Abstract
Insulin-like growth factors (Igfs) are key regulators of key biological processes such as embryonic development, growth, and tissue repair and regeneration. The role of Igf in myogenesis is well documented and, in zebrafish, promotes fin and heart regeneration. However, the mechanism of action of Igf in muscle repair and regeneration is not well understood. Using adult zebrafish extraocular muscle (EOM) regeneration as an experimental model, we show that Igf1 receptor blockage using either chemical inhibitors (BMS754807 and NVP-AEW541) or translation-blocking morpholino oligonucleotides (MOs) reduced EOM regeneration. Zebrafish EOMs regeneration depends on myocyte dedifferentiation, which is driven by early epigenetic reprogramming and requires autophagy activation and cell cycle reentry. Inhibition of Igf signaling had no effect on either autophagy activation or cell proliferation, indicating that Igf signaling was not involved in the early reprogramming steps of regeneration. Instead, blocking Igf signaling produced hypercellularity of regenerating EOMs and diminished myosin expression, resulting in lack of mature differentiated muscle fibers even many days after injury, indicating that Igf was involved in late re-differentiation steps. Although it is considered the main mediator of myogenic Igf actions, Akt activation decreased in regenerating EOMs, suggesting that alternative signaling pathways mediate Igf activity in muscle regeneration. In conclusion, Igf signaling is critical for re-differentiation of reprogrammed myoblasts during late steps of zebrafish EOM regeneration, suggesting a regulatory mechanism for determining regenerated muscle size and timing of differentiation, and a potential target for regenerative therapy.
Introduction
Loss of skeletal muscle mass, whether from degenerative disease, muscular dystrophy, denervation or trauma, is a major cause of morbidity and one of the top public health burdens [1]. In mammals, muscle injury leads to satellite cell activation and repair of focal injury, but de novo regeneration is not observed [2–4]. Degenerative muscle conditions result in atrophy, fibrosis and loss of muscle function [5]. Whether loss of muscle function is the result of severe muscle injury or degeneration, recovery of muscle function would require replacement of lost muscle tissue, i.e. de novo regeneration.
Our lab has discovered that in adult zebrafish, extraocular muscles (EOMs)–a subtype of skeletal muscle–can undergo de novo regeneration that is driven by myocyte reprogramming and dedifferentiation [6]. We have further characterized the early steps of EOM reprogramming, revealing important roles for epigenetic alterations, FGF signaling and autophagy in regulating proliferation by reprogrammed myoblasts [7–9].
In this work, we investigated the role of Igf signaling in zebrafish EOM regeneration. Igf family members are growth factors that play important roles in zebrafish fin [10] and heart [11] regeneration, myogenesis and muscle repair (particularly in birds and mammals) [12–16], and autophagy regulation [17–20]. Igf and Igf receptors are expressed in EOMs [21, 22] suggesting a role in EOM plasticity and force regulation. In fact, single IGF injection or sustained administration in rabbit [23], chicken [24, 25] and non-human primates [26] increased both EOM mass and force. Therefore, we hypothesized that it might also be important in EOM regeneration, and particularly in early reprogramming events.
Our data reveal that both pharmacologic and genetic inhibition of Igf signaling disrupt EOM regeneration. Interestingly, neither cell proliferation nor autophagy activation were affected by Igf signaling inhibition, indicating that it does not regulate myocyte reprogramming. Instead, histologic analysis and myosin staining of regenerating muscles indicate that Igf promotes later events in the regeneration process, i.e. myoblast terminal differentiation and fusion. We also discovered that the Akt pathway is not the target of Igf signaling in this process. We conclude that Igf signaling has an evolutionarily conserved role in the response of skeletal muscles to devastating injury, and that in zebrafish EOMs, Igf signaling is important for re-differentiation of the regenerating muscle.
Materials and methods
Zebrafish (Danio rerio) rearing and surgeries
All animal work was performed in compliance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research, and approved by the University of Michigan Committee on the Use and Care of Animals, protocol 06034. Sexually mature adult (4–18 month old) zebrafish were spawned in our fish facility and raised per standard protocol at 28°C with a 14-h light/10-h dark alternating cycle.
Adult zebrafish were anesthetized (0.05% Tricaine Methanosulfate) and approximately 50% of the right lateral rectus (LR) muscle was surgically excised, i.e. myectomy, as described [6]. The contralateral side (left side) was used as internal uninjured control. The remaining muscle following surgery (48.42% ± 4.9%, average ± S.D.) is represented in the graph figures as a gray area (i.e. a baseline for regeneration). Fish were euthanized using anesthesia overdose followed by decapitation, and the length of the regenerating muscle was quantified by craniectomy as described previously [6]. Regeneration is represented as the relative size of the injured (right) LR muscle normalized to the length of the uninjured (left) LR control muscle (representing 100%—immediately following myectomy, the ratio is approximately 50%). All experiments were performed using 5 fish per experimental group and/or time point, unless stated otherwise in the text and/or figure legend.
Drug treatments
BMS754807 (ChemieTek, Indianapolis, IN), an Igf1r kinase inhibitor [27], was dissolved in DMSO as a 10 mM stock and added to fish water to a final concentration of 5μM. To confirm the specificity of IGF signaling blockade, the unrelated inhibitor NVP-AEW541 (MedChem Express, Princeton, NJ), another structurally distinct Igf1r inhibitor [28], was dissolved in DMSO as a 10 mM stock and added to fish water to a final concentration of 5 μM. Up to 5 fish were treated in 1 liter of water, tanks were maintained at 28.5°C, and drug solutions were replaced every 24 h. Drug treatments were initiated immediately after surgery and no mortality was observed.
Morpholino oligonucleotide injection and electroporation
Microinjection of morpholino oligonucleotides (MOs; Gene-Tools, LLC, Philomath, OR), a widely used technique to perform knockdown experiments in adult zebrafish [29–31], was used. Briefly, lissamine-tagged MOs were directly microinjected into the right LR muscle followed by square-wave electroporation (6 to 10 pulses at 48 V/cm, BTX ECM830 electroporator; Harvard Apparatus, Holliston, MA). Microinjections were performed 3 hours prior to LR injury, and MO uptake was confirmed via lissamine fluorescence prior to myectomy. The MO sequence for igf1ra and igf1rb genes was previously validated in zebrafish [32, 33], and a standard MO targeting a human beta-globin intron mutation was used as control. Experiments were performed using 5 fish per experimental group, unless stated otherwise in the text and/or figure legend. No mortality was detected during the experimental process.
Specimen processing
Zebrafish heads were excised and fixed in 4% paraformaldehyde (PFA) overnight at 4°C. Decalcification was performed using either Morse’s solution. Fixed and decalcified tissues were cryopreserved with 20% sucrose in PBS, embedded in OCT (Fisher Scientific), frozen and evaluated microscopically using coronal frozen sections (12 μm). Paraffin sections (5 μm) were obtained at 5 or 7 dpi and H&E stained following standard techniques as described previously [6].
Autophagy assessment
Adult zebrafish were incubated in 500 nM LysoTracker Red DND-99 (L-7528, Thermo Fisher Scientific, Waltham, MA) for one h and then washed in fresh fish water (3 x 20 min). During the induction of autophagy the soluble form (LC3-I) is conjugated to a form (LC3-II) that accumulates in the autophagosomes [34]. Transgenic fish expressing GFP-tagged Lc3 (GFP-Lc3) [35] were used to visualize Lc3 after LR muscle myectomy.
Then fish were processed for craniectomy as described. LysoTracker Red staining and GFP-Lc3 accumulation were quantified measuring the fluorescence intensity of the area corresponding to the regenerating muscle in pictures taken from craniectomized fish with ImageJ software (http://rsbweb.nih.gov/ij/). Pictures were taken at the same time and with the same microscope settings to minimize experimental variability.
EdU incorporation assays
Cellular proliferation was assessed by intra-peritoneal (IP) injections of 5-ethynyl-2’-deoxyuridine (EdU) and standard detection methods [36]. Fish were anesthetized and injected with EdU (20 μl, 10 mM EdU in PBS) at 23 hours post injury (hpi) and sacrificed four hours later (27 hpi). For each experiment, 4 fish per group were analyzed. The injured muscle of each fish was analyzed and EdU+ or total (DAPI) nuclei were counted from, at least, 3 nonconsecutive sections per muscle, representing approximately 1958 total nuclei (range 1273–2449) per muscle. Cell proliferation is represented as the percentage of EdU+ nuclei in the injured muscle.
Immunolabeling
Myosin immunohistochemistry was performed as described [6]. Negative control experiments were performed in which either primary or secondary antibodies were omitted. Briefly, slides with coronal frozen sections (12 μm) were washed in PBS for 5 min and placed in blocking solution (5% goat serum in PBS + 0.2% Tween, PBST) for 30 min. Slides were incubated in a humidity chamber overnight at 4°C in primary antibody (mouse monoclonal anti-myosin heavy chain, F59, Developmental Studies Hybridoma Bank (DSHB), University of Iowa, Iowa City, Iowa) diluted to 1:50 in PBST+1% goat serum and washed again 4 times for 5 min in PBST. Then, slides were incubated in the dark with Alexafluor 647-conjugated goat anti-mouse secondary antibody (Invitrogen) diluted 1:1000 in PBST + 1% goat serum. After 3 5-min PBS washes, slides were coverslipped using ProLong Diamond Antifade Reagent with DAPI to stain the nuclei. Sections from 7 different fish per experiment were stained and imaged.
Western blots
Western blots were performed following standard protocols. Injured or control (uninjured) LR muscles from 10 to 15 fish were pooled and homogenized in lysis buffer containing protease (cOmplete, Roche Diagnostics Corporation, Indianapolis, IN) and phosphatase (PhosSTOP, Roche Diagnostics Corporation) inhibitors. The transgenic α actin-EGFP fish were used to visualize the muscles. Samples were sonicated and centrifuged at 10,000 g for 10 min at 4°C. Supernatant was collected and protein concentration determined using the Pierce™ BCA Protein Assay Kit (Thermo Scientific, Rockford, IL) and bovine serum albumin (BSA) as standard. Equal amounts of protein (20 μg) were loaded on 7.5% SDS polyacrylamide gels covered with a 3.9% stacking polyacrylamide gel and separated at 130 V for 1 h using Mini-Protean III (Bio-Rad, Hercules, CA). Proteins were then electroblotted onto PVDF membranes (Bio-Rad) by wet transfer (Mini Trans-Blot® Cell, Bior-rad) at 100 W for 1 h. Membranes were blocked for 1 h at room temperature with 5% BSA in TBST and incubated overnight at 4°C with primary antibody diluted in blocking solution. Anti-γ-tubulin antibody (1:10000, T5326) was obtained from Sigma-Aldrich, anti-Akt (1:1000, #9272) and anti-phospho-Akt (Ser473) (1:2000, #4060) were purchased from Cell Signaling Technology. Membranes were washed in TBST and incubated with IgG-horseradish peroxidase conjugate secondary antibody (1:10000, anti-mouse #7076 and anti-rabbit #7074 from Cell Signaling Technology) at room temperature for 1 h. Detection of signal was done using WesternBright ECL HRP substrate (advansta, Menlo Park, CA) and an Azure c500 Western Blot Imaging System (azure biosystems, Dublin, CA). Densitometric quantification of the bands was done with ImageJ software (http://rsbweb.nih.gov/ij/). The intensity of the protein of interest is normalized to the intensity of the tubulin band and represented in relative units (R.U.).
Statistics
For the time course experiment (Fig 1C), differences among time points for each fish group were analyzed by one-way analysis of variance (ANOVA) while differences between fish groups at each time point were analyzed by Student’s t-test. Thus, when more than 2 groups were compared, ANOVA (p < 0.05) followed by Newman-Keuls multiple comparisons test (p < 0.05) was performed. Comparisons between 2 groups were analyzed by Student’s t-test (*p < 0.05; **p < 0.01; ***p < 0.001). All tests were performed using the statistical software Prism 7.0a (GraphPad, LaJolla, CA, USA) for Mac OS X (Apple, Cupertino, CA, USA).
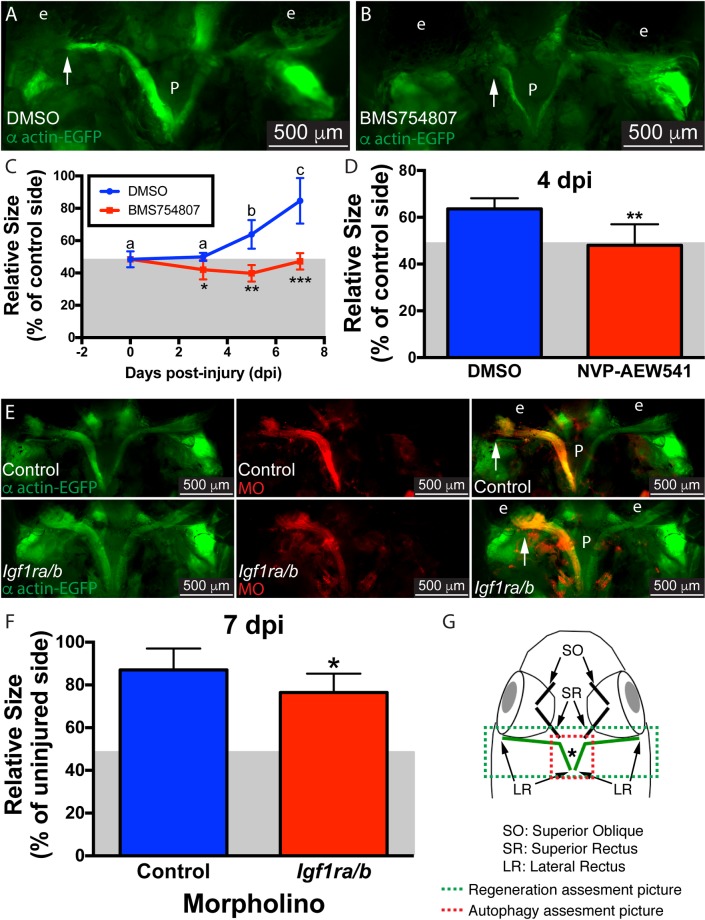
Fig 1. Inhibition of Igf1r impairs muscle regeneration.
Myectomized α-actin-EGFP fish treated with the Igf1r inhibitor BMS754807 (B) or DMSO (A) for 5 days. At selected time points (3, 5, and 7 dpi), the length of the regenerating muscle was measured as described (C), values are averages ± SD (n = 5–6). For each group (DMSO or BMS754807), differences among time points were analyzed by ANOVA. Different letters (lower case over DMSO group, there was no statistically significant difference for the BMS754807 group) indicate significant differences among time points (P < 0.05, Newman-Keuls multiple comparisons test). For each time point, differences between DMSO and BMS754807 treated fish were analyzed by Student’s t-test (*p < 0.05; **p < 0.01; ***p <0.001). To confirm our findings, α-actin-EGFP were treated with the unrelated NVP-AEW541 Igf1R inhibitor. At 4 dpi the regenerating muscle was measured as before showing similar results (D); values are averages ± SD (Student's t-test, **p < 0.01, n = 5). To knock down Igf1r, lissamine-tagged MOs (red) against both Igf1r paralogs (a and b) were microinjected into α-actin-EGFP (green) fish muscles prior myectomy. MOs were detected through the whole regenerating muscle, including the distal ends (arrowhead). Control MO (up) and Igfra/b MO (down) injected fish are shown (E). The length of the regenerating muscle was measured as described (F); values are averages ± SD (Student’s t-test, *p < 0.05, n = 10). Diagram of a craniectomized zebrafish head (G); muscles visualized by this technique are shown, and LR muscles are highlighted in green. Green and red boxes show approximately the picture used for regeneration or LysoTracker Red and GFP-Lc3 (shown in Fig 2) assessment, respectively. The white arrows mark the growing end of the regenerating muscle. P, pituitary; e, eye. Gray box in panels C, D and F represent the 50% muscle length as baseline following myectomy.
Results
Inhibition of Igf signaling impairs adult zebrafish muscle regeneration
Based on its known roles in muscle biology [12–16, 37–39], including EOMs [21–26], as well as in zebrafish heart and fin regeneration [10, 11], we decided to test the role of Igf signaling in the EOM regeneration of adult zebrafish. We used the drug BMS754807, selective inhibitor of Igf1r kinase [27] that has been shown to be effective in zebrafish [40–42]. Transgenic α-actin-EGFP fish were myectomized and daily treated with fresh BMS754807 for 3 days (Fig 1A and 1B). The measured length of the regenerating muscle was significantly lower in BMS754807 treated fish compared with control (Fig 1C—a gray area represents the residual muscle left after surgery [48.42 ± 4.9%, average ± S.D.] in the figures), revealing a role for Igf in EOM regeneration. To differentiate between a transient and a persistent action of Igf, we extended the drug treatments to 5 and 7 dpi. The length of the regenerating muscle was always lower in the treated fish (Fig 1C). The control fish (DMSO treated) showed a significant increase in muscle length over time while the treated fish did not show any significant muscle growth during the experimental period (Fig 1C), suggesting a key role of Igf in EOM regeneration. To confirm the specificity of the drug treatment, we utilized NVP-AEW541, an optimized Igf1r kinase inhibitor non-related to BMS754807 [28]. The lateral rectus of transgenic α-actin-EGFP fish was injured as before and fish were treated with NVP-AEW541 for 4 days. The regenerating muscle length was significantly lower in the NVP-AEW541 treated fish (Fig 1D), validating our previous results.
The key elements of Igf signaling are well conserved throughout evolution. However, due to the genome duplication in teleosts [43], two Igf1 receptor genes (igf1ra and igf1rb) have been described in zebrafish [44, 45]. To further test whether Igf signaling plays a role in muscle regeneration, Igf1ra/b expression was knocked down using previously described translation-blocking antisense MOs [32, 33], which were delivered via microinjection followed by electroporation prior to myectomy. LR muscle regeneration at 7 dpi was measured as before (Fig 1E) showing that knockdown of Igf1r expression effectively reduced LR muscle regeneration (Fig 1F).
Myocyte dedifferentiation is not regulated by Igf signaling
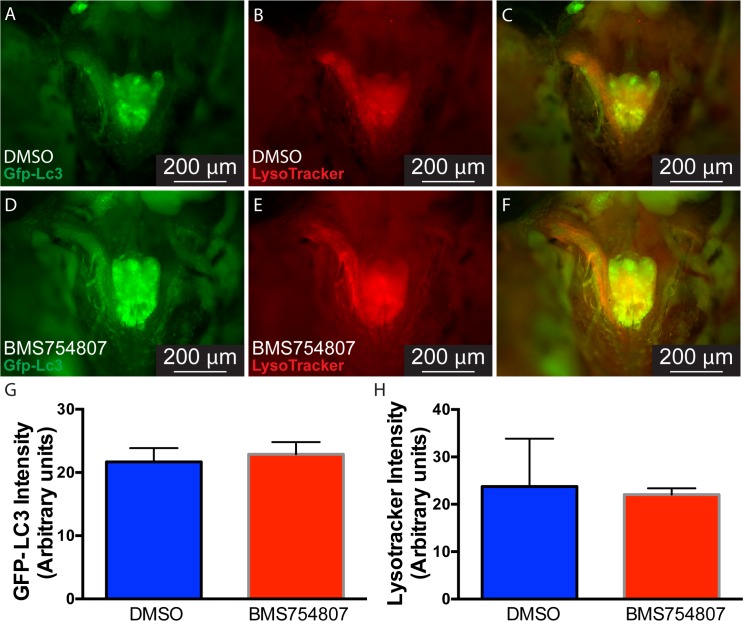
As previously determined, zebrafish EOMs activate autophagy after myectomy to degrade the sarcomeric contractile machinery and recycle specific myonuclei [7]. Map1lc3a/b is a protein ubiquitously distributed in the cytoplasm. After autophagy induction, the soluble form (Lc3-I) is conjugated to a form (Lc3-II) that localizes to the autophagosome membrane and accumulates within it [34]. LysoTracker Red, a vital dye that accumulates in autolysosomes and other acidic compartments, was used to stain transgenic GFP-Lc3 fish that were myectomyzed and treated with BMS754807 as before. Both GFP-Lc3 (Fig 2A and 2D) and LysoTracker Red (Fig 2B and 2E) clearly accumulated at 18 hpi in the injured muscle of BMS754807 treated and control (DMSO) fish, indicating autophagy activation. When GFP-Lc3 (Fig 2G) and LysoTracker Red (Fig 2H) fluorescence intensity was measured, no statistically significant differences were found indicating that, in EOM regeneration, autophagy activation is not under control of Igf signaling.
Fig 2. Inhibition of Igf signaling does not affect autophagy activation in the regenerating muscle.
GFP-LC3 (A and D) fish were myectomyzed and LysoTracker Red (B and E) was used to label autophagy in the regenerating LR in fish treated with DMSO (A-C) or BMS754807 (D-F). C and F show the merging of A-B and D-E, respectively. GFP-LC3 (G) and LysoTracker (H) fluorescence intensity of the regenerating muscle were measured and no statistically significant difference between DMSO and BMS754807 treated fish was found. Values represent average ± SD (Student’s t-test, significance set at P < 0.05, n = 5). P, pituitary.
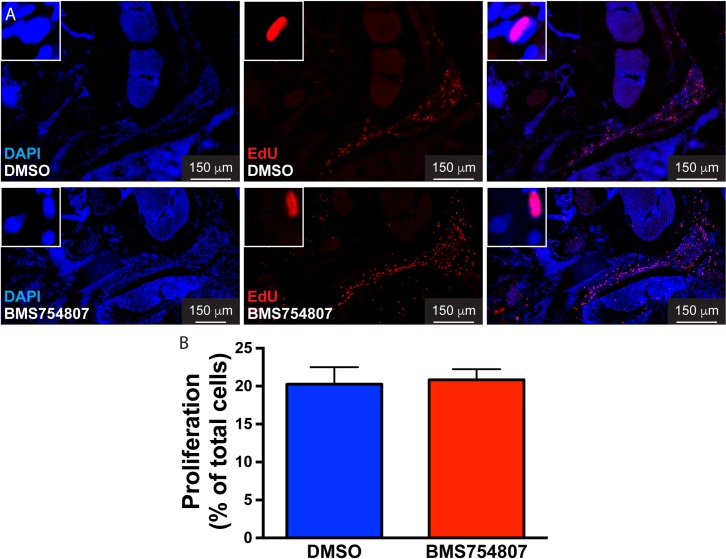
Myocyte dedifferentiation to myoblasts is required for the regeneration of adult zebrafish extraocular muscles. Myocyte dedifferentiation results in cell cycle reentry leading to a proliferative burst at 24–48 hpi. Then a gradual decline in proliferation occurs, followed by myoblast migration and myocyte re-differentiation [6]. Igf signaling could be important either in the early dedifferentiation steps leading to proliferation, and/or in the subsequent steps of migration and re-differentiation. In order to assess the role of Igf signaling in dedifferentiation, we used proliferation as readout. To label S-phase, zebrafish were treated with EdU at 23 hpi. EdU incorporation into dedifferentiated myocytes was assessed at 27 hpi. The percentage in the regenerating muscle of EdU positive cells was not affected by the treatment with the Igf1r inhibitor BMS754807 (Fig 3A and 3B). When combined, these observations suggest that myocyte reprogramming and dedifferentiation after injury is not regulated by Igf signaling.
Fig 3. Inhibition of Igf signaling does not modify cell proliferation in the regenerating muscle.
The role of Fgf in cell proliferation was assessed at 27 hours post-injury treating fish with DMSO or BMS754807 (A). DAPI, blue; EdU, red. Cell proliferation in the injured muscles from DMSO or BMS754807 treated fish was not statistically different (B). Values represent average ± SEM (Student’s t-test, significance set at P < 0.05, n = 4).
Inhibition of Igf signaling interferes with muscle re-differentiation
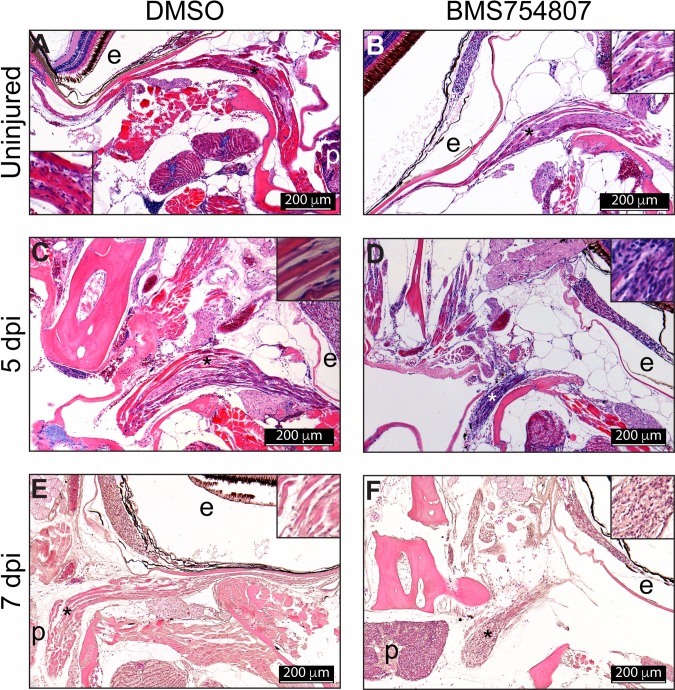
In the absence of data supporting a contribution of Igf signaling to the dedifferentiation of injured myocytes during EOM regeneration, we performed a histological analysis of the regenerating EOMs (Fig 4). Hematoxylin and eosin (H&E) staining was performed at 5 and 7 dpi in fish treated with the Igf1r inhibitor BMS754807. The analysis of control fish (DMSO treated fish) showed a progressive re-differentiation of the muscle after injury displaying the typical muscle fiber structure (Fig 4A and 4C). Contrarily, the regenerating muscle of BMS754807 treated fish did not show differentiated muscle fibers and there was a general hypercellularity (Fig 4B and 4D). Thus, the histological analysis of the regenerating EOMs suggests a role of IGF in re-differentiating injured muscles.
Fig 4. Histological analysis of the regenerating muscle.
Paraffin sections (5 μm) H&E staining of regenerating muscles from DMSO (A, C) and BMS754807 (B, D) treated fish. Sections of regenerating muscle at 5 (A, B) and 7 dpi (C, D). The asterisk marks the approximate position of the inset. Images are representative examples from 5 fish analyzed per treatment and time point. P, pituitary; e, eye. The S1 Fig shows a zebrafish coronal section diagram as a reference for the position of the pictures shown in this figure.
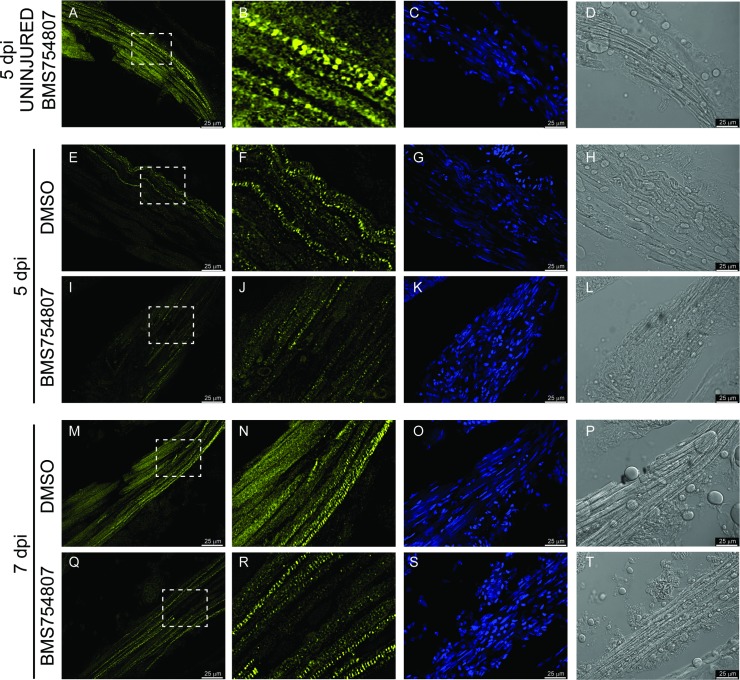
To further test for the impairment of re-differentiation in regenerating EOMs, zebrafish were treated with BMS754807 as before and stained for heavy chain myosin (MHC, Fig 5), a marker of differentiated muscle fibers [46, 47]. The injured muscle of DMSO treated control fish shows an increase in MHC staining from 5 dpi (Fig 5E and 5F) to 7dpi (Fig 5M and 5N). In fact, by 7 dpi, the MHC staining of a DMSO control fish (Fig 5Q and 5R) injured muscle is very similar to that of an uninjured muscle (Fig 5A and 5B). Conversely, the MHC staining of injured muscles of BMS754807 treated fish was clearly less intense both at 5 dpi (Fig 5I and 5J) and 7dpi (Fig 5Q and 5R) dpi, when compared to that of DMSO control fish, indicating a defect, or at least a delay, in muscle differentiation when Igf signaling was inhibited. Moreover, nuclear DAPI staining supports our previous observations of hypercellularity (Fig 5K and 5S compared to Fig 5G and 5O) and reveals the presence of elongated nuclei at 7 dpi, typical of muscle fibers[48, 49], only in DMSO treated fish (Fig 5O) but not in BMS754807 treated fish (Fig 5S). All combined, these results indicate that, when Igf signaling is inhibited, regenerating EOMs are not able to properly re-differentiate muscle fibers from reprogrammed and dedifferentiated myoblasts.
Fig 5. Myosin staining of the regenerating muscle.
The effect of Igf signaling inhibition on EOM regeneration was analyzed using myosin expression as a marker of muscle differentiation. Uninjured EOMs of BMS754807 treated fish showed high levels of myosin staining (A, B), that were not different of those of a DMSO treated control fish. Myosin staining (yellow) of DMSO control fish at 5 dpi (E, F) and 7 dpi (M, N) reveal higher protein levels than the myosin staining of BMS754807 treated fish at 5 dpi (I, J) and 7 dpi (Q, R). The dashed box shows the approximate position of the magnification shown in B, F, J, N and R. DAPI staining of the corresponding myosin staining picture (C, G, K, O and S) and DIC images (D, H, L, P and T) are also shown. DAPI staining shows hypercellularity in BMS754807 (K, S compared to C, G, O) and typical elongated muscle nuclei in DMSO 7 dpi (O) and Uninjured EOMs (C). Pictures are representative examples of 7 fish per group, time and treatment.
Role of Akt in zebrafish muscle regeneration
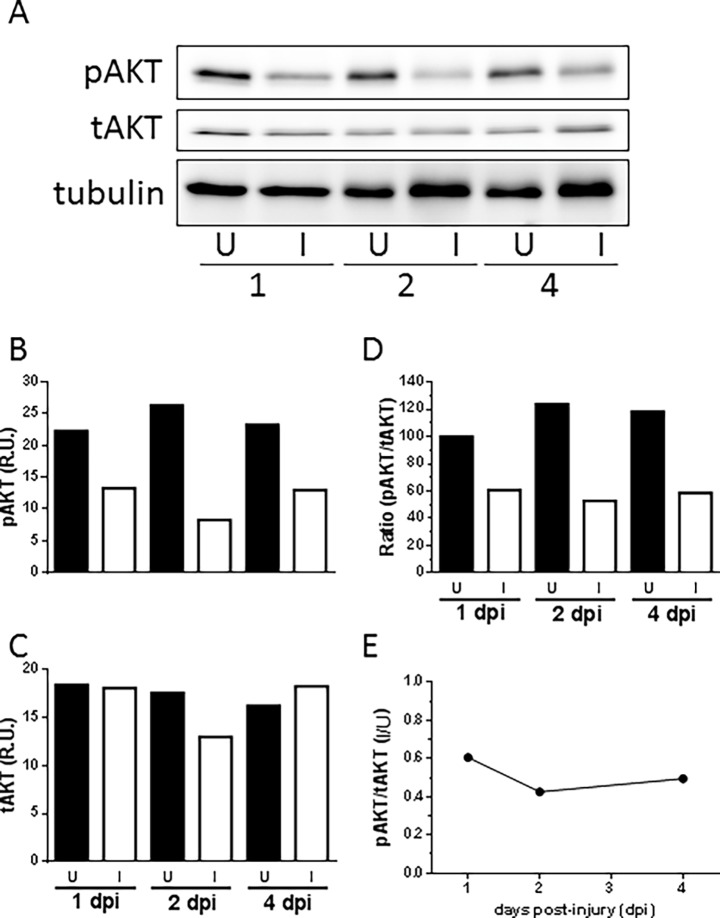
The kinase Akt, also known as PKB (protein kinase B), is considered the main mediator of IGF actions in muscle development and regeneration. Upon ligand binding, Igf1r phosphorylates insulin receptor substrate (IRS). Phosphorylated IRS activates phosphatidylinositol-3-kinase (PI3K) which generates phosphoinositide-3,4,5-triphosphate (PIP3). PIP3 acts as a docking site for two kinases, phosphoinositide-dependent kinase 1 (PDK1) and Akt. The phosphorylation of Akt by PDK1 leads to its activation [50]. To determine the role of this pathway in EOM regeneration, the time course of Akt activation in the injured muscle was examined by western blot (Fig 6). Both forms of Akt, phosphorylated (pAkt) and total (tAkt), could be detected at 1, 2 and 4 dpi in uninjured muscles by western blot (Fig 6A). Following myectomy, phosphorylated and total Akt were still detectable by western blot in the injured muscle at 1, 2 and 4 dpi (Fig 6A). However, the levels of phosphorylated (active) Akt were lower than in uninjured muscles at 1 and 2 dpi (Fig 6A), as revealed by the band intensity quantification of phosphorylated (active, Fig 6B) and total Akt (Fig 5C); and the ratio between them (Fig 5D). The levels of phosphorylated (active) Akt remained low in injured muscles even 4 days after myectomy (Fig 5A, 5B and 5D), when the injured muscle was already regenerating (Fig 1). These results suggest that the actions of Igf in zebrafish muscle regeneration are not mediated by Akt.
Fig 6. Role of Akt in the regenerating muscle.
The activation of Akt in injured muscles (non-BMS754807 treated fish) was assessed by western blot in a time course experiment (A). Immunoblotting was performed with anti-phosphorylated Akt antibody. Total amounts of Akt were monitored by reprobing membranes with anti-Akt antibody. Note that phosphorylated Akt (pAkt) was rapidly and persistently reduced in the injured muscle. Tubulin was used as a loading control. The densitometric quantification of the Akt bands is shown (B, C). The intensity of pAkt (B) and tAkt bands (C) was normalized to the tubulin content. The ratio between pAkt and tAkt was used to represent the fraction of active Akt (D). For comparative purposes, the pAkt/tAkt ratio of the injured muscle was divided by the pAkt/tAkt ratio of the uninjured muscle at each time point (E). U, uninjured muscle; I, injured muscle; R.U., relative units.
Discussion
This work focuses on the regulatory role of Igf in zebrafish extraocular muscle (EOM) regeneration. In adult zebrafish, EOM regeneration begins with myocyte dedifferentiation followed by proliferation and migration of reprogrammed myoblasts, and eventually redifferentiation into myocytes that fuse to form myofibers [6]. As expected, Igf signaling inhibition impaired muscle regeneration (Fig 1), supporting the described role of Igf in promoting zebrafish tissue regeneration [10, 11], EOM plasticity and force regulation [23–25] and muscle repair more generally [12–16, 37–39].
We identified autophagy as an essential early step of EOM regeneration[7]. Given the role of Igf signaling promoting [18] or inhibiting [17] autophagy, we tested whether Igf inhibition altered autophagy in EOM regeneration. Surprisingly, using both LysoTracker Red staining and GFP-Lc3 accumulation, we found that autophagy activation is Igf-independent (Fig 2). The most important early step of regeneration is cell cycle reentry by dedifferentiated myoblasts, leading to a proliferative burst [6]. Our results reveal that Igf signaling is dispensable for this early step as well (Fig 3). A histologic analysis of injured EOMs in which Igf signaling had been inhibited revealed hypercellularity and relative absence of differentiated muscle fibers even days following injury (Fig 4), suggesting defects in the late steps of EOM regeneration. Since Igf is known to play a role in myoblast terminal differentiation [51–53], we tested the hypothesis that Igf signaling is necessary for redifferentiation of proliferating myoblasts. Staining for myosin, a classical marker of muscle differentiation [46, 47], we discovered that indeed myocyte redifferentiation was inhibited or severely delayed, providing a mechanistic explanation for the observed delay in EOM regeneration.
Since Akt is considered a primary mediator of Igf signaling in myogenesis [50], we tested for the presence of phospho-Akt in injured EOMs (untreated). Interestingly, phospho-Akt levels decreased after injury (Fig 6), making it extremely unlikely that Akt mediates Igf actions in adult de novo EOM regeneration. Interestingly, in old-age transgenic mice expressing Igf-1, muscle reparative capacities were similar to those of young mice [54] but with no effect on Akt activation [55]. Therefore, Igf signaling in the context of adult skeletal muscle regeneration must function through a different pathway in both mice and zebrafish. The mitogen-activated protein kinase/extracellular signal-regulated receptor kinase (MAPK/ERK) signaling pathway was reported as an alternative mediator of Igf actions in rat muscle [56–58]. We had previously described that Erk2 was selectively activated during the early steps of zebrafish EOM regeneration [8], but the data reported in the present manuscript reveal a late role for Igf signaling. Therefore, it is unlikely that the Erk pathway mediates Igf actions in EOM regeneration. These results suggest the existence of a yet-to-be-identified alternate pathway for Igf signaling in adult muscle repair and regeneration.
In conclusion, using an in vivo model of zebrafish EOMs, we report that Igf signaling is important for the redifferentiating steps of muscle regeneration, and that this role may be shared with muscle reparative pathways in adult mammals. Despite the obvious differences among the models, studying adult zebrafish EOM regeneration may facilitate a mechanistic understanding of a skeletal muscle’s response to injury and highlight opportunities for novel therapeutic development. Furthermore, understanding the biological mechanisms underlying early de-differentiation vs. late re-differentiation of injured adult tissues enhance our understanding of regenerative pathways in general, irrespective of tissue type.
Supporting information
Diagrams of coronal zebrafish head sections from DMSO (A, C) and BMS754807 (B, D) treated fish. Sections of regenerating muscle at 5 (A, B) and 7 dpi (C, D). Dashed box shows the approximate location of the picture shown in Fig 4. For reference, the asterisk is approximately located in the same position than in Fig 4. Approximate position of the sectioning plane (G).
(TIF)
Acknowledgments
We thank Amy Stevenson, Amy Robbins, Alyssa Benson, Joseph Barden and Chitra Parthasarathy for their expertise in zebrafish husbandry.
Data Availability
All relevant data are within the paper.
Funding Statement
This study was supported by the Alliance for Vision Research, a Physician-Scientist Award from Research to Prevent Blindness, Inc. (RPB), grant R01 EY022633 from the NEI of the NIH (AK), and an unrestricted grant from RPB to the Department of Ophthalmology and Visual Sciences. This research utilized the Vision Research Core (P30 EY007003), the Cancer Center Research Core (P30 CA046592), and the Michigan Diabetes Research Center (P30 DK020572) at the University of Michigan. AK is supported by the Mrs. William Davidson Emerging Scholar Award from the A. Alfred Taubman Medical Research Institute. The Zebrafish International Resource Center is supported by grant P40 RR012546 from the NIH NCRR. Funders had no role in study design, data collection and analysis, decision to publish, or manuscript preparation.
References
- 1.Larkindale J, Yang W, Hogan PF, Simon CJ, Zhang Y, Jain A, et al. Cost of illness for neuromuscular diseases in the United States. Muscle Nerve. 2014;49(3):431–8. doi: 10.1002/mus.23942 . [DOI] [PubMed] [Google Scholar]
- 2.Zammit PS, Relaix F, Nagata Y, Ruiz AP, Collins CA, Partridge TA, et al. Pax7 and myogenic progression in skeletal muscle satellite cells. J Cell Sci. 2006;119(Pt 9):1824–32. doi: 10.1242/jcs.02908 . [DOI] [PubMed] [Google Scholar]
- 3.Yablonka-Reuveni Z, Rivera AJ. Temporal expression of regulatory and structural muscle proteins during myogenesis of satellite cells on isolated adult rat fibers. Dev Biol. 1994;164(2):588–603. doi: 10.1006/dbio.1994.1226 ; PubMed Central PMCID: PMCPMC4128087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yablonka-Reuveni Z, Day K, Vine A, Shefer G. Defining the transcriptional signature of skeletal muscle stem cells. J Anim Sci. 2008;86(14 Suppl):E207–16. doi: 10.2527/jas.2007-0473 ; PubMed Central PMCID: PMCPMC4450102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wallace GQ, McNally EM. Mechanisms of muscle degeneration, regeneration, and repair in the muscular dystrophies. Annu Rev Physiol. 2009;71:37–57. doi: 10.1146/annurev.physiol.010908.163216 . [DOI] [PubMed] [Google Scholar]
- 6.Saera-Vila A, Kasprick DS, Junttila TL, Grzegorski SJ, Louie KW, Chiari EF, et al. Myocyte Dedifferentiation Drives Extraocular Muscle Regeneration in Adult Zebrafish. Invest Ophth Vis Sci. 2015;56(8):4977–93. PubMed PMID: WOS:000362882700095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saera-Vila A, Kish PE, Louie KW, Grzegorski SJ, Klionsky DJ, Kahana A. Autophagy Regulates Cytoplasmic Remodeling During Cell Reprogramming in a Zebrafish Model of Muscle Regeneration. Autophagy. 2016;12(10):1864–75. doi: 10.1080/15548627.2016.1207015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saera-Vila A, Kish PE, Kahana A. Fgf regulates dedifferentiation during skeletal muscle regeneration in adult zebrafish. Cell Signal. 2016;28(9):1196–204. PubMed PMID: WOS:000380595500007. doi: 10.1016/j.cellsig.2016.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Louie KW, Saera-Vila A, Kish PE, Colacino JA, Kahana A. Temporally distinct transcriptional regulation of myocyte dedifferentiation and Myofiber growth during muscle regeneration. BMC Genomics. 2017;18(1):854 doi: 10.1186/s12864-017-4236-y . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chablais F, Jazwinska A. IGF signaling between blastema and wound epidermis is required for fin regeneration. Development. 2010;137(6):871–9. PubMed PMID: WOS:000274887300003. doi: 10.1242/dev.043885 [DOI] [PubMed] [Google Scholar]
- 11.Huang Y, Harrison MR, Osorio A, Kim J, Baugh A, Duan CM, et al. Igf Signaling Is Required for Cardiomyocyte Proliferation during Zebrafish Heart Development and Regeneration. Plos One. 2013;8(6). PubMed PMID: WOS:000321424400088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coleman ME, Demayo F, Yin KC, Lee HM, Geske R, Montgomery C, et al. Myogenic Vector Expression of Insulin-Like Growth-Factor-I Stimulates Muscle-Cell Differentiation and Myofiber Hypertrophy in Transgenic Mice. J Biol Chem. 1995;270(20):12109–16. PubMed PMID: WOS:A1995QY73600062. [DOI] [PubMed] [Google Scholar]
- 13.Engert JC, Berglund EB, Rosenthal N. Proliferation precedes differentiation in IGF-I-stimulated myogenesis. J Cell Biol. 1996;135(2):431–40. PubMed PMID: WOS:A1996VP22900011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Florini JR, Ewton DZ, Roof SL. Insulin-Like Growth Factor-I Stimulates Terminal Myogenic Differentiation by Induction of Myogenin Gene-Expression. Mol Endocrinol. 1991;5(5):718–24. PubMed PMID: WOS:A1991FM56700014. doi: 10.1210/mend-5-5-718 [DOI] [PubMed] [Google Scholar]
- 15.Vandenburgh HH, Karlisch P, Shansky J, Feldstein R. Insulin and Igf-I Induce Pronounced Hypertrophy of Skeletal Myofibers in Tissue-Culture. Am J Physiol. 1991;260(3):C475–C84. PubMed PMID: WOS:A1991FC25000012. [DOI] [PubMed] [Google Scholar]
- 16.Tollefsen SE, Lajara R, McCusker RH, Clemmons DR, Rotwein P. Insulin-like growth factors (IGF) in muscle development. Expression of IGF-I, the IGF-I receptor, and an IGF binding protein during myoblast differentiation. J Biol Chem. 1989;264(23):13810–7. . [PubMed] [Google Scholar]
- 17.Jia GH, Cheng G, Gangahar DM, Agrawal DK. Insulin-like growth factor-1 and TNF-alpha regulate autophagy through c-jun N-terminal kinase and Akt pathways in human atherosclerotic vascular smooth cells. Immunol Cell Biol. 2006;84(5):448–54. PubMed PMID: WOS:000240005500005. doi: 10.1111/j.1440-1711.2006.01454.x [DOI] [PubMed] [Google Scholar]
- 18.Renna M, Bento CF, Fleming A, Menzies FM, Siddiqi FH, Ravikumar B, et al. IGF-1 receptor antagonism inhibits autophagy. Hum Mol Genet. 2013;22(22):4528–44. PubMed PMID: WOS:000326675300007. doi: 10.1093/hmg/ddt300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Troncoso R, Diaz-Elizondo J, Espinoza SP, Navarro-Marquez MF, Oyarzun AP, Riquelme JA, et al. Regulation of cardiac autophagy by insulin-like growth factor 1. IUBMB Life. 2013;65(7):593–601. doi: 10.1002/iub.1172 . [DOI] [PubMed] [Google Scholar]
- 20.Bains M, Florez-McClure ML, Heidenreich KA. Insulin-like growth factor-I prevents the accumulation of autophagic vesicles and cell death in Purkinje neurons by increasing the rate of autophagosome-to-lysosome fusion and degradation. J Biol Chem. 2009;284(30):20398–407. doi: 10.1074/jbc.M109.011791 ; PubMed Central PMCID: PMCPMC2740464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Feng CY, von Bartheld CS. Expression of insulin-like growth factor 1 isoforms in the rabbit oculomotor system. Growth Horm IGF Res. 2011;21(4):228–32. doi: 10.1016/j.ghir.2011.06.001 ; PubMed Central PMCID: PMCPMC3140565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fischer MD, Gorospe JR, Felder E, Bogdanovich S, Pedrosa-Domellof F, Ahima RS, et al. Expression profiling reveals metabolic and structural components of extraocular muscles. Physiol Genomics. 2002;9(2):71–84. doi: 10.1152/physiolgenomics.00115.2001 . [DOI] [PubMed] [Google Scholar]
- 23.McLoon LK, Christiansen SP. Increasing extraocular muscle strength with insulin-like growth factor II. Invest Ophthalmol Vis Sci. 2003;44(9):3866–72. . [DOI] [PubMed] [Google Scholar]
- 24.Chen J, von Bartheld CS. Role of exogenous and endogenous trophic factors in the regulation of extraocular muscle strength during development. Invest Ophthalmol Vis Sci. 2004;45(10):3538–45. doi: 10.1167/iovs.04-0393 . [DOI] [PubMed] [Google Scholar]
- 25.Li T, Wiggins LM, von Bartheld CS. Insulin-like growth factor-1 and cardiotrophin 1 increase strength and mass of extraocular muscle in juvenile chicken. Invest Ophthalmol Vis Sci. 2010;51(5):2479–86. doi: 10.1167/iovs.09-4414 ; PubMed Central PMCID: PMCPMC2868486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Willoughby CL, Christiansen SP, Mustari MJ, McLoon LK. Effects of the sustained release of IGF-1 on extraocular muscle of the infant non-human primate: adaptations at the effector organ level. Invest Ophthalmol Vis Sci. 2012;53(1):68–75. doi: 10.1167/iovs.11-8356 ; PubMed Central PMCID: PMCPMC3292383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wittman MD, Carboni JM, Yang Z, Lee FY, Antman M, Attar R, et al. Discovery of a 2,4-disubstituted pyrrolo[1,2-f][1,2,4]triazine inhibitor (BMS-754807) of insulin-like growth factor receptor (IGF-1R) kinase in clinical development. J Med Chem. 2009;52(23):7360–3. doi: 10.1021/jm900786r . [DOI] [PubMed] [Google Scholar]
- 28.Garcia-Echeverria C, Pearson MA, Marti A, Meyer T, Mestan J, Zimmermann J, et al. In vivo antitumor activity of NVP-AEW541-A novel, potent, and selective inhibitor of the IGF-IR kinase. Cancer Cell. 2004;5(3):231–9. . [DOI] [PubMed] [Google Scholar]
- 29.Craig SEL, Thummel R, Ahmed H, Vasta GR, Hyde DR, Hitchcock PF. The Zebrafish Galectin Drgal1-L2 Is Expressed by Proliferating Muller Glia and Photoreceptor Progenitors and Regulates the Regeneration of Rod Photoreceptors. Invest Ophth Vis Sci. 2010;51(6):3244–52. PubMed PMID: WOS:000277846500056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ramachandran R, Fausett BV, Goldman D. Ascl1a regulates Muller glia dedifferentiation and retinal regeneration through a Lin-28-dependent, let-7 microRNA signalling pathway. Nat Cell Biol. 2010;12(11):1101–U106. PubMed PMID: WOS:000283711500014. doi: 10.1038/ncb2115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thummel R, Bai S, Sarras MP, Song PZ, McDermott J, Brewer J, et al. Inhibition of zebrafish fin regeneration using in vivo electroporation of morpholinos against fgfr1 and msxb. Dev Dynam. 2006;235(2):336–46. PubMed PMID: WOS:000234672400005. [DOI] [PubMed] [Google Scholar]
- 32.Bohnsack BL, Gallina D, Kahana A. Phenothiourea Sensitizes Zebrafish Cranial Neural Crest and Extraocular Muscle Development to Changes in Retinoic Acid and IGF Signaling. Plos One. 2011;6(8). PubMed PMID: WOS:000294128100004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schlueter PJ, Royer T, Farah MH, Laser B, Chan SJ, Steiner DF, et al. Gene duplication and functional divergence of the zebrafish insulin-like growth factor 1 receptors. Faseb J. 2006;20(8):1230–2. PubMed PMID: WOS:000240210300035. doi: 10.1096/fj.05-3882fje [DOI] [PubMed] [Google Scholar]
- 34.Tanida I, Ueno T, Kominami E. LC3 and Autophagy. Methods Mol Biol. 2008;445:77–88. doi: 10.1007/978-1-59745-157-4_4 . [DOI] [PubMed] [Google Scholar]
- 35.He CC, Bartholomew CR, Zhou WB, Klionsky DJ. Assaying autophagic activity in transgenic GFP-Lc3 and GFP-Gabarap zebrafish embryos. Autophagy. 2009;5(4):520–6. PubMed PMID: WOS:000266118900010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Salic A, Mitchison TJ. A chemical method for fast and sensitive detection of DNA synthesis in vivo. P Natl Acad Sci USA. 2008;105(7):2415–20. PubMed PMID: WOS:000253469900032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Edwall D, Schalling M, Jennische E, Norstedt G. Induction of insulin-like growth factor I messenger ribonucleic acid during regeneration of rat skeletal muscle. Endocrinology. 1989;124(2):820–5. doi: 10.1210/endo-124-2-820 . [DOI] [PubMed] [Google Scholar]
- 38.Krishan K, Dhoot GK. Changes in some troponin and insulin-like growth factor messenger ribonucleic acids in regenerating and denervated skeletal muscles. J Muscle Res Cell Motil. 1996;17(5):513–21. . [DOI] [PubMed] [Google Scholar]
- 39.Levinovitz A, Jennische E, Oldfors A, Edwall D, Norstedt G. Activation of insulin-like growth factor II expression during skeletal muscle regeneration in the rat: correlation with myotube formation. Mol Endocrinol. 1992;6(8):1227–34. doi: 10.1210/mend.6.8.1406701 . [DOI] [PubMed] [Google Scholar]
- 40.Onuma TA, Ding YH, Abraham E, Zohar Y, Ando H, Duan C. Regulation of Temporal and Spatial Organization of Newborn GnRH Neurons by IGF Signaling in Zebrafish. J Neurosci. 2011;31(33):11814–24. PubMed PMID: WOS:000293950300009. doi: 10.1523/JNEUROSCI.6804-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kamei H, Ding YH, Kajimura S, Wells M, Chiang P, Duan CM. Role of IGF signaling in catch-up growth and accelerated temporal development in zebrafish embryos in response to oxygen availability. Development. 2011;138(4):777–86. PubMed PMID: WOS:000286590400019. doi: 10.1242/dev.056853 [DOI] [PubMed] [Google Scholar]
- 42.Dai W, Bai Y, Hebda L, Zhong X, Liu J, Kao J, et al. Calcium deficiency-induced and TRP channel-regulated IGF1R-PI3K-Akt signaling regulates abnormal epithelial cell proliferation. Cell Death Differ. 2014;21(4):568–81. PubMed PMID: WOS:000332784800007. doi: 10.1038/cdd.2013.177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Volff JN. Genome evolution and biodiversity in teleost fish. Heredity (Edinb). 2005;94(3):280–94. doi: 10.1038/sj.hdy.6800635 . [DOI] [PubMed] [Google Scholar]
- 44.Maures T, Chan SJ, Xu B, Sun H, Ding J, Duan C. Structural, biochemical, and expression analysis of two distinct insulin-like growth factor I receptors and their ligands in zebrafish. Endocrinology. 2002;143(5):1858–71. doi: 10.1210/endo.143.5.8768 . [DOI] [PubMed] [Google Scholar]
- 45.Ayaso E, Nolan CM, Byrnes L. Zebrafish insulin-like growth factor-I receptor: molecular cloning and developmental expression. Mol Cell Endocrinol. 2002;191(2):137–48. . [DOI] [PubMed] [Google Scholar]
- 46.Diehl AG, Zareparsi S, Qian M, Khanna R, Angeles R, Gage PJ. Extraocular muscle morphogenesis and gene expression are regulated by Pitx2 gene dose. Invest Ophthalmol Vis Sci. 2006;47(5):1785–93. doi: 10.1167/iovs.05-1424 . [DOI] [PubMed] [Google Scholar]
- 47.Doherty JT, Lenhart KC, Cameron MV, Mack CP, Conlon FL, Taylor JM. Skeletal muscle differentiation and fusion are regulated by the BAR-containing Rho-GTPase-activating protein (Rho-GAP), GRAF1. J Biol Chem. 2011;286(29):25903–21. doi: 10.1074/jbc.M111.243030 ; PubMed Central PMCID: PMCPMC3138304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ovalle WK, Nahirney PC. Netter's Essential Histology. 1st ed: Elsevier; 2007 18th September 2007. [Google Scholar]
- 49.Berridge BR, Van Vleet JF, Herman E. Cardiac, Vascular, and Skeletal Muscle Systems In: Haschek WM, Rousseaux CG, Wallig MA, Bolon B, Ochoa R, editors. Haschek and Rousseaux's Handbook of Toxicologic Pathology (Third Edition). III: Academic Press; 2013. p. 1567–665. [Google Scholar]
- 50.Schiaffino S, Mammucari C. Regulation of skeletal muscle growth by the IGF1-Akt/PKB pathway: insights from genetic models. Skelet Muscle. 2011;1(1):4 doi: 10.1186/2044-5040-1-4 ; PubMed Central PMCID: PMCPMC3143906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Allen RE, Boxhorn LK. Regulation of skeletal muscle satellite cell proliferation and differentiation by transforming growth factor-beta, insulin-like growth factor I, and fibroblast growth factor. J Cell Physiol. 1989;138(2):311–5. doi: 10.1002/jcp.1041380213 . [DOI] [PubMed] [Google Scholar]
- 52.Greene EA, Allen RE. Growth factor regulation of bovine satellite cell growth in vitro. J Anim Sci. 1991;69(1):146–52. . [DOI] [PubMed] [Google Scholar]
- 53.McFarland DC, Pesall JE, Gilkerson KK. The influence of growth factors on turkey embryonic myoblasts and satellite cells in vitro. Gen Comp Endocrinol. 1993;89(3):415–24. doi: 10.1006/gcen.1993.1049 . [DOI] [PubMed] [Google Scholar]
- 54.Musaro A, McCullagh K, Paul A, Houghton L, Dobrowolny G, Molinaro M, et al. Localized Igf-1 transgene expression sustains hypertrophy and regeneration in senescent skeletal muscle. Nat Genet. 2001;27(2):195–200. doi: 10.1038/84839 . [DOI] [PubMed] [Google Scholar]
- 55.Barton ER, Morris L, Musaro A, Rosenthal N, Sweeney HL. Muscle-specific expression of insulin-like growth factor I counters muscle decline in mdx mice. J Cell Biol. 2002;157(1):137–48. doi: 10.1083/jcb.200108071 ; PubMed Central PMCID: PMCPMC2173262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Haddad F, Adams GR. Inhibition of MAP/ERK kinase prevents IGF-I-induced hypertrophy in rat muscles. J Appl Physiol (1985). 2004;96(1):203–10. doi: 10.1152/japplphysiol.00856.2003 . [DOI] [PubMed] [Google Scholar]
- 57.Shi H, Scheffler JM, Zeng C, Pleitner JM, Hannon KM, Grant AL, et al. Mitogen-activated protein kinase signaling is necessary for the maintenance of skeletal muscle mass. Am J Physiol Cell Physiol. 2009;296(5):C1040–8. doi: 10.1152/ajpcell.00475.2008 . [DOI] [PubMed] [Google Scholar]
- 58.Murgia M, Serrano AL, Calabria E, Pallafacchina G, Lomo T, Schiaffino S. Ras is involved in nerve-activity-dependent regulation of muscle genes. Nat Cell Biol. 2000;2(3):142–7. doi: 10.1038/35004013 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Diagrams of coronal zebrafish head sections from DMSO (A, C) and BMS754807 (B, D) treated fish. Sections of regenerating muscle at 5 (A, B) and 7 dpi (C, D). Dashed box shows the approximate location of the picture shown in Fig 4. For reference, the asterisk is approximately located in the same position than in Fig 4. Approximate position of the sectioning plane (G).
(TIF)
Data Availability Statement
All relevant data are within the paper.