Abstract
Objective
Attention deficit hyperactivity disorder (ADHD) is associated with deficits in motor learning and sleep. In healthy adults, overnight motor skill learning improvement is associated with sleep spindle activity in the sleep EEG. This association is poorly characterized in children, particularly in pediatric ADHD.
Method
Polysomnographic sleep was monitored in seven children with ADHD and fourteen typically developing controls. All children trained on a validated motor sequence task (MST) in the evening with retesting the following morning. Analyses focused on MST precision (speed-accuracy trade-off). NREM Stage 2 sleep EEG power spectral analyses focused on spindle-frequency EEG activity in the sigma (12–15 Hz) band.
Results
The ADHD group demonstrated a selective decrease in power within the sigma band. Evening MST precision was lower in ADHD, yet no difference in performance was observed following sleep. Moreover, ADHD-status moderated the association between slow sleep spindle activity (12–13.5 Hz) and overnight improvement; spindle-frequency EEG activity was positively associated with performance improvements in children with ADHD but not in controls.
Conclusions
These data highlight the importance of sleep in supporting next day behavior in ADHD, while indicating that differences in sleep neurophysiology may, in part, underlie cognitive deficits in this population.
Keywords: Sleep, ADHD, Motor learning, Adolescence, Sleep spindles
Introduction
Attention deficit hyperactivity disorder (ADHD) is associated with simultaneous deficits in daytime learning (Adi-Japha, Fox, & Karni, 2011) and nocturnal sleep (Owens et al., 2013). Whereas sleep supports learning in adults, particularly for motor skills (Walker, Brakefield, Morgan, Hobson, & Stickgold, 2002), this association is less well understood in adolescents, those with ADHD in particular. This pilot study examines whether distinct profiles of sleep-dependent learning are observed in ADHD and typically developing children.
The current study focused on a procedural memory paradigm known to demonstrate sleep-dependent improvement in adults. Whereas sleep deprivation impairs overnight improvement in motor skill learning (Fischer, Hallschmid, Elsner, & Born, 2002), both nocturnal sleep (Walker et al., 2002) and daytime naps (Nishida & Walker, 2007) consolidate and stabilize performance. A neural marker of sleep-related overnight improvement is found in the non-rapid eye movement Stage 2 Sleep EEG: the thalamocortical sleep spindle (a synchronous burst in the 12–15 Hz range (Nishida & Walker, 2007)). Sleep-spindle-frequency EEG activity in the sigma band (12–15 Hz) over motor cortex predicts overnight gains in motor learning in healthy adults, and indexes the degree of motor skill learning deficits in circumstances such as schizophrenia (Wamsley et al., 2013).
While one recent study examined sleep-dependent procedural motor skill learning in pediatric ADHD (Prehn-Kristensen et al., 2011), the authors did not perform spectral analysis of the sleep EEG. Indeed, whether sleep spindles are atypical in children with ADHD is unclear. When compared to traditional polysomnography, relatively little is known about the micro-features of the sleep EEG in this population. Whereas one recent study identified lower slow wave activity and higher spindle-frequency sigma activity in ADHD (Prehn-Kristensen et al., 2013), another group demonstrated a converse increase in slow wave activity over central cortices (Ringli et al., 2013). Neither of these latter studies involved a motor-learning paradigm. Thus, the current pilot study therefore aimed to add to this growing literature while testing the hypothesis that overnight motor learning ability in ADHD may be sensitive to spindle-frequency EEG activity expressed during sleep.
Materials and Methods
The local institutional review board approved the study. Informed consent was obtained from all parents, with assent from all children. Each child received monetary compensation for participating in the study.
Participants
26 children (aged 10–12.9 years) entered the study. Five children were excluded from analysis for technical issues (n=2) or attrition (n=3), yielding a final sample of 7 children with ADHD and 14 typically developing controls (TDC). The sample was primarily male, yet was balanced for gender, with 2 girls in the ADHD group, and 4 girls in the TDC group. Complete demographics are reported in Table 1.
Table 1.
Demographic Variables
| ADHD n = 7 | Controls n = 14 | p | |
|---|---|---|---|
| Gender (# Girls) | 2 | 2 | 1.000 |
| Age (years) | 11.9 ± 0.9 | 11.7 ± 0.9 | .62 |
|
|
|||
| DISC-IV | |||
|
|
|||
| ADHD Symptoms | 11.3 ± 3.4 | 0.86 ± 1.2 | < .001 |
| Behavioral Impairments | 6.6 ± 1.9 | 0.00 ± 0.0 | < .001 |
| Symptoms + Impairments Impairments | 17.9 ± 4.1 | 0.86 ± 1.2 | < .001 |
|
|
|||
| BDI | |||
|
|
|||
| Raw Score | 5.1 ± 4.0 | 5.00 ± 4.0 | .94 |
| T-Score | 39.2 ± 4.3 | 39.15 ± 4.2 | .95 |
|
|
|||
| WRAML-2 | |||
|
|
|||
| Verbal Memory | 112.3 ± 9.8 | 116.21 ± 10.5 | .42 |
| Visual | 98.7 ± 15.1 | 97.64 ± 11.5 | .86 |
| Screening Memory | 106.9 ± 11.3 | 108.86 ± 11.3 | .71 |
|
|
|||
| KBIT-2 | |||
|
|
|||
| Verbal | 111.6 ± 13.3 | 119.21 ± 10.6 | .17 |
| Nonverbal | 105.9 ± 13.3 | 115.05 ± 9.3 | .079 |
| IQ | 110.3 ± 14.1 | 120.07 ± 9.4 | .072 |
|
|
|||
| WRAT4 | |||
|
|
|||
| Word Reading | 99.2 ± 15.2 | 115.21 ± 15.9 | .067 |
| Spelling | 107.0 ± 12.7 | 116.29 ± 16.3 | .31 |
| Math Computation | 101.8 ± 18.4 | 120.20 ± 11.6 | .018 |
Note: Data reported as mean ± sd. Significance from 2-tailed X2 tests, or independent samples t-tests as appropriate. Definitions: DISC-IV: Diagnostic Interview Schedule for Children Version IV. WRAML-2: Wide Range Assessment of Memory and Learning, Second Edition. KBIT-2: Kaufman Brief Intelligence Test, Second Edition. WRAT4: Wide Range Achievement Test 4.
A brief parental telephone interview screened for current past medical/psychiatric conditions, family history of medical/psychiatric conditions, current use of psychoactive agents, and abnormal sleep habits. Diagnosis of ADHD was confirmed using the “ADHD” and “other DSM-IV Diagnostic Categories” of the Diagnostic Interview Schedule for Children, Fourth Edition (DISC-IV) (Shaffer, Fisher, Lucas, Dulcan, & Schwab-Stone, 2000) (Table 1). Exclusion criteria included: parental or child report of irregular sleep patterns (e.g., self-reported sleep schedules that vary by greater than 3 hours across a week, self-reported excessive daytime sleepiness, frequent napping (≥ 3 times/week), a morningness/eveningness score (Smith, Reilly, & Midkiff, 1989) of greater than 2 standard deviations above or below the mean values of subjects in the same grade (age) and of the same sex, based on our laboratory normative data pool); travel beyond two time zones within 2 months of the study; diagnosis of a learning disability, mental retardation, dyslexia, pervasive developmental disorder; a personal and/or family history of narcolepsy.
Neuropsychological assessment characterized children on intelligence/achievement, attention/impulse control, learning/memory, response speed/productivity, and problem solving/cognitive control. Trained staff administered the Kaufman Brief Intelligence Test, Second Edition (KBIT-2) (Kaufman & Kaufman, 2004), the Wide Range Assessment of Memory and Learning, Second Edition (WRAML-2) (Sheslow & Adams, 2003), and the Wide Range Achievement Test, Fourth Edition (WRAT4) (Wilkinson & Robertson, 2006). Both groups were in normative ranges (Table 1), while TDC children had higher math computation scores compared to ADHD peers (p=.018).
Procedures
At-Home Protocol
Participants were monitored by wrist actigraphy (Sadeh, Sharkey, & Carskadon, 1994) and completed a sleep-wake diary for one week before and throughout the study. During this time participants were asked to maintain a 10-hour sleep schedule set to habitual rise time (call-ins to a laboratory answering machine ensured compliance) to ensure stable sleep patterns prior to the start of the study for all children. Participants abstained from caffeine 12 hours before bedtime for the entire course of the study. Those in the ADHD group were withdrawn from psychostimulants for two weeks before and throughout the in-lab study.
In-Lab Protocol
Participants spent two consecutive nights in the laboratory on the same schedule as above: (i) an adaptation night to acclimate to the laboratory and screen for sleep-disordered breathing; (ii) the primary experimental night. On the adaptation night participants arrived approximately 3.5 hours before scheduled bedtime and were monitored overnight using polysomnography (see below). Participants returned the next evening, were provided dinner, and were again prepared for polysomnography. Approximately 80 minutes before bedtime, each participant began a cognitive test battery including a declarative memory assessment (Ellenbogen, Hulbert, Stickgold, Dinges, & Thompson-Schill, 2006) (not reported here), followed by the Motor Sequence Task (MST) (Nishida & Walker, 2007; Walker et al., 2002; Walker et al., 2003), described in detail below. MST training occurred approximately 60 minutes before bed; morning testing on the MST began after breakfast, approximately 75 minutes after waking.
Motor Sequence Task
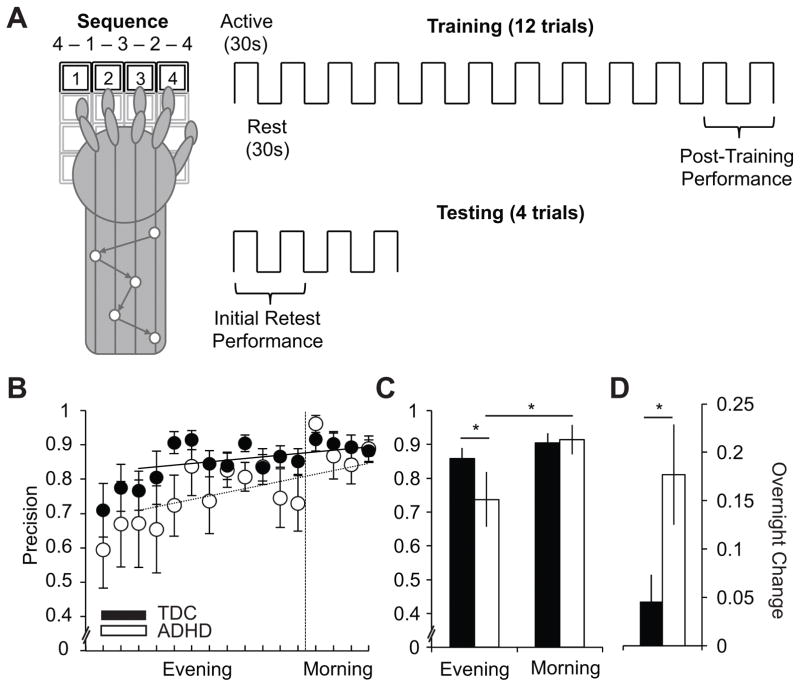
To assess procedural skill learning, participants completed a Motor Sequence Task (Figure 2, upper-panel; MST), well documented as sleep-dependent in adults (Nishida & Walker, 2007; Walker et al., 2002). The MST required participants to tap a 5-element sequence (4-1-3-2-4) on the numeric keys of a computer keyboard with the fingers of their non-dominant hand “as quickly and accurately as possible” for 30 seconds. Participants could see the sequence on the screen; however, no feedback was given with respect to accuracy. Evening training consisted of 12, 30-second trials interspersed with 30-second periods of rest. Morning testing consisted of 4, 30-second trials, with equivalent intervening rest. “MST Precision” was calculated as the proportion of total keystrokes that were not errors. This metric represents a trade-off between speed (number of correct 5-digit sequences typed) and accuracy (number of errors made). As in prior studies, evening performance was defined as the average of the final two evening trials; morning performance was defined as the average of the first two trials (Nishida & Walker, 2007; Walker et al., 2002; Walker et al., 2003; Walker & Stickgold, 2005; Walker, Stickgold, Alsop, Gaab, & Schlaug, 2005; Walker, Stickgold, Jolesz, & Yoo, 2005). A within-subject measure of overnight improvement was derived as the absolute difference between morning and evening scores.
Figure 2. Motor Sequence Task (MST) Behavior.
Motor learning was measured using the Motor Sequence Task (MST). (A) MST protocol: Evening training (right-top panel) consisted of 12 rounds of 30-second trials of repeatedly tapping the motor sequence “4 - 1 - 3 - 2 - 4” on the numeric keys of the keyboard with the non-dominant hand (schematic shown in left panel) alternated with 30-second bouts of rest. Morning testing consisted of 4 rounds of tapping and rest, as before. Evening performance was quantified as the average of the final two training trials, and morning performance as the average of the first two testing trials. MST schematic adapted and redrawn from Walker, et al., 2003 (Walker et al., 2003). (B) MST precision is reported trial-by-trial (left panel) and using evening and morning summary scores (right panel) for the TDC (black-fill) and ADHD (white-fill) groups, respectively. Lines of best fit for ADHD (dotted) and TDC (solid) are fit to the final 10 trials of training, and project forward to morning, to predict precision gains based on practice alone. (C–D) Histograms plotting evening and morning summary scores for each group (C), and within-subject overnight change scores (D). All error bars indicate standard error of the mean. Significance values are derived from appropriate statistics reported in the text and are indicated as: + = p < 0.10, * = p < 0.05.
Polysomnographic Recording
Sleep on both nights was monitored by polysomnography (PSG) using a Grass Comet XL system (Astro-Med, Inc., West Warwick, RI) measuring the electroencephalogram (EEG) at central and occipital derivations (C3/A2, C4/A1, O1/A2, and O2/A1), right and left electrooculogram (EOG), electromyogram (EMG), and electrocardiogram (ECG). Sleep stages were visually scored by trained technicians (blind to ADHD condition; inter-rater reliability ≥ 85%) in 30-second epochs from C3/A2 according to standard criteria (Rechtschaffen & Kales, 1968). Respiratory measures were applied on the adaptation night to screen to sleep-disordered breathing. An Apnea-Hypopnea Index (the number of apnea or hypopnea events per hour of sleep) was derived according to standard criteria (Iber, Ancoli-Israel, Chesson, & Quan, 2007).
Power Spectral Analysis of Sleep EEG
The current analyses focus upon sleep spindle frequencies in Stage 2 sleep due to their documented role in supporting overnight MST improvement (Nishida & Walker, 2007; Walker et al., 2002). A power spectral analysis of the Stage 2 NREM sleep EEG was performed following established procedures (Kurth et al., 2010; Mander, Santhanam, Saletin, & Walker, 2011; Ringli et al., 2013; van der Helm et al., 2011). In short, following semi-automated artifact detection fast fourier transforms on hamming-windowed 5-second segments yielded power spectral densities with 0.2 Hz resolutions. To normalize amplitude differences among individuals, the power spectrum of each channel was divided by the total integrated power (0.6–32 Hz), yielding relative power spectral densities, which were matched with corresponding sleep stages.
Relative power was assessed for the average of the C3/A2 and C4/A1 derivations, reflecting activity over primary motor cortex, motivated by prior associations with overnight MST improvement (Nishida & Walker, 2007). Group differences in sleep EEG spectra were first examined across the entire frequency range (0.6–32 Hz), and subsequently using a priori defined bands of interest in the sigma band: slow sleep spindle activity (12–13.5 Hz) and fast sleep spindle activity (13.5–15 Hz). While the primary focus of this analysis was placed on sleep spindle frequency activity, slow wave activity SWA (1–4.6 Hz) was also derived as a comparison and to test prior reports of SWA changes in ADHD (Ringli et al., 2013).
Statistical Analysis
Group differences in the EEG power density spectra were examined using bootstrapped independent-sample t-tests. This method makes no assumptions regarding the distributional qualities of the EEG frequency spectrum and is described in detail elsewhere (Tarokh & Carskadon, 2010a, 2010b). In short, for each 0.2 Hz frequency bin, 5000 random group assignments were sampled with replacement from the data, yielding a bootstrapped distribution for each group. A difference distribution, S2-S1 was used to test statistical significance of the actual difference between groups. If the true difference exceeded the 97.5th percentile of the bootstrapped distribution, it was considered statistically significant (equivalent to a two-tailed alpha level of .05). To limit erroneous Type-1 errors from testing many frequencies, significant frequencies not surrounded by at least 2 other significant frequencies were discarded.
Performance data were analyzed in StataSE 13.0 (StataCorp LP, College Station, TX) using mixed-effects models including a within-subjects factor condition (Evening/Morning) and a between-subjects factor group (ADHD/TDC). Primary focus was placed upon the interaction of group and condition; that is, whether ADHD-status moderates the impact of sleep. Exploratory simple main-effects decomposed the interaction to examine (i) whether the groups differed statistically in the morning or evening conditions and (ii) whether overnight changes occurred within-each group independently. Effect sizes are reported as appropriate.
Multiple regression analyses were used to test whether the relationship between prior night sleep EEG activity and overnight behavioral change differed between the ADHD and TDC groups. Each model included three parameters: (i) Relative EEG power, (ii) Group (ADHD/TDC), and (iii) the interaction of group and EEG power; that is, did ADHD status moderate the relationship between sleep EEG activity and overnight learning. Simple-slopes decomposed the interaction. Robust standard errors accommodated potential effects of outliers in a heterogeneous and small sample.
Results
Group Sleep Variables
Polysomnographic sleep variables are reported in Table 2. The ADHD group spent less time in bed as evident by a lower Total Dark Time (p=.025). Despite this difference, both groups demonstrated total sleep time of nearly 10 hours thus suggesting no major differences in sleep opportunity. A non-significant trend suggested fewer minutes in REM sleep in the ADHD group (p=.069); however, this trend was eliminated when expressed as a percentage of total sleep time (p=.10). No other differences in sleep were observed. No evidence for sleep-disordered breathing was observed in these participants; AHI was low for both the TDC (1.21±0.22) and ADHD (1.41±0.16) groups; further, AHI did not distinguish the groups (p=.54).
Table 2.
Polysomnographic Sleep Statistics
| ADHD n = 7 | Controls n = 14 | p | |
|---|---|---|---|
| Total Dark Time (min.) | 590 ± 15 | 602 ± 9 | .025 |
| Sleep Period Time (min.) | 563 ± 11 | 579 ± 19 | .051 |
| Total Sleep Time (min.) | 541 ± 21 | 561 ± 23 | .064 |
| Sleep Efficiency (%) | 96.0 ± 3.0 | 96.8 ± 1.7 | .44 |
| Latency to Sleep Onset (min.) | 18 ± 15 | 17 ± 10 | .76 |
| Latency to REM Sleep (min.) | 144 ± 64 | 124 ± 55 | .48 |
| Wake After Sleep Onset (min.) | 16 ± 16 | 10 ± 9 | .28 |
| Wake After Final Awakening (min.) | 8 ± 17 | 6 ± 16 | .76 |
|
|
|||
| Wake (min.) | 42 ± 28 | 32 ± 18 | .33 |
| NREM Stage 1 (min.) | 35 ± 19 | 30 ± 9 | .38 |
| NREM Stage 2 (min.) | 205 ± 29 | 222 ± 41 | .36 |
| NREM Stage 3 (min.) | 74 ± 28 | 66 ± 22 | .56 |
| NREM Stage 4 (min.) | 125 ± 21 | 124 ± 30 | .95 |
| REM Sleep (min.) | 108 ± 22 | 119 ± 18 | .069 |
| Slow Wave Sleep (min.) | 199 ± 41 | 190 ± 40 | .66 |
|
|
|||
| Wake (% TST) | 9.0 ± 5.7 | 5.9 ± 3.6 | .31 |
| NREM Stage 1 (% TST) | 6.5 ± 3.5 | 5.3 ± 1.5 | .28 |
| NREM Stage 2 (% TST) | 38.1 ± 6.4 | 39.5 ± 6.9 | .65 |
| NREM Stage 3 (% TST) | 13.5 ± 6.8 | 11.7 ± 3.6 | .43 |
| NREM Stage 4 (% TST) | 23.2 ± 4.3 | 22.3 ± 5.9 | .72 |
| REM Sleep (% TST) | 17.3 ± 3.7 | 19.8 ± 2.9 | .10 |
| Slow Wave Sleep (% TST) | 36.7 ± 7.0 | 34.0 ± 7.3 | .43 |
|
|
|||
| Stage 2 EEG Spectral Power | |||
|
|
|||
| Slow Wave Activity (% μV2) | .57 ± .058 | .57 ± .031 | .99 |
| Slow Sleep Spindle Activity (% μV2) | .024 ± .012 | .037 ± .011 | .023 |
| Fast Sleep Spindle Activity (% μV2) | .0059 ± .0014 | .011 ± .0062 | .049 |
Note: Data reported as mean ± sd. Definitions: Time in Bed: elapsed time from lights out to lights on. Sleep Period Time (SPT): elapsed time from sleep onset to final awakening. Total sleep time (TST): total time scored sleep within SPT. Sleep Efficiency: (TST/SPT)*100. Slow Wave Sleep: Stages 3+4 collapsed. Significance from 2-tailed independent-samples t-tests.
Sleep Power Spectra by Group
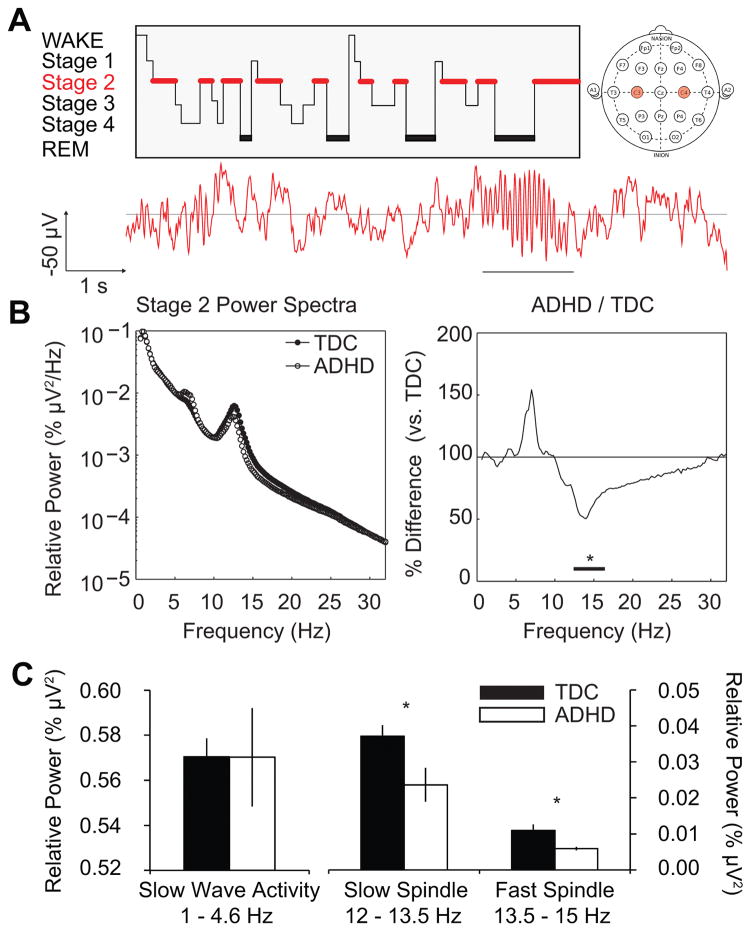
Stage 2 EEG analyses revealed a suppression of power in the spindle-related sigma band (12.4–16.4 Hz) in ADHD relative to TDC (Figure 1, bootstrapped p’s<.05). While theta activity (~4–8 Hz) was marginally higher in ADHD, this difference was not statistically significant. No other significant differences in the power spectra between ADHD and TDCs occurred. These results were confirmed by examining a priori bands of interest. Slow wave activity did not differentiate groups (t(19)=-0.01, p=.99, d=-0.0048) while the ADHD group expressed lower power in the slow (t(19)=-2.48, p=.023, d=-1.15) and fast (t(19)=-2.11, p=.049, d=-0.98) spindle bands, with the greatest deficit observed for slow spindle activity.
Figure 1. All-night NREM Stage 2 Power Spectra.
(A) Top-Left: A schematic “hypnogram” (redrawn and adapted from (Abel, Havekes, Saletin, & Walker, 2013)) demonstrating a prototypical night of sleep, as staged by polysomnography. Red highlights NREM Stage 2 sleep, from which all EEG analyses derive. Top-Right: A pictorial depiction of the standard electrode placements for EEG. Power spectra are averaged for the left and right central derivations (C3 and C4, labeled in red), respectively, corresponding to primary motor cortex. Bottom: Representative 10-seconds of Stage 2 NREM EEG. Time and amplitude scale as indicated. Underlined portion highlights a prototypical sleep spindle frequency event. (B) Left: Relative EEG power during Stage 2 sleep for the typically developing controls (black circles) and ADHD group (white circles), respectively from 0.6 Hz to 32 Hz at 0.2 Hz resolution (frequency on abscissa). Each participant’s power spectrum is normalized to his or her total power across this range yielding relative (%) values, plotted here on a base-10 logarithmic scale. Right: Stage 2 power in the ADHD group plotted as a percentage of the typically developing group; frequency axis as before. Black bar indicates frequencies of significant difference between groups. (C) Integrated Stage 2 power extracted for the slow wave, slow spindle, and fast spindle bands, respectively, using a priori frequency definitions. Significance values are derived from appropriate statistics reported in the text and are indicated as: + = p < 0.10, * = p < 0.05.
MST Behavioral Performance
MST precision—representing the trade-off between speed and accuracy—improved following sleep (Figure 2), as indicated by a main-effect of condition (Wald-χ2=17.56, p<.0001, d=.67). The magnitude of this overnight improvement in precision differed by ADHD status, as shown by an interaction of group and condition (Wald-χ2=6.08, p=.014, d=1.14). Specifically, MST precision improved overnight for the ADHD group (Wald-χ2=16.61, p<.0001, d=1.33), but not for TDCs (Wald-χ2=2.23, p=.14, d=.079). As a result, while the ADHD group had impaired MST precision in the evening vis-à-vis TDC (Wald-χ2=3.90, p=.048, d=1.33), this effect was ameliorated in the morning (Simple effect: Wald-χ2=0.02, p=.88, d=.34).
Sleep–Behavior Relationships
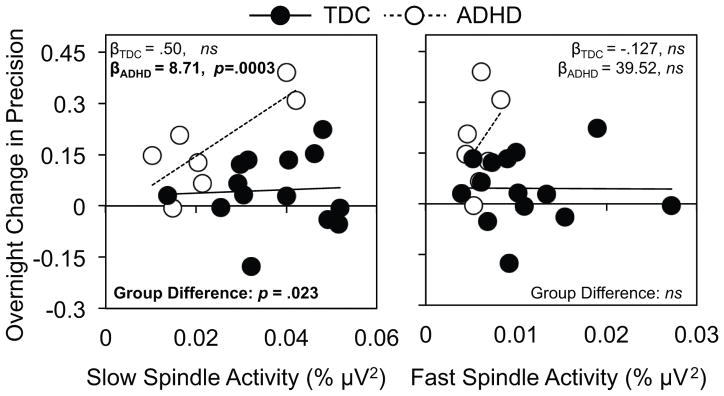
We next examined whether the group differences in slow and fast spindle-frequency activity accounted for differential overnight improvement in MST precision in ADHD; these results are illustrated in Figure 3. ADHD status significantly moderated the association between slow spindle EEG frequency activity and overnight improvement in MST precision (β=-8.22, p=.023). Specifically, MST precision was positively associated with slow spindle activity for the ADHD group (β=8.71, p=.003), but not for TDCs (β=.50, p=.82). That is, despite having lower slow spindle activity, children with ADHD appear more sensitive than TDCs to the cognitive benefits it supports. These associations were not mirrored for fast spindle activity (p’s>.05). In light of the potential (albeit not statistically significant) group difference in IQ (ADHD group: 110.3±14.1; TDCs: 120.07±9.4), we repeated this analysis with IQ included as a covariate in the model. Despite controlling for IQ, we confirmed the moderating effect of ADHD-status on the association between slow sleep spindle activity and overnight gains in MST precision (β=-8.72, p=.026); thus, a significant effect remained in the ADHD group with no effect for the TDC group (β=.435, p=.85).
Figure 3. Association between EEG spindle activity and overnight precision gains.
Scatterplots of the association between individual levels of slow (12–13.5 Hz; left) and fast (13.5–15 Hz; right) spindle activity during NREM Stage 2 sleep and overnight change in MST precision (proportion of keystrokes not associated with errors). Lines of best fit for the TDC group (black circles) are plotted as solid lines with those for the ADHD group (white circles) plotted as dotted lines. Group-wise simple slopes and the difference between these slopes are extracted from the regression models reported in the text. ns = p > 0.05.
Discussion
These initial findings provide a series of novel insights. First, despite no differences in sleep structure, sleep spindle EEG activity appears attenuated in 10–13 year old children with ADHD. Second, despite deficits in motor skill precision present in children with ADHD prior to sleep (Adi-Japha et al., 2011), no group difference was observed in the morning. Third, the degree of successful overnight improvement in ADHD children is associated with the magnitude of sleep spindle EEG frequency activity retained. Together these data underscore a necessity to ensure healthy sleep in this population, while indicating a need for future research examining mechanisms underlying these associations.
Traditional polysomnography has yet to reveal a consistent sleep signature of ADHD (Gruber, 2009; Owens et al., 2013). Our finding of reduced spindle-frequency EEG activity adds to recent quantitative spectral analyses (Prehn-Kristensen et al., 2013; Ringli et al., 2013) in demonstrating that micro-features of the sleep EEG may aid in distinguishing children with ADHD. One limitation of early, inconsistent reports of fewer spindles in ADHD (Khan & Rechtschaffen, 1978; Kiesow & Surwillo, 1987) is that sleep EEG sigma frequencies shift during development (Jenni & Carskadon, 2004; Kurdziel, Duclos, & Spencer, 2013) making automated spindle detection in this age-range challenging. The current study examined the entire range of EEG frequencies, circumventing this limitation. Further studies will continue to elucidate how sleep EEG may differentiate children with and without ADHD.
It is intriguing that no association emerged between overnight gains in MST precision and spindle-frequency EEG activity for the typically developing children, despite spindle-dependent effects in healthy adults (Nishida & Walker, 2007; Wamsley et al., 2013), consistent with other sleep-dependent memory studies in children (Wilhelm, Diekelmann, & Born, 2008). Despite this null finding in typically developing children, those with ADHD demonstrated an adult-like relationship despite an overall decrease in sleep spindle activity. Thus, the success of spindle production may be functionally protective in children with ADHD, as those with higher (more TDC-like) spindle activity benefited by exhibiting a concomitantly higher improvement in MST precision the next day. Conversely, those with a greater spindle deficit demonstrated lower overnight improvement in MST precision. The magnitude of the spindle activity deficit in a child with ADHD may therefore provide an index of their behavioral deficits, while also providing a mechanistic target within sleep for intervention. For example, non-invasive stimulation of the brain using techniques such as transcranial direct current stimulation (tDCS) (Prehn-Kristensen et al., 2014) or sensorimotor rhythm (SMR) neurofeedback (Hoedlmoser et al., 2008) can manipulate sleep EEG in a non-pharmacological manner, potentiating slow wave as well as sleep spindle frequency activity above baseline levels. These studies have demonstrated benefits in declarative memory from this modulation of the sleep EEG; however, they have not yet been extended to the motor learning domain described here.
This study is not without limitations. First, as the adaptation night may include EEG-altering first-night effects (Agnew, Webb, & Williams, 1966), the current study cannot preclude the possibility that the differences in sleep EEG reported reflect not a stable difference in ADHD but rather a difference in learning-dependent changes in EEG (Huber, Ghilardi, Massimini, & Tononi, 2004). Second, the current sample was unable to dissociate between symptom presentations of ADHD. To do so will require sample sizes greater than here or other sleep studies (Prehn-Kristensen et al., 2011; Prehn-Kristensen et al., 2014). In the context of the small sample size of the current study, we note that the strength of the effects reported (e.g., d=1.14 with respect to the group difference in overnight performance gains) indicate a potentially powerful difference in sleep-dependent cognition in ADHD, meriting a more robust sample. Specifically, a large within-group effect of sleep was observed in the ADHD group (d=1.33) yet a small effect was observed for TDCs (d=0.079). Third, our subjects were recruited to be free of disordered sleep. Thus, the current study cannot address how sleep disorders in ADHD (e.g., sleep disordered breathing (Sedky, Bennett, & Carvalho, 2014) or atypical Cyclic Alternating Pattern (Akinci et al., 2015)) may impact overnight improvements in motor learning. Fourth, the current study does not replicate a group-difference in slow wave activity (Ringli et al., 2013). The lack of frontal EEG derivations limits our present ability to address whether a stable slow wave activity difference is present in ADHD. Slow wave activity transitions from posterior- to anterior-dominance during adolescence (Kurth et al., 2012); thus, future studies employing denser EEG montages will be necessary to more adequately address this issue. Finally, the current participants were withdrawn from psychostimulants throughout the protocol, limiting the ecological validity of our participants’ sleep. Stimulants prescribed for ADHD (e.g., dextroamphetamine) may disrupt sleep (Andersen et al., 2009), although the nature of this disruption, particularly with respect to the frequencies described here, is unclear (Surman & Roth, 2011). More generally, the potential interaction of ADHD medications and sleep in mitigating behavioral deficits in ADHD warrants further examination.
In conclusion, while sleep disturbance is a common complaint in childhood ADHD, traditional polysomnography has been inconclusive in identifying a neurophysiological signature in sleep that indexes cognitive and behavioral deficits. These data join with other recent reports to indicate that microstructural properties of the sleep EEG not commonly examined in a clinical study —here sleep spindle frequency activity—may, in part, regulate next-day behavioral function in ADHD. Future clinical and basic studies will elucidate the extent to which these sleep EEG oscillations relate to the broader spectrum of symptoms common to ADHD, and whether interventions targeting such sleep neurophysiology may therapeutically aid in the treatment of this and other neurodevelopmental disorders.
Acknowledgments
This work was funded by support from the Periodic Breathing Foundation (to MAC), the Johns Hopkins Second Decade Society (to JMS) and NIMH T32MH019927 (to JMS). The authors thank Robert Daly of the Periodic Breathing Foundation, as well as Drs. Jeffrey Ellenbogen, Robert Stickgold, and Robert Thomas for their guidance in study design, Drs. Tifenn Raffray, Eliza Van Reen and Bryce Mander for considerations in preparation of this manuscript, and laboratory staff—particularly Henry Arantes, Dave Bushnell, Jennifer King, Denise Maceroni, and Erin McInrue—for assistance in collecting the data. The authors finally offer thanks to the participants in this study and their families for the contribution of their time and effort.
References
- Abel T, Havekes R, Saletin JM, Walker MP. Sleep, plasticity and memory from molecules to whole-brain networks. Curr Biol. 2013;23(17):R774–788. doi: 10.1016/j.cub.2013.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adi-Japha E, Fox O, Karni A. Atypical acquisition and atypical expression of memory consolidation gains in a motor skill in young female adults with ADHD. Res Dev Disabil. 2011;32(3):1011–1020. doi: 10.1016/j.ridd.2011.01.048. [DOI] [PubMed] [Google Scholar]
- Agnew HW, Jr, Webb WB, Williams RL. The first night effect: an EEG study of sleep. Psychophysiology. 1966;2(3):263–266. doi: 10.1111/j.1469-8986.1966.tb02650.x. [DOI] [PubMed] [Google Scholar]
- Akinci G, Oztura I, Hiz S, Akdogan O, Karaarslan D, Ozek H, Akay A. Sleep Structure in Children With Attention-Deficit/Hyperactivity Disorder. J Child Neurol. 2015;30(11):1520–1525. doi: 10.1177/0883073815573318. [DOI] [PubMed] [Google Scholar]
- Andersen ML, Margis R, Frey BN, Giglio LM, Kapczinski F, Tufik S. Electrophysiological correlates of sleep disturbance induced by acute and chronic administration of D-amphetamine. Brain Res. 2009;1249:162–172. doi: 10.1016/j.brainres.2008.10.023. [DOI] [PubMed] [Google Scholar]
- Ellenbogen JM, Hulbert JC, Stickgold R, Dinges DF, Thompson-Schill SL. Interfering with theories of sleep and memory: sleep, declarative memory, and associative interference. Curr Biol. 2006;16(13):1290–1294. doi: 10.1016/j.cub.2006.05.024. [DOI] [PubMed] [Google Scholar]
- Fischer S, Hallschmid M, Elsner AL, Born J. Sleep forms memory for finger skills. Proc Natl Acad Sci U S A. 2002;99(18):11987–11991. doi: 10.1073/pnas.182178199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber R. Sleep characteristics of children and adolescents with attention deficit-hyperactivity disorder. Child Adolesc Psychiatr Clin N Am. 2009;18(4):863–876. doi: 10.1016/j.chc.2009.04.011. [DOI] [PubMed] [Google Scholar]
- Hoedlmoser K, Pecherstorfer T, Gruber G, Anderer P, Doppelmayr M, Klimesch W, Schabus M. Instrumental conditioning of human sensorimotor rhythm (12–15 Hz) and its impact on sleep as well as declarative learning. Sleep. 2008;31(10):1401–1408. [PMC free article] [PubMed] [Google Scholar]
- Huber R, Ghilardi MF, Massimini M, Tononi G. Local sleep and learning. Nature. 2004;430(6995):78–81. doi: 10.1038/nature02663. [DOI] [PubMed] [Google Scholar]
- Iber C, Ancoli-Israel S, Chesson A, Quan S, editors. The AASM manual for the scoring of sleep and associated events: rules, terminology, and technical specification. 1. Westchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- Jenni OG, Carskadon MA. Spectral analysis of the sleep electroencephalogram during adolescence. Sleep. 2004;27(4):774–783. [PubMed] [Google Scholar]
- Kaufman AS, Kaufman NL. Kaufman Brief Intelligence Test. 2. Bloomington, MN: Pearson, Inc; 2004. [Google Scholar]
- Khan A, Rechtschaffen A. Sleep patterns and sleep spindles in hyperkinetic children. Sleep Research. 1978;7:137. [Google Scholar]
- Kiesow NA, Surwillo WW. Sleep spindles in the EEGs of hyperactive children. Psychol Rep. 1987;60(1):139–144. doi: 10.2466/pr0.1987.60.1.139. [DOI] [PubMed] [Google Scholar]
- Kurdziel L, Duclos K, Spencer RM. Sleep spindles in midday naps enhance learning in preschool children. Proc Natl Acad Sci U S A. 2013;110(43):17267–17272. doi: 10.1073/pnas.1306418110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurth S, Jenni OG, Riedner BA, Tononi G, Carskadon MA, Huber R. Characteristics of sleep slow waves in children and adolescents. Sleep. 2010;33(4):475–480. doi: 10.1093/sleep/33.4.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurth S, Ringli M, Lebourgeois MK, Geiger A, Buchmann A, Jenni OG, Huber R. Mapping the electrophysiological marker of sleep depth reveals skill maturation in children and adolescents. Neuroimage. 2012;63(2):959–965. doi: 10.1016/j.neuroimage.2012.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mander BA, Santhanam S, Saletin JM, Walker MP. Wake deterioration and sleep restoration of human learning. Curr Biol. 2011;21(5):R183–184. doi: 10.1016/j.cub.2011.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida M, Walker MP. Daytime naps, motor memory consolidation and regionally specific sleep spindles. PLoS One. 2007;2(4):e341. doi: 10.1371/journal.pone.0000341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens J, Gruber R, Brown T, Corkum P, Cortese S, O’Brien L, … Weiss M. Future research directions in sleep and ADHD: report of a consensus working group. J Atten Disord. 2013;17(7):550–564. doi: 10.1177/1087054712457992. [DOI] [PubMed] [Google Scholar]
- Prehn-Kristensen A, Molzow I, Munz M, Wilhelm I, Muller K, Freytag D, … Baving L. Sleep restores daytime deficits in procedural memory in children with attention-deficit/hyperactivity disorder. Res Dev Disabil. 2011;32(6):2480–2488. doi: 10.1016/j.ridd.2011.06.021. [DOI] [PubMed] [Google Scholar]
- Prehn-Kristensen A, Munz M, Goder R, Wilhelm I, Korr K, Vahl W, … Baving L. Transcranial oscillatory direct current stimulation during sleep improves declarative memory consolidation in children with attention-deficit/hyperactivity disorder to a level comparable to healthy controls. Brain Stimul. 2014;7(6):793–799. doi: 10.1016/j.brs.2014.07.036. [DOI] [PubMed] [Google Scholar]
- Prehn-Kristensen A, Munz M, Molzow I, Wilhelm I, Wiesner CD, Baving L. Sleep promotes consolidation of emotional memory in healthy children but not in children with attention-deficit hyperactivity disorder. PLoS One. 2013;8(5):e65098. doi: 10.1371/journal.pone.0065098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rechtschaffen A, Kales A, editors. A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. Los Angeles, CA: Brain Information Service/Brain Research Institute, University of California; 1968. [Google Scholar]
- Ringli M, Souissi S, Kurth S, Brandeis D, Jenni OG, Huber R. Topography of sleep slow wave activity in children with attention-deficit/hyperactivity disorder. Cortex. 2013;49(1):340–347. doi: 10.1016/j.cortex.2012.07.007. [DOI] [PubMed] [Google Scholar]
- Sadeh A, Sharkey KM, Carskadon MA. Activity-based sleep-wake identification: an empirical test of methodological issues. Sleep. 1994;17(3):201–207. doi: 10.1093/sleep/17.3.201. [DOI] [PubMed] [Google Scholar]
- Sedky K, Bennett DS, Carvalho KS. Attention deficit hyperactivity disorder and sleep disordered breathing in pediatric populations: a meta-analysis. Sleep Med Rev. 2014;18(4):349–356. doi: 10.1016/j.smrv.2013.12.003. [DOI] [PubMed] [Google Scholar]
- Shaffer D, Fisher P, Lucas CP, Dulcan MK, Schwab-Stone ME. NIMH Diagnostic Interview Schedule for Children Version IV (NIMH DISC-IV): description, differences from previous versions, and reliability of some common diagnoses. J Am Acad Child Adolesc Psychiatry. 2000;39(1):28–38. doi: 10.1097/00004583-200001000-00014. [DOI] [PubMed] [Google Scholar]
- Sheslow D, Adams W. Wide Range Assessment of Memory and Learning, Second Edition administration and technical manual. Lutz, FL: Psychological Assessment Resources; 2003. [Google Scholar]
- Smith CS, Reilly C, Midkiff K. Evaluation of three circadian rhythm questionnaires with suggestions for an improved measure of morningness. J Appl Psychol. 1989;74(5):728–738. doi: 10.1037/0021-9010.74.5.728. [DOI] [PubMed] [Google Scholar]
- Surman CB, Roth T. Impact of stimulant pharmacotherapy on sleep quality: post hoc analyses of 2 large, double-blind, randomized, placebo-controlled trials. J Clin Psychiatry. 2011;72(7):903–908. doi: 10.4088/JCP.11m06838. [DOI] [PubMed] [Google Scholar]
- Tarokh L, Carskadon MA. Developmental changes in the human sleep EEG during early adolescence. Sleep. 2010a;33(6):801–809. doi: 10.1093/sleep/33.6.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarokh L, Carskadon MA. Sleep electroencephalogram in children with a parental history of alcohol abuse/dependence. J Sleep Res. 2010b;19(1 Pt 2):165–174. doi: 10.1111/j.1365-2869.2009.00763.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Helm E, Yao J, Dutt S, Rao V, Saletin JM, Walker MP. REM sleep depotentiates amygdala activity to previous emotional experiences. Curr Biol. 2011;21(23):2029–2032. doi: 10.1016/j.cub.2011.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker MP, Brakefield T, Morgan A, Hobson JA, Stickgold R. Practice with Sleep Makes Perfect: Sleep-Dependent Motor Skill Learning. Neuron. 2002;35:205–211. doi: 10.1016/s0896-6273(02)00746-8. [DOI] [PubMed] [Google Scholar]
- Walker MP, Brakefield T, Seidman J, Morgan A, Hobson JA, Stickgold R. Sleep and the time course of motor skill learning. Learn Mem. 2003;10(4):275–284. doi: 10.1101/lm.58503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker MP, Stickgold R. It’s practice, with sleep, that makes perfect: implications of sleep-dependent learning and plasticity for skill performance. Clin Sports Med. 2005;24(2):301–317. ix. doi: 10.1016/j.csm.2004.11.002. [DOI] [PubMed] [Google Scholar]
- Walker MP, Stickgold R, Alsop D, Gaab N, Schlaug G. Sleep-dependent motor memory plasticity in the human brain. Neuroscience. 2005;133(4):911–917. doi: 10.1016/j.neuroscience.2005.04.007. [DOI] [PubMed] [Google Scholar]
- Walker MP, Stickgold R, Jolesz FA, Yoo SS. The functional anatomy of sleep-dependent visual skill learning. Cereb Cortex. 2005;15(11):1666–1675. doi: 10.1093/cercor/bhi043. [DOI] [PubMed] [Google Scholar]
- Wamsley EJ, Shinn AK, Tucker MA, Ono KE, McKinley SK, Ely AV, … Manoach DS. The effects of eszopiclone on sleep spindles and memory consolidation in schizophrenia: a randomized placebo-controlled trial. Sleep. 2013;36(9):1369–1376. doi: 10.5665/sleep.2968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm I, Diekelmann S, Born J. Sleep in children improves memory performance on declarative but not procedural tasks. Learn Mem. 2008;15(5):373–377. doi: 10.1101/lm.803708. [DOI] [PubMed] [Google Scholar]
- Wilkinson GS, Robertson GJ. Wide Range Achievement Test 4 professional manual. Lutz, FL: Psychological Assessment Resources; 2006. [Google Scholar]