Abstract
Purpose: We determined the correlation between the Fitbit One and Actical accelerometer for quantifying the 3-day step count and activity levels in community-dwelling individuals with stroke. Method: Twelve participants with a mean age of 62.6 (SD 9.3) years wore both the Fitbit One and the Actical on the non-paretic ankle for 3 days. Regression analyses were performed to examine concurrent validity between the devices for step counts and sedentary, light, moderate, and vigorous activity. The relative error of the Fitbit One compared with the Actical in measuring step count was calculated. Results: Participants spent about 80% of their days being sedentary. The associations between the Fitbit One and the Actical were r>0.80 for step count and light-intensity activity across the 3 days of free-living activity. The overall relative error in measuring step count was 3.8%, with differences between those with walking speeds of less than 0.58 metres per second and 0.58 metres per second or more (27.4% [SD 34.2] vs. –8.0% [SD 10.7], p<0.001). Conclusions: The Fitbit One was associated with the Actical accelerometer in measuring step count and light-intensity activity during free-living activity after stroke, but had lower error in capturing step count for those with faster walking speeds. The Fitbit One may not be valid for capturing higher intensity activity after stroke.
Key Words: exercise, fitness trackers, outcome assessment, rehabilitation, stroke
Abstract
Objectif : sur une période de trois jours, déterminer la corrélation entre le capteur d'activité Fitbit One et l'accéléromètre Actical pour quantifier le nombre de pas et le niveau d'activité de personnes ayant subi un accident vasculaire cérébral (AVC) et qui sont revenues en milieu ambulatoire. Méthodologie : au total, 12 participants, d'un âge moyen de 62,6 ans (ÉT 9,3), ont porté à la fois le Fitbit One et l'Actical sur leur cheville non parétique pendant trois jours. Les chercheurs ont effectué des analyses de régression pour examiner la validité convergente entre les appareils pour calculer le nombre de pas et l'activité sédentaire, légère, modérée et vigoureuse. Ils ont mesuré l'erreur relative du Fitbit One pour calculer le nombre de pas par rapport à celle de l'Actical. Résultats : Les participants consacraient environ 80 % de leur journée à des activités sédentaires. L'association entre le Fitbit One et l'Actical étaient de r>0,80 pour le calcul du nombre de pas et l'activité de faible intensité au cours des trois jours d'activités libres. L'erreur relative globale pour le calcul du nombre de pas s'élevait à 3,8 % et comportait des différences entre les personnes qui marchaient à une vitesse inférieure à 0,58 mètre par seconde et celles qui marchaient à au moins 0,58 mètre par seconde (27,4 [ÉT 34,2 %] par rapport à −8,0 [ÉT 10,7 %], p<0,001). Conclusions : Les chercheurs ont associé le capteur d'activité Fitbit One à l'accéléromètre Actical pour calculer le nombre de pas et l'activité d'intensité légère dans le cadre des activités libres après un AVC, mais le taux d'erreur dans le calcul du nombre de pas était plus faible chez les personnes qui marchaient plus vite. Le Fitbit One n'est peut-être pas valide pour capter les activités d'intensité plus élevée après un AVC.
Mots clés : accident vasculaire cérébral, évaluation des résultats cliniques, moniteur d'activité physique, réadaptation
Recently published data have projected that more individuals are living with the effects of stroke than previously estimated1 and that residual neurological deficits can contribute to low physical activity.2 Physical fitness interventions have been shown to improve walking ability,3 and published scientific statements have recommended more than 150 minutes of moderate- to vigorous-intensity activity per week.4 Yet despite the evidence and recommendations, individuals with stroke are the least active group compared with those with other chronic conditions.5
In physical activity research, the time engaged in activity has commonly been quantified using accelerometers that track acceleration along multiple axes when worn near the body's centre of mass.6 The Actical accelerometer (Philips Respironics, Baltimore, MD) has been used in research settings to provide data that can be integrated with time to calculate velocity and displacement information and thereby quantify physical activity and the time spent at varying levels of intensity (light, moderate, vigorous).7
However, accelerometers may not accurately detect accelerations in individuals with stroke, who commonly walk at slower speeds. A few studies have examined whether placing such devices on alternative parts of the body may enhance their accuracy when used with people with stroke. Accelerometers worn on the paretic or non-paretic hip reliably quantified activity counts and energy expenditure,8 but sensitivity was potentially further improved when the devices were worn at distal limb segments (e.g., the ankle), where higher accelerations occurred with slower walking speeds.9 These findings are important because if physical therapists can accurately capture physical activity in their clients with stroke, they can make informed and individualized recommendations to meet targets for physical activity.
Mounting evidence has suggested that capturing sedentary time, not just active time, is also important. Individuals with stroke spend approximately 81% of their day being sedentary, compared with 71% for healthy, age-matched adults.10 Sedentary behaviour represents more than simply the absence of activity; it in fact encompasses a unique set of behaviours (sitting time accumulated through office work, watching television, driving) and health consequences that have garnered attention in recent years.11,12
Until recently, sophisticated accelerometry-sensor technologies have not been widely adopted outside the research realm, in part because of their relatively high cost, complex software, and less user-friendly interface; these factors have limited the options available to physical therapists and stroke survivors for objectively measuring free-living physical activity patterns. However, activity trackers, such as the Fitbit One (Fitbit Inc., San Francisco, CA), have now penetrated the consumer market and may be more user-friendly and cost-effective alternatives.
Among healthy younger adults who wore the device as recommended on the hip, the Fitbit One was accurate and reliable for measuring step count during a range of treadmill walking speeds (0.84–1.78 m/sec).13,14 More recently, exploratory work has examined whether device placement affects the accuracy of capturing step count in individuals with compromised walking ability. In a study involving 42 adults older than age 65 years,15 the relative error in step count between the Fitbit One and videographic footage was compared across slow walking speeds (0.3–0.9 m/sec) and between placement location (hip vs. ankle). The relative error was consistently less than 10% when the Fitbit One was worn at the ankle but was low only at the faster walking speeds when worn at the hip.15 Similarly, an ankle-worn Fitbit One was accurate in capturing step count in 43 people with stroke at walking speeds as slow as 0.4 metres per second.16
Both of these studies were conducted in controlled laboratory settings, and walking speed was externally paced by metronome and visual markings on the floor. No study has yet evaluated the validity of the Fitbit One for quantifying levels of physical activity or sedentary time during free-living activity in individuals with stroke.
The purpose of this study was to (1) determine the correlation between the Fitbit One and Actical accelerometer for quantifying 3-day step count and time spent being sedentary and engaged in light, moderate, and vigorous activity after stroke and (2) examine the factors associated with the relative error between these devices. We hypothesized that there would be strong correlations (r>0.80) between the devices in quantifying step count and time spent at various levels of activity.
Methods
This study was part of a larger randomized controlled trial examining the effectiveness of a community stroke exercise programme (Hamilton Integrated Research Ethics Board 14–388, approved June 2014). All participants provided informed, written consent.
Participants
Participants with stroke were eligible for the larger study if they were aged at least 18 years, not actively engaged in rehabilitation, able to walk 10 metres or more independently with or without the use of an assistive device, able to follow instructions, able to be active for 60 minutes or more with rest breaks, and medically cleared to exercise.
Devices
The Actical is a small (29×37×11 mm), lightweight (16 g), omnidirectional accelerometer that measures activity counts using vibrations. These vibrations are converted into step counts and time spent at four activity levels, defined using metabolic equivalents (METS): sedentary (1 MET), light (<3 METS), moderate (3–6 METS), and vigorous (>6 METS).7 We used 15-second epochs (time intervals), as had been done previously to measure free-living physical activity after stroke.8
The Fitbit One is a small (48×19×10 mm), lightweight (8 g) triaxial accelerometer that uses proprietary algorithms to count steps, distance travelled, floors climbed, and time spent at four activity levels (sedentary, lightly active, fairly active, very active).14 Specific information regarding the thresholds for activity intensity (e.g., METS) was unavailable from the device manufacturer or support staff. Activity was collected in 60-second epochs. A previous study had validated accelerometer-based step count using 15-second epochs against the Fitbit One's 60-second epoch in older adults.17
Testing procedure
The participants' characteristics were recorded, including their age, sex, BMI, amount of time post-stroke, stroke severity (using the National Institutes of Health Stroke Scale [NIHSS]18), motor impairment (using the Chedoke-McMaster Stroke Assessment Impairment Inventory19), cognitive ability (using the Montreal Cognitive Assessment [MoCA]),20 and self-reported physical activity (using the Rapid Assessment of Physical Activity21). The Berg Balance Scale (BBS),22 10-metre walk,23 and 6-minute walk test (6MWT)24 were performed to assess functional balance, self-paced 10-metre walk gait speed, and ambulatory capacity, respectively.
The devices were configured for each participant on the basis of age, sex, height, and weight. Because ankle positioning (vs. hip positioning) of the Fitbit One has been shown to be more accurate in capturing step count in individuals with slower walking speeds,15,16 the participants wore both devices on the lateral aspect of the non-paretic ankle (see Figure 1). The devices were secured using a nylon strap. The participants wore them for 3 consecutive days (from Friday to Sunday to capture both weekdays and weekend days) and were to remove them before bathing, showering, or swimming (when they would be submerged in water) or if their skin became irritated. The study personnel gave the participants verbal and written instructions to promote their adherence to the protocol and a log in which the participants could write the times when they removed each device.
Figure 1.
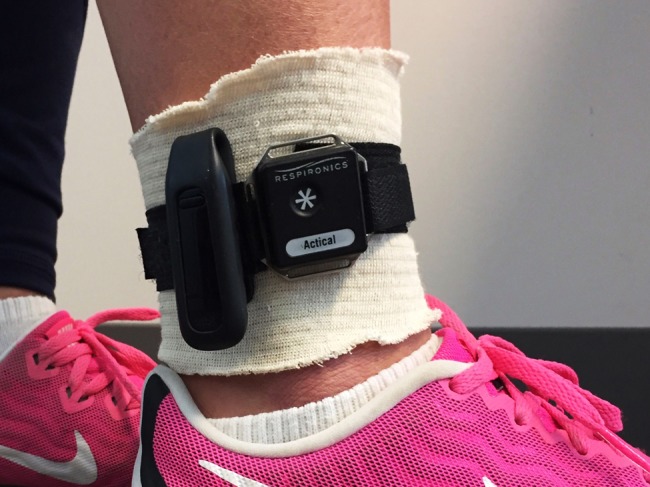
Placement of the Fitbit and Actical devices on the non-paretic ankle using a nylon strap.
Each device recorded a participant's step count and time spent being sedentary and during light, moderate, and vigorous activity (in minutes). Participants also completed a survey to evaluate their day-to-day use of and comfort level with the technology and their specific experience with the devices (difficulties encountered, adequacy of instruction received, and likelihood of using activity trackers in the future).
Statistical analysis
Descriptive statistics (mean [SD] for continuous variables, frequency counts for non-continuous variables) were performed on all measures. Regression analyses were performed for each day to determine whether the correlations between the Fitbit and Actical values (for each day for step count, sedentary time, and time spent in light, moderate, and vigorous activity) were more than 0.80. For comparison purposes, the levels for sedentary, light, moderate, and vigorous activity on the Actical were equated to sedentary, lightly active, fairly active, and very active levels on the Fitbit.
The relative error of the Fitbit, relative to the Actical, in measuring step count was calculated as (Actical—Fitbit step count) / Actical step count×100%. Multivariable regression analysis was performed to examine the factors that could contribute to the variance in relative error, including participants' characteristics (age, sex, BMI), stroke severity (using the NIHSS and Chedoke-McMaster Stroke Assessment Impairment Inventory leg motor recovery score), functional ability (using the BBS, MoCA, and self-paced 10 m walk gait speed), and physical activity levels (using the Rapid Assessment of Physical Activity). First, bivariate correlations were conducted between the relative error and each of these candidate variables. Variables with a significance level of p<0.20 were entered into the regression model. Multi-collinearity was examined using tolerance and variance inflation factors. We conducted our statistical analysis using the IBM SPSS Statistics, version 20.0 (IBM Corporation, Armonk, NY).
Results
A total of 12 participants were enrolled in this study; their characteristics are shown in Table 1. Participants rated their mean perceived comfort with technology as 4.6 (SD 1.8) on a 7-point scale, where 1=not comfortable at all, I never use technology, and 7=very comfortable, I use technology very frequently. Most participants (n=9; 75%) reported that they used computers for email and Internet browsing, and tablet devices were least commonly used (n=4; 33%) for gaming and emergencies. Three participants reported that they had used activity-tracking devices in the past (pedometer, n=2; heart rate monitor, n=1).
Table 1.
Participants' Characteristics
| Characteristic | Mean (SD); range* |
|---|---|
| Age, y | 62.6 (9.3); 38–73 |
| Sex, male/female; no. (%) | 7 (58)/5 (42) |
| Time post-stroke, mo | 19.6 (28.0); 3–107 |
| National Institutes of Health Stroke Scale score | 3.3 (1.6); 1–6 |
| Montreal Cognitive Assessment score | 21.2 (4.4); 13–28 |
| Chedoke-McMaster Stroke Assessment score | |
| Arm | 4.2 (1.8); 1–6 |
| Hand | 4.7 (1.7); 2–6 |
| Leg | 5.4 (0.67); 4–6 |
| Foot | 4.5 (1.6); 2–7 |
| No assistive device, cane, or walker, no. (%) | 6 (50)/2 (17)/4 (33) |
| Self-paced 10 m walk gait speed, m/s | 0.73 (0.27); 0.13–1.10 |
| 6 min walk test distance, m | 250.7 (106.0); 31–366 |
| Berg Balance Scale score | 45.8 (7.2); 33–55 |
| Rapid Assessment of Physical Activity score | 3.3 (1.4); 1–6 |
Except where otherwise indicated.
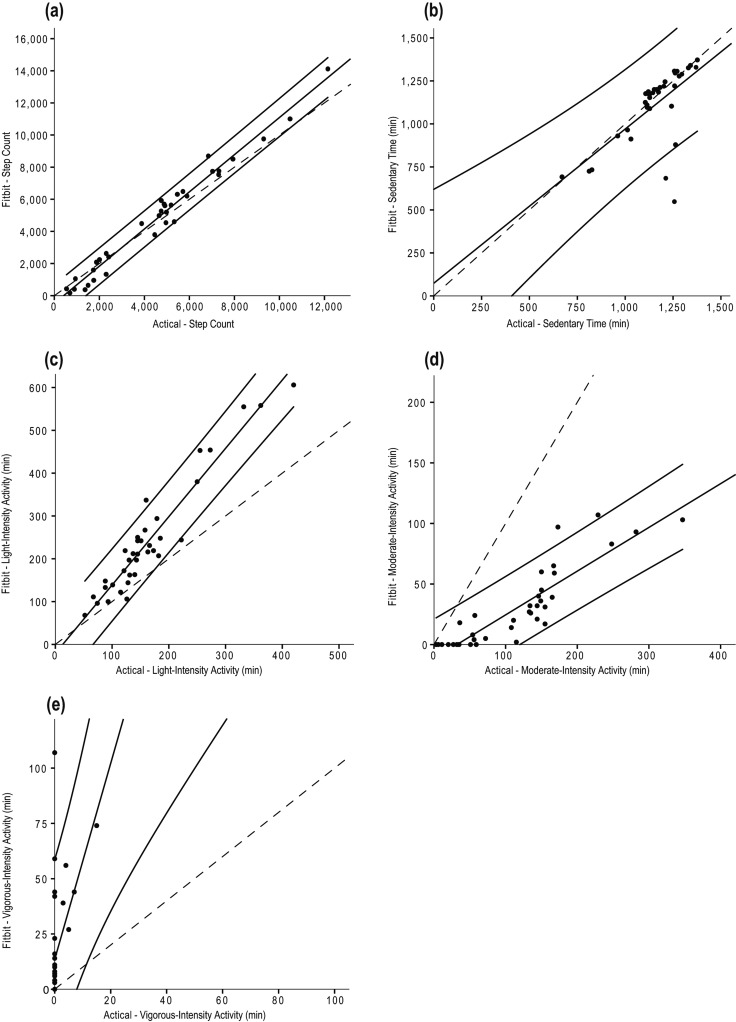
Total step count and total number of minutes spent in sedentary, light, moderate, and vigorous activity are displayed in Table 2. Participants spent most of their days being sedentary (79% and 80% of the day, as recorded by the Actical and Fitbit One, respectively). The associations between the Actical accelerometer and the Fitbit One are presented for each day in Table 3 (with the exception of Days 2 and 3 for vigorous-intensity activity because there were 0 minutes of recorded activity). Correlations for all 3 days of free-living activity were more than 0.80 for step count and time spent in light-intensity activity only (see Table 3). Scatterplots for the correlations between the Fitbit and Actical devices are presented in Figure 2.
Table 2.
Daily Step Count and Time Spent at Each Activity Level for Each Device
| Mean (SD) |
||
|---|---|---|
| Measure | Actical | Fitbit One |
| Step count | 4,673 (2,933) | 4,453 (2,398) |
| Time, min | ||
| Sedentary | 1,156 (134) | 1,110 (176) |
| Light | 165 (68) | 242 (120) |
| Moderate | 117 (72) | 31 (28) |
| Vigorous | 0.9 (1.7) | 18 (19) |
Table 3.
Associations between Actical and Fitbit Values for Daily Step Count and Time Spent at Each Activity Level
| Actical correlation with Fitbit values |
r (lower 1-sided 95% CI) |
p-value for r>0.80 |
|---|---|---|
| Step count | ||
| Day 1 | 0.99 (0.97) | <0.001* |
| Day 2 | 0.99 (0.96) | <0.001* |
| Day 3 | 0.97 (0.92) | <0.001* |
| Time spent sedentary, min | ||
| Day 1 | 0.94 (0.83) | 0.03* |
| Day 2 | 0.78 (0.45) | 0.59 |
| Day 3 | 0.41 (–0.11) | 0.98 |
| Time spent in light-intensity activity, min | ||
| Day 1 | 0.95 (0.85) | 0.01* |
| Day 2 | 0.91 (0.92) | 0.001* |
| Day 3 | 0.97 (0.90) | 0.001* |
| Time spent in moderate-intensity activity, min | ||
| Day 1 | 0.90 (0.73) | 0.13 |
| Day 2 | 0.91 (0.74) | 0.10 |
| Day 3 | 0.83 (0.56) | 0.39 |
| Time spent in vigorous-intensity activity, min† | ||
| Day 1 | 0.86 (0.63) | 0.28 |
p<0.05 for r>0.80.
Unable to analyze Days 2 and 3 because times recorded=0.
Figure 2.
Scatterplots for the correlations between the Fitbit and Actical devices for (a) step count; (b) sedentary time; (c) light-intensity activity; (d) moderate-intensity activity; and (e) vigorous-intensity activity.
Note: Solid lines represent regression lines and 95% prediction bands; dashed lines represent lines of identity.
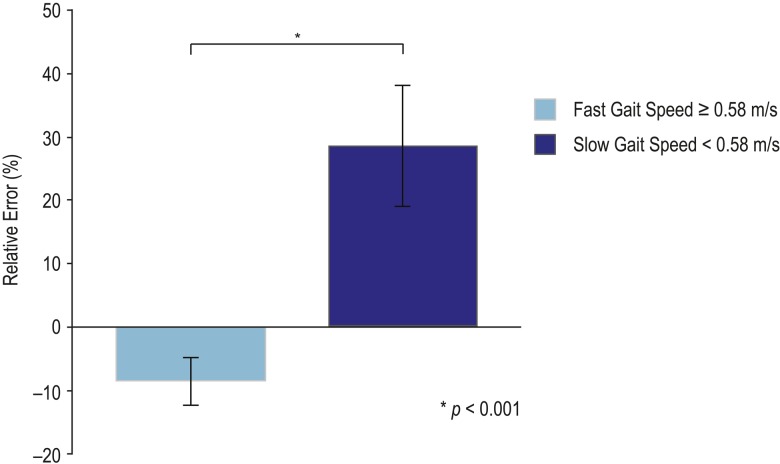
Overall, the mean relative error in step count was 3.8 (SD 27.0%). Because a previous study found that a threshold walking speed of 0.58 metres per second differentiated Fitbit accuracy in individuals with stroke,25 we dichotomized our sample into fast and slow subgroups on the basis of a gait speed of 0.58 metres or more per second and less than 0.58 metres per second, respectively. The relative error was lower in the fast than in the slow walking speed subgroup (p<0.001) (see Figure 3).
Figure 3.
Relative error between participants with faster (≥0.58 m/s) and slower (<0.58 m/s) gait speeds.
The relative error was associated with age, BBS score, Chedoke-McMaster Stroke Assessment Impairment Inventory leg impairment score, MoCA score, and 10 m walk gait speed (see Table 4). These variables, with the exception of the BBS because of its high correlation with gait speed and the Rapid Assessment of Physical Activity score because of its high correlation with the MoCA, were entered into a multivariable regression model, which explained 69% of the variance of the relative error in step count (see Table 5).
Table 4.
Bivariate Correlations, with Relative Error in Step Count as the Dependent Variable
| Characteristic | r (95% CI) | p-value |
|---|---|---|
| Age | 0.36 (0.18, 0.54) | 0.03 |
| Sex | −0.01 (−0.36, 0.32) | 0.93 |
| BMI | −0.07 (−0.33, 0.21) | 0.70 |
| National Institutes of Health Stroke Scale score |
0.18 (−0.21, 0.54) | 0.28 |
| Chedoke-McMaster Stroke Assessment Impairment Inventory leg score |
−0.31 (−0.69, 0.20) | 0.07 |
| Berg Balance Scale score | −0.63 (−0.79, −0.35) | <0.001 |
| Self-paced 10 m walk gait speed | −0.73 (−0.86, −0.53) | <0.001 |
| Montreal Cognitive Assessment score | −0.34 (−0.58, −0.10) | 0.04 |
| Rapid Assessment of Physical Activity score |
−0.32 (−0.63, 0.03) | 0.06 |
Table 5.
Multivariable Regression to Examine Correlates with Relative Error in Step Count
| Correlate | Unstandardized β (SE) | Standardized β | 95% CI | p-value |
|---|---|---|---|---|
| Model R2=0.69, F4,31=17.58 | – | – | – | <0.001 |
| Age | 0.64 (0.33) | 0.21 | −0.04, 1.31 | 0.06 |
| Chedoke-McMaster Stroke Assessment Impairment Inventory leg score | 6.88 (5.25) | 0.17 | −3.84, 17.59 | 0.20 |
| Montreal Cognitive Assessment score | −2.36 (0.64) | −0.38 | −3.67, −1.05 | 0.001 |
| Self-paced 10 m walk gait speed | −76.02 (13.44) | −0.75 | −103.44, −48.61 | <0.001 |
Most participants (n=10; 83%) reported that they had received adequate instruction about using the devices. There were no complications from using the Fitbit One, but five people (41%) reported discomfort from the Actical strap when they wore it overnight. The participants' mean confidence in the ability of the devices to capture their activity level was 5.6 (SD 1.4) out of 7 (where 1=not confident at all and 7=very confident). Although seven participants (58%) did not find that their physical activity levels changed because they were wearing the devices, the remaining five people (42%) reported slight and moderate increases in their activity and were interested in using the Fitbit in the future.
Discussion
The results of the current study confirm that physical activity levels are very low in individuals with stroke, who are sedentary for the majority of their day. Daily step count and minutes spent in light activity, as measured by the commercially available Fitbit One, were correlated with the research-based Actical, but not for higher levels of physical activity.
Both devices were comparable for quantifying the number of steps taken, suggesting that an ankle-worn Fitbit One can capture step count in individuals with stroke. These findings corroborate those of previous studies with older adults15 and persons with stroke and traumatic brain injuries,16,25 which compared using activity monitors with the standard method of a hand-tallied step count. This study builds on these previous studies, which were conducted in controlled laboratory settings, by collecting activity data in open environments, during habitual activities, and over multiple days. Our findings suggest that the Fitbit One, with its lower price point and user-friendly interface, may be a useful tool for people with stroke for capturing free-living step count.
Our findings confirmed that daily step count was low after stroke, comparable in magnitude to several previous reports (3,500–5,000 steps).26–28 Indeed, although our participants would be classified as having mild severity of stroke according to their NIHSS scores, other functional measures suggested otherwise. The gait speed of our sample represented only 55% of the meta-analytic values reported for age-matched healthy individuals,29 6MWT distance was 52% of predicted values,30 and MoCA scores suggested the presence of cognitive impairment.18,31 The step count totals demonstrated by our sample were lower than the 6,000 steps per day recommended for persons with physical disabilities32 and consistent with the threshold of 5,000 steps per day or fewer that would be considered a sedentary lifestyle.33 Our findings indicate that effective strategies are needed to promote physical activity after stroke and reduce the risk of secondary immobility-related consequences.
We also confirmed that our participants spent the majority of their day (approximately 80%) being sedentary; this parallels previous findings.10 Sedentary behaviour has been associated with poorer cardiometabolic health in the general population, such as higher waist circumference and increased lipid, insulin, and C-reactive protein levels,33,34 and it has been recognized as a growing public health concern.12 Although the association between sedentary behaviour and negative health outcomes is not as clearly established as this in people with stroke, it remains critically important for these individuals to engage in regular physical activity to counteract sedentary behaviour and the elevated risk of secondary cardiac complications and recurrent events. Participants in the current study failed to meet recommended activity targets. This is of concern given that they may arguably represent a higher functioning and more active subset of the stroke population because they were participating in a community-based exercise trial. Indeed, step counts in individuals with stroke have been reported to be even lower (2,500–3,000).35,36
Time spent at light-intensity activity measured by the Fitbit One was strongly associated with Actical-derived values, but this association was not consistently observed for sedentary time across the 3 days. When we inspected the scatterplots, we noted two values that fell beyond the 95% prediction bands that influenced the strength of the associations (see Figure 2c). Whether these outlier values were a true reflection of the values recorded or the result of artifact is not known. Further investigation is warranted to establish the correlation between these devices for capturing light-intensity activity.
There were no associations between the devices for time spent in moderate- or vigorous-intensity activity. The Fitbit One tended to underestimate the minutes spent in moderate-intensity activity and overestimate that spent in vigorous-intensity activity. This disparity may be a result of the method by which each device categorizes and captures activity. The bandwidths that the Actical uses to classify activity intensity were known (e.g., 3–6 METs for moderate-intensity activity), but such information for the Fitbit One is proprietary and could not be obtained. Because the Fitbit One recorded less time spent in moderate-intensity activity and correspondingly more time spent in both light- and vigorous-intensity activity, we speculate that it uses a smaller bandwidth for moderate activity than the Actical, which may account for the greater amount of time spent in the extreme categories of intensity.
The disparity may also be partially explained by differences in epoch length, whereby the 60-second epoch used by the Fitbit One may not detect very short bouts of activity, which would otherwise have been captured by the more sensitive 15-second epoch of the Actical. Because Fitbit devices were originally intended for use in the general population, the step detection algorithm may not be suitable for individuals who present with compromised function. Future research may match epoch lengths between the devices for a more equitable comparison.
The 3.8% relative error observed in this study was higher than that in a previous study (1.3%),14 but this disparity is likely attributable to differences in study participants (young healthy adults) and setting (controlled treadmill walking). We observed that the relative error was associated with slower walking speed, older age, and greater impairment in cognition. Cognitive impairment in older adults was associated with lower levels of activity,37,38 which can contribute to slower walking speeds and greater error in activity tracking. Walking speed may be a factor associated with the device's accuracy in quantifying steps counts, although the evidence is unclear.
Earlier studies have reported threshold values for gait speed for greater Fitbit accuracy (Fitbit Tracker, Fitbit Ultra) compared with hand-tallied step counts (>0.56 m/sec for older adults,17 >0.58 m/sec for individuals with stroke and traumatic brain injuries25), and other studies have reported that speed-associated differences in error may be mitigated by positioning the Fitbit One at the ankle.15,16 Anticipating that participants in the current study would present with slower walking speeds, we also positioned the devices at the ankle to endeavour to enhance agreement, but we nevertheless observed differences in the degree of error related to walking speed.
Nonetheless, the current study contributes novel and clinically relevant evidence related to activity tracking because it is the first study to examine the measurement properties of the popular Fitbit device in people with stroke outside a controlled laboratory setting and over 3 days of free living, where more variability in movement and activity can occur. The inconsistencies between our study and earlier studies highlight the need for further research, particularly as affordable, mass-market devices continue to gain popularity.
These findings have implications for physical therapists working in stroke rehabilitation settings, who are well positioned to design and develop exercise programmes for this vulnerable population. Published evidence-based guidelines recommend regular physical activity after stroke,4 and clients may seek advice from their therapist about activity trackers that may help them achieve these goals. Using devices to monitor activity levels may be an integral component of promoting daily physical activity in this population. The participants in our study enjoyed using these activity-tracking devices, many experienced minimal to no complications, and several felt that the devices provided external motivation for activity change and would consider using them in the future. Wearing an activity monitor on the ankle is arguably not common practice, but if evidence continues to emerge supporting ankle-worn devices after stroke, users may consider adapting the standard protocol to ensure greater accuracy.
Despite the participants' willingness to use the Fitbit One and their comfort with it, as well as its agreement with a research-based accelerometer in quantifying free-living step count, the device may misrepresent the time spent engaged in moderate- and vigorous-intensity activities. Further research is warranted to better understand the bandwidth for capturing activity levels and the device's accuracy in detecting activity in individuals across a broad spectrum of functional abilities. It is important for stroke rehabilitation professionals to remain current with the measurement parameters of ever-changing activity-tracking technologies so that they can provide informed recommendations to their clients.
This study has a few limitations. First, we were unable to obtain technical specifications from the Fitbit manufacturer about activity-intensity thresholds, and between-device differences in measuring activity at various levels of intensity may be partially explained by differences in acceleration cut-points. Certainly, it would be mutually beneficial for the research and commercial sectors to collaborate with full disclosure, with the aim of using such devices with individuals across all levels of function. Second, because this study focused only on the Fitbit One, we cannot comment on whether these findings can be carried over to other devices in the Fitbit product line. Finally, the sample size was small, but because the present study is the first to use a Fitbit device to capture free-living activity over 3 full days, our results provide insight into the use of this popular device for tracking activity after stroke.
Conclusion
Popular, commercially available devices have the potential to track activity and motivate stroke survivors to engage in regular, daily physical activity. The Fitbit One may be used to measure step count in individuals with stroke, but conventional, research-based accelerometers may be more accurate in capturing higher intensity activity.
Key Messages
What is already known on this topic
Physical activity levels are low after stroke. Previous studies have examined the validity of commercially available trackers for quantifying step count in people with stroke under controlled walking conditions (treadmill or paced corridor walking) but with conflicting findings. Faster walking speeds have been associated with lower rates of error, but positioning these devices at the ankle appears to enhance sensitivity, even at slow speeds.
What this study adds
This is the first study to use a Fitbit One to capture free-living activity over 3 days in people living in the community after stroke and to compare activity levels with those measured by an Actical accelerometer. Despite positioning the devices at the ankle, there were differences in quantifying activity at higher intensities. Agreement between the devices for measuring step count was greater in individuals with gait speed of 0.58 metres or more per second.
References
- 1. Krueger H, Koot J, Hall RE, et al. . Prevalence of individuals experiencing the effects of stroke in Canada: trends and projections. Stroke. 2015;46(8):2226–31. http://dx.doi.org/10.1161/STROKEAHA.115.009616 Medline:26205371 [DOI] [PubMed] [Google Scholar]
- 2. Gresham GE, Fitzpatrick TE, Wolf PA, et al. . Residual disability in survivors of stroke—the Framingham study. N Engl J Med. 1975;293(19):954–6. http://dx.doi.org/10.1056/NEJM197511062931903 Medline:1178004 [DOI] [PubMed] [Google Scholar]
- 3. Saunders DH, Sanderson M, Hayes S, et al. . Physical fitness training for stroke patients. Cochrane Database Syst Rev. 2016;(3):CD003316 Medline:27010219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Billinger SA, Arena R, Bernhardt J, et al. ; American Heart Association Stroke Council, Council on Cardiovascular and Stroke Nursing, Council on Lifestyle and Cardiometabolic Health, Council on Epidemiology and Prevention, Council on Clinical Cardiology. Physical activity and exercise recommendations for stroke survivors: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45(8):2532–53. http://dx.doi.org/10.1161/STR.0000000000000022 Medline:24846875 [DOI] [PubMed] [Google Scholar]
- 5. Ashe MC, Miller WC, Eng JJ, et al. ; Physical Activity and Chronic Conditions Research Team. Older adults, chronic disease and leisure-time physical activity. Gerontology. 2009;55(1):64–72. http://dx.doi.org/10.1159/000141518 Medline:18566534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yang CC, Hsu YL. A review of accelerometry-based wearable motion detectors for physical activity monitoring. Sensors (Basel). 2010;10(8):7772–88. http://dx.doi.org/10.3390/s100807772 Medline:22163626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. John D, Freedson P. ActiGraph and Actical physical activity monitors: a peek under the hood. Med Sci Sports Exerc. 2012;44(1 Suppl. 1):S86–9. http://dx.doi.org/10.1249/MSS.0b013e3182399f5e Medline:22157779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rand D, Eng JJ, Tang PF, et al. . How active are people with stroke? Use of accelerometers to assess physical activity. Stroke. 2009;40(1):163–8. http://dx.doi.org/10.1161/STROKEAHA.108.523621 Medline:18948606 [DOI] [PubMed] [Google Scholar]
- 9. Dobkin BH, Xu X, Batalin M, et al. . Reliability and validity of bilateral ankle accelerometer algorithms for activity recognition and walking speed after stroke. Stroke. 2011;42(8):2246–50. http://dx.doi.org/10.1161/STROKEAHA.110.611095 Medline:21636815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tieges Z, Mead G, Allerhand M, et al. . Sedentary behavior in the first year after stroke: a longitudinal cohort study with objective measures. Arch Phys Med Rehabil. 2015;96(1):15–23. http://dx.doi.org/10.1016/j.apmr.2014.08.015 Medline:25220942 [DOI] [PubMed] [Google Scholar]
- 11. Askim T, Bernhardt J, Salvesen O, et al. . Physical activity early after stroke and its association to functional outcome 3 months later. J Stroke Cerebrovasc Dis. 2014;23(5):e305–12. http://dx.doi.org/10.1016/j.jstrokecerebrovasdis.2013.12.011 Medline:24529353 [DOI] [PubMed] [Google Scholar]
- 12. Owen N, Healy GN, Matthews CE, et al. . Too much sitting: the population health science of sedentary behavior. Exerc Sport Sci Rev. 2010;38(3):105–13. http://dx.doi.org/10.1097/JES.0b013e3181e373a2 Medline:20577058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Diaz KM, Krupka DJ, Chang MJ, et al. . Fitbit®: An accurate and reliable device for wireless physical activity tracking. Int J Cardiol. 2015;185:138–40. http://dx.doi.org/10.1016/j.ijcard.2015.03.038 Medline:25795203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Takacs J, Pollock CL, Guenther JR, et al. . Validation of the Fitbit One activity monitor device during treadmill walking. J Sci Med Sport. 2014;17(5):496–500. http://dx.doi.org/10.1016/j.jsams.2013.10.241 Medline:24268570 [DOI] [PubMed] [Google Scholar]
- 15. Simpson LA, Eng JJ, Klassen TD, et al. . Capturing step counts at slow walking speeds in older adults: comparison of ankle and waist placement of measuring device. J Rehabil Med. 2015;47(9):830–5. http://dx.doi.org/10.2340/16501977-1993 Medline:26181670 [DOI] [PubMed] [Google Scholar]
- 16. Klassen TD, Simpson LA, Lim SB, et al. . “Stepping up” activity poststroke: ankle-positioned accelerometer can accurately record steps during slow walking. Phys Ther. 2016;96(3):355–60. Medline:26251478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Phillips LJ, Petroski GF, Markis NE. A comparison of accelerometer accuracy in older adults. Res Gerontol Nurs. 2015;8(5):213–9. http://dx.doi.org/10.3928/19404921-20150429-03 Medline:25942386 [DOI] [PubMed] [Google Scholar]
- 18. Brott T, Adams HP Jr, Olinger CP, et al. . Measurements of acute cerebral infarction: a clinical examination scale. Stroke. 1989;20(7):864–70. http://dx.doi.org/10.1161/01.STR.20.7.864 Medline:2749846 [DOI] [PubMed] [Google Scholar]
- 19. Gowland C, Stratford P, Ward M, et al. . Measuring physical impairment and disability with the Chedoke-McMaster Stroke Assessment. Stroke. 1993;24(1):58–63. http://dx.doi.org/10.1161/01.STR.24.1.58 Medline:8418551 [DOI] [PubMed] [Google Scholar]
- 20. Nasreddine ZS, Phillips NA, Bédirian V, et al. . The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695–9. http://dx.doi.org/10.1111/j.1532-5415.2005.53221.x Medline:15817019 [DOI] [PubMed] [Google Scholar]
- 21. Topolski TD, LoGerfo J, Patrick DL, et al. . The Rapid Assessment of Physical Activity (RAPA) among older adults. Prev Chronic Dis. 2006;3(4):A118 Medline:16978493 [PMC free article] [PubMed] [Google Scholar]
- 22. Berg KO, Wood-Dauphinee SL, Williams JI, et al. . Measuring balance in the elderly: validation of an instrument. Can J Public Health. 1992;83(2 Suppl. 2):S7–11. Medline:1468055 [PubMed] [Google Scholar]
- 23. Flansbjer UB, Holmbäck AM, Downham D, et al. . Reliability of gait performance tests in men and women with hemiparesis after stroke. J Rehabil Med. 2005;37(2):75–82. http://dx.doi.org/10.1080/16501970410017215 Medline:15788341 [DOI] [PubMed] [Google Scholar]
- 24. ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166(1):111–7. http://dx.doi.org/10.1164/ajrccm.166.1.at1102 Medline:12091180 [DOI] [PubMed] [Google Scholar]
- 25. Fulk GD, Combs SA, Danks KA, et al. . Accuracy of 2 activity monitors in detecting steps in people with stroke and traumatic brain injury. Phys Ther. 2014;94(2):222–9. http://dx.doi.org/10.2522/ptj.20120525 Medline:24052577 [DOI] [PubMed] [Google Scholar]
- 26. Prajapati SK, Gage WH, Brooks D, et al. . A novel approach to ambulatory monitoring: investigation into the quantity and control of everyday walking in patients with subacute stroke. Neurorehabil Neural Repair. 2011;25(1):6–14. http://dx.doi.org/10.1177/1545968310374189 Medline:20829413 [DOI] [PubMed] [Google Scholar]
- 27. Mansfield A, Wong JS, Bryce J, et al. . Use of accelerometer-based feedback of walking activity for appraising progress with walking-related goals in inpatient stroke rehabilitation: a randomized controlled trial. Neurorehabil Neural Repair. 2015;29(9):847–57. http://dx.doi.org/10.1177/1545968314567968 Medline:25605632 [DOI] [PubMed] [Google Scholar]
- 28. Bowden MG, Balasubramanian CK, Behrman AL, et al. . Validation of a speed-based classification system using quantitative measures of walking performance poststroke. Neurorehabil Neural Repair. 2008;22(6):672–5. http://dx.doi.org/10.1177/1545968308318837 Medline:18971382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bohannon RW, Williams Andrews A. Normal walking speed: a descriptive meta-analysis. Physiotherapy. 2011;97(3):182–9. http://dx.doi.org/10.1016/j.physio.2010.12.004 Medline:21820535 [DOI] [PubMed] [Google Scholar]
- 30. Enright PL, Sherrill DL. Reference equations for the six-minute walk in healthy adults. Am J Respir Crit Care Med. 1998;158(5 Pt 1):1384–7. http://dx.doi.org/10.1164/ajrccm.158.5.9710086 Medline:9817683 [DOI] [PubMed] [Google Scholar]
- 31. Damian AM, Jacobson SA, Hentz JG, et al. . The Montreal Cognitive Assessment and the Mini-Mental State Examination as screening instruments for cognitive impairment: item analyses and threshold scores. Dement Geriatr Cogn Disord. 2011;31(2):126–31. http://dx.doi.org/10.1159/000323867 Medline:21282950 [DOI] [PubMed] [Google Scholar]
- 32. Tudor-Locke C, Craig CL, Aoyagi Y, et al. . How many steps/day are enough? For older adults and special populations. Int J Behav Nutr Phys Act. 2011;8:80 Medline:21798044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tudor-Locke C, Craig CL, Thyfault JP, et al. . A step-defined sedentary lifestyle index: <5000 steps/day. Appl Physiol Nutr Metab. 2013;38(2):100–14. http://dx.doi.org/10.1139/apnm-2012-0235 Medline:23438219 [DOI] [PubMed] [Google Scholar]
- 34. Healy GN, Matthews CE, Dunstan DW, et al. . Sedentary time and cardio-metabolic biomarkers in US adults: NHANES 2003-06. Eur Heart J. 2011;32(5):590–7. http://dx.doi.org/10.1093/eurheartj/ehq451 Medline:21224291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zalewski KR, Dvorak L. Barriers to physical activity between adults with stroke and their care partners. Top Stroke Rehabil. 2011;18(Suppl. 1):666–75. http://dx.doi.org/10.1310/tsr18s01-666 Medline:22120035 [DOI] [PubMed] [Google Scholar]
- 36. Michael KM, Allen JK, Macko RF. Reduced ambulatory activity after stroke: the role of balance, gait, and cardiovascular fitness. Arch Phys Med Rehabil. 2005;86(8):1552–6. http://dx.doi.org/10.1016/j.apmr.2004.12.026 Medline:16084807 [DOI] [PubMed] [Google Scholar]
- 37. Narazaki K, Matsuo E, Honda T, et al. . Physical fitness measures as potential markers of low cognitive function in Japanese community-dwelling older adults without apparent cognitive problems. J Sports Sci Med. 2014;13(3):590–6. Medline:25177186 [PMC free article] [PubMed] [Google Scholar]
- 38. Kerr J, Marshall SJ, Patterson RE, et al. . Objectively measured physical activity is related to cognitive function in older adults. J Am Geriatr Soc. 2013;61(11):1927–31. http://dx.doi.org/10.1111/jgs.12524 Medline:24219194 [DOI] [PMC free article] [PubMed] [Google Scholar]