Abstract
Scarring following burn injury and its accompanying aesthetic and functional sequelae still pose major challenges. Hypertrophic scarring (HTS) can greatly impact patients’ quality of life related to appearance, pain, pruritus and even loss of function of the injured body region. The identification of molecular events occurring in the evolution of the burn scar has increased our knowledge; however, this information has not yet translated into effective treatment modalities. Although many of the pathophysiologic pathways that bring about exaggerated scarring have been identified, certain nuances in burn scar formation are starting to be recognized. These include the effects of neurogenic inflammation, mechanotransduction, and the unique interactions of burn wound fluid with fat tissue in the deeper dermal layers, all of which may influence scarring outcome. Tension on the healing scar, pruritus, and pain all induce signaling pathways that ultimately result in increased collagen formation and myofibroblast phenotypic changes. Exposure of the fat domes in the deep dermis is associated with increased HTS, possibly on the basis of altered interaction of adipose-derived stem cells and the deep burn exudate. These pathophysiologic patterns related to stem cell-cytokine interactions, mechanotransduction, and neurogenic inflammation can provide new avenues of exploration for possible therapeutic interventions.
Hypertrophic scarring (HTS) is a major concern in deep partial thickness burn injuries. A scar generally forms within weeks of insult, and studies indicate that a wound that takes longer than 2–3 weeks to heal is characterized by an increased risk of hypertrophic scar formation.1 The incidence of HTS occurrence following burn injuries has been reported to range from 32 to 94%.2,3 This condition can be associated with significant pain and limited movement when present over joints. Aside from these symptomatic and functional elements, the ultimate unsightly aesthetic appearance of hypertrophic scars can be a great psychological burden for affected patients.2
Normal wound healing relies upon a series of steps including inflammation at the site of injury, cell migration, proliferation, differentiation, angiogenesis, reepithelialization, synthesis, and remodeling of the extracellular matrix (ECM).4–9 The three stages of inflammation, proliferation, and remodeling in wound healing are present in all types of burn injuries, but the period of each stage differs and carries dramatic downstream consequences.10 Superficial burns typically undergo a complete recovery with minimal scarring within 14 days of injury. In deep partial thickness, as well as full thickness burns, healing involves processes such as reepithelialization and contraction. In partial thickness, burns reepithelialization begins due to keratinocyte migration from viable skin appendages in the dermis a few hours after the injury occurs. In deeper burns with a diminished number of skin appendages, healing takes place from the edges rather than the center of the wound,3,8,10,11 thus more contraction is necessary for closure. This additional contraction is provided by the continuous activation of the transforming growth factor beta (TGF-β) pathway, which generally stalls the remodeling phase of wound healing and can eventually lead to formation of a hypertrophic scar.10
Burn wounds are a specific type of injury which, unlike other types of wounds, are characterized by profound systemic effects.10,12,13 Burn injury affects virtually every body system leading to changes in heart, lung, liver, kidney, GI tract, lymphoid organ and bone marrow function,12 and sometimes multiple organ dysfunction syndrome (MODS).13 Studies have shown that inflammatory mediators responsible for these systemic effects include tumor necrosis factor alpha (TNF-α), interleukins 6, 8, and 1-beta (IL-6, IL-8, IL-1β).14,15 These mediators are released at the site of the initial burn injury,13 and their serum levels correlate with the surface area of the burn wound.16–18 Elevated serum levels of TNF-α, IL-6 and IL-8 have been linked to an increased risk of developing burn wound infections, MODS, and ultimately death.13–15
The systemic and local events at the injury site are tightly interwoven in burn pathophysiology, which is unique to this type of wound and can dramatically affect its healing. Although early surgical intervention is highly recommended in the management of burn wounds,12 debridement and closure is often delayed because of the patient’s critical health condition that needs to be stabilized before surgery.10 Profound changes in body metabolism following burn injury are characterized by a hypermetabolic/catabolic state.13 These factors predispose to delayed wound healing with a high rate of abnormal scar formation. Moreover, burns often involve a larger surface area as compared to other types of wounds, which leads to extended scarring.
Wound healing in burn injury is also highly affected by a pronounced immune imbalance. Early-stage burn trauma is characterized by bone marrow suppression with an impaired function of both myeloid and lymphoid immune cells.10,12 Suppressed immune function predisposes the burn wound to infection which is often resistant to empiric therapy and can evolve into sepsis. Both systemic and local infectious complications further delay wound healing. Conversely, studies have demonstrated elevated expression of heat shock proteins (HSPs) in neutrophils following severe burn trauma, leading to increased oxidative activity and decelerated apoptosis of these cells.19 The inflammatory phase of wound healing thus becomes prolonged, and is characterized by excessive exposure of the wound to inflammatory mediators and growth factors. A correlation between prolonged neutrophil infiltration of the burn wound and increased serum levels of TGF-β1 has also been described.20
Thus, multiple factors contribute to hypertrophic scar formation in burn wounds. Characteristics of HTS are commonly associated with collagen overproduction and an altered ratio of type I to type III collagen.4 Moreover, decreased matrix metalloproteinase (MMP) activity results in the prevention of appropriate collagen degradation.4,21 In addition, increased growth factor production including previously mentioned TGF-β, connective tissue growth factor (CTGF), and vascular endothelial growth factor (VEGF) plays a significant role in hypertrophic scar development.6,21,22 Balanced inflammation, angiogenesis, and granulation tissue formation are essential phases of proper scar formation. Maintaining physiological levels of growth factors in the wound bed is critical to scar outcome as elevated levels of TGF-β, CTGF, and VEGF have been shown to promote hypergranulation and HTS.21,22
It is apparent that the depth of a burn wound correlates with corresponding anatomical structure signaling cascades. At the superficial level, nerve endings are stimulated resulting in a transitory neurogenic inflammation that can impact the burn wound outcome. In addition, mechanoreceptors within the superficial cells signal the impact of outside vector forces on the wound, translating into increased collagen production. As the burn wound deepens anatomically, distinct fat compartments are exposed, which also carry unique signaling profiles resulting in fibroblast to myofibroblast conversion and a potential for HTS formation. Lastly, in all anatomic areas described, mesenchymal stem cells (MSCs) may be involved in regeneration and healing. However, the process is complex and the optimal timing of stem cell stimulation and potential therapeutic usage is still not clear. Thus, these nuanced situations related to burn depth and recent scientific discovery have been chosen as background for the discussion of burn-related HTS.
HYPERTROPHIC SCAR DEVELOPMENT
Hypertrophic scar development is dependent on the key regulator TGF-β and its downstream effectors. This pathway functions to recruit and stimulate fibroblasts to produce a new structural framework through the deposition of collagen.6 During both the normal and abnormal wound healing process, cyto-/chemokines are released at the site of injury, causing various cell types (neutrophils, monocytes, endothelial cells, fibroblasts, MSCs, etc.) to migrate to the wound bed within 24–72 hours.4,7 It has been suggested that the chemokine SDF-1 (stromal cell-derived factor 1) through the interaction with its receptor CXCR4 plays an important role in this process.9,23 Fibroblasts subsequently proliferate and differentiate into myofibroblasts, cells having a smooth muscle cell-like phenotype, through mechanical activation and chemical inflammatory mediators.
Myofibroblasts are the primary cells responsible for wound contraction and remodeling.6,9,21,24–26 They play a key role in the initial inflammatory stage of wound healing by secreting their own cytokines (IL-6, IL-10, and TGF-β) which serve to enhance the inflammatory response. Myofibroblasts contain bundles of microfilaments connected to extracellular fibronectin that collectively serve as a contractile mechanism to generate force around the ECM. This force is maintained over time, and further contraction is reinforced by continual deposition of collagen.24,27 Although myofibroblasts are necessary for wound closure, during normal wound healing they undergo massive apoptosis or deactivation after epithelialization is complete.26,28,29 However, in chronic pathophysiology such as HTS, excessive ECM deposition by myofibroblasts can persist for years, as these cells fail to undergo normal apoptosis.24,25
The mechanisms of apoptosis resistance in HTS fibroblasts/myofibroblasts are complex. Linge et al. previously demonstrated that normal scar fibroblasts undergo significant apoptosis during collagen contraction while HTS fibroblasts showed resistance to apoptosis in an in vitro model of wound healing.30 One proposed mechanism of fibroblasts dysfunction is the overexpression of tissue transglutaminase, an enzyme that stabilizes newly formed ECM. The reduction of transglutaminase activity in a collagen gel permitted HTS fibroblast apoptosis on gel contraction.30 Myofibroblast apoptosis has been linked to the presence of IL-1β, as studies have shown that apoptosis levels increase as the amounts of IL-1β increase.27 Van De Water et al. suggest that persistent myofibroblast function in HTS is due to a feed-forward loop based on prolonged inflammation and activation of TGFβ1.25 The presence of a focal adhesion protein Hic-5 has been shown to be necessary for the production of TGF-β1 and maintenance of the HTS myofibroblast phenotype.25,31 Genetic silencing of Hic-5 reduces myofibroblast characteristics such as expression of alpha-smooth muscle actin (α-SMA), collagen I production and contraction.31 Hic-5 is induced by TGF-β1 and remains consistently high in HTS myofibroblasts. Thannickal and Horowitz proposed two converging pathways by which TGF-β1 elicits an apoptosis-resistant phenotype for HTS myofibroblasts.32 The first pathway transduces cell adhesion signals through integrin-linked kinase and focal adhesion kinase (FAK), while the second pathway depends on p38 mitogen-activated protein kinases (MAPK) and PI3 kinase/AKt-dependent signaling.33 The CCN family of matricellular proteins has been shown to promote apoptosis or myofibroblast senescence through interactions with ECM proteins and integrins.34,35 Overall, it appears that the persistence of myofibroblasts in the setting of HTS is due to a combination of feed-forward pathways that integrate the mechanical environment, extracellular growth factor activation and signaling, and intracellular tension and gene expression.25
In addition to the factors described above, CTGF appears to persist in the setting of HTS, and may be an important target in its prevention.21,36 Increasing evidence from Tredgets group also indicates that HTS develops after varied interactions between TGF-β1, T helper 1/2/3 cytokines, decorin and MMPs acting in different phases of the wound healing process.37
As noted above, there is a correlation of the depth of injury and the associated signaling response—this is one of a few of the more exciting areas of ongoing HTS research. These areas include:
Neurogenic inflammation and its contribution to HTS.
The phenomenon of mechanotransduction, or the mechanism by which mechanical force is translated into a biochemical reaction that may precede HTS.
The anatomic association of HTS with fat domes emanating from the deep dermis.
The possible use of stem cell therapies for HTS treatment.
These new research areas are discussed below.
Neurogenic inflammation/itch/pruritus
Following initial resuscitation and early homeostatic control, the greater challenges in the long term management of thermal injuries include pain (67.6%), itching (73.3%), and abnormal appearance (75.2%) according to burn therapists.38 Itching/pruritus of the affected area begins at the time of wound closure, but intensifies after 3 months and continues for over a year. Although it has been traditionally viewed as a side effect of wound healing, pruritus is in fact an important part of its pathophysiology. The tremendous discomfort caused by pruritus and the subsequent scratching of the wound is thought to trigger a cellular process known as neurogenic inflammation. This enhances hypertrophic scar formation in areas with extensive thermal damage, thus, pruritus should be minimized.
Peripheral nerve damage is speculated to play a key role in wound related pruritus and hypertrophic scar formation. Stimulation of sensory nerves results in the release of neuropeptides— extracellular messengers which may act as neurotransmitters, hormones, and paracrine factors.39,40 In the normal wound healing setting, neuropeptides modulate key steps such as vasodilation, inflammatory response, and proliferation of epithelial, vascular, and connective tissue cells.39,41–43 Substance P (SP) is a neuropeptide that is released from nerve endings at the site of injury and induces inflammation, mediates angiogenesis, keratinocyte proliferation, and fibrogenesis.41,43,44 It plays an important role in the maturation phase of wound healing, where collagen degradation must be regulated.45 Substance P has been found to modulate the activity of MMPs and tissue inhibitors of metalloproteinases in fibroblasts.46 Conversely, it may also promote HTS by inducing fibroblast proliferation.39,47 Substance P levels were found to be greater in wounds resulting from acute partial and fullthickness burns injuries, as compared to superficial burns and unburned skin,48 and even greater in hypertrophic scars forming after deep burn injuries.49 Along with central neuropeptides, such as the corticotropin releasing factor, SP can also initiate a systemic stress response through multiple pathways including the sympathetic nervous system, hypothalamic-pituitary axis, the renin-angiotensin system, and a release of a variety of stress hormones.50–52
Mechanical stress such as skin stretching and itching during wound healing stimulates mechanosensitive nociceptors on sensory unmyelinated Ae and C fibers of the skin.53–55 Stimulation of these fibers leads to the release of neuropeptides such as SP, calcitonin-gene related peptide, neurokinin A and vasoactive intestinal peptide, which mediate a variety of effects at the wound bed, known as neurogenic inflammation.39 Neuropeptides modulate the activities of keratinocytes, Langerhans cells, mast cells, dermal endothelial cells, fibroblasts, and immune cells. As noted above, they affect multiple cell functions including proliferation, cytokine production, antigen presentation, sensory neurotransmission, mast cell degradation, and vasodilation. Activated endothelial cells induce vasodilation and permeability of vessels. Mast cells secrete histamine which also causes vasodilation and an increase in of vessel permeability through conjugation with its H1 receptors, as well as the induction of pruritus when the binding occurs on C-fibers. The clinical signs of neurogenic inflammation are thus commonly seen as erythema, edema, and pruritus.54,56
Simultaneously, neuropeptides up-regulate the production of TGF-β and nerve growth factor (NGF) in fibroblasts and other cell types. This neurogenic inflammation initiates the dysfunctional cascade as previously described: up-regulation of TGF-β triggers differentiation of fibroblasts into myofibroblasts as well as stimulating their production of collagen. Overexpression of NGF leads to excessive release of neuropeptides from nerve fibers, and the cycle repeats over and over again, even without additional mechanical stress.54,57 Additionally, it has been shown that the activity of neutral endopeptidase is decreased in HTS, which leads to an increased SP concentration, and ultimately results in a greater neuroinflammatory response.49
In summary, pruritus and the physical disruption that occurs as a result of scratching a healing wound increases the release of neuropeptides, which cause neurogenic inflammation and ultimately stimulate abnormal collagen production. The accumulation of cells in the ECM of immature wound beds is associated with increased activity of inflammatory and immune cells. Langerhans cells, macrophages, and activated T-cells prevent normal tissue repair. At the same time, neuropeptides, specifically SP, promote a neuroinflammatory response. This further promotes cell proliferation, cytokine and growth factor accumulation, which result in hyperemia, pruritus and ECM overproduction.43
Current treatments of severe pruritus include antihistamines, opioid antagonists, and over-the-counter topical treatments—all of which have varied effectiveness. Stabilizing the wound bed by protecting it from sweat, biomechanical forces, and interface movement may limit pruritus in healing wounds.
Mechanotransduction
As discussed above, mechanical cues are important factors in the development of HTS. Not only does mechanical stimulation cause neurogenic inflammation in the wound bed, but it also triggers a set of cellular and extracellular events, ultimately resulting in abnormal ECM accumulation and scar formation. It is a widely accepted fact that HTS typically occurs in areas that experience high tension (anterior chest, scapular region, shoulder, etc.), and it has been long known that mechanical stress is closely related to hypertrophic scar formation.3,4,21,58–60 It is also well known that incisions closed under higher tension result in greater scar formation when compared to incisions closed under lower tension.4,59 The phenomena of conversion of mechanical signals into biochemical responses is referred to as mechanotransduction.
There are several interrelated pathways by which cells can be mechanically stimulated including integrin-matrix interactions, cytoskeletal strain, growth factor receptors, G protein-coupled receptors, and stretch ion channels.21,59–61 Cells interact with the ECM through transmembrane integrins, which further associate with various binding proteins and kinases to trigger downstream intracellular effects.21,59,60,62 Alterations in the physical microenvironment after tissue injury result in the activation of multiple intra/intercellular pathways that ultimately work to reestablish mechanical homeostasis. Myofibroblasts pick up mechanical signals and respond by causing wound contraction.6,21,24–26 The mechanical signals are thought to influence myofibroblast formation, function, extracellular growth factor activation, and intracellular transcription factor regulation.25
Mechanical stimulation has been shown to induce fibroblast production of collagen, expression of α-SMA (a marker for HTS), activation of myofibroblasts, and the release of pro-fibrotic cytokines.21,24,59,62,63 Fibroblasts and keratinocytes are important for tension perception. Mechanoreceptors in these cells are responsible for the initiation of intercellular and matrix-cellular signal transmission.21,59,64,65 Integrins have been shown to specifically activate pro-fibrotic cytokine cascades.21,62,66,67 Fibroblasts interact with collagen fibrils through various integrins, which in turn regulate fibroblast differentiation and collagen production. It has been shown that mechanical stress and members of the TGF-β superfamily activate the α11β1 integrin heterodimer, which is a collagen receptor regulating fibroblast to myofibroblast differentiation.68
Latent TGF-β-binding proteins have been observed tethering TGF-β to the ECM. These latent proteins are anchored to fibrillin microfibrils—physical force activation mediated through integrins may then release and activate the tethered TGF-β which can then impact downstream effector proteins Smad 2 and 3 to induce several profibrotic mechanisms, including collagen production and fibroblast to myofibroblast differentiation.59–61
The TGF-β pathway in turn can control integrin expression and activity through the Smad effector proteins, demonstrating the interplay between cytokine and mechanotransduction signaling.60,67 FAK, a nonreceptor protein tyrosine kinase, regulates survival, motility, inflammatory signaling, and collagen production in fibroblasts. Focal adhesion complexes containing FAK also target MAPK, which respond to extracellular signals and regulate numerous intracellular pathways including cell proliferation, survival, and gene expression.59,60,63,69 Mechanical strain also promotes keratinocyte proliferation and migration which is another feature of HTS.70,71
Mechanotransduction pathways in wound healing are complex, and the above discussed pathway is not the only one involved in the transmission of mechanical signals. FAK also targets RhoGTPase. RhoGTPase and its downstream effector, ROCK, are important components of the Rho/ROCK signaling pathway that is known to regulate fibroblast and keratinocyte responses to mechanical forces.60,72 RhoGTPases influence cell tension, motility, adherence and cytoskeletal dynamics, in addition to stimulating myofibroblast differentiation.63,73 The characteristic for myofibroblasts expression of α-SMA is dependent on environmental stiffness and requires factors from Rho/ROCK pathway, as well as the myocardin-related transcription factor A (MRTF-A).74 Blocking Rho/ROCK signaling has been shown to inhibit matrix stiffening-induced α-SMA expression in myofibroblasts,74 and specific inhibition of ROCK has been shown to reduce granulation tissue contraction.25
Another pathway essential to tissue regeneration is the Hippo signaling pathway, which contains the two key downstream transcription coactivators: Yes-associated protein (YAP) and Transcriptional coactivator with PDZ-binding motif (TAZ). Studies done with full thickness wounds have shown that the knockdown of YAP and TAZ significantly delays the rate of wound closure and reduces the amount of TGF-β1 expression in fibroblast cells.75 These factors also modulate the expression of TGF-β1 signaling pathway components, such as Smad2 and Smad7.75–77 Specifically, YAP is capable of interacting with Smad7 to enhance its ability to inhibit the TGF-β pathway.76 Although the specific mechanism of TAZ modulation of the TGF-β pathway has not been discovered, TAZ has been shown to be as effective as YAP in controlling TGF-β1 signaling. The YAP/TAZ pathway may be modulating the wound healing process through increasing the production of TGF-β1 and other TGF-β-related signaling factors, such as CTGF and the Smad proteins. Nuclear localization of YAP/TAZ facilitates crosstalk with the TGF-β1/Smad pathway to ultimately influence wound closure, cell proliferation, and collagen formation.63,75
It is also important to consider the intrinsic mechanotransduction pathway mediated by the megakaryoblastic leukemia factor-1 (MKL-1) and MRTF-A. As previously mentioned, mechanical stimuli from the environment are converted to fibrogenic signals that promote myofibroblast differentiation.25,74,78 MRTF-A acts as an actin dynamics sensor and serves a central role in activating expression of fibrotic genes. Mice deficient in MRTF-A show reduced myofibroblast differentiation and attenuated scar formation.74 Nuclear localization of MRTF-A activates the fibrotic phenotype through α-SMA gene expression.25
Intracellular mechanotransduction is also regulated by stretch activated calcium-dependent ion channels, as they play key roles in the regulation of cytoskeletal function. Mechanical stimulation leads to an influx of Ca2+ through these channels, triggering a sequence of events that ultimately leads to fibrotic gene expression.63,74 Calcium plays a central role in wound healing by acting as an intracellular messenger in multiple cells, including fibroblasts. Studies have shown calcium influx and calcium mediated signaling in fibroblasts in response to mechanical stimulation.55,79,80 Thus, a decrease in intracellular calcium leads to impaired signaling in response to mechanoreceptor and neuropeptide receptor activity.55,81,82
Thus, multiple interrelated signaling pathways participate in the complex mechanism of intracellular mechanotransduction. These signal pathways are mediated by integrin-matrix interactions, growth factor receptors (TGF-β), G protein-coupled receptors, mechanoresponsive ion channels and cytoskeletal strain responses.59–61
In summary, external mechanical stresses on a wound can trigger an unrelenting cycle of repeated fibrotic gene activation resulting in excessive collagen production and fibroblast differentiation. The intermittent repetition of this pathway contributes to the outcome of HTS. Furthermore, in the wound bed, external vector forces influence the collagen bundle size and the directionality of collagen and other ECM compounds in scar tissue.21
Therefore, stabilizing the wound environment by preventing external forces from initiating pathways that lead to exaggerated scarring can help maintain normal repair processes and collagen production. Compression garments, occlusive and adhesive skin taping, and silicone gel sheets have been commonly used to reduce tension, form an occlusion on the scar surface, and increase pressure on the scar.21,55,59,83 Studies involving the use of silicone sheeting and compression bandages have demonstrated a level of success in limiting hypertrophic scar formation, as both therapies target mechanical offloading of the wound environment.59,83 Improved designs for dressings that control the biomechanics of the wound bed have the potential to improve healing and further reduce scar formation.
Dermal cones/fat domes
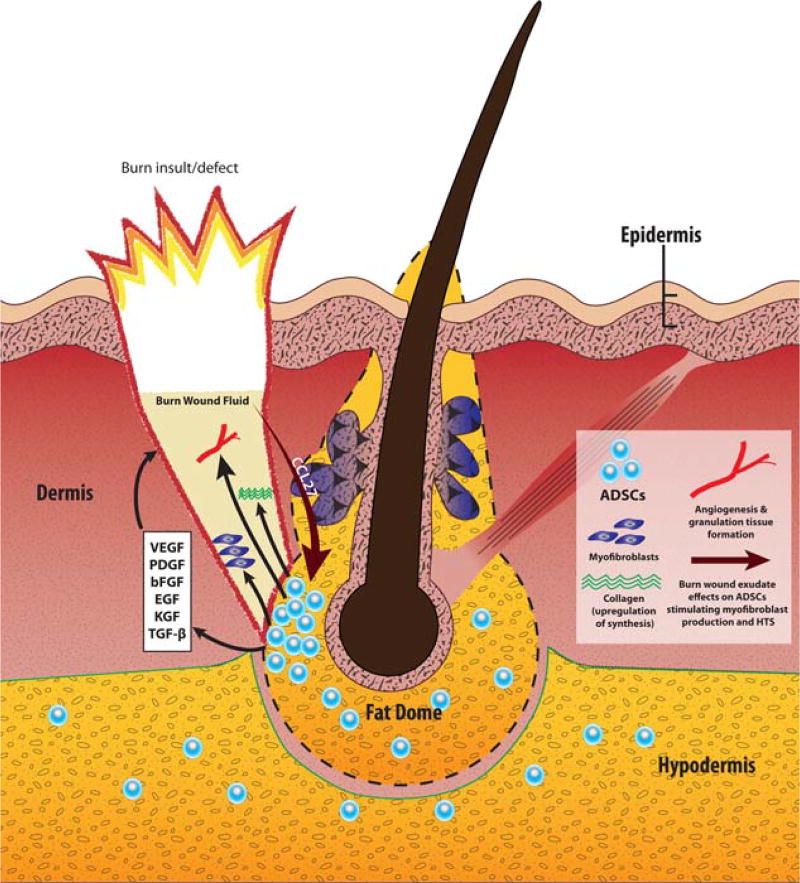
As the burn wound depth increases, structures other than sensitive nerve endings and mechanoreceptors begin to play a role in healing and scar formation. Perhaps one of the more interesting relevant structures, the dermal cone, has been increasingly investigated. These cones are specific anatomical units within the otherwise homogenous collagen/elastin dermal matrix.84 They consist of a lower portion, which contains fat tissue (referred to as the fat dome) and the deep aspect of the hair follicle, as well as an upper portion that contains other skin appendages (Figure 1). The cone has a very small diameter at the surface of the epidermis, and the most visible aspect at this level is the hair shaft. At the bottom of the dermis, the cone can be up to 2–3 mm in diameter and this is represented mainly by the fat dome that merges with the adipose tissue of the hypodermis. The fat dome is thought to be the place where the endogenous MSCs, namely adipose-derived stem cells (ADSCs), of the skin reside. Overall, the cone is of varying diameter and contains skin appendages, vessels, and nerves.85
Figure 1.
The dermal cone/fat dome structure and its role in burn-related HTS pathogenesis. Burn wounds that reach the depth of the fat dome structure are more prone to develop HTS. The fat dome is thought to be a main reservoir of ADSCs. Interactions of the burn wound fluid with ADSCs shape the healing process, and in some instances direct it down the HTS pathway. Via chemokine CCL27, burn wound fluid influences residual ADSCs in the fat dome to secrete a variety of growth factors that promote collagen synthesis, angiogenesis and granulation tissue formation in the wound bed. Additionally, fat dome ADSCs are one of the putative sources of aggressive myofibroblasts that are highly characteristic of HTS. Abbreviations: HTS - hypertrophic scarring; ADSCs - adipose-derived stem cells; VEGF - vascular endothelial growth factor; PDGF - platelet-derived growth factor; bFGF - basic fibroblast growth factor; EGF - epidermal growth factor; KGF - keratinocyte growth factor; TGF-β - transforming growth factor beta; CCL27 - chemokine CCL27. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Currently, HTS pathogenesis is being linked to the exposure of dermal cones. These cones exists in areas where HTS occurs such as the neck, chest, abdomen, back, buttock, arm, forearm, dorsum of the hand, thigh, leg, dorsum of the foot, ear helical rim, and ear lobe.86 Conversely, HTS does not often occur in the areas where dermal cones are absent, including the forehead, scalp, concha, eyelid, palm, and sole.84,86 Dermal cones are also absent in the early human fetus, as well as the rat and rabbit, all of which also do not form hypertrophic scars from injury.86
In a study on red Duroc pigs, Zhu et al. evaluated dermal cones from deep partial-thickness wounds.87 All that remains of the dermis after deep partial-thickness injury is the fat dome structure.86 Findings from this study demonstrated that 11 collagen genes and 7 collagen types were overexpressed in the experimental (deep partial-thickness wounded) group. These findings suggest that exposure of the fat dome somehow influences collagen gene expression and promotes the fibroproliferative phenotype seen in HTS.87 Based on these findings, it is important to recognize and continue to investigate the role of dermal cones in HTS.
As noted above, the dermal/hypodermal fat contains a pool of MSCs that may play their own role in wound healing. For instance, Ulrich and colleagues have proposed that one of the contributing factors to the formation of HTS in the setting of full thickness wounds is the migration of α-SMA positive cells from subcutaneous fat into the wound area.88 Thus, a potential explanation for the role of the dermal cones in HTS is that the fat domes within the cones may be producing cells with this aggressive myofibroblast phenotype. Additionally, a clinical study performed by Li et al. on the relationship between the dermis, fat domes within the dermal cones and postburn HTS demonstrated a positive correlation between the degree of dermal defect and HTS after burn injury. The authors speculate that disruption of the fat dome structure may induce scar development, specifically the exposure of the fat dome combined with a dermal defect may lead to hypertrophic scar formation.89 The impact of fatty tissue within the cones on the development of HTS has not been fully determined, but based on these findings, may prove to be a significant factor.
Considering the relationship between exposure of fat tissue in the wound and scar formation, it is apparent that the situations leading to HTS are usually negated by early skin grafting. It should be noted that skin grafting on fat in wounds with a conserved hypodermis results in less contraction than grafting on fascia.90 Thus, the decision relating to skin grafting in deep dermal burns is critical. In burn wounds left to heal without intervention, the exposure of fat contributes to HTS.
Mesenchymal/adipose-derived stem cells and stem cell therapies
While the role of exposed fat tissue and inherent MSCs/ADSCs in burn wound healing is not yet completely understood, therapy with exogenous stem cells has been explored as a new promising modality of fibrosis/scarring/contracture prevention and treatment.91–97 MSCs are defined as self-renewing multipotent stem cells that can differentiate into various lineages of mesenchymal origin.7 Among the many subsets of MSCs, ADSCs are of particular interest because this is the primary type of stem cell found in the dermal cones and hypodermis. Thus, investigation of their role in HTS would be beneficial not only for developing new avenues of treatment, but also for understanding the pathophysiology of scar development. Besides, when used as an exogenous treatment, ADSCs have the benefit of relatively easy harvesting from readily available adipose tissue.
Stem cells play a significant role in all major stages of wound healing.7,94,96 Endogenous stem cells migrate to the site of injury during the initial inflammatory stage, where they elicit immunomodulating effects.7,98 In the subsequent wound healing stages, MSCs have been shown to improve wound closure through various mechanisms including promotion of reepithelialization, a positive effect on granulation tissue formation and angiogenesis. The paracrine effects of MSCs are mediated by a variety of known mediators of tissue repair including growth factors such as VEGF, platelet-derived growth factor, basic fibroblast growth factor, epidermal growth factor, keratinocyte growth factor, and TGF-β.7,99
The positive effects of MSCs on HTS reduction have been demonstrated by a number of studies.91,95,97 Bone marrow-derived MSCs (BMSCs) have been found to not only decrease expression of myofibroblast markers, but also down-regulate the synthesis collagen type I in murine dermal fibroblasts in vitro.95 BMSCs have also been shown to increase vascular density as well as enhance the reepithelialization rate of full-thickness burn wounds in a murine model.96,100 There is evidence that application of MSCs on the surface of deep burn wounds can decrease inflammatory cell infiltration and further accelerate the process of angiogenesis.7 Adipose-derived stem cells have been reported to improve wound healing and decrease scarring.93,97,101 However, a recent literature search reveals some studies reporting the opposite, e.g., induction of a pro-fibrotic phenotype by stem cells.102–104 Therefore, more research is needed in this area.
In a recent study, van den Broek et al. examined burn wound exudate and its effect on ADSCs, keratinocytes, and fibroblasts.105 They demonstrated that burn wound exudate significantly increased ADSC secretion of VEGF and IL-6, a pro-inflammatory cytokine. In addition to an increased inflammatory response, these cytokines promote granulation and angiogenesis in the wound bed. All three responses, especially excess granulation, have been linked to the production of hypertrophic scars in deeper burns. Additionally, the authors showed that the skin-specific chemokine CCL27 present in burn exudate increases the secretion of granulation promoting mediators from ADSCs, especially in full thickness burn wounds.105 Adipose-derived stem cells have also been found to promote vascularization and wound healing through VEGF secretion.106 Sultan et al. demonstrated that fat grafting after a burn injury leads to an increase in VEGF and a decrease in TGF-β1 secretion in a mouse model.91 Additionally, high levels of VEGF are also associated with endothelial cell survival, migration and proliferation, which may lead to excessive angiogenesis107 which is ultimately associated with HTS.108
It should be considered that exposure of deep dermis fat domes and the interactions of resident ADSCs with factors being released at the wound site can contribute to the increased likelihood of HTS in full thickness burn wounds.84 Burn wound exudate may trigger inherent ADSCs to produce granulation tissue forming factors in deeper burns where adipose tissue (in the form of fat domes) is exposed. Because superficial burn wounds do not reach the fat dome layer, the presence of inherent ADSCs is greatly reduced. However, it has not been established whether introducing new levels of ADSCs (and therefore VEGF) for the treatment of superficial acute wounds may put the patient at risk for HTS.
Recently, a new paradigm in MSC biology has been described. It appears that, similarly to macrophages, MSCs can polarize into two distinct phenotypes—the proinflammatory M1 and the anti-inflammatory M2.98 This polarization is mediated by different toll-like receptors (TLRs), which respond to various substances in the extracellular milieu and promote MSC phenotype switching accordingly to the current needs of the organism. Specifically, stimulation of TLR3 would switch the cell to an anti-inflammatory phenotype, whereas TLR4 would promote pro-inflammatory gene expression. The two phenotypes have been shown to have different effects in terms of cell migration, cytokine secretion, lymphocyte activation, TGF-β expression, and ECM deposition. These findings can potentially explain the opposite effects of MSCs on wound healing/scar formation reported by different studies, as the phenotype switch depends on the presence of biologically active substances in the wound bed.
Thus, the role of MSCs in wound healing requires further investigation to obtain a better understanding of how these cells interact with the wound milieu and in what setting their effects would be beneficial for healing/scar formation. The appropriate use of MSCs as a therapeutic modality needs to be defined, and factors like timing of possible stem cell therapies and their suitability for burn wounds of varying severity have to be determined.
It is now evident that different depths of burns may require different therapeutic modalities to optimize wound healing. Treatment with MSCs/ADSCs may be beneficial to the healing of certain types of burn injuries, while potentially causing HTS in others. Anti-VEGF antibodies have been explored as a therapy to prevent HTS in healing burn wounds.109,110 Conversely, levels of VEGF may be one of the most important indicators of whether stem cells could be used, as ADSCs have been shown to increase VEGF production in the wound bed. Chronic, nonhealing wounds are associated with low levels of VEGF due to protease degradation, which can lead to delays in the repair process.111,112 These types of wounds may benefit from the addition of ADSCs to stimulate the production of VEGF, which in turn can initiate the wound healing process. Conversely, the findings of Spiekman et al. suggest that ADSC treatments can diminish established hypertrophic scars and keloids via the inhibition of TGF-β1 mediated fibroblast differentiation.94 Thus, ADSCs interrupt the cycle of excess collagen formation and are capable of modulating existing HTS. These authors also found reduced collagen deposition after the use of ADSCs, which is more likely to correlate with a normal wound healing phenotype. Adipose-derived stem cells were also able to control healing processes through the up-regulation of MMPs, which are capable of remodeling collagen deposition at the wound site.94 Spiekman et al. also found that the use of ADSCs reduced gene expression of collagen III in scar fibroblasts.94
Thus, the chronic wound or the wound already manifesting with HTS or contractures may well benefit from the addition of fat tissue components in the form of fat grafts or isolated ADSCs. The benefit in acute burn wounds, however, has yet to be determined and may depend on burn wound depth.
TREATMENT CONSIDERATIONS
Based on these less described nuances of hypertrophic burn scar development, newer therapies have been introduced aiming at HTS prevention. Specifically, it is evident that managing pruritus of healing burn wounds is not only a major relief for patients, but also essential for HTS prevention. Treatment should involve protection of exposed sensory nerves as well as their hydration.3,55 Aside from the use of topical and systemic medications, material such as silicone may be able to control pruritus and neurogenic inflammation due to its moisturizing and occlusive properties as well as the possibility of active agent impregnation of the silicone gel. Silicone sheeting may also be beneficial in addressing the issue of mechanotransduction by stabilizing the scar surface.54,113 However, current silicone sheeting provides little tension relief and results have varied,114 thus development of new renditions of “stretchable silicone” that may be able to provide improved stability through its elastic characteristics, is desirable.3 A stabilizing tensile dressing can significantly reduce scar formation,58 presumably by influencing multiple mechanotransduction signaling pathways.
Compression dressing and paper tape have shown efficacy in reducing the amount of scarring in postburn injuries, leading to significant improvements in scar appearance.63,113,115–118 Additionally, Reno et al. have reported an increase in fibroblast/myofibroblast apoptosis when utilizing compression therapy on hypertrophic scars in vitro.119 The same effects have been achieved to a certain extent by traditional pressure therapy, which appears to suppress dermal myofibroblasts and inhibit keratinocyte proliferation in hypertrophic scar tissue.120 It may also be beneficial to impregnate the silicone gel of the dressing with substances that can act at a molecular level to manage other mechanisms involved in hypertrophic scar formation, including neurogenic inflammation, cellular signaling and growth factor activity, namely the TGF-β pathway and VEGF.3,63,110 Wound protection and hydration with a silicone-based dressing currently seems to be reasonably effective in controlling pruritus and limiting HTS, but newer renditions that involve continuous rather than intermittent use of silicone sheeting may prove better for countering ongoing mechanical forces on the developing scar.
New therapies should seek to optimize a combination of cytokines and cells, including ADSCs and may require refinement based on the severity of the burn. Additionally, a therapeutic combination of cells, such as that seen in stromal vascular fractions, may be preferable to a pure ADSC treatment for balanced regeneration and scar control.121
In comparison to the treatment of acute wounds where excess granulation tissue and vascularity may be a problem, the treatment of established scar contractures with stem cell combinations appears to be better defined. In a series of 694 consecutive patients with HTS caused by burns, trauma or surgery, Klinger et al. showed improvement, and in some cases excellent resolution, of pain, limitation in movement and scar hypertrophy following injection of uncultured centrifuged fat.92 Skin became softer, more flexible and fat grafted areas had improved function. Similarly, Sultan et al. showed significant improvement in the skin of irradiated mice following human fat grafting to the areas. Results showed improvement in appearance of irradiated skin. At 4 and 8 weeks, the fat grafted murine models had lower Smad3 expression than the control group. Moreover, results of the study showed that after 8 weeks there was a significantly lower scar index in the fat grafted mice as compared to the saline-injected control group.91 Thus, as opposed to the potential risk in some acute healing wounds, the increased vascularity and decreased inflammation and fibrosis contributed to by adipose progenitor cells appear to be beneficial in these chronic cases.
CONCLUSION
Hypertrophic scarring is a serious complication of burn trauma, characterized by long-lasting adverse functional, aesthetic, and psychological outcomes for the patient. The pathophysiology behind HTS is complex and not fully understood, however, one factor that plays a crucial role is the depth of the burn wound. Superficial burns heal quickly with minimal scarring. As the burn wound depth increases, affecting structures deeper to the epidermis, additional aspects of healing come into play. Sensory nerve exposure leads to constant stimulation of these nerve endings with a subsequent release of neuropeptides and development of neurogenic inflammation. The deeper is the wound, the more mechanical strain it experiences during healing. This mechanical tension is sensed by the cells and converted into biochemical signals stimulating myofibroblast differentiation and collagen synthesis. This phenomena is known as mechanotransduction, and multiple pathways have been shown to play a role here, including TGF-β1, Rho/ROCK, YAP/TAZ, and MKL-1/MRTF-A signaling. With increased depth, research has focused on specific skin structures called dermal cones/fat domes, which contain residual stem cells and can significantly affect the healing process. Interactions of stem cells within the dome with factors being released at the wound site may contribute to HTS. Studies on residual stem cell physiology will lead us to a better understanding of their role in wound healing and scar formation/prevention. Thus, the rise in the therapeutic use of injected/transplanted stem cells becomes theoretically justified as we gain more insight to the mechanisms at play.
These recently revealed pathophysiologic nuances to burn scar formation potentially present new therapeutic avenues for HTS prevention and treatment. The combination of old therapeutic techniques with newer improved strategies and technologies that address exposed nerve endings, wound tension stabilization, and controlled lipoaspirate/ADSC usage may ultimately provide improved methods of HTS management.
Acknowledgments
Source of Funding: The project described was supported by the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant UL1 TR000154. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Conflicts of Interest: None.
References
- 1.Procter F. Rehabilitation of the burn patient. Indian journal of plastic surgery : official publication of the Association of Plastic Surgeons of India. 2010;43:S101–13. doi: 10.4103/0970-0358.70730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lawrence JW, Mason ST, Schomer K, Klein MB. Epidemiology and impact of scarring after burn injury: a systematic review of the literature. J Burn Care Res. 2012;33:136–46. doi: 10.1097/BCR.0b013e3182374452. [DOI] [PubMed] [Google Scholar]
- 3.Wolfram DTA, Pulzl P, Piza-Katzer H. Hypertrophic scars and keloids - a review of their pathphysiology, risk factors, and therapeutic management. Dermatol Surg. 2009;35:171–81. doi: 10.1111/j.1524-4725.2008.34406.x. [DOI] [PubMed] [Google Scholar]
- 4.Tiwari VK. Burn wound: How it differs from other wounds? Indian journal of plastic surgery : official publication of the Association of Plastic Surgeons of India. 2012;45:364–73. doi: 10.4103/0970-0358.101319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Evers LH, Bhavsar D, Mailander P. The biology of burn injury. Exp Dermatol. 2010;19:777–83. doi: 10.1111/j.1600-0625.2010.01105.x. [DOI] [PubMed] [Google Scholar]
- 6.Mace JE, Park MS, Mora AG, Chung KK, Martini W, White CE, et al. Differential expression of the immunoinflammatory response in trauma patients: burn vs. non-burn. Burns. 2012;38:599–606. doi: 10.1016/j.burns.2011.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Drost AC, Burleson DG, Cioffi WG, Jr, Jordan BS, Mason AD, Jr, Pruitt BA., Jr Plasma cytokines following thermal injury and their relationship with patient mortality, burn size, and time postburn. J Trauma. 1993;35:335–9. doi: 10.1097/00005373-199309000-00001. [DOI] [PubMed] [Google Scholar]
- 8.Kowal-Vern A, Walenga JM, Hoppensteadt D, Sharp-Pucci M, Gamelli RL. Interleukin-2 and interleukin-6 in relation to burn wound size in the acute phase of thermal injury. J Am Coll Surg. 1994;178:357–62. [PubMed] [Google Scholar]
- 9.Ogura H, Hashiguchi N, Tanaka H, Koh T, Noborio M, Nakamori Y, et al. Long-term enhanced expression of heat shock proteins and decelerated apoptosis in polymorphonuclear leukocytes from major burn patients. J Burn Care Rehabil. 2002;23:103–9. doi: 10.1097/00004630-200203000-00006. [DOI] [PubMed] [Google Scholar]
- 10.Sakallioglu AE, Basaran O, Ozdemir BH, Arat Z, Yucel M, Haberal M. Local and systemic interactions related to serum transforming growth factor-beta levels in burn wounds of various depths. Burns. 2006;32:980–5. doi: 10.1016/j.burns.2006.04.018. [DOI] [PubMed] [Google Scholar]
- 11.Wilgus TA, Ferreira AM, Oberyszyn TM, Bergdall VK, Dipietro LA. Regulation of scar formation by vascular endothelial growth factor. Lab Invest. 2008;88:579–90. doi: 10.1038/labinvest.2008.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sarrazy V, Billet F, Micallef L, Coulomb B, Desmouliere A. Mechanisms of pathological scarring: role of myofibroblasts and current developments. Wound repair and regeneration : official publication of the Wound Healing Society [and] the European Tissue Repair Society. 2011;19(Suppl 1):s10–5. doi: 10.1111/j.1524-475X.2011.00708.x. [DOI] [PubMed] [Google Scholar]
- 13.Ding J, Hori K, Zhang R, Marcoux Y, Honardoust D, Shankowsky HA, et al. Stromal cell-derived factor 1 (SDF-1) and its receptor CXCR4 in the formation of postburn hypertrophic scar (HTS) Wound repair and regeneration : official publication of the Wound Healing Society [and] the European Tissue Repair Society. 2011;19:568–78. doi: 10.1111/j.1524-475X.2011.00724.x. [DOI] [PubMed] [Google Scholar]
- 14.Gabbiani G. The myofibroblast in wound healing and fibrocontractive diseases. J Pathol. 2003;200:500–3. doi: 10.1002/path.1427. [DOI] [PubMed] [Google Scholar]
- 15.Desmouliere A, Redard M, Darby I, Gabbiani G. Apoptosis mediates the decrease in cellularity during the transition between granulation tissue and scar. Am J Pathol. 1995;146:56–66. [PMC free article] [PubMed] [Google Scholar]
- 16.Linge C, Richardson J, Vigor C, Clayton E, Hardas B, Rolfe K. Hypertrophic scar cells fail to undergo a form of apoptosis specific to contractile collagen-the role of tissue transglutaminase. J Invest Dermatol. 2005;125:72–82. doi: 10.1111/j.0022-202X.2005.23771.x. [DOI] [PubMed] [Google Scholar]
- 17.Baum J, Duffy HS. Fibroblasts and myofibroblasts: what are we talking about? Journal of cardiovascular pharmacology. 2011;57:376–9. doi: 10.1097/FJC.0b013e3182116e39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Van De Water L, Varney S, Tomasek JJ. Mechanoregulation of the Myofibroblast in Wound Contraction, Scarring, and Fibrosis: Opportunities for New Therapeutic Intervention. Adv Wound Care (New Rochelle) 2013;2:122–41. doi: 10.1089/wound.2012.0393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dabiri G, Tumbarello DA, Turner CE, Van de Water L. Hic-5 promotes the hypertrophic scar myofibroblast phenotype by regulating the TGF-beta1 autocrine loop. The Journal of investigative dermatology. 2008;128:2518–25. doi: 10.1038/jid.2008.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thannickal VJ, Horowitz JC. Evolving concepts of apoptosis in idiopathic pulmonary fibrosis. Proceedings of the American Thoracic Society. 2006;3:350–6. doi: 10.1513/pats.200601-001TK. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thannickal VJ, Lee DY, White ES, Cui Z, Larios JM, Chacon R, et al. Myofibroblast differentiation by transforming growth factor-beta1 is dependent on cell adhesion and integrin signaling via focal adhesion kinase. J Biol Chem. 2003;278:12384–9. doi: 10.1074/jbc.M208544200. [DOI] [PubMed] [Google Scholar]
- 22.Jun JI, Lau LF. The matricellular protein CCN1 induces fibroblast senescence and restricts fibrosis in cutaneous wound healing. Nature cell biology. 2010;12:676–85. doi: 10.1038/ncb2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Holmes A, Abraham DJ, Sa S, Shiwen X, Black CM, Leask A. CTGF and SMADs, maintenance of scleroderma phenotype is independent of SMAD signaling. J Biol Chem. 2001;276:10594–601. doi: 10.1074/jbc.M010149200. [DOI] [PubMed] [Google Scholar]
- 24.Zhu Z, Ding J, Shankowsky HA, Tredget EE. The molecular mechanism of hypertrophic scar. J Cell Commun Signal. 2013;7:239–52. doi: 10.1007/s12079-013-0195-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Forbes-Duchart L, Cooper J, Nedelec B, Ross L, Quanbury A. Burn therapists’ opinion on the application and essential characteristics of a burn scar outcome measure. J Burn Care Res. 2009;30:792–800. doi: 10.1097/BCR.0b013e3181b47cc2. [DOI] [PubMed] [Google Scholar]
- 26.Altun V, Hakvoort TE, van Zuijlen PP, van der Kwast TH, Prens EP. Nerve outgrowth and neuropeptide expression during the remodeling of human burn wound scars. A 7-month follow-up study of 22 patients. Burns. 2001;27:717–22. doi: 10.1016/s0305-4179(01)00026-2. [DOI] [PubMed] [Google Scholar]
- 27.Ziche M, Morbidelli L, Masini E, Amerini S, Granger HJ, Maggi CA, et al. Nitric oxide mediates angiogenesis in vivo and endothelial cell growth and migration in vitro promoted by substance P. J Clin Invest. 1994;94:2036–44. doi: 10.1172/JCI117557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fong G, Backman LJ, Hart DA, Danielson P, McCormack B, Scott A. Substance P enhances collagen remodeling and MMP-3 expression by human tenocytes. J Orthop Res. 2013;31:91–8. doi: 10.1002/jor.22191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cury PR, Canavez F, de Araujo VC, Furuse C, de Araujo NS. Substance P regulates the expression of matrix metalloproteinases and tissue inhibitors of metalloproteinase in cultured human gingival fibroblasts. J Periodontal Res. 2008;43:255–60. doi: 10.1111/j.1600-0765.2007.01022.x. [DOI] [PubMed] [Google Scholar]
- 30.Kahler CM, Herold M, Wiedermann CJ. Substance P: a competence factor for human fibroblast proliferation that induces the release of growth-regulatory arachidonic acid metabolites. J Cell Physiol. 1993;156:579–87. doi: 10.1002/jcp.1041560318. [DOI] [PubMed] [Google Scholar]
- 31.Papp A, Valtonen P. Tissue substance P levels in acute experimental burns. Burns. 2006;32:842–5. doi: 10.1016/j.burns.2006.01.018. [DOI] [PubMed] [Google Scholar]
- 32.Scott JR, Muangman PR, Tamura RN, Zhu KQ, Liang Z, Anthony J, et al. Substance P levels and neutral endopeptidase activity in acute burn wounds and hypertrophic scar. Plast Reconstr Surg. 2005;115:1095–102. doi: 10.1097/01.prs.0000156151.54042.da. [DOI] [PubMed] [Google Scholar]
- 33.Scholzen T, Armstrong CA, Bunnett NW, Luger TA, Olerud JE, Ansel JC. Neuropeptides in the skin: interactions between the neuroendocrine and the skin immune systems. Exp Dermatol. 1998;7:81–96. doi: 10.1111/j.1600-0625.1998.tb00307.x. [DOI] [PubMed] [Google Scholar]
- 34.Zheng Z, Lamotte RH, Grigg P. Comparison of responses to tensile and compressive stimuli in C-mechanosensitive nociceptors in rat hairy skin. Somatosens Mot Res. 2002;19:109–13. doi: 10.1080/08990220120113095. [DOI] [PubMed] [Google Scholar]
- 35.Zegarska B, Lelinska A, Tyrakowski T. Clinical and experimental aspects of cutaneous neurogenic inflammation. Pharmacol Rep. 2006;58:13–21. [PubMed] [Google Scholar]
- 36.Yamaoka J, Di ZH, Sun W, Kawana S. Changes in cutaneous sensory nerve fibers induced by skin-scratching in mice. J Dermatol Sci. 2007;46:41–51. doi: 10.1016/j.jdermsci.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 37.Scott JR, Muangman P, Gibran NS. Making sense of hypertrophic scar: a role for nerves. Wound repair and regeneration : official publication of the Wound Healing Society [and] the European Tissue Repair Society. 2007;15:S27–31. doi: 10.1111/j.1524-475X.2007.00222.x. [DOI] [PubMed] [Google Scholar]
- 38.Widgerow AD. Cellular/extracellular matrix cross-talk in scar evolution and control. Wound repair and regeneration : official publication of the Wound Healing Society [and] the European Tissue Repair Society. 2011;19:117–33. doi: 10.1111/j.1524-475X.2010.00662.x. [DOI] [PubMed] [Google Scholar]
- 39.Ko KS, Arora PD, McCulloch CA. Cadherins mediate intercellular mechanical signaling in fibroblasts by activation of stretch-sensitive calcium-permeable channels. J Biol Chem. 2001;276:35967–77. doi: 10.1074/jbc.M104106200. [DOI] [PubMed] [Google Scholar]
- 40.Munger JS, Huang X, Kawakatsu H, Griffiths MJ, Dalton SL, Wu J, et al. The integrin alpha v beta 6 binds and activates latent TGF beta 1: a mechanism for regulating pulmonary inflammation and fibrosis. Cell. 1999;96:319–28. doi: 10.1016/s0092-8674(00)80545-0. [DOI] [PubMed] [Google Scholar]
- 41.Carracedo S, Lu N, Popova SN, Jonsson R, Eckes B, Gullberg D. The fibroblast integrin alpha11beta1 is induced in a mechanosensitive manner involving activin A and regulates myofibroblast differentiation. J Biol Chem. 2010;285:10434–45. doi: 10.1074/jbc.M109.078766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Leask A, Abraham DJ. TGF-beta signaling and the fibrotic response. FASEB J. 2004;18:816–27. doi: 10.1096/fj.03-1273rev. [DOI] [PubMed] [Google Scholar]
- 43.Zouq NK, Keeble JA, Lindsay J, Valentijn AJ, Zhang L, Mills D, et al. FAK engages multiple pathways to maintain survival of fibroblasts and epithelia: differential roles for paxillin and p130Cas. J Cell Sci. 2009;122:357–67. doi: 10.1242/jcs.030478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yano S, Komine M, Fujimoto M, Okochi H, Tamaki K. Mechanical stretching in vitro regulates signal transduction pathways and cellular proliferation in human epidermal keratinocytes. J Invest Dermatol. 2004;122:783–90. doi: 10.1111/j.0022-202X.2004.22328.x. [DOI] [PubMed] [Google Scholar]
- 45.Harvey KA, Paranavitana CN, Zaloga GP, Siddiqui RA. Diverse signaling pathways regulate fibroblast differentiation and transformation through Rho kinase activation. Journal of cellular physiology. 2007;211:353–63. doi: 10.1002/jcp.20939. [DOI] [PubMed] [Google Scholar]
- 46.Haudek SB, Gupta D, Dewald O, Schwartz RJ, Wei L, Trial J, et al. Rho kinase-1 mediates cardiac fibrosis by regulating fibroblast precursor cell differentiation. Cardiovasc Res. 2009;83:511–8. doi: 10.1093/cvr/cvp135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huang X, Yang N, Fiore VF, Barker TH, Sun Y, Morris SW, et al. Matrix stiffness-induced myofibroblast differentiation is mediated by intrinsic mechanotransduction. Am J Respir Cell Mol Biol. 2012;47:340–8. doi: 10.1165/rcmb.2012-0050OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee MJ, Ran Byun M, Furutani-Seiki M, Hong JH, Jung HS. YAP and TAZ regulate skin wound healing. The Journal of investigative dermatology. 2014;134:518–25. doi: 10.1038/jid.2013.339. [DOI] [PubMed] [Google Scholar]
- 49.Ferrigno O, Lallemand F, Verrecchia F, L’Hoste S, Camonis J, Atfi A, et al. Yes-associated protein (YAP65) interacts with Smad7 and potentiates its inhibitory activity against TGF-beta/Smad signaling. Oncogene. 2002;21:4879–84. doi: 10.1038/sj.onc.1205623. [DOI] [PubMed] [Google Scholar]
- 50.Duscher D, Maan ZN, Wong VW, Rennert RC, Januszyk M, Rodrigues M, et al. Mechanotransduction and fibrosis. J Biomech. 2014;47:1997–2005. doi: 10.1016/j.jbiomech.2014.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Crider BJ, Risinger GM, Jr, Haaksma CJ, Howard EW, Tomasek JJ. Myocardin-related transcription factors A and B are key regulators of TGF-beta1-induced fibroblast to myofibroblast differentiation. The Journal of investigative dermatology. 2011;131:2378–85. doi: 10.1038/jid.2011.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Glogauer M, Arora P, Yao G, Sokholov I, Ferrier J, McCulloch CA. Calcium ions and tyrosine phosphorylation interact coordinately with actin to regulate cytoprotective responses to stretching. J Cell Sci. 1997;110:11–21. doi: 10.1242/jcs.110.1.11. [DOI] [PubMed] [Google Scholar]
- 53.Roosterman D, Goerge T, Schneider SW, Bunnett NW, Steinhoff M. Neuronal control of skin function: the skin as a neuroimmunoendocrine organ. Physiol Rev. 2006;86:1309–79. doi: 10.1152/physrev.00026.2005. [DOI] [PubMed] [Google Scholar]
- 54.Yagmur C, Akaishi S, Ogawa R, Guneren E. Mechanical receptor-related mechanisms in scar management: a review and hypothesis. Plastic and reconstructive surgery. 2010;126:426–34. doi: 10.1097/PRS.0b013e3181df715d. [DOI] [PubMed] [Google Scholar]
- 55.Wong VW, Akaishi S, Longaker MT, Gurtner GC. Pushing back: wound mechanotransduction in repair and regeneration. The Journal of investigative dermatology. 2011;131:2186–96. doi: 10.1038/jid.2011.212. [DOI] [PubMed] [Google Scholar]
- 56.Matsumura H, Engrav LH, Gibran NS, Yang TM, Grant JH, Yunusov MY, et al. Cones of skin occur where hypertrophic scar occurs. Wound Repair Regen. 2001;9:269–77. doi: 10.1046/j.1524-475x.2001.00269.x. [DOI] [PubMed] [Google Scholar]
- 57.Zhu KQ, Engrav LH, Gibran NS, Cole JK, Matsumura H, Piepkorn M, et al. The female, red Duroc pig as an animal model of hypertrophic scarring and the potential role of the cones of skin. Burns. 2003;29:649–64. doi: 10.1016/s0305-4179(03)00205-5. [DOI] [PubMed] [Google Scholar]
- 58.Engrav LH, Tuggle CK, Kerr KF, Zhu KQ, Numhom S, Couture OP, et al. Functional genomics unique to week 20 post wounding in the deep cone/fat dome of the Duroc/Yorkshire porcine model of fibroproliferative scarring. PloS one. 2011;6:e19024. doi: 10.1371/journal.pone.0019024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhu KQ, Carrougher GJ, Couture OP, Tuggle CK, Gibran NS, Engrav LH. Expression of collagen genes in the cones of skin in the Duroc/Yorkshire porcine model of fibroproliferative scarring. J Burn Care Res. 2008;29:815–27. doi: 10.1097/BCR.0b013e3181848141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ulrich MM, Verkerk M, Reijnen L, Vlig M, van den Bogaerdt AJ, Middelkoop E. Expression profile of proteins involved in scar formation in the healing process of full-thickness excisional wounds in the porcine model. Wound repair and regeneration : official publication of the Wound Healing Society [and] the European Tissue Repair Society. 2007;15:482–90. doi: 10.1111/j.1524-475X.2007.00255.x. [DOI] [PubMed] [Google Scholar]
- 61.Li ZY, Su HT, Lu SL, Huang LB, Yang XB, Shao TB, et al. Clinical study on the relationship among the dermis, fat dome and postburn hyperplastic scar formation. Zhonghua Shao Shang Za Zhi. 2004;20:343–6. [PubMed] [Google Scholar]
- 62.Rose LF, Wu JC, Carlsson AH, Tucker DI, Leung KP, Chan RK. Recipient wound bed characteristics affect scarring and skin graft contraction. Wound repair and regeneration : official publication of the Wound Healing Society [and] the European Tissue Repair Society. 2015;23:287–96. doi: 10.1111/wrr.12267. [DOI] [PubMed] [Google Scholar]
- 63.Sultan SM, Barr JS, Butala P, Davidson EH, Weinstein AL, Knobel D, et al. Fat grafting accelerates revascularisation and decreases fibrosis following thermal injury. Journal of plastic, reconstructive & aesthetic surgery : JPRAS. 2012;65:219–27. doi: 10.1016/j.bjps.2011.08.046. [DOI] [PubMed] [Google Scholar]
- 64.Maxson S, Lopez EA, Yoo D, Danilkovitch-Miagkova A, Leroux MA. Concise review: role of mesenchymal stem cells in wound repair. Stem cells translational medicine. 2012;1:142–9. doi: 10.5966/sctm.2011-0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Waterman RS, Tomchuck SL, Henkle SL, Betancourt AM. A new mesenchymal stem cell (MSC) paradigm: polarization into a pro-inflammatory MSC1 or an Immunosuppressive MSC2 phenotype. PloS one. 2010;5:e10088. doi: 10.1371/journal.pone.0010088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gnecchi M, Zhang Z, Ni A, Dzau VJ. Paracrine mechanisms in adult stem cell signaling and therapy. Circ Res. 2008;103:1204–19. doi: 10.1161/CIRCRESAHA.108.176826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wu Y, Huang S, Enhe J, Ma K, Yang S, Sun T, et al. Bone marrow-derived mesenchymal stem cell attenuates skin fibrosis development in mice. International wound journal. 2014;11:701–10. doi: 10.1111/iwj.12034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Leonardi D, Oberdoerfer D, Fernandes MC, Meurer RT, Pereira-Filho GA, Cruz P, et al. Mesenchymal stem cells combined with an artificial dermal substitute improve repair in full-thickness skin wounds. Burns. 2012;38:1143–50. doi: 10.1016/j.burns.2012.07.028. [DOI] [PubMed] [Google Scholar]
- 69.Yun IS, Jeon YR, Lee WJ, Lee JW, Rah DK, Tark KC, et al. Effect of human adipose derived stem cells on scar formation and remodeling in a pig model: a pilot study. Dermatol Surg. 2012;38:1678–88. doi: 10.1111/j.1524-4725.2012.02495.x. [DOI] [PubMed] [Google Scholar]
- 70.Zhang GY, Li X, Chen XL, Li ZJ, Yu Q, Jiang LF, et al. Contribution of epidermal stem cells to hypertrophic scars pathogenesis. Med Hypotheses. 2009;73:332–3. doi: 10.1016/j.mehy.2008.10.037. [DOI] [PubMed] [Google Scholar]
- 71.van den Broek LJ, Kroeze KL, Waaijman T, Breetveld M, Sampat-Sardjoepersad SC, Niessen FB, et al. Differential response of human adipose tissue-derived mesenchymal stem cells, dermal fibroblasts, and keratinocytes to burn wound exudates: potential role of skin-specific chemokine CCL27. Tissue Eng Part A. 2014;20:197–209. doi: 10.1089/ten.tea.2013.0123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kuo YR, Wang CT, Cheng JT, Kao GS, Chiang YC, Wang CJ. Adipose-Derived Stem Cells Accelerate Diabetic Wound Healing Through the Induction of Autocrine and Paracrine Effects. Cell transplantation. 2016;25:71–81. doi: 10.3727/096368915X687921. [DOI] [PubMed] [Google Scholar]
- 73.Olsson AK, Dimberg A, Kreuger J, Claesson-Welsh L. VEGF receptor signalling - in control of vascular function. Nat Rev Mol Cell Biol. 2006;7:359–71. doi: 10.1038/nrm1911. [DOI] [PubMed] [Google Scholar]
- 74.van der Veer WM, Niessen FB, Ferreira JA, Zwiers PJ, de Jong EH, Middelkoop E, et al. Time course of the angiogenic response during normotrophic and hypertrophic scar formation in humans. Wound repair and regeneration : official publication of the Wound Healing Society [and] the European Tissue Repair Society. 2011;19:292–301. doi: 10.1111/j.1524-475X.2011.00692.x. [DOI] [PubMed] [Google Scholar]
- 75.Yue YG, Jiang CW, Li PY, Zhou S. Influence of the VEGF antibody targeted vascular therapy on the expression of collagen type I in hyperplastic. Zhonghua Shao Shang Za Zhi. 2006;22:427–30. [PubMed] [Google Scholar]
- 76.Lauer G, Sollberg S, Cole M, Flamme I, Sturzebecher J, Mann K, et al. Expression and proteolysis of vascular endothelial growth factor is increased in chronic wounds. J Invest Dermatol. 2000;115:12–8. doi: 10.1046/j.1523-1747.2000.00036.x. [DOI] [PubMed] [Google Scholar]
- 77.Spiekman M, Przybyt E, Plantinga JA, Gibbs S, van der Lei B, Harmsen MC. Adipose tissue-derived stromal cells inhibit TGF-beta1-induced differentiation of human dermal fibroblasts and keloid scar-derived fibroblasts in a paracrine fashion. Plast Reconstr Surg. 2014;134:699–712. doi: 10.1097/PRS.0000000000000504. [DOI] [PubMed] [Google Scholar]
- 78.Akaishi S, Ogawa R, Hyakusoku H. Keloid and hypertrophic scar: neurogenic inflammation hypotheses. Med Hypotheses. 2008;71:32–8. doi: 10.1016/j.mehy.2008.01.032. [DOI] [PubMed] [Google Scholar]
- 79.Friedstat JS, Hultman CS. Hypertrophic burn scar management: what does the evidence show? A systematic review of randomized controlled trials. Ann Plast Surg. 2014;72:S198–201. doi: 10.1097/SAP.0000000000000103. [DOI] [PubMed] [Google Scholar]
- 80.Widgerow AD. Hypertrophic scar evolution and management. Wound Healing Southern Africa. 2013;6:79–86. [Google Scholar]
- 81.Gurtner GC, Dauskardt RH, Wong VW, Bhatt KA, Wu K, Vial IN, et al. Improving cutaneous scar formation by controlling the mechanical environment: large animal and phase I studies. Ann Surg. 2011;254:217–25. doi: 10.1097/SLA.0b013e318220b159. [DOI] [PubMed] [Google Scholar]
- 82.Atkinson JA, McKenna KT, Barnett AG, McGrath DJ, Rudd M. A randomized, controlled trial to determine the efficacy of paper tape in preventing hypertrophic scar formation in surgical incisions that traverse Langer’s skin tension lines. Plast Reconstr Surg. 2005;116:1648–56. doi: 10.1097/01.prs.0000187147.73963.a5. discussion 57-8. [DOI] [PubMed] [Google Scholar]
- 83.Reno F, Sabbatini M, Lombardi F, Stella M, Pezzuto C, Magliacani G, et al. In vitro mechanical compression induces apoptosis and regulates cytokines release in hypertrophic scars. Wound Repair Regen. 2003;11:331–6. doi: 10.1046/j.1524-475x.2003.11504.x. [DOI] [PubMed] [Google Scholar]
- 84.Li-Tsang CW, Feng B, Huang L, Liu X, Shu B, Chan YT, et al. A histological study on the effect of pressure therapy on the activities of myofibroblasts and keratinocytes in hypertrophic scar tissues after burn. Burns. 2015;41:1008–16. doi: 10.1016/j.burns.2014.11.017. [DOI] [PubMed] [Google Scholar]
- 85.Diao JS, Xia WS, Guo SZ. Bevacizumab: a potential agent for prevention and treatment of hypertrophic scar. Burns. 2010;36:1136–7. doi: 10.1016/j.burns.2010.01.014. [DOI] [PubMed] [Google Scholar]
- 86.Rigotti G, Marchi A, Galie M, Baroni G, Benati D, Krampera M, et al. Clinical treatment of radiotherapy tissue damage by lipoaspirate transplant: a healing process mediated by adipose-derived adult stem cells. Plastic and reconstructive surgery. 2007;119:1409–22. doi: 10.1097/01.prs.0000256047.47909.71. discussion 23-4. [DOI] [PubMed] [Google Scholar]
- 87.Klinger M, Caviggioli F, Klinger FM, Giannasi S, Bandi V, Banzatti B, et al. Autologous fat graft in scar treatment. J Craniofac Surg. 2013;24:1610–5. doi: 10.1097/SCS.0b013e3182a24548. [DOI] [PubMed] [Google Scholar]
- 88.Ulrich MM, et al. Expression profile of proteins involved in scar formation in the healing process of full-thickness excisional wounds in the porcine model. Wound Repair Regen. 2007;15:482–90. doi: 10.1111/j.1524-475X.2007.00255.x. [DOI] [PubMed] [Google Scholar]
- 89.Li ZY, et al. Clinical study on the relationship among the dermis, fat dome and postburn hyperplastic scar formation. Zhonghua Shao Shang Za Zhi. 2004;20:343–6. [PubMed] [Google Scholar]
- 90.Rose LF, et al. Recipient wound bed characteristics affect scarring and skin graft contraction. Wound Repair Regen. 2015;23:287–96. doi: 10.1111/wrr.12267. [DOI] [PubMed] [Google Scholar]
- 91.Sultan SM, et al. Fat grafting accelerates revascularisation and decreases fibrosis following thermal injury. J Plast Reconstr Aesthet Surg. 2012;65:219–27. doi: 10.1016/j.bjps.2011.08.046. [DOI] [PubMed] [Google Scholar]
- 92.Klinger M, et al. Autologous fat graft in scar treatment. J Craniofac Surg. 2013;24:1610–5. doi: 10.1097/SCS.0b013e3182a24548. [DOI] [PubMed] [Google Scholar]
- 93.Lam MT, et al. Effective delivery of stem cells using an extracellular matrix patch results in increased cell survival and proliferation and reduced scarring in skin wound healing. Tissue Eng Part A. 2013;19:738–47. doi: 10.1089/ten.tea.2012.0480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Spiekman M, et al. Adipose tissue-derived stromal cells inhibit TGF-beta1-induced differentiation of human dermal fibroblasts and keloid scar-derived fibroblasts in a paracrine fashion. Plast Reconstr Surg. 2014;134:699–712. doi: 10.1097/PRS.0000000000000504. [DOI] [PubMed] [Google Scholar]
- 95.Wu Y, et al. Bone marrow-derived mesenchymal stem cell attenuates skin fibrosis development in mice. Int Wound J. 2014;11:701–10. doi: 10.1111/iwj.12034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ghieh F, et al. The use of stem cells in burn wound healing: a review. Biomed Res Int. 2015;2015:684084. doi: 10.1155/2015/684084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhang Q, et al. Intralesional injection of adipose-derived stem cells reduces hypertrophic scarring in a rabbit ear model. Stem Cell Res Ther. 2015;6:145. doi: 10.1186/s13287-015-0133-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Waterman RS, et al. A new mesenchymal stem cell (MSC) paradigm: polarization into a pro-inflammatory MSC1 or an Immunosuppressive MSC2 phenotype. PLoS One. 2010;5:e10088. doi: 10.1371/journal.pone.0010088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gnecchi M, et al. Paracrine mechanisms in adult stem cell signaling and therapy. Circ Res. 2008;103:1204–19. doi: 10.1161/CIRCRESAHA.108.176826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Leonardi D, et al. Mesenchymal stem cells combined with an artificial dermal substitute improve repair in full-thickness skin wounds. Burns. 2012;38:1143–50. doi: 10.1016/j.burns.2012.07.028. [DOI] [PubMed] [Google Scholar]
- 101.Yun IS, et al. Effect of human adipose derived stem cells on scar formation and remodeling in a pig model: a pilot study. Dermatol Surg. 2012;38:1678–88. doi: 10.1111/j.1524-4725.2012.02495.x. [DOI] [PubMed] [Google Scholar]
- 102.Zhang GY, et al. Contribution of epidermal stem cells to hypertrophic scars pathogenesis. Med Hypotheses. 2009;73:332–3. doi: 10.1016/j.mehy.2008.10.037. [DOI] [PubMed] [Google Scholar]
- 103.van den Bogaerdt AJ, et al. Collagen cross-linking by adipose-derived mesenchymal stromal cells and scar-derived mesenchymal cells: are mesenchymal stromal cells involved in scar formation? Wound Repair Regen. 2009;17:548–58. doi: 10.1111/j.1524-475X.2009.00501.x. [DOI] [PubMed] [Google Scholar]
- 104.Ding J, et al. Deep dermal fibroblast profibrotic characteristics are enhanced by bone marrow-derived mesenchymal stem cells. Wound Repair Regen. 2013;21:448–55. doi: 10.1111/wrr.12046. [DOI] [PubMed] [Google Scholar]
- 105.van den Broek LJ, et al. Differential response of human adipose tissue-derived mesenchymal stem cells, dermal fibroblasts, and keratinocytes to burn wound exudates: potential role of skin-specific chemokine CCL27. Tissue. Eng Part A. 2014;20:197–209. doi: 10.1089/ten.tea.2013.0123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kuo YR, et al. Adipose-derived stem cells accelerate diabetic wound healing through the induction of autocrine and paracrine effects. Cell Transplant. 2016;25:71–81. doi: 10.3727/096368915X687921. [DOI] [PubMed] [Google Scholar]
- 107.Olsson AK, et al. VEGF receptor signalling—in control of vascular function. Nat Rev Mol Cell Biol. 2006;7:359–71. doi: 10.1038/nrm1911. [DOI] [PubMed] [Google Scholar]
- 108.van der Veer WM, et al. Time course of the angiogenic response during normotrophic and hypertrophic scar formation in humans. Wound Repair Regen. 2011;19:292–301. doi: 10.1111/j.1524-475X.2011.00692.x. [DOI] [PubMed] [Google Scholar]
- 109.Yue YG, et al. Influence of the VEGF antibody targeted vascular therapy on the expression of collagen type I in hyperplastic. Zhonghua Shao Shang Za Zhi. 2006;22:427–30. [PubMed] [Google Scholar]
- 110.Diao JS, Xia WS, Guo SZ. Bevacizumab: a potential agent for prevention and treatment of hypertrophic scar. Burns. 2010;36:1136–7. doi: 10.1016/j.burns.2010.01.014. [DOI] [PubMed] [Google Scholar]
- 111.Lauer G, et al. Expression and proteolysis of vascular endothelial growth factor is increased in chronic wounds. J Invest Dermatol. 2000;115:12–8. doi: 10.1046/j.1523-1747.2000.00036.x. [DOI] [PubMed] [Google Scholar]
- 112.Johnson KE, Wilgus TA. Vascular endothelial growth factor and angiogenesis in the regulation of cutaneous wound repair. Adv Wound Care (New Rochelle) 2014;3:647–61. doi: 10.1089/wound.2013.0517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Akaishi S, et al. The tensile reduction effects of silicone gel sheeting. Plast Reconstr Surg. 2010;126:109e–11e. doi: 10.1097/PRS.0b013e3181df7073. [DOI] [PubMed] [Google Scholar]
- 114.Friedstat JS, Hultman CS. Hypertrophic burn scar management: what does the evidence show? a systematic review of randomized controlled trials. Ann Plast Surg. 2014;72:S198–S201. doi: 10.1097/SAP.0000000000000103. [DOI] [PubMed] [Google Scholar]
- 115.Atkinson JA, et al. A randomized, controlled trial to determine the efficacy of paper tape in preventing hypertrophic scar formation in surgical incisions that traverse Langer’s skin tension lines. Plast Reconstr Surg. 2005;116:1648–56. doi: 10.1097/01.prs.0000187147.73963.a5. discussion 1657-8. [DOI] [PubMed] [Google Scholar]
- 116.Engrav LH, et al. 12-Year within-wound study of the effectiveness of custom pressure garment therapy. Burns. 2010;36:975–83. doi: 10.1016/j.burns.2010.04.014. [DOI] [PubMed] [Google Scholar]
- 117.Li-Tsang CW, Zheng YP, Lau JC. A randomized clinical trial to study the effect of silicone gel dressing and pressure therapy on posttraumatic hypertrophic scars. J Burn Care Res. 2010;31:448–57. doi: 10.1097/BCR.0b013e3181db52a7. [DOI] [PubMed] [Google Scholar]
- 118.Steinstraesser L, et al. Pressure garment therapy alone and in combination with silicone for the prevention of hypertrophic scarring: randomized controlled trial with intraindividual comparison. Plast Reconstr Surg. 2011;128:306e–313e. doi: 10.1097/PRS.0b013e3182268c69. [DOI] [PubMed] [Google Scholar]
- 119.Reno F, et al. In vitro mechanical compression induces apoptosis and regulates cytokines release in hypertrophic scars. Wound Repair Regen. 2003;11:331–6. doi: 10.1046/j.1524-475x.2003.11504.x. [DOI] [PubMed] [Google Scholar]
- 120.Li-Tsang CW, et al. A histological study on the effect of pressure therapy on the activities of myofibroblasts and keratinocytes in hypertrophic scar tissues after burn. Burns. 2015;41:1008–16. doi: 10.1016/j.burns.2014.11.017. [DOI] [PubMed] [Google Scholar]
- 121.Rigotti G, et al. Clinical treatment of radiotherapy tissue damage by lipoaspirate transplant: a healing process mediated by adipose-derived adult stem cells. Plast Reconstr Surg. 2007;119:1409–22. doi: 10.1097/01.prs.0000256047.47909.71. discussion 1423-4. [DOI] [PubMed] [Google Scholar]