Abstract
Background
Obesity is a risk factor for postmenopausal breast cancer incidence and pre- and postmenopausal breast cancer mortality, which may be explained by several metabolic and hormonal factors (sex hormones, insulin resistance, and inflammation) that are biologically related. Differential effects of dietary composition on weight loss and these metabolic factors may occur in insulin-sensitive vs. insulin-resistant obese women.
Objective
To examine the effect of diet composition on weight loss and metabolic, hormonal and inflammatory factors in overweight/obese women stratified by insulin resistance status in a 1-year weight loss intervention.
Methods and Results
Nondiabetic women who were overweight/obese (n = 245) were randomly assigned to a lower fat (20% energy), higher carbohydrate (65% energy) diet; a lower carbohydrate (45% energy), higher fat (35% energy) diet; or a walnut-rich (18% energy), higher fat (35% energy), lower carbohydrate (45% energy) diet. All groups lost weight at follow-up (P < 0.0001), with mean (SEM) percent loss of 9.2 (1.1)% in lower fat, 6.5 (0.9)% in lower carbohydrate, and 8.2 (1.0)% in walnut-rich groups at 12 months. The diet × time × insulin resistance status interaction was not statistically significant in the model for overall weight loss, although insulin sensitive women at 12 months lost more weight in the lower fat vs. lower carbohydrate group (7.5 kg vs 4.3 kg, P = 0.06), and in the walnut-rich vs. lower carbohydrate group (8.1 kg vs 4.3 kg, P = 0.04). Sex hormone binding globulin increased within each group except in the lower carbohydrate group at 12 months (P < 0.01). C-reactive protein and interleukin-6 decreased at follow-up in all groups (P < 0.01).
Conclusions
Findings provide some support for differential effects of diet composition on weight loss depending on insulin resistance status. Prescribing walnuts is associated with weight loss comparable to a standard lower fat diet in a behavioral weight loss intervention. Weight loss itself may be the most critical factor for reducing the chronic inflammation associated with increased breast cancer risk and progression.
Clinical Trial Registration
Keywords: Diet composition, Insulin resistance, Walnuts, Breast cancer, Weight loss, Biomarkers
1. Introduction
Obesity is a risk factor for postmenopausal breast cancer incidence and pre- and postmenopausal breast cancer mortality [1-3]. Several mechanisms have been proposed to explain the adverse effect of obesity on the risk and progression of breast cancer. One possible mechanism relates to the effect of excess adiposity on circulating reproductive steroid hormones, because adipose tissue is an important extragonadal source of estrogens from precursor adrenal androgens. Endogenous circulating estrogen levels are higher in obese postmenopausal women than in women who are not overweight, and higher circulating estrogen levels are also a risk factor for breast cancer incidence and recurrence [4, 5]. Further, obesity is associated with lower levels of sex hormone binding globulin (SHBG), which increases the bioavailable estrogen fraction [6].
Another possible mechanism relates to insulin and interactions between insulin and other metabolic factors associated with adiposity and weight gain [7]. Insulin and insulin-like growth factor - 1 stimulate mammary cell proliferation and promote tumor development by inhibiting apoptosis in cell culture [8]. Obesity also is associated with chronic inflammation and increased cytokine production, a key causative factor in insulin resistance and hyperinsulinemia [9]. Further, insulin stimulates the synthesis of sex steroids and inhibits SHBG synthesis, so the effects of these various metabolic and hormonal factors (sex hormones, insulin resistance, and inflammation) are biologically related [10].
Optimal macronutrient distribution of weight loss diets has not been established, and successful weight loss has been shown to be achieved with either a low fat or low carbohydrate diet in the context of energy restriction [11, 12]. Recent review panels and dietary guidelines recommend a range of energy intake from dietary carbohydrate and fat [13, 14], with the only specific limitation targeting saturated fat (<10% of energy intake) [15]. Cancer control guidelines have historically recommended a low fat diet, but data from observational studies and clinical trials support the current conclusions that evidence linking higher fat intake with risk for breast cancer is not strongly supportive [1, 2, 16]. A dietary pattern that is higher in carbohydrate, especially if provided mainly from highly refined food choices, is associated with increased cardiometabolic risk factors, including hyperinsulinemia [17]. In previous short-term studies, it was observed that insulin sensitive individuals lost more weight in response to lower fat vs. lower carbohydrate intake, and individuals who were insulin resistant or secreting higher levels of insulin lost more weight in response to a lower carbohydrate vs. lower fat intake [18, 19].
The source and type of dietary fatty acids also may affect metabolic and hormonal factors associated with obesity. Regular consumption of walnuts, which are rich in polyunsaturated fatty acids and bioactive food components, has been associated with a reduction in inflammatory markers in addition to improved lipid profile in observational and feeding studies [20, 21]. Numerous clinical studies of nut consumption, primarily focused on the effects on cardiovascular disease risk factors and inflammatory markers, have observed minimal or no effect on body weight despite the potential additional energy intake contributed by the addition of nuts (including walnuts) to the diet [22, 23]. The specific effects of nut consumption in the context of a weight loss intervention have been examined in a few previously-published studies [24-28], which have tested the effects of almonds, pistachios and peanuts and have had mixed results. Also, a Mediterranean diet (which includes walnuts as one component of this dietary pattern) has been shown to promote weight loss in addition to favorable effects on lipids, fasting glucose and insulin levels [29, 30].
This study was designed to examine the effect of three dietary approaches on weight loss, metabolic factors, and hormonal and inflammatory markers in overweight and obese women stratified by insulin resistance status, within a 1-year behavioral weight loss intervention. The diets compared were a lower fat, higher carbohydrate diet; a lower carbohydrate, higher fat diet; and a walnut-rich, higher fat, lower carbohydrate diet. Differential weight loss and metabolic response to variable macronutrient content of the diet is highly relevant to breast cancer given that these metabolic and hormonal factors may indeed explain the link between obesity and breast cancer risk and progression.
2. Methods
2.1. Study Population
The overall rationale and context of this randomized controlled trial has been published previously [10], and the plasma lipid responses at the 6-month interim follow-up time point have been previously reported [31]. As previously described [31], study participants were nondiabetic overweight and obese women. The UCSD institutional review board approved the study protocol, and all participants provided written informed consent.
Participants were randomly assigned to one of three study arms: lower fat (20% energy), higher carbohydrate (65% energy) diet; lower carbohydrate (45% energy), higher fat (35% energy) diet; or walnut-rich (18% energy), higher fat (35% energy), lower carbohydrate (45% energy) diet. The randomization used a sequence generated by the study statistician, stratified by menopausal status (older/younger than 55 years as a proxy) and insulin resistance status, which was calculated from the homeostasis model assessment - insulin resistance (HOMA-IR) index ([fasting glucose, mmol/L] × [insulin, mIU /L]/22.5) with HOMA-IR >3.0 considered indicative of insulin resistance [28]. Anthropometric measurements and a fasting (>6 hours) blood sample collection were conducted at clinic visits at baseline and 6 and 12 months.
2.2 Intervention
Details about the diet prescription and intervention have been previously published [31]. Briefly, the overall goal of the dietary guidance was to promote a 500-1000 kcal/day deficit relative to expenditure. Participants assigned to the walnut-rich diet study group were instructed to consume an average of 42 g (1.5 oz) walnuts per day, within their reduced-energy diet. All participants were also encouraged to aim for an average of at least 60 minutes/day of purposeful exercise at a moderate level of intensity.
2.3. Measurements
At baseline and follow-up clinic visits, weight, height (baseline only), and waist circumference were measured and questionnaires were collected. The 3-minute step test, which has high reliability and is sensitive to change [33], measured heart rate during the first 30 seconds of recovery from stepping, and was used to assess aerobic fitness. Self-reported physical activity data were collected using the Global Physical Activity Questionnaire [34].
2.4. Laboratory Measures
The Kodak Ektachem Analyzer system (Johnson & Johnson Clinical Diagnostics, Rochester, NY, USA) was used to measure glucose, total cholesterol, triglycerides, and high-density lipoprotein cholesterol (HDL-C) with enzymatic methods, and low-density lipoprotein cholesterol (LDL-C) values were calculated [35]. Commercially prepared quality control samples, and laboratory participation in the College of American Pathologists Quality Assurance Program, was utilized to monitor accuracy and precision. The inter-batch and intra-batch CV for these analytes ranged from 0 to 8%. Insulin was measured by Arup Laboratories (Salt Lake City, UT, USA) using the ADVIA Centaur assay, a double antibody immunoassay with chemiluminescent detection. The inter-batch and intra-batch CV was 3.3% and 2.3%, respectively.
Serum estradiol was measured at the Endocrine Research Laboratory at the University of Southern California (Los Angeles, CA, USA) by radioimmunoassay after organic solvent extraction and celite column-chromatography; procedural losses were monitored by addition of tritiated standard to each sample prior to the extraction [36]. The assay sensitivity is 2 pg/ml, and the interassay CVs are 11%, 13% and 12% at 15, 36 and 101 pg/mL, respectively. Follicle stimulating hormone (FSH, baseline only) and serum SHBG were measured by Arup Laboratories (Salt Lake City, UT, USA) using chemiluminescent sandwich techniques, and results are determined via a calibration curve which is instrument-specifically generated. The inter-batch and intra-batch CVs were 3.2% and 0.6% for FSH, and for SHBG, 1.7% and 0.6%, respectively. High-sensitivity C-reactive protein (CRP) and interleukin-6 (IL-6) were measured at the Laboratory for Clinical Biochemistry Research, University of Vermont (Colchester, VT, USA). CRP was assayed using a polystyrene-enhanced turbidimetric in vitro immunoassay, and the inter-assay CV range was 2.1-5.7%. IL-6 was measured using a solid phase quantitative sandwich ELISA technique (R & D Systems, Inc., Minneapolis, MN, USA). The method inter-assay CV is 9%.
Red blood cell (RBC) fatty acids were measured using gas liquid chromatography methodology (Wake Forest School of Medicine Lipid and Lipoprotein Analytic Laboratory (Winston-Salem, NC, USA). We present the percent of total fatty acids attributable to the fatty acid of interest. The inter-batch and intra-batch CV were 6.0% and 5.5% for linoleic, and for linolenic 15.3% and 9.8%, respectively.
2.5. Statistical Analysis
Study outcomes (weight, percent weight loss, metabolic factors and biomarkers) were examined in longitudinal mixed effects models that assumed unstructured covariance. Predictors (diet group assignment, study time, baseline insulin resistance status) were modeled as fixed effects, and a random cluster term accounted for cluster-specific effects of the behavioral intervention meeting groups. Two-way interactions (e.g., diet by time, diet by insulin resistance status) and the three-way interaction (diet by time by insulin resistance status) were also included as predictors in the models. Contrasts between outcomes for diets at each study time point, and between time points within each study diet group, were also examined in the models.
Triglycerides, CRP, insulin, and estradiol were log transformed in analysis to control for skew in their distributions, but untransformed means are presented for clarity. One subject whose IL-6 values were extremely high at follow-up was excluded from analysis of IL-6. Estradiol is presented stratified by menopausal status, with postmenopausal status defined as FSH >20 IU/L, which was usually consistent with self-reported menopausal status.
Any analyte for which a significant main effect for insulin resistance status was observed is presented also by strata of insulin resistance status. Contrasts estimated the effects of each diet on insulin sensitive and insulin resistant subjects at follow-up. Change in insulin resistance status was tested with chi-square tests. As an exploratory analysis, we examined the relationship between baseline weight and baseline estradiol levels in postmenopausal women using a regression model. Alpha for type 1 error was set at 0.05. All analyses were conducted in SAS version 9.4 (SAS Institute, Cary, NC, USA).
3. Results
Characteristics of the study participants at enrollment are presented in Table 1. Weekly minutes of moderate/vigorous physical activity were similar and recovery heart rate improved in all diet study groups over the study, and there were no differences between groups (data not shown).
Table 1. Baseline characteristics of study participants (n = 245).
| Variable | Value |
|---|---|
| Age (years), mean (range) | 50 (22-72) |
| Race/ethnicity, n (%) | |
| White non-Hispanic | 181 (73.9) |
| Hispanic | 42 (17.1) |
| African American | 12 (4.9) |
| Asian American | 4 (1.6) |
| Mixed or other | 6 (2.5) |
| BMI, mean (range), (kg/m2) | 33.5 (27-40) |
| Insulin resistance status, (n [%]) | |
| Insulin sensitive | 119 (48.6) |
| Insulin resistant | 126 (51.4) |
3.1. Weight Loss
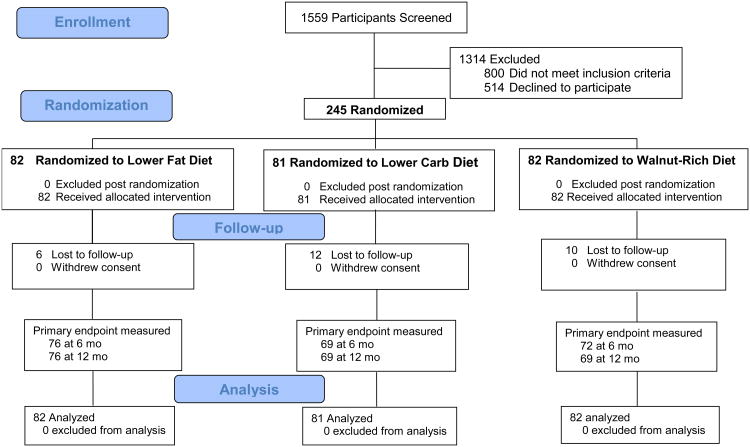
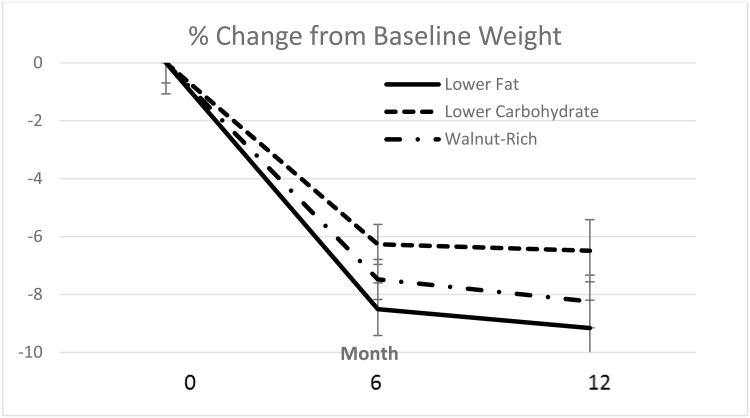
Weight data were available for 214 (87%) of the women at 12 months (Figure 1). As shown in Figure 2, each of the three diet groups demonstrated significant weight loss at follow-up time points (P < 0.001 for each time effect compared with baseline). There was a marginally significant diet × time interaction at 12 months for the lower fat vs. lower carbohydrate groups at 12 months (P = 0.06), with a 2.3 kg greater average weight loss in the lower fat vs. lower carbohydrate group. Percent loss at 12 months was 9.2 (1.1)% in lower fat, 6.5 (0.9)% in lower carbohydrate, and 8.2 (1.0)% in walnut-rich diet groups, with P < 0.001 for time effect but no difference between groups.
Fig. 1.
Flow chart of participants in the study.
Fig. 2.
Percent weight loss by diet group assignment. Values shown are means and standard errors.
Insulin resistance status was significantly associated with weight (likelihood ratio P < 0.001 for models with vs. without insulin resistance status); insulin resistant study participants were a mean of 5.4 kg heavier than insulin sensitive subjects at study entry (P = 0.03). The diet × time × insulin resistance status interaction was marginally statistically significant for the walnut-rich vs. lower carbohydrate groups in the model for weight change (P = 0.06), suggesting differential diet-related weight loss by insulin resistance status. Insulin resistant women did not show diet-related differential weight loss (Table 2). At 12 months, among insulin sensitive subjects, differences between the lower carbohydrate group were -3.6 kg for lower fat diet group subjects (P = 0.06), and -4.0 kg for walnut-rich diet group subjects (P = 0.04).
Table 2. Weight and waist circumference change by diet group and insulin resistance status.
| Insulin Sensitive | Insulin Resistant** | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Lower Fat Diet | Lower Carb Diet | Walnut-Rich Diet | Lower Fat Diet | Lower Carb Diet | Walnut-Rich Diet | |
| Baseline | ||||||
| n | 39 | 39 | 41 | 43 | 42 | 41 |
| Weight (kg) (Mean [SEM]) | 86.7 (1.6) | 87.4 (2.2) | 87.3 (1.7) | 92.3 (1.5) | 92.4 (1.7) | 92.8 (1.9) |
| Waist (cm) (Mean [SEM]) | 104 (2) | 104 (1) | 104 (1) | 108 (1) | 109 (1) | 109 (1) |
| 12 months | ||||||
| n | 37 | 37 | 35 | 39 | 32 | 34 |
| Weight change (kg) (Mean [SEM]) | -7.5 (1.3) | -4.3 (1.0) | -8.1 (1.2)* | -8.8 (1.5) | -7.0 (1.4) | -6.8 (1.4) |
| % Weight change | -8.7 (1.5) | -5.5 (1.1) | -9.2 (1.3) | -9.6 (1.5) | -7.6 (1.5) | -7.2 (1.4) |
| % Who lost ≥5% | 65 | 59 | 62 | 65 | 53 | 52 |
|
| ||||||
| Waist change (cm) (Mean [SEM]) | -9 (1) | -4 (1) | -6 (1) | -7 (1) | -8 (2) | -7 (2) |
Diet effect compared with lower carbohydrate diet, holding time and insulin resistance status constant, P < 0.05.
Insulin resistance status effect: Insulin resistant women were on average 5.4 kg heavier than insulin sensitive women, P = 0.03, and had waist circumference 2 cm larger, P = 0.01.
3.2. Metabolic Factors and Biomarkers
None of the cardiometabolic factors and biomarkers differed between diet arms at study entry and we therefore present baseline data aggregated across the three diet arms in Tables 3 and 4 (full subgroup data are available as Supplementary material). Insulin levels were significantly lower at 12 months in the lower fat group, compared with baseline (P < 0.05). At study end, 30% of subjects categorized as insulin resistant at baseline no longer met the HOMA-IR criteria for being insulin resistant. Triglycerides and CRP decreased from baseline at study end in all three groups (P < 0.03). Although HDL-C decreased at 6 months in lower fat diet group subjects (data not shown) as previously reported [31], HDL-C had increased from baseline in each of the diet arms at study end (Table 3). LDL-C decreased significantly at 12 months in the walnut-rich group (P < 0.01). IL-6 decreased from baseline to 12 months in each of the diet groups (P < 0.001).
Table 3. Biomarker summary data (mean [SEM]).
| Biomarkers | All Baseline (n = 245) | Lower Fat Diet 12 Months (n = 68) | Lower Carb Diet 12 Months (n = 61) | Walnut-Rich Diet 12 Months (n = 65) |
|---|---|---|---|---|
| % Insulin resistant | 51.4 | 32.4* | 39.3* | 40.0* |
| Insulin (μIU/mL)a | 14.5 (0.5) | 12.2 (0.8)* | 13.0 (1.0) | 13.8 (1.2) |
| Glucose (mg/dL)b | 97 (1) | 92 (1)* | 93 (2) | 93 (1)* |
| HOMA-IR | 4 (0.1) | 3 (0.2)* | 3 (0.3)* | 3 (0.3) |
| HOMA-Beta | 160 (6) | 158 (9) | 162 (10) | 164 (10) |
| Triglycerides (mg/dL)c | 124 (4) | 116 (6)* | 112 (7)* | 101 (6)* |
| HDL cholesterol (mg/dL)d | 59 (1) | 63 (2)* | 61 (2)* | 67 (2)* |
| LDL cholesterol (mg/dL)d | 122 (2) | 114 (4) | 115 (3)* | 115 (4)* |
| CRP (μg/mL)e | 4.86 (0.34) | 3.95 (0.72)* | 2.89 (0.49)* | 2.94 (0.45)* |
| IL-6 (pg/mL) | 2.62 (0.13) | 1.49 (0.16)* | 1.79 (0.36) * | 1.50 (0.20)* |
| SHBG (nmol/L) | 56 (2) | 73 (5)* | 64 (5) | 69 (5)* |
| Estradiol (pg/mL)f | ||||
| Premenopausal (n = 100) | 127 (8) | 140 (25) | 91 (16) | 119 (23) |
| Postmenopausal (n = 145 | 24 (2) | 25 (8) | 26 (7)* | 27 (7) |
| Linoleic acid (%) | 13.3 (0.2) | 11.7 (0.2) * | 12.0 (0.3) * | 12.3 (0.3) *,** |
| α-Linolenic acid (%) | 0.12 (0.005) | 0.14 (0.01)* | 0.15 (0.01) | 0.22 (0.03)*,** |
Time effect within diet compared with baseline (significant diet × time interaction), P < 0.05.
Diet effect compared with lower carbohydrate diet at a given time, P < 0.05.
Multiply by 6.945 to obtain pmol/L.
Multiply by 0.0555 to obtain mmol/L
Multiply by 0.0113 to obtain mmol/L.
Multiply by 0.0259 to obtain mmol/L.
Multiply by 9.524 to obtain nmol/L.
Multiply by 3.671 to obtain pmol/L. Postmenopausal was defined as FSH > 20 IU/L (and includes two subjects who reported themselves premenopausal at baseline)
Table 4. Biomarkers stratified by insulin resistance status (mean [SEM]) for analytes that differed by insulin resistance status.
| Biomarkers | All Insulin Sensitive at Baseline (n = 119) | Lower Fat Diet 12 Months (n = 33) | Lower Carb Diet 12 Months (n = 34) | Walnut-Rich Diet 12 Months (n = 35) |
|---|---|---|---|---|
| Insulin Sensitive Subjects | ||||
|
| ||||
| Insulin (μIU/mL)a | 8.9 (0.2) | 9.5 (0.7) | 10.5 (1.1)* | 9.1 (0.6) |
| Triglycerides (mg/dL)b | 101 (5) | 103 (8) | 99 (9) | 84 (5) |
| HDL cholesterol (mg/dL)c | 63 (1) | 66 (3) | 65 (3)* | 71 (3)* |
| CRP (μg/mL)d | 3.67 (0.36) | 2.85 (0.57)* | 2.92 (0.81) | 2.95 (0.69)* |
| IL-6 (pg/mL) | 2.30 (0.20) | 1.23 (0.21)* | 1.63 (0.35)*,** | 1.58 (0.35)* |
| SHBG (nmol/L) | 63 (3) | 88 (7)* | 71 (7) | 80 (6)* |
|
| ||||
| Insulin Resistant Subjects | ||||
|
| ||||
| Insulin (μIU/mL)a | 19.8 (0.7) | 14.7 (1.3)* | 16.2 (1.4) | 19.3 (2.0) |
| Triglycerides (mg/dL)b | 145 (6) | 129 (8) | 129 (10) | 122 (11)* |
| HDL cholesterol (mg/dL)c | 55 (1) | 60 (3)* | 57 (3)* | 61 (2) |
| CRP (μg/mL)d | 6.00 (0.54) | 5.00 (1.27)* | 2.85 (0.42)* | 2.92 (0.56)* |
| IL-6 (pg/mL) | 2.93 (0.17) | 1.73 (0.24)* | 2.00 (1.68)* | 1.50 (0.19)* |
| SHBG (nmol/L) | 50 (3) | 59 (5)* | 54 (7) | 57 (7) |
Time effect within diet compared with baseline, P < 0.05.
One extreme outlier for IL-6 was excluded from the lower carbohydrate diet group data.
Multiply by 6.945 to obtain pmol/L.
Multiply by 0.0113 to obtain mmol/L.
Multiply by 0.0259 to obtain mmol/L.
Multiply by 9.524 to obtain nmol/L.
SHBG increased significantly from baseline within each of the three diet groups except in lower carbohydrate diet subjects at 12 months (P < 0.01). Estradiol did not change with weight loss in postmenopausal women, although it was significantly associated with baseline weight (P = 0.01) in an exploratory regression model. In premenopausal women, a small decrease in estradiol was observed at 12 months (P = 0.03 time effect in a mixed model).
At 12 months, linoleic acid was significantly lower than at baseline (P < 0.001), and the walnut-rich diet group subjects had higher levels than those in the other diet groups (diet by time effect, P = 0.04). Alpha-linolenic acid, on the other hand, increased significantly from baseline at follow-up, and those in the walnut-rich diet group had a higher level than those in the reference lower carbohydrate diet group (P < 0.001 for diet effect, P < 0.001 for time effect, P < 0.01 for diet by time interaction). Significant group by time interactions were observed for linoleic acid, alpha-linolenic acid and HDL-C but not for any of the other analytes.
Some of the analytes differed by insulin resistance status (significant main effect P < 0.001 for insulin, triglycerides and SHBG; P < 0.01 for HDL-C and CRP; P < 0.05 for IL-6 and gamma-tocopherol) and these data are presented in Table 4. Insulin, triglycerides, CRP, and IL-6 were higher in insulin resistant than in insulin sensitive women, whereas HDL-C and SHBG were lower in insulin resistant women than in insulin sensitive women. No significant 3-way interactions were observed for lipids, inflammatory markers or SHBG, but there was a significant 3-way interaction (diet by time by insulin resistance status) for insulin level.
4. Discussion
Findings from this study provide some support for differential effects of diet composition on weight loss and metabolic and hormonal factors depending on insulin resistance status. At 12 months, insulin sensitive women lost more weight if assigned to the walnut-rich vs. lower carbohydrate diet group. However, insulin resistant women did not show diet-related differential weight loss at follow-up. Study participants in each of the study arms (lower fat, lower carbohydrate, and walnut-rich diet) demonstrated significant weight loss at 12 months, and the overall degree of weight reduction achieved (∼8% of initial weight) has been shown to reduce risk of diabetes and cardiovascular disease risk factors in previous large randomized studies [37, 38].
Results from this study may be compared to two previous reports in which weight loss in response to lower fat vs. lower carbohydrate diets were examined along with indicators of insulin sensitivity, although there are differences in study duration and target samples. In a 4-month diet intervention study [18], obese nondiabetic insulin sensitive women (defined as fasting insulin <10 μmL) lost more weight on a lower fat (20% energy), higher carbohydrate (60% energy) diet than on a lower carbohydrate (40% energy), higher fat (40% energy) diet, and insulin resistant women (defined as fasting insulin >15 μmL) lost more weight on the lower carbohydrate vs. lower fat diet. In a 6-month diet intervention study [19], individuals exhibiting greater insulin secretion in response to an oral glucose load lost more weight when assigned to a lower carbohydrate (40% energy), higher fat (30% energy) vs. higher carbohydrate (60% energy), lower fat (20% energy) diet. However, those investigators did not find differential weight loss when HOMA-IR (rather than post-glucose load insulin secretion) was used to categorize insulin resistance status, and no differences in weight loss across these two diet arms were observed in individuals exhibiting lower insulin secretion in response to a glucose load. Taken together, current evidence thus suggests that differential degree of weight loss in response to higher vs. lower carbohydrate diets depending on insulin resistance status may be evident in the initial period of diet modification and weight loss. In the long term (e.g., greater than 6 months) and with continued energy restriction and weight reduction (which is associated with improved insulin sensitivity), dietary macronutrient composition is not an important determinant of weight loss in women with differing initial insulin resistance status.
Overall weight loss was generally similar across the diet groups, although the walnut-rich diet was associated with greater weight loss at 12 months than a lower carbohydrate, higher fat diet with similar levels of total fat and carbohydrate. The specific effects of nut consumption in the context of a weight loss intervention have been examined in a few previously-published randomized studies and have had mixed results. Wein et al. [24] found a formula-based low-calorie diet enriched with 84 g/day almonds (vs. isocaloric with carbohydrate replacement) to promote a greater reduction in weight and BMI (18% vs. 11%) in a 12-week weight reduction program involving 65 overweight or obese adults. In another study, the effect of a higher-fat reduced-energy diet with 16% energy from peanuts (approximately 38 g/day) compared to an isocaloric higher-carbohydrate diet for 6 weeks followed by a 4-week weight maintenance phase was examined [25]. Weight loss was similar in the two diet arms, although the peanut-containing arm had more favorable effects on cardiovascular disease risk factors. Participants in another study were prescribed an isocaloric reduced-energy diet that included a daily afternoon snack of 53 g pistachios or 56 g pretzels in a 12-week weight loss intervention [26]. There was a trend but not a significant difference in weight change, although the pistachio group did exhibit a significantly greater reduction in BMI and plasma triglyceride concentration. The two most recently published studies involving almonds in the context of a weight loss intervention report divergent results. Foster et al. [27] compared a reduced-energy almond-enriched diet (56 g/day) with a reduced-energy nut-free diet in 123 overweight and obese adults in the context of an 18-month group-based behavioral weight loss program. Participants in the almond-enriched study arm lost less weight than the nut-free group at 6 months (5.5 vs. 7.4%) and there were no differences at 18 months. Abazarfard et al. [28] similarly compared a reduced-energy almond-enriched diet (50 g/day) to an isocaloric reduced-energy nut-free diet in 108 overweight and obese women and found greater weight loss in the almond-enriched group compared to the nut-free group (3.68 vs. 1.27 kg, respectively) at 3 months.
As consumption of nuts is one characteristic of a Mediterranean dietary pattern, walnuts have been a component of two diets prescribed to test the effect of a Mediterranean diet on weight loss and selected lipid and metabolic factors. Although relevant, the greater complexity of these prescribed diets constrains comparisons with the present study, due to several differences in diet composition and foods prescribed. In a 2-year randomized controlled trial involving 322 obese subjects, Shai et al. [29] found that both a low carbohydrate diet and a Mediterranean diet promoted more weight loss, in addition to more favorable effects on lipids (with the low carbohydrate diet) and on glycemic control (with the Mediterranean diet), compared to a standard low fat diet. The Mediterranean diet in that study is described as being rich in vegetables and low in red meat, with poultry and fish replacing beef and lamb, in addition to 30-45 g of olive oil and a handful of nuts (5-7 nuts, <20 g) each day. In another study of the effect of a Mediterranean diet on weight loss, Austel et al. [30] found a diet that included canola oil, walnuts and walnut oil, in addition to two portion-controlled sweet snacks (chocolate, ice cream and cake) promoted more weight loss compared to a wait-list control group at 12 weeks (5.2 kg vs. 0.4 kg, respectively) in 212 overweight or obese subjects.
Several mechanisms may explain why prescribing energy-dense walnuts is generally not associated with weight gain that might be expected in the previous studies and may also explain the weight loss observed in this study [22, 23, 39]. Nuts may promote satiety, which could modulate appetite and promote dietary compensation; e.g., total energy intake may be spontaneously reduced due to greater satiety and satiation association with nut consumption. Also, walnuts have recently been shown to contribute less metabolizable energy in the human biological system than is calculated by proximate analysis and standardized Atwater estimates [40]. Walnuts are rich in polyunsaturated fatty acids, and these have been suggested to improve weight reduction by influencing appetite and satiety perception [17]. Prescribing walnuts as a component of a weight loss diet intervention is associated with weight loss that is comparable to a standard lower fat diet (and better than a higher fat, lower carbohydrate diet without walnuts) in the context of a behavioral weight loss program.
As we previously reported [31], lipid responses differed across the diet groups in the initial period of the weight loss intervention. At 12 months, however, improvements in lipid levels in the lower fat and lower carbohydrate diet groups closed the gap, as study participants in all groups had further weight loss and good weight loss maintenance over time. Changes in the RBC fatty acids of interest, linoleic acid and alpha-linolenic acid, indicate good adherence in the walnut-rich diet group, as observed in previous walnut feeding and walnut-rich diet intervention studies [20, 41].
SHBG increased in association with weight loss, particularly in the lower fat and walnut-rich diet groups, which may be explained in part by reduced insulin levels with weight loss. As an important determinant of bioavailable estradiol, increased SHBG levels, regardless of change in total estradiol, suggest that the weight loss achieved by either a lower fat or walnut-rich diet may reduce breast cancer risk and progression.
Changes in body weight and selected metabolic factors in overweight breast cancer survivors in response to a lower fat vs. lower carbohydrate diet has been investigated in two previous weight loss interventions [42, 43]. Thomson et al. [42] found no differences in weight loss or changes in the total cholesterol/HDL-C ratio, insulin and HOMA-IR in response to a lower fat (25% energy), higher carbohydrate (60% energy) vs. lower carbohydrate (35% energy), higher fat (40% energy) diet, although triglycerides were significantly reduced only in the low carbohydrate diet group. Similarly, Thompson et al. [43] did not observe differential weight loss in response to a low fat (18% energy), high carbohydrate (64% energy) vs. a low carbohydrate (33% energy), high fat (48% energy) diet, although greater improvement in levels of triglycerides and HDL-C was observed in the low carbohydrate diet group. Insulin resistance status was not examined in either of these studies, and both were of shorter duration (6 months) and compared greater extremes of macronutrient composition than were tested in this study.
At baseline, insulin resistant women had higher levels of several metabolic factors and biomarkers of relevance to risk for breast cancer, including insulin and inflammatory markers, and lower SHBG, compared to insulin sensitive women. The inflammatory markers were reduced in response to the weight loss intervention in all diet groups at study end. These findings suggest that weight loss itself, rather than diet composition, may be the most critical factor for reducing the chronic inflammation that has been suggested to be a key factor in promoting insulin resistance [9].
This study has several strengths. One strength is the low rate of drop-out and missing data, a recognized problem in the interpretation of results of many studies of diet and weight loss, which minimizes ambiguity in drawing inferences from this study. A limitation is that when participants were divided into individual diet groups and further stratified by insulin resistance status, statistical power to detect differences across these subgroups was reduced because of small subsamples. These results may not be generalizable to men, because the sample consisted of women. Another limitation is the lack of detailed information about dietary intake, and variability in adherence is likely because this was a free-living population. Differential adherence may contribute to differential response that was observed across the study diet groups. However, the weight loss demonstrated by most study participants suggests that most were adhering to a reduced-energy diet.
In conclusion, findings from this study suggest that insulin resistance status may be associated with differential effects of macronutrient diet composition on weight loss and metabolic and hormonal factors. Prescribing walnuts is associated with weight loss that is comparable to a standard low fat diet in the context of a behavioral weight loss program. Weight loss itself, rather than diet composition, may be the most critical factor for reducing the chronic inflammation that has been suggested to be a key factor in promoting insulin resistance and thus increasing breast cancer risk and progression.
Supplementary Material
Acknowledgments
The authors thank the Data and Safety Monitoring Committee (Richard Schwab, MD, Jeanne Nichols, PhD, and Sonia Jain, PhD). We also thank the laboratories of Matthew Davis, MS, Wake Forest School of Medicine, for conducting the fatty acid analysis; Frank Stanczyk, PhD, University of Southern California, for conducting the estradiol analysis; and Elaine Cornell, University of Vermont, for conducting the CRP and IL-6 analysis. We thank Hava-Shoshana Barkai, MS, RD, and Lea Jacinto for operational support and Lita Hinton for assistance with manuscript preparation.
Funding: This study was supported by the National Cancer Institute (NIH) grant, CA155435, and the California Walnut Commission.
Abbreviations
- BMI
body mass index
- CRP
C-reactive protein
- CV
coefficient of variation
- FSH
follicle stimulating hormone
- HDL-C
high-density lipoprotein cholesterol
- HOMA-IR
homeostasis model assessment-insulin resistance index
- IL-6
interleukin-6
- LDL-C
low-density lipoprotein cholesterol
- n
number
- RBC
red blood cell
- SHBG
sex hormone binding globulin
Footnotes
Conflict of Interest: The authors declare no conflict of interest.
Disclosures: None.
Author Contributions: CLR designed and led the trial effort with major contributions from SWF, BP and LN, and SWF and LN ran the analysis. BP and ELQ directed and coordinated the study, and DDH directed the sample collections and laboratory analysis. CLR, SWF, DDH, and LN drafted the article and all authors reviewed and revised for important intellectual content.
References
- 1.Food, Nutrition, Physical Activity, and the Prevention of Cancer: a Global Perspective. Washington DC: AICR; 2007. World Cancer Research Fund/American Institute for Cancer Research. [Google Scholar]
- 2.Food, Nutrition, Physical Activity, and the Prevention of Breast Cancer. Washington DC: AICR; 2010. World Cancer Research Fund/American Institute for Cancer Research. [Google Scholar]
- 3.Protani M, Coory M, Martin JH. Effect of obesity on survival of women with breast cancer: systematic review and meta-analysis. Breast Cancer Res Treat. 2010;123:627–35. doi: 10.1007/s10549-010-0990-0. [DOI] [PubMed] [Google Scholar]
- 4.Clemons M, Goss P. Estrogen and the risk of breast cancer. N Engl J Med. 2001;344:276–85. doi: 10.1056/NEJM200101253440407. [DOI] [PubMed] [Google Scholar]
- 5.Rock CL, Flatt SW, Laughlin GA, Gold EB, Thomson CA, Natarajan L, et al. Reproductive steroid hormones and recurrence-free survival in women with a history of breast cancer. Cancer Epidemiol Biomarkers Prev. 2008;17:614–20. doi: 10.1158/1055-9965.EPI-07-0761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Verkasalo PK, Thomas HV, Appleby PN, Davey GK, Key TJ. Circulating levels of sex hormones and their relation to risk factors for breast cancer: a cross-sectional study in 1092 pre-and postmenopausal women (United Kingdom) Cancer Causes Control. 2001;12:47–59. doi: 10.1023/a:1008929714862. [DOI] [PubMed] [Google Scholar]
- 7.Goodwin PJ, Ennis M, Pritchard KI, Trudeau ME, Koo J, Madarnas Y, et al. Fasting insulin and outcome in early-stage breast cancer: results of a prospective cohort study. J Clin Oncol. 2002;20:42–51. doi: 10.1200/JCO.2002.20.1.42. [DOI] [PubMed] [Google Scholar]
- 8.Kaaks R, Lukanova A. Energy balance and cancer: the role of insulin and insulin-like growth factor-I. Proc Nutr Soc. 2001;60:91–106. doi: 10.1079/pns200070. [DOI] [PubMed] [Google Scholar]
- 9.Grimble RF. Inflammatory status and insulin resistance. Current Opinion Clin Nutri Metab Care. 2002;5:551–9. doi: 10.1097/00075197-200209000-00015. [DOI] [PubMed] [Google Scholar]
- 10.Patterson RE, Rock CL, Kerr J, Natarajan L, Marshall SJ, Pakiz B, et al. Metabolism and breast cancer risk: frontiers in research and practice. J Acad Nutr Diet. 2013;113:288–96. doi: 10.1016/j.jand.2012.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Foster GD, Wyatt HR, Hill JO, Makris AP, Rosenbaum DL, Brill C, et al. Weight and metabolic outcomes after 2 years on a low-carbohydrate versus low-fat diet. Ann Intern Med. 2010;153:147–157. doi: 10.1059/0003-4819-153-3-201008030-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sacks FM, Bray GA, Carey VJ, Smith SR, Ryan DH, Anton SD, et al. Comparison of weight-loss diets with different compositions of fat, protein, and carbohydrates. N Engl J Med. 2009;360:859–73. doi: 10.1056/NEJMoa0804748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.IOM. National Academy Press. Washington, DC: National Academy Press; 2002. Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids. [DOI] [PubMed] [Google Scholar]
- 14.Eckel RH, Jakicic JM, Ard JD, de Jesus JM, Houston Miller N, Hubbard VS, et al. 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:S76–99. doi: 10.1161/01.cir.0000437740.48606.d1. [DOI] [PubMed] [Google Scholar]
- 15.US Department of Agriculture. 2015–2020 Dietary Guidelines for Americans. (8th) 2015 Dec; [Google Scholar]
- 16.Thomson CA. Diet and breast cancer: understanding risks and benefits. Nutr Clin Prac. 2012;27:636–650. doi: 10.1177/0884533612454302. [DOI] [PubMed] [Google Scholar]
- 17.Abete I, Astrup A, Martinez JA, Thorsdottir I, Zulet MA. Obesity and the metabolic syndrome: role of different dietary macronutrient distribution patterns and specific nutritional components on weight loss and maintenance. Nutr Rev. 2010;68:214–31. doi: 10.1111/j.1753-4887.2010.00280.x. [DOI] [PubMed] [Google Scholar]
- 18.Cornier MA, Donahoo WT, Pereira R, Gurevich I, Westergren R, Enerback S, et al. Insulin sensitivity determines the effectiveness of dietary macronutrient composition on weight loss in obese women. Obes Res. 2005;13:703–9. doi: 10.1038/oby.2005.79. [DOI] [PubMed] [Google Scholar]
- 19.Pittas AG, Das SK, Hajduk CL, Golden J, Saltzman E, Stark PC, et al. A low-glycemic load diet facilitates greater weight loss in overweight adults with high insulin secretion but not in overweight adults with low insulin secretion in the CALERIE Trial. Diabetes Care. 2005;28:2939–41. doi: 10.2337/diacare.28.12.2939. [DOI] [PubMed] [Google Scholar]
- 20.Ros E, Nunez I, Perez-Heras A, Serra M, Gilabert R, Casals E, et al. A walnut diet improves endothelial function in hypercholesterolemic subjects: a randomized crossover trial. Circulation. 2004;109:1609–14. doi: 10.1161/01.CIR.0000124477.91474.FF. [DOI] [PubMed] [Google Scholar]
- 21.Banel DK, Hu FB. Effects of walnut consumption on blood lipids and other cardiovascular risk factors: a meta-analysis and systematic review. Am J Clin Nutr. 2009;90:56–63. doi: 10.3945/ajcn.2009.27457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rajaram S, Sabate J. Nuts, body weight and insulin resistance. Br J Nutr. 2006;96(Suppl 2):S79–86. doi: 10.1017/bjn20061867. [DOI] [PubMed] [Google Scholar]
- 23.Sabate J, Cordero-Macintyre Z, Siapco G, Torabian S, Haddad E. Does regular walnut consumption lead to weight gain? Br J Nutr. 2005;94:859–64. doi: 10.1079/bjn20051567. [DOI] [PubMed] [Google Scholar]
- 24.Wien MA, Sabate JM, Ikle DN, Cole SE, Kandeel FR. Almonds vs complex carbohydrates in a weight reduction program. Int J Obesity. 2003;27:1365–72. doi: 10.1038/sj.ijo.0802411. [DOI] [PubMed] [Google Scholar]
- 25.Pelkman CL, Fishell VK, Maddox DH, Pearson TA, Mauger DT, Kris-Etherton PM. Effects of moderate-fat (from monounsaturated fat) and low-fat weight-loss diets on the serum lipid profile in overweight and obese men and women. Am J Clin Nutr. 2004;79:204–12. doi: 10.1093/ajcn/79.2.204. [DOI] [PubMed] [Google Scholar]
- 26.Li Z, Song R, Nguyen C, Zerlin A, Karp H, Naowamondhol K, et al. Pistachio nuts reduce triglycerides and body weight by comparison to refined carbohydrate snack in obese subjects on a 12-week weight loss program. J Am Coll Nutr. 2010;29:198–203. doi: 10.1080/07315724.2010.10719834. [DOI] [PubMed] [Google Scholar]
- 27.Foster GD, Shantz KL, Vander Veur SS, Oliver TL, Lent MR, Virus A, et al. A randomized trial of the effects of an almond-enriched, hypocaloric diet in the treatment of obesity. Am J Clin Nutr. 2012;96:249–54. doi: 10.3945/ajcn.112.037895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abazarfard Z, Salehi M, Keshavarzi S. The effect of almonds on anthropometric measurements and lipid profile in overweight and obese females in a weight reduction program: a randomized controlled clinical trial. J Res Med Sci. 2014;19:457–64. [PMC free article] [PubMed] [Google Scholar]
- 29.Shai I, Schwarzfuchs D, Henken Y, Shahar DR, Witkow S, Greenberg I, et al. Weight loss with a low-carbohydrate, Mediterranean, or low-fat diet. N Eng J Med. 2008;359:229–41. doi: 10.1056/NEJMoa0708681. [DOI] [PubMed] [Google Scholar]
- 30.Austel A, Ranke C, Wagner N, Gorge J, Ellrott T. Weight loss with a modified Mediterranean-type diet using fat modification: a randomized controlled trial. Eur J Clin Nutr. 2015;69:878–84. doi: 10.1038/ejcn.2015.11. [DOI] [PubMed] [Google Scholar]
- 31.Le T, Flatt SW, Natarajan L, Pakiz B, Quintana EL, Heath DD, et al. Effects of diet composition and insulin resistance status on plasma lipid levels in a weight loss intervention in women. J Am Heart Assoc. 2016;5:e002771. doi: 10.1161/JAHA.115.002771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bonora E, Targher G, Alberiche M, Bonadonna RC, Saggiani F, Zenere MB, et al. Homeostasis model assessment closely mirrors the glucose clamp technique in the assessment of insulin sensitivity: studies in subjects with various degrees of glucose tolerance and insulin sensitivity. Diabetes Care. 2000;23:57–63. doi: 10.2337/diacare.23.1.57. [DOI] [PubMed] [Google Scholar]
- 33.McArdle W, K F, Katch V. Exercise Physiology: Energy, Nutrition, and Human Performance. 6th. Lippincott Williams & Wilkins; 2006. [Google Scholar]
- 34.Cleland CL, Hunter RF, Kee F, Cupples ME, Sallis JF, Tully MA. Validity of the global physical activity questionnaire (GPAQ) in assessing levels and change in moderate-vigorous physical activity and sedentary behaviour. BMC Public Health. 2014;14:1255. doi: 10.1186/1471-2458-14-1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 36.Abrahamson PE, Tworoger SS, Aiello EJ, Bernstein L, Ulrich CM, Gilliland FD, et al. Associations between the CYP17, CYPIB1, COMT and SHBG polymorphisms and serum sex hormones in post-menopausal breast cancer survivors. Breast Cancer Res Treat. 2007;105:45–54. doi: 10.1007/s10549-006-9426-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jensen MD, Ryan DH, Apovian CM, Ard JD, Comuzzie AG, Donato KA, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation. 2014;129:S102–38. doi: 10.1161/01.cir.0000437739.71477.ee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Look AHEAD Research Group. Pi-Sunyer X, Blackburn G, Brancati FL, Bray GA, Bright R, et al. Reduction in weight and cardiovascular disease risk factors in individuals with type 2 diabetes: one-year results of the look AHEAD trial. Diabetes Care. 2007;30:1374–83. doi: 10.2337/dc07-0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mattes RD, Dreher ML. Nuts and healthy body weight maintenance mechanisms. Asia Pacific J Clin Nutri. 2010;19:137–41. [PubMed] [Google Scholar]
- 40.Baer DJ, Gebauer SK, Novotny JA. Walnuts consumed by healthy adults provide less available energy than predicted by the Atwater factors. J Nutr. 2016;146:9–13. doi: 10.3945/jn.115.217372. [DOI] [PubMed] [Google Scholar]
- 41.Estruch R, Ros E, Salas-Salvado J, Covas MI, Corella D, Aros F, et al. Primary prevention of cardiovascular disease with a Mediterranean diet. N Engl J Med. 2013;368:1279–90. doi: 10.1056/NEJMc1806491. [DOI] [PubMed] [Google Scholar]
- 42.Thomson CA, Stopeck AT, Bea JW, Cussler E, Nardi E, Frey G, et al. Changes in body weight and metabolic indexes in overweight breast cancer survivors enrolled in a randomized trial of low-fat vs. reduced carbohydrate diets. Nutr Cancer. 2010;62:1142–52. doi: 10.1080/01635581.2010.513803. [DOI] [PubMed] [Google Scholar]
- 43.Thompson HJ, Sedlacek SM, Paul D, Wolfe P, McGinley JN, Playdon MC, et al. Effect of dietary patterns differing in carbohydrate and fat content on blood lipid and glucose profiles based on weight-loss success of breast-cancer survivors. Breast Cancer Res. 2012;14:R1. doi: 10.1186/bcr3082. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.