transcriptome for H. glycines was mined for transcripts related to the endosymbiont “Candidatus Cardinium hertigii”. All transcripts annotated with the species designation ‘Cardinium endosymbiont’ were extracted from the transcriptome and combined into a file. A database was then constructed from the complete proteome of the closest available sequenced bacterial isolate, Cardinium hertigii cEper1 isolated from Encarsia pergandiella29. Then all putative Cardinium-associated sequences were mapped against the proteome database using BLASTX at an e-value cutoff of 1e–5 to confirm their identity as putative endosymbiont-associated transcripts. The resulting transcripts were then used for gene ontology analysis.
Gene ontology analysis of endosymbiont-associated sequences from the H. glycines transcriptome
Gene ontology (GO) analysis was performed to identify the putative function of endosymbiont-associated sequences within SCN. To do this endosymbiont-associated sequences from the SCN transcriptome were used in the research tool BLAST2GO73. This tool uses a similarity searches to assign GO annotation to sequence data lacking well-characterized GO annotation. In BLAST2GO BLASTX was performed at an e-value cutoff of 1e-5 and the top available BLAST hit used to pull available GO annotation. Once available GO annotation was assigned to the 468 endosymbiont-associated transcripts the results were examined for their potential role in SCN biology.
Variation of known SCN effectors
The protein sequences for the 72 known SCN effector sequences17–19 were aligned using MUSCLE74 and then a maximum likelihood tree was constructed based on sequence homology in MEGA775. MUSCLE (multiple sequence comparison by log-expectation) is a high accuracy tool for protein alignment. Effectors with bootstrap values greater than 50 were grouped into stylet-secreted effector families (SSEFs). Predicted transcript peptide sequences from the SCN transcriptome were then mapped to known SCN effector protein sequences using BLASTP at an e-value cutoff of 1e–5 and quantified for each known effector. Variants of known SCN effectors in a SSEF were pooled for quantification.
Effector alternative splicing analysis
De novo alternative splicing analysis represents a challenging task since a complete H. glycines genome is not available to assess exon and intron relationship73. However, it is possible to associate known genes of interest and build associated relationships to infer alternative splicing events by comparing known regions of overlap and extract exons associated with specific alternative splicing isoforms. This alternative splicing analysis relies on the transcripts that are assembled with the Trinity pipeline25. The alternative splicing quantification is then carried out with the kallisto tool76 using the preprocessed reads and the pseudo alignment on the assembled transcripts, which allows the analysis to be computationally more efficient, without losing its quality. Using these quantified transcripts, sleuth tool was employed to determine statistically significant differentially expressed transcripts77. From the list of 72 known SCN effector genes, the inferred alternative splicing relationship is built based on significant overlap between effector sequences and transcripts, defined by sequence identity of greater than 85%. The overlap and sequence identity are determined using the BLASTN tool, with the gap penalty parameter set to 078. This high sequence identity threshold is used because a true alternative splicing event is expected to have a significant exon overlap between the effector sequences and transcripts. The reason that a higher identity threshold is not used is because the SCN population used as a source for the effector genes is different from the SCN population used as a source for transcriptomic data obtained in this study. Combining the high sequence identity threshold and zero gap penalty in the BLASTN search, thus, allows for alternative splicing events of known exons from the effector genes to be identified, while not allowing the discovery of new relationships. New relationships that will be missed due to the data and methodology limitation are primarily the intron retention events and require the assembled genome as a reference. Using the identified associated alternative spliced transcripts, protein functional analysis is done by predicting the domain architectures and characterizing protein domains using InterPro79. Since it is expected for the reading frame to change, all 6 reading frames (forward and reverse) are assessed for the domain architectures and protein functions. In summary, this approach allows one to functionally characterize the differential expression changes for alternative spliced transcripts. These functionally characterized differentially expressed transcripts were compared between different nematode life stages and host interactions.
Effector prediction
The effector prediction pipeline started with all predicted peptides from the SCN transcriptome. First, sequences represented in the gland cell transcriptome were subjected to two different prediction tools: SignalP67 and N-Preffector, developed in this study. For the SignalP-based prediction, peptides were run through SignalP 4.0 and TMHMM68 to predict signal peptides and transmembrane helices, respectively. Predicted peptides containing a signal peptide and lacking a transmembrane domain were then filtered based on their expression between the ppJ2 and pJ2 life stages of the nematode, with those peptides showing a minimum 4-fold up-regulation into the pJ2 life stage retained. Finally, nuclear localization signals were predicted using NLStradamus80. For N-Preffector based prediction, predicted peptides were run through a machine-learning algorithm trained on 72 known H. glycines effector sequences and 150 known non-effector sequences from H. glycines in addition to the original sequences (gram negative bacteria) in which the Preffector model was trained81. For each protein sequence, N-Preffector calculates a vector of length-invariant features; the feature vector is then used as an input for the classification model. Feature categories that were considered are: residue composition, sequence/structure information, and physico-chemical properties of proteins. To select highly correlated features with the class and not correlated with each other, Preffector utilizes the correlation-based feature selection (CFS) method82. Our goal was to minimize the number of proteins erroneously misclassified as effectors, i.e., false positives, while trying to maximize the number of predicted real effectors, using the same exact protocol utilized in Preffector. N-Preffector achieves this through a more stringent classification criterion. Given an SVM model M and a training data of size n, for each training example xk, let be its decision value predicted by the SVM model, and be its true annotation of being an effector or non-effector. Given the SVM model M, the prediction probability for a training example xk is defined as
| 1 |
The coefficients A(i) and B(i) are estimated during the SVM training process by minimizing the log-likelihood function. Those peptides predicted by N-Preffector at or above a 0.9 confidence score cutoff were then filtered based on expression, retaining peptides with a minimum 4-fold up-regulation from the ppJ2 to the pJ2 life stages. Nuclear localization signals were then predicted using NLStradamus for the remaining peptides80.
Data Availability
Raw sequence reads are available under the Short Read Archive (SRA) accession no. SRP122521. This Transcriptome Shotgun Assembly project has been deposited at DDBJ/EMBL/GenBank under the accession GFZZ00000000. The version described in this paper is the first version, GFZZ01000000.
Electronic supplementary material
Acknowledgements
We would like to acknowledge William Spollen and Christopher Bottoms of the University of Missouri Informatics Research Core Facility for sharing their expertise with bioinformatics analysis. We thank summer NSF-REU interns Ying Lin, Eric Penton, Harrison Hennessey for assistance with annotation and Robert Heinz for maintenance of nematode cultures. This work was supported by USDA-NIFA-AFRI Awards 2012-67013-19345 (to TJB, MGM, and ELD) and 2015-67013-23511 (to TJB, MGM, and DK). MNG was supported in part by a MU Life Sciences Graduate Research Fellowship.
Author Contributions
M.G.M., D.K., T.J.B., and E.L.D. conceived the project and designed the research; M.G.M. and D.K. supervised the experiments and bioinformatics analysis; M.G. performed the experiments; M.G., A.D., and N.J. conducted the bioinformatics analysis; M.G., A.D., N.J., D.K., and M.G.M. wrote the manuscript; all authors contributed to data analysis, reviewed and commented on the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-20536-5.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Dmitry Korkin, Email: dkorkin@wpi.edu.
Melissa G. Mitchum, Email: goellnerm@missouri.edu
References
- 1.Allen TW, et al. Soybean yield loss estimates due to diseases in the United States and Ontario, Canada, from 2010 to 2014. Plant Health Prog. 2017;18:19–27. [Google Scholar]
- 2.Mitchum, M. G. et al. Nematode effector proteins: an emerging paradigm of parasitism. New Phytol. 199, 879–894 (2013). [DOI] [PubMed]
- 3.Gheysen G, Mitchum MG. How nematodes manipulate plant development pathways for infection. Curr Opin Plant Biol. 2011. 2013;14:415–421. doi: 10.1016/j.pbi.2011.03.012. [DOI] [PubMed] [Google Scholar]
- 4.Quentin M, Abad P, Favery B. Plant parasitic nematode effectors target host defense and nuclear functions to establish feeding cells. Front Plant Sci. 2013;4:53. doi: 10.3389/fpls.2013.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Toruño TY, Stergiopoulos I, Coaker G. Plant-pathogen effectors: cellular probes interfering with plant defenses in spatial and temporal manners. Ann Rev Phytopath. 2016;54:419–441. doi: 10.1146/annurev-phyto-080615-100204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saucet SB, Shirasu K. Molecular parasitic plant-host interactions. PLoS Pathog. 2016;12:e1005978. doi: 10.1371/journal.ppat.1005978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abad P, et al. Genome sequence of the metazoan plant-parasitic nematode Meloidogyne incognita. Nat Biotechnol. 2008;26:909–915. doi: 10.1038/nbt.1482. [DOI] [PubMed] [Google Scholar]
- 8.Cotton JA, et al. The genome and life-stage specific transcriptomes of Globodera pallida elucidate key aspects of plant parasitism by a cyst nematode. Genome Biol. 2014;15:R43. doi: 10.1186/gb-2014-15-3-r43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eves-van den Akker S, et al. The genome of the yellow potato cyst nematode, Globodera rostochiensis, reveals insights into the basis of parasitism and virulence. Genome Biol. 2016;17:124. doi: 10.1186/s13059-016-0985-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kikuchi T, et al. Genomic insights into the origin of parasitism in the emerging plant pathogen Bursaphelenchus xylophilus. Plos Pathogens. 2011;7:e1002219. doi: 10.1371/journal.ppat.1002219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zheng J, et al. The Ditylenchus destructor genome provides new insights into the evolution of plant parasitic nematodes. Proc Royal Soc Biol Sci. 2016;283:20160942. doi: 10.1098/rspb.2016.0942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Phillips WS, et al. The draft genome of Globodera ellingtonae. J Nematol. 2017;49:127–128. doi: 10.21307/jofnem-2017-054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fosu-Nyarko J, Nicol P, Naz F, Gill R, Jones MGK. Analysis of the transcriptome of the infective stage of the beet cyst nematode. H. schachtii. Plos One. 2016;11:e0147511. doi: 10.1371/journal.pone.0147511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hageman A, Bauters L, Kyndt T, Rahman MM, Gheysen G. Identification of candidate effector genes in the transcriptome of the rice root knot nematode Meloidogyne graminicola. Mol Plant Pathol. 2013;14:379–390. doi: 10.1111/mpp.12014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kumar M, et al. De novo transcriptome sequencing and analysis of the cereal cyst nematode, Heterodera avenae. Plos One. 2014;9:e96311. doi: 10.1371/journal.pone.0096311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Petitot A, et al. Dual RNA-seq reveals Meloidogyne graminicola transcriptome and candidate effectors during the interaction with rice plants. Mol Plant Pathol. 2016;17:860–874. doi: 10.1111/mpp.12334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bekal S, et al. A SNARE-like protein and biotin are implicated in soybean cyst nematode virulence. Plos One. 2015;10:e0145601. doi: 10.1371/journal.pone.0145601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gao B, et al. The parasitome of the phytonematode Heterodera glycines. Mol Plant-Microbe Interact. 2003;16:720–726. doi: 10.1094/MPMI.2003.16.8.720. [DOI] [PubMed] [Google Scholar]
- 19.Noon JB, et al. Eighteen new candidate effectors of the phytonematode Heterodera glycines produced specifically in the secretory esophageal gland cells during parasitism. Phytopathol. 2015;105:1362–72. doi: 10.1094/PHYTO-02-15-0049-R. [DOI] [PubMed] [Google Scholar]
- 20.Gardner M, Verma A, Mitchum MG. Emerging roles of cyst nematode effectors in exploiting plant cellular processes. In: Escobar C, Fenoll C, editors. Advances in Botanical Research. Oxford: Elsevier Publishing; 2015. pp. 259–291. [Google Scholar]
- 21.Eves-van den Akker S, Lilley CJ, Jones JT, Urwin PE. Identification and characterization of a hyper-variable apoplastic effector gene family of the potato cyst nematodes. PLoS Pathog. 2014;10:e1004391. doi: 10.1371/journal.ppat.1004391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jones JDG, Dangl JL. The plant immune system. Nature. 2006;444:323–329. doi: 10.1038/nature05286. [DOI] [PubMed] [Google Scholar]
- 23.Na R, Gijzen M. Escaping host immunity: new tricks for plant pathogens. Plos Pathogens. 2016;12:e1005631. doi: 10.1371/journal.ppat.1005631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Simão F, Waterhouse R, Ioannidis P, Kriventseva E, Zdobnov E. BUSCO: assessing genome assembly and annotation completeness with single-copy orthologs. Bioinformatics. 2015;31:3210–3212. doi: 10.1093/bioinformatics/btv351. [DOI] [PubMed] [Google Scholar]
- 25.Haas BJ, et al. De novo transcript sequence reconstruction from RNA-seq using the Trinity platform for reference generation and analysis. Nat Prot. 2013;8:1494–1512. doi: 10.1038/nprot.2013.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bekal S, Domier LL, Niblack TL, Lambert KN. Discovery and initial analysis of novel viral genomes in the soybean cyst nematode. J GenVirol. 2011;92:1870–1879. doi: 10.1099/vir.0.030585-0. [DOI] [PubMed] [Google Scholar]
- 27.Bekal S, et al. A novel flavivirus in the soybean cyst nematode. J GenVirol. 2014;95:1272–1280. doi: 10.1099/vir.0.060889-0. [DOI] [PubMed] [Google Scholar]
- 28.Noel GR, Atibalentja N. ‘Candidatus Paenicardinium endonii’, an endosymbiont of the plant-parasitic nematode Heterodera glycines (Nemata: Tylenchida), affiliated to the phylum. Bacteroidetes. Intl J System Evol Microbiol. 2006;56:1697–1702. doi: 10.1099/ijs.0.64234-0. [DOI] [PubMed] [Google Scholar]
- 29.Penz T, et al. Comparative genomics suggests an independent origin of cytoplasmic incompatibility in Cardinium hertigii. Plos Genetics. 2012;8:e1003012. doi: 10.1371/journal.pgen.1003012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Santos-Garcia D, et al. The genome of Cardinium cBtQ1 provides insights into genome reduction, symbiont motility, and its settlement in Bemisia tabaci. Gen Biol Evol. 2014;6:1013–1030. doi: 10.1093/gbe/evu077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Endo BY. The ultrastructure and distribution of an intracellular bacterium-like microorganism in tissues of larvae of the soybean cyst nematode. Heterodera glycines. J Ultrastruct Res. 1979;67:1–14. doi: 10.1016/S0022-5320(79)80012-X. [DOI] [PubMed] [Google Scholar]
- 32.Ruark CL, et al. Soybean cyst nematode culture collections and field populations from North Carolina and Missouri reveal high incidences of infection by viruses. PLoS One. 2017;12:e0171514. doi: 10.1371/journal.pone.0171514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Finn RD, et al. InterPro in 2017 – beyond protein family and domain annotations. Nuc Acids Res. 2017;45(D1):D190–D199. doi: 10.1093/nar/gkw1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang J, et al. Dual roles for the variable domain in protein trafficking and host-specific recognition of Heterodera glycines CLE effector proteins. New Phytol. 2010;187:1003–1017. doi: 10.1111/j.1469-8137.2010.03300.x. [DOI] [PubMed] [Google Scholar]
- 35.Wang J, Joshi S, Korkin D, Mitchum MG. Variable domain I of nematode CLEs directs post-translational targeting of CLE peptides to the extracellular space. Plant Signal Behav. 2010;5:1633–1635. doi: 10.4161/psb.5.12.13774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Elling AA, Davis EL, Hussey RS, Baum TJ. Active uptake of cyst nematode parasitism proteins into the plant cell nucleus. Intl J Parasitol. 2007;37:1269–1279. doi: 10.1016/j.ijpara.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 37.Baldacci-Cresp F, et al. (Homo)glutathione deficiency impairs root-knot nematode development in Medicago truncatula. Plos Pathogens. 2012;8:e1002471. doi: 10.1371/journal.ppat.1002471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rehman S, et al. A secreted SPRY domain-containing protein (SPRYSEC) from the plant-parasitic nematode Globodera rostochiensis interacts with a CC-NB-LRR protein from a susceptible tomato. Mol Plant-Microbe Interact. 2009;22:330–40. doi: 10.1094/MPMI-22-3-0330. [DOI] [PubMed] [Google Scholar]
- 39.Nakamura Y, et al. Prevalence of Cardinium bacteria in planthoppers and spider mites and taxonomic revision of “Candidatus Cardinium hertigii” based on detection of a new Cardinium group from biting midges. Appl Environ Microbiol. 2009;75:6757–6763. doi: 10.1128/AEM.01583-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Eleftherianos I, Atri J, Accetta J, Castillo JC. Endosymbiotic bacteria in insects: guardians of the immune system? Front Physiol. 2013;4:46. doi: 10.3389/fphys.2013.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Patel N, et al. A nematode effector protein similar to annexins in host plants. J Exp Bot. 2010;61:235–248. doi: 10.1093/jxb/erp293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lu S, Duanhua T, Borchardt-Wier H, Wang X. Alternative splicing: a novel mechanism of regulation identified in the chorismate mutase gene of the potato cyst nematode Globodera rostochiensis. Mol Biochem Parasitol. 2008;162:1–15. doi: 10.1016/j.molbiopara.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 43.Noon J, et al. A Plasmodium- like virulence effector of the soybean cyst nematode suppresses plant innate immunity. New Phytol. 2016;212:444–460. doi: 10.1111/nph.14047. [DOI] [PubMed] [Google Scholar]
- 44.Thorpe P, et al. Genomic characterization of the effector complement of the potato cyst nematode Globodera pallida. BMC Genomics. 2014;15:923. doi: 10.1186/1471-2164-15-923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Robertson L, Robertson WM, Jones JT. Direct analysis of the secretions of the potato cyst nematode Globodera rostochiensis. Parasitol. 1999;119:167–176. doi: 10.1017/S0031182099004448. [DOI] [PubMed] [Google Scholar]
- 46.Robertson L, et al. Cloning, expression and functional characterization of a preoxiredoxin from the potato cyst nematode Globodera rostochiensis. Mol Biochem Parasitol. 2000;111:41–49. doi: 10.1016/S0166-6851(00)00295-4. [DOI] [PubMed] [Google Scholar]
- 47.Guo X, et al. Identification of cyst nematode B-type CLE peptides and modulation of the vascular stem cell pathway for feeding cell formation. Plos Pathogens. 2017;13:e1006142. doi: 10.1371/journal.ppat.1006142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ali S, et al. Analysis of Globodera rostochiensis effectors reveals conserved functions of SPRYSEC proteins in suppressing and eliciting plant immune responses. Front Plant Sci. 2015;6:623. doi: 10.3389/fpls.2015.00623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Diaz-Granados A, Petrescu A-J, Goverse A, Smant G. SPRYSEC effectors: a versatile protein-binding platform to disrupt plant innate immunity. Front Plant Sci. 2017;7:1575. doi: 10.3389/fpls.2016.01575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jaouannet M, et al. The root-knot nematode calreticulin Mi-CRT is a key effector in plant defense suppression. Mol Plant-Microbe Interact. 2013;26:97–105. doi: 10.1094/MPMI-05-12-0130-R. [DOI] [PubMed] [Google Scholar]
- 51.Ganji S, Jenkins J, Wubben M. Molecular characterization of the reniform nematode C-type lectin gene family reveals a likely role in mitigating environmental stresses during plant parasitism. Gene. 2014;537:268–78. doi: 10.1016/j.gene.2013.12.048. [DOI] [PubMed] [Google Scholar]
- 52.Gúzman P. The prolific ATL family of RING-H2 ubiquitin ligases. Plant Signal Behav. 2012;7:1014–1021. doi: 10.4161/psb.20851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cosgrove D. Loosening of plant cell walls by expansins. Nature. 2000;407:321–326. doi: 10.1038/35030000. [DOI] [PubMed] [Google Scholar]
- 54.Minic Z, Jouanin L. Plant glycoside hydrolases involved in cell wall polysaccharide degradation. Plant Physiol Biochem. 2006;7:435–449. doi: 10.1016/j.plaphy.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 55.Weinstein L, Albersheim P. Purification and partial characterization of a wall-degrading endo-arabanase and an arabinosidase from. Bacillus subtilis. Plant Physiol. 1979;63:425–432. doi: 10.1104/pp.63.3.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Qin L, et al. Plant degradation: A nematode expansin acting on plants. Nature. 2004;427:30. doi: 10.1038/427030a. [DOI] [PubMed] [Google Scholar]
- 57.Wieczorek K, et al. Expansins are involved in the formation of nematode-induced syncytia in roots of Arabidopsis thaliana. Plant J. 2006;48:98–112. doi: 10.1111/j.1365-313X.2006.02856.x. [DOI] [PubMed] [Google Scholar]
- 58.De Boer JM, et al. Developmental expression of secretory β-1,4-endoglucanases in the subventral esophageal glands of Heterodera glycines. Mol Plant-Microbe Interact. 1999;12:663–669. doi: 10.1094/MPMI.1999.12.8.663. [DOI] [PubMed] [Google Scholar]
- 59.Bolger, A.M., Lohse, M., & Usadel, B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. btu170 (2014). [DOI] [PMC free article] [PubMed]
- 60.Grabherr MG, et al. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nature Biotechnol. 2011;29:644–652. doi: 10.1038/nbt.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Langmead B, Salzberg S. Fast gapped-read alignment with Bowtie2. Nature Methods. 2012;9:357–359. doi: 10.1038/nmeth.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Benson DA, Karsh-Mizrachi I, Lipman DJ, Ostell J, Wheeler DL. GenBank. Nuc Acids Res. 2005;1:D34–8. doi: 10.1093/nar/gki063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Magrane, M. & UniProt Consortium. UniProt knowledgebase: a hub of integrated protein data. Database. bar009 (2011). [DOI] [PMC free article] [PubMed]
- 64.Altschul SF, et al. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nuc Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Finn RD, Clements J, Eddy SR. HMMER web server: interactive sequence similarity searching. Nuc Acids Res. 2011;39:W29–W37. doi: 10.1093/nar/gkr367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Punta M, et al. The Pfam protein families database. Nuc Acids Res. 2012;40:D290–301. doi: 10.1093/nar/gkr1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Petersen TN, Brunak S, von Heijne G, Neilsen H. SignalP 4.0: discriminating signal peptides from transmembrane regions. Nature Methods. 2011;8:785–786. doi: 10.1038/nmeth.1701. [DOI] [PubMed] [Google Scholar]
- 68.Sonnhammer, E., von Heijne, G., & Krogh, A. A hidden Markov model for predicting transmembrane helices in protein sequences. Proceedings of the Sixth International Conference on Intelligent Systems for Molecular Biology; June 28 – July 1; Quebec, Canada. Menlo Park: AAAI Press (1998). [PubMed]
- 69.Howe KL, et al. WormBase 2016: expanding to enable helminth genomic research. Nuc Acids Res. 2016;44:D774–80. doi: 10.1093/nar/gkv1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Li B, Dewey CN. RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinformatics. 2011;12:323. doi: 10.1186/1471-2105-12-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chandramohan, R., Wu, P., Phan, J., & Wang, M. Benchmarking RNA-Seq quantification tools. Conference Proceedings of the IEEE Engineering in Medicine and Biology Society. 10.1109/EMBC.2013.6609583 (2016). [DOI] [PMC free article] [PubMed]
- 72.Robinson MD, McCarthy DJ, Smyth GK. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26:139–40. doi: 10.1093/bioinformatics/btp616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Conesa A, et al. A survey of best practices for RNA-seq data analysis. Gen Biol. 2016;17.1:16. doi: 10.1186/s13059-016-0881-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Edgar RC. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nuc Acids Res. 2004;32:1792–1797. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kumar, S., Stecher, G. & Tamura, K. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol. msw054 (2016). [DOI] [PMC free article] [PubMed]
- 76.Bray NL, Pimentel H, Melsted P, Pachter L. Near-optimal probabilistic RNA-seq quantification. Nature Biotechnol. 2016;34:525–527. doi: 10.1038/nbt.3519. [DOI] [PubMed] [Google Scholar]
- 77.Pimentel, H. J., Bray, N., Puente, S., Melsted, P. & Pachter, L. Differential analysis of RNA-Seq incorporating quantification uncertainty. bioRxiv. 058164 (2016). [DOI] [PubMed]
- 78.Madden, T. The BLAST sequence analysis tool. In: Beck J, et al., editors. The NCBI Handbook. Bethesda: National Center for Biotechnology Information (2013).
- 79.Mitchell A, et al. The InterPro protein families database: the classification resource after 15 years. Nuc Acids Res. 2015;43:D213–D221. doi: 10.1093/nar/gku1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nguyen BAN, Pogoutse A, Provart N, Moses AM. NLStradamus: a simple Hidden Markov Model for nuclear localization signal prediction. BMC Bioinformatics. 2009;10:202. doi: 10.1186/1471-2105-10-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dhroso, A., Eidson, S. & Korkin, D. Genome-wide prediction of bacterial effectors across six secretion system types using a feature-based supervised learning framework. bioRxiv, 255604 (2018). [DOI] [PMC free article] [PubMed]
- 82.Hall MA. Correlation-based feature selection for machine learning [dissertation] Hamilton (NZ): The University of Waikato; 1999. [Google Scholar]
- 83.Federhen S. The NCBI taxonomy database. Nuc Acids Res. 2011;40:D136–D143. doi: 10.1093/nar/gkr1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Vaughan TG. IcyTree: rapid browser-based visualization of phylogenetic trees and networks. Bioinformatics. 2017;33:2392–2394. doi: 10.1093/bioinformatics/btx155. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Raw sequence reads are available under the Short Read Archive (SRA) accession no. SRP122521. This Transcriptome Shotgun Assembly project has been deposited at DDBJ/EMBL/GenBank under the accession GFZZ00000000. The version described in this paper is the first version, GFZZ01000000.