Abstract
The hypothalamic-pituitary-adrenal (HPA) axis is activated in response to stressors and is controlled by neurons residing in the paraventricular nucleus of the hypothalamus (PVN). Although gonadal steroid hormones can influence HPA reactivity to stressors, the exact mechanism of action is not fully understood. It is known, however, that estrogen receptor β (ERβ) inhibits HPA reactivity and decreases anxiety-like behavior in rodents. Since ERβ is co-expressed with oxytocin (OT) in neurons of the PVN, an ERβ-selective agonist was utilized to test the whether ERβ decreases stress-induced HPA reactivity and anxiety-like behaviors via an OTergic pathway. Adult gonadectomized male and female rats were administered diarylpropionitrile, or vehicle, peripherally for 5 days. When tested for anxiety-like behavior on the elevated plus maze (EPM), diarylpropionitrile-treated males and females significantly increased time on the open arm of the EPM compared to vehicle controls indicating that ERβ reduces anxiety-like behaviors. One week after behavioral evaluation, rats were subjected to a 20 minute restraint stress. Treatment with diarylpropionitrile reduced CORT and ACTH responses in both males and females. Subsequently, another group of animals was implanted with cannulae directed at the lateral ventricle. One week later, rats underwent the same protocol as above but with the additional treatment of intracerebroventricular infusion with an OT antagonist (des Gly-NH2 d(CH2)5 [Tyr(Me)2, Thr4] OVT) or VEH, 20 minutes prior to behavioral evaluation. OT antagonist treatment blocked the effects of diarylpropionitrile on the display of anxiety-like behaviors and plasma CORT levels. These data indicate that ERβ and OT interact to modulate the HPA reactivity and the display of anxiety-like behaviors.
Keywords: Oxytocin, paraventricular nucleus, anxiety, HPA axis, diarylpropionitrile, 3beta diol, estrogen receptor
Introduction
In female rodents, the response of the hypothalamo-pituitary-adrenal (HPA) axis to stress is greater than that of males, as evidenced by a larger and more prolonged secretion of adrenocorticotropic hormone (ACTH) and adrenal corticosterone (CORT) [1- 3]. Much of this sex difference is attributed to activational effects stemming from sex differences in circulating testosterone (T) and estradiol (E2), since adult gonadectomy reduces, and hormone replacement reinstates, the sex difference [4 - 7]. In particular, studies show that E2 enhances, whereas T inhibits, HPA axis reactivity [8 -11], although some studies also have shown E2-mediated inhibition of the HPA response to stress [12, 13]. It is known that E2 and T act by binding the classic estrogen receptors α or β (ERα, ERβ) or the androgen receptor (AR) in neuropeptide-containing cells located within, or projecting to, the paraventricular nucleus (PVN) [14-17], the principal site for regulation of the HPA axis.
Estrogen receptors are localized within the PVN and surrounding hypothalamic regions, albeit with differing patterns specific for ERα and ERβ. Whereas few ERα-expressing neurons are found in the PVN proper [18], ERα is expressed by GABA containing neurons in the periPVN region [14]. By contrast, ERβ is highly expressed by OT-containing neurons in the parvocellular PVN of both rats and mice [17- 20). Within the rat PVN, approximately 85% of OT-containing neurons co-express ERβ (18). Furthermore, in wild-type mice, exogenous E2 increases OT expression in the brain, but this increase is not observed in ERβ knockout mice (ERβKO) [21, 22]. Thus, substantial overlap in the anatomical distribution of OT and ERβ indicate the potential for interactions in the control of neuroendocrine function and behavior.
Estrogen Receptor β knockout mice [23, 24] and OT knockout mice [25, 26] display increased anxiety-like behavior and enhanced stress-induced plasma CORT levels, suggesting that both ERβ and oxytocin are normally involved in the control of the adult stress response [27- 30]. Moreover, activation of ERβ by a variety of ERβ agonists attenuates stress-induced hypothalamic-pituitary-adrenal (HPA) activity and decreases the display of anxiety-like behaviors in rodents [31, 32]. Correspondingly, an endogenous ERβ ligand, 5α androstane 3β,17β diol, a metabolite of the non-aromatizable androgen, dihydrotestosterone, has similarly been shown to increase PVN OT mRNA expression, likely through direct actions of ERβ on the OT promoter [33]. Nonetheless, the degree to which ERβ and OT regulatory mechanisms intersect in the control of HPA activity and anxiety-like behaviors has not yet been explored.
Oxytocin is a hypothalamic neuropeptide that was originally shown to regulate parturition. Release of OT from parvocellular PVN neurons that project to the median eminence and release OT into the hypophyseal portal vessels to enhance HPA function and increase adrenal glucocorticoid release by modulating the actions of CRF at the level of the anterior pituitary [34]. However, OT neurons in the PVN also provide the predominant OTergic projections to the forebrain where OT is released in response to psychological and physiological stressors [35, 36] to exert anxiolytic actions and enable social interactions that may otherwise be avoided [37]. When applied to the PVN, OT acts to inhibit HPA axis activity [38] apparently through modulation of CRH neuron activity. Although baseline diurnal rhythms of CORT do not differ between OTKO and wild-type (WT) mice [25, 26], OTKO mice do display more anxiety-related behavior and have a greater plasma CORT response to a stressor as compared to their WT counterparts [25,30], further supporting a specific role for OT in the HPA reactivity to stress.
Oxytocin receptors are expressed at high levels in limbic brain regions [39]. Following testing of female OTKO and WT mice on the elevated plus maze, c-Fos expression in the medial amygdala of female OTKO mice was greater than that observed in WT counterparts [25]. The medial amygdala is a limbic region important for the processing of psychogenic stress and anxiety and also contains OT receptor expressing neurons [39]. Moreover, following restraint stress, upregulation of CRH mRNA in the PVN was observed with OTKO mice exhibiting a greater increase in CRH mRNA than did WT mice [40]. Correspondingly, central administration of an oxytocin antagonist, increases anxiety-like behaviors and HPA function as indicated by increased levels of plasma ACTH and CORT [38] supporting the hypothesis that central release of OT in response to stress may act to attenuate the stress-induced increase in HPA reactivity, thereby enabling social interactions beneficial to the animal such as reproduction and feeding [37].
In the current experiments, we tested the hypothesis that activation of ERβ neurons with a selective ERβ agonist inhibits the reactivity of the HPA-axis and reduces anxiety-like behaviors through activation of OT-containing neurons in both males and females. To our knowledge, this is the first study directly comparing effects of the ERβ agonist, Rdiarylpropionitrile (R-DPN) on neuroendocrine stress responses and stress related behaviors across both sexes. Our studies demonstrate a sex difference in the response to ERβ activation and further,,,activation of ERβ utilizes an oxytocinergic pathway to temper anxiety-like behaviors and HPA activation.
Methods
Animals
Male and female adult Sprague-Dawley rats were purchased from Charles River Laboratories (San Diego, CA) and housed at the laboratory animal research facility at Colorado State University. A total of 118 animals were used in these studies. Animals were group housed in temperature and humidity controlled rooms on a 12:12 light:dark cycle (lights on at 0600h) and placed onto a phytoestrogen free diet (AIN-93G modified, DYETS Inc, Allentown PA, with corn oil substituted for soy oil) with water and food available ad libitum. Young adult rats were bilaterally gonadectomized under isoflurane anesthesia one week after arrival as described [41]. Following gonadectomy, animals were returned to their home cage and allowed to awaken prior to the administration of post-operative buprenorphine hydrochloride (0.05 mg/kg). 7 days later, animals began an experimental treatment regimen consisting of daily subcutaneous (S.C) injections of the biologically active R-isomer of the ERβ agonist diarylpropionitrile (R-DPN, 2mg/kg S.C.)[31] or vehicle (VEH; 27% hydroxypropyl β-cyclodextran in 0.9% saline S.C.). Beginning two days preceding the initiation of peripheral R-DPN treatment, animals were handled for 3-5 minutes daily and then daily throughout the course of the study. All behavioral tests and restraint testing were performed in the morning between 0800 to 1200h in order to avoid the diurnal rise in corticosterone that occurs in the afternoon in rodents. All animal protocols followed NIH guidelines and were approved by the Animal Care and Use Committee at Colorado State University.
Experiment 1. Effect of R-DPN on anxiety and depressive-like behaviors and hormonal response to restraint stress in gonadectomized male and female rats
Male and female Sprague-Dawley rats (30 males, 32 females) were gonadectomized and one week later, were started on a regimen of once daily injections of R-DPN (2mg/kg BW, S.C.) or vehicle (250 ul/animal). 2-4 hrs after the 5th injection, animals were tested for behaviors in the elevated plus maze (EPM, see below). Following testing, animals were returned to their home cage. Animals were allowed to rest for two days and following the 7th injection, animals were introduced to the forced swim test (FST, see below). Behaviors in the FST were scored 2-4 hrs after the 8th daily injection the following day. Following the FST, animals were returned to their home cage. Restraint testing (see below) was initiated 4 days later, 2-4 hrs after the 12th injection and trunk blood was collected by decapitation immediately following the 20 min. restraint stress. Control animals were killed immediately after being removed from their home cage and trunk blood collected.
Experiment 2. Effect of central treatment with an OT antagonist on ERβ agonist effects
One week following gonadectomy, a separate group of 56 male and female rats received an intracerebroventricular (ICV) cannula directed at the lateral ventricle. Five days later, animals were started on a treatment regimen of 5 daily injections of either R-DPN (2mg/kg) or VEH. On the fifth day, animals received a central infusion of either an oxytocin receptor antagonist (des Gly-NH2 d(CH2)5 [Tyr(Me)2, Thr4] OVT; Sigma) or VEH (aCSF) 3-4 hrs after R-DPN treatment and 20 minutes prior to being tested on the elevated plus maze. Animals were returned to their home cage and then killed by decapitation 15 minutes following the conclusion of the 5 minute behavioral test.
Elevated Plus Maze
Animals were placed on the EPM, a non-invasive measure of anxiety-like behavior, for 5 minutes as previously described [31]. The maze consists of two closed arms and two open arms (each 40cm h × 10cm w × 50 cm l) forming a cross, with a square center platform (15 × 15 cm). All surfaces are made of black plexiglass, and each arm of the maze is placed on a support column raising the apparatus 40 cm above the floor. The rats were placed in the center of the elevated plus maze facing an open arm and allowed to spontaneously locomote for 5 minutes. The EPM apparatus was cleaned with dilute Nolvosan solution and dried between each trial. Animals were returned to their home cage and the colony room following testing if they were to be used further (expt 1). In experiment 2, animals were returned to the home cage and sacrificed 15 min. later.
Forced Swim Test
The forced swim test is a two-day test consisting of a 10 minute training on Day 1 using a glass cylinder containing fresh 23 ± 2 °C tap water filled to a 10 inches depth as previously described [31] with modifications. Each animal was tested once on the first day followed by a 5 minute test on the second day. The time the animal spent immobile or struggling was determined from the 2nd 5 minute probe trial. Immobility was described as the postural position of floating in the water, while struggling was defined by the rapid movement of all limbs and/or the attempt to climb the glass walls. After the swim test, each animal was towel-dried and returned to a cage pre-heated with a heating pad. Animals were allowed to dry for at least 10 min before returning to the home cage.
Behavioral Scoring
All tests were videotaped and later scored by an individual blind to treatment, sex, or genotype. On the elevated plus maze task, the parameters scored included the number of open and closed arm entries, time spent in the open and closed arms, numbers of head dips from the open arms, and time spent grooming while in the closed arms. Other parameters measured included the total number of entries to the arms, and the percentage of open arm entries. In the forced swim task, the total time spent immobile and total time spent struggling were measured.
Restraint stress
The restraint procedure was applied to animals in experiment 1 beginning 4 days after the end of behavior testing. Animals were maintained on daily injections of R-DPN (2 mg/kg BW) following behavioral testing, as described above. 2-4 hrs after the last injection of R-DPN, animals were placed into a plexiglass restraint tube for 20 minutes (stress group). Control animals (non-stress) were killed immediately following removal from their home cage. To collect trunk blood animals were rapidly decapitated within 1 minute of initial disturbance to prevent rises in CORT due to handling. Trunk blood was collected into pre-chilled tubes containing EDTA (0.5M) and aprotinin (4μg/ml, 100μl). Samples were centrifuged and plasma was collected and stored at −20°C until assayed for CORT and ACTH by RIA as described previously (14,15). All restraint studies were performed in the morning, between the hours of 0800 and 1200 when circulating CORT and ACTH are at their diurnal nadir.
Intracerebroventricular Cannulation
A separate group of gonadectomized animals were implanted one week following gonadectomy, with a stainless steel guide cannula (Plastics One, Roanoke, VA) targeted to the lateral ventricle using the following stereotaxic coordinates in relation to Bregma : A/P, -0.3 mm; M/L, 1.5mm; D/V: -3.8mm; with incisor bar set at -3.3 mm. Successful cannulation of the lateral ventricle was assessed by the reflux of CSF from the cannula following placement into the ventricle. Following sacrifice, all brains were checked histologically for a cannula tract targeting the lateral ventricle. Animals were single housed after cannulation and following a post-surgical recovery period of 5 days, animals began receiving daily treatment with either R-DPN (2mg/kg; S.C) or VEH (27% β-cyclodextran S.C.) for 5 consecutive days. On the fifth day, an internal infusion cannula (4.0mm length to extend 0.2mm past end of the guide) was placed into the guide cannula, and animals received a central infusion of either an oxytocin receptor antagonist (des Gly-NH2 d(CH2)5 [Tyr(Me)2, Thr4] OVT; 1 ug/5 ul infused over 2 min, Sigma Aldrich, St. Louis, MO) or VEH (artificial CSF; 5 ul infused over 2 min) 20 minutes prior to being tested on the elevated plus maze.
Radioimmunoassay of Plasma Hormones
Plasma levels of corticosterone were measured as described previously [14,15]. Samples were diluted 1:25 in 0.01M PBS and corticosterone binding proteins were heat denatured at 65°C for 1 h. All samples were run in duplicate. Standard curves were constructed from dilutions of corticosterone (4-pregnen-11β, 21-diol-3, 20-dione; 5–500 ng/ml; Steraloids) dissolved in 95% ethanol and air dried. Rabbit anti-corticosterone serum (ICN Biomedicals, Costa Mesa, CA) was used at a final dilution of 1:2000, according to manufacturer's protocol. H3 corticosterone was purchased from Amersham (Wood Dale, IL, now GE Healthcare). The limit of detection for the assay was 5.4 ng/ml and the intra-assay coefficient of variation was 5.3%.
Plasma ACTH levels were determined as described previously (31). The standard curve ranging from 5 to 2000 pg ACTH was constructed from an initial 1μg/ml dilution of rat ACTH (NHPP, NIDDK, Harbor UCLA Medical Center, Torrance, CA) dissolved in acidified albumin-saline. All samples were run in duplicate. ACTH antiserum (Immunostar, Hudson, WI) was used at a final dilution of 1:6000 in 2% normal rabbit serum. 125I ACTH (MP Biomedicals, Solon, OH) was used as the tracer. The limit of detection for the assay was 3.15 pg/ml and the intra-assay coefficient of variation was 1.1%.
Data Analysis
All data were analyzed using either a two-way analysis of variance (ANOVA; sex × treatment as factors) or, three way ANOVA (sex × peripheral treatment × central treatment). Posthoc comparisons were made using a Bonferroni (All-Pairwise) Multiple Comparison Test. Significance was set at p<0.05.
Results
Experiment 1: ERβ agonist alters anxiety and depressive-like and the CORT and ACTH response to restraint stress behaviors in male and female rats
Elevated Plus Maze
Data gathered from testing in the elevated plus maze were analyzed by a two way ANOVA (sex × treatment) (Figure 1). The overall pattern of DPN treatment showed a marked reduction in behavioral anxiety. Main effects of Treatment were seen in:
Figure 1.
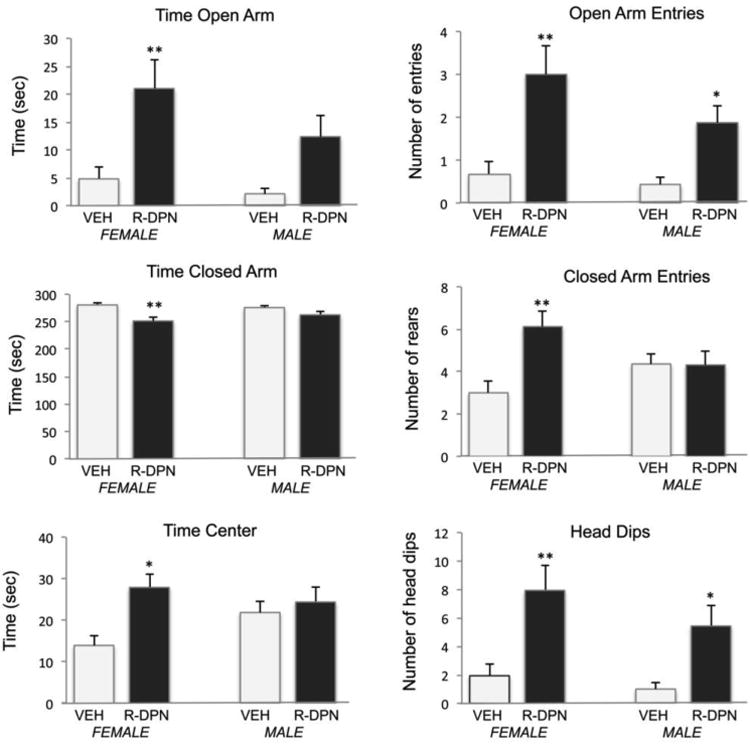
Effect of R-DPN treatment of gonadectomized male and female rats on individual behaviors in the elevated plus maze. Animals were treated with R-DPN for 5 days and then placed into the elevated plus maze for 5 minutes. Specific behaviors are indicated above each graph. Each bar represents the mean +/- SEM of 14-16 animals. Results from 2-way ANOVA are described in results section. * indicates p<0.05 vs same sex – vehicle treated group. ** indicates p<0.01 vs same sex-vehicle treated group.
1) Time in the Open Arm [F(1,63) = 14.91, p<0.001]; 2) Time in Closed Arms [F(1,63) = 13.81, p<0.001]; 3) Time in the Center Space [F(1,63) = 7.63, p<0.01]; 4) Entries into the Open Arm [F(1,63) = 19.72, p<0.0001]; 5) Entries into the Closed Arms [F(1,63) = 5.73, p<0.05]; 6) Head dips from the Open Arm [F(1,63) = 17.93, p<0.0001].
In addition, a significant interaction between sex and treatment was seen for entries into the closed arms [F(1,63) = 7.29, p<0.01], wherein females treated by DPN, but not males, made more entries than controls. There were no other significant interaction effects and there was no treatment effect on rearing behavior in the closed arm (data not shown).
Overall, R-DPN treatment increased measures of anxiolysis in a consistent fashion. R-DPN also decreased anxiogenic behaviors (e.g. closed arm time and entries) in females. Moreover, post hoc analysis showed that the effects of R-DPN across EPM behaviors were largely restricted to females except for R-DPN effect on entries into the open arms and head dips where significance was found in females and males.
Forced Swim Test
Two way ANOVA (sex × treatment) of behaviors in the forced swim test revealed a significant main effect of sex for time spent struggling [F1,58 = 5.81; p<0. 02; figure 2]. Post hoc analysis showed that R-DPN treatment increased struggling time in females (p<0.05), but not males. There were no significant differences for time spent immobile (Figure 2).
Figure 2.
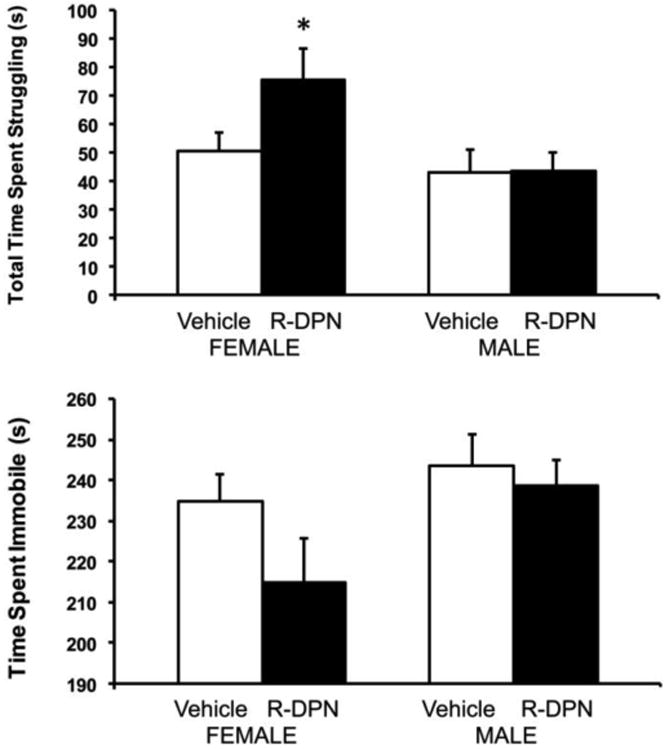
Effect of R-DPN treatment of gonadectomized female and male rats on behavior in the forced swim test 2 days after the EPM. Animals were treated with RDPN for 7 days and then trained in the FST. A 5 min test session occurred on the next day after 8 daily injections of R-DPN. Time struggling (panel A) and time immobile (panel B) are shown. Each bar represents the mean +/- SEM of 14-16 animals / group. * indicates p<0.05 vs same sex vehicle-treated group.
Restraint Stress: Plasma corticosterone levels
Three-way ANOVA revealed significant main effects of Sex [F(1,63) = 46.90, p<0.0 01], R-DPN treatment [F(1,63) = 13.88, p<0.001], and Stress [F(1,63) = 184.99, p<0.00001], as well as a Treatment × Stress interaction, [F(1,63) = 11.16, p<0.01]. As shown in Figure 3A, all groups undergoing restraint stress displayed significantly elevated levels of CORT compared to non-stressed groups, regardless of treatment. R-DPN treatment significantly lowered post-restraint stress plasma CORT levels compared to VEH-treated counterparts in both males and females and the response was somewhat greater in females. Additionally, males had significantly lower pre and post-stress plasma CORT levels than females.
Figure 3.
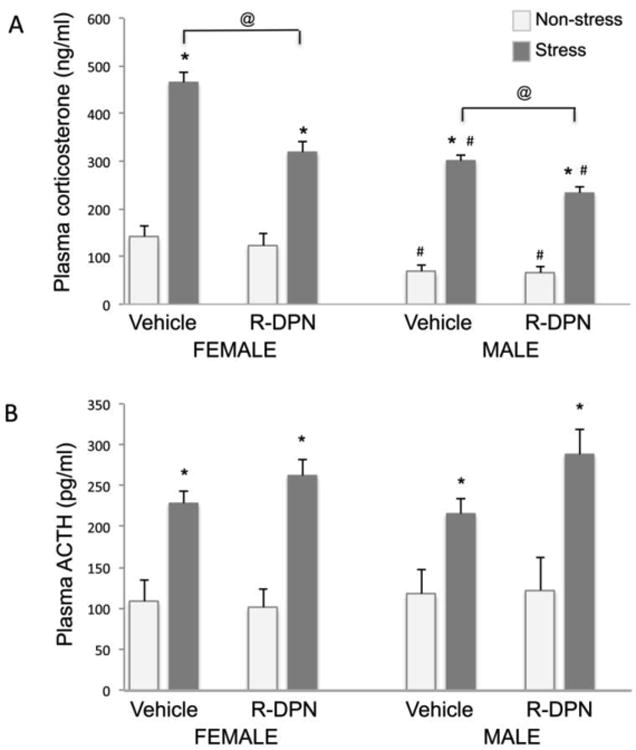
Effect of R-DPN treatment of gonadectomized female and male rats on plasma corticosterone (panel A) and ACTH (panel B) levels in non-stress and stress (20 min. restraint) conditions after 12 daily injections of R-DPN. Each bar represents the mean +/- SEM of 6-8 individuals. *= p<0.05 vs non-stress control. @ indicates p<0.05 vs vehicle treated/stress group, # = p<0.05 vs similar group of opposite sex.
Restraint Stress: Plasma ACTH levels
Three way ANOVA of ACTH levels revealed a significant main effect of Stress [F(1,63) = 53.31, p<0.00001] only. (Figure 3B). Regardless of treatment, plasma samples from all animals undergoing restraint stress contained significantly higher plasma ACTH levels as compared to non-stressed controls. There was no effect of R-DPN treatment or sex on plasma ACTH levels.
Experiment 2: Effects of ICV treatment with oxytocin antagonist on ERβ agonist effects
Elevated plus maze
Based on the results of experiment 1, we next examined the possibility that oxytocinergic pathways mediate the effects of ERβ on behavior in the EPM. The oxytocin receptor antagonist, (des Gly-NH2 d(CH2)5 [Tyr(Me)2, Thr4] OVT, was delivered ICV to R-DPN treated male and female rats 20 minutes prior to behavioral assessment in the EPM as this test showed reliable anxiolytic effects of R-DPN in both males and females in Expt 1. Data are represented graphically in Figure 4.
Figure 4.
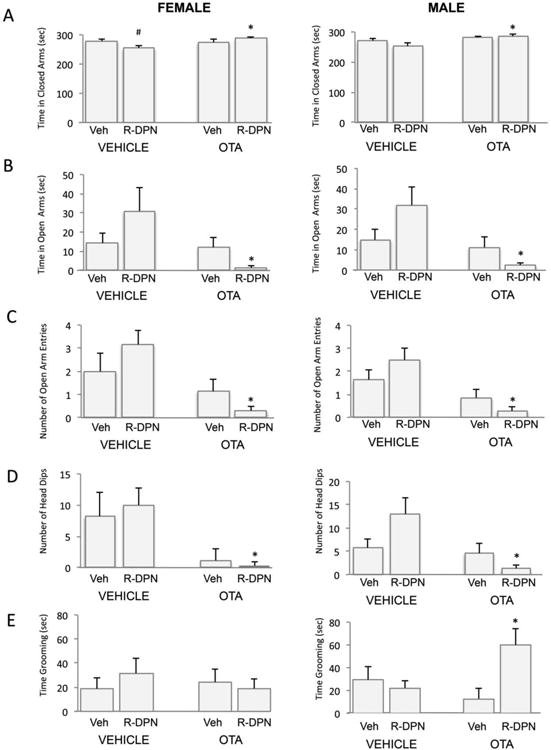
Effect of ICV treatment with an oxytocin receptor antagonist (OTA) and an ERβ agonist (R-DPN), given to gonadectomized female and male rats, on behaviors in the elevated plus maze. Animals were tested for 5 min in the EPM following 5 days of treatment with R-DPN. OTA was given 20 min prior to testing. A description of the results of 3 way and 2 way ANOVA are described in the results section. Panel A shows results for time in closed arm. Panel B shows results for Time in open arm. Panel C shows results for number of open arm entries. Panel D shows results for number of head dips. Panel E shows results for grooming behaviors. Each bar represents the mean +/- SEM of 6-8 individuals.. # indicates significant difference (p<0.05) versus vehicle-treated control following post hoc analysis. * indicates p<0.05 vs. central vehicle treated/ peripheral R-DPN group following post hoc analysis.
Time in Closed Arms
3 Way ANOVA showed that peripheral R-DPN treatment caused a reduction in the time spent in the closed arms by animals treated centrally with vehicle, as was observed in Expt 1, but this was not observed in the R-DPN animals also receiving central OTA treatment. Results of the 3-way ANOVA showed a significant central × peripheral treatment interaction [F1,49=7.94, p<0.05] and a significant main effect of central treatment [F1,49=10.68, p<0.01]. We also analyzed data by 2 way ANOVA for males or females. Subsequent two way ANOVA (peripheral × central treatment) of data from females showed a significant interaction [F1,23=5.32, p<0.05] where DPN reduced levels in vehicle treated animals and did not alter levels in OTA treated animals (Fig 4A). In males, two way ANOVA revealed a significant main effect of OTA treatment (F1,26 = 8.20; p <0.01) where OTA increased closed arm time.
Time in Open Arms
3-way ANOVA revealed that R-DPN treatment caused an increase in time spent in the open arms by animals receiving central delivery of VEH, which was not present following central OTA treatment. This is illustrated by a significant central × peripheral treatment interaction [F1,49=9.13, p<0.05] and a significant main effect of central OTA treatment [F1,49=13.68, p<0.01]. Results of subsequent two way ANOVA (Figure 4B; R-DPN × OTA) of data from females or males showed a significant interaction effect [F1,23=4.29; p<0.05 for females; F1,26=4.85; p<0.05 for males] characterized by elevated open arm time following RDPN treatment in central vehicle treated groups, but a decrease following R-DPN treatment in the central OTA treated groups. Both females and males also showed a main effect of OTA treatment [F1,23 = 5.84; p<0.05 for females; F1,26 = 8.0 2; p<0.05 for males], where OTA treatment reduced open arm time compared to central vehicle treatment.
Open arm entries
Results of a 3 way ANOVA indicate that R-DPN treatment caused an increase in the number of open arm entries displayed by animals treated centrally with vehicle whereas there was a reduction in the number of open arm entries displayed by animals treated centrally with OTA. Overall levels of open arm entries were lower in all OTA treated groups compared to those receiving ICV VEH. Results of the 3-way ANOVA showed a significant central × peripheral treatment interaction [F1,49=6.42, p<0.05] and a significant main effect of central treatment [F1,49=24.07, p<0.01]. Subsequent two way ANOVA (OTA × R-DPN treatments) on data obtained from female rats showed a significant main effect of OTA treatment [F 1,23=11.17, p<0.01] in that there was a decrease in the number of open arm entries observed following OTA pretreatment compared with vehicle groups. There was no main effect of RDPN in females. Post hoc analysis showed a significant reduction in open arm entries in the OTA/R-DPN treated group. In males, two way ANOVA revealed a significant central × peripheral treatment interaction (F1,26=78.89; p<0.01] as well as significant main effects of central OTA [F1,26=49.78, p<0.01] and peripheral R-DPN treatment [F1,26=123.0; p<0.01]. The data in males was consistent with the trends shown in females in that R-DPN treatment increased open arm entries in ICV vehicle treated animals but significantly reduced it in ICV OTA treated animals as indicated by the significant interaction effect in the two way ANOVA. Moreover, ICV OTA reduced open arm entries compared to ICV vehicle treated groups.
Head Dips
An increase in the number of head dips from the open arm is also considered to reflect a decrease in anxiety-like behavior. 3 way ANOVA (sex × central × peripheral treatments) revealed that R-DPN treatment increased the number of head dips on the open arm of male and female rats treated centrally with vehicle whereas there was no effect of R-DPN in the animals treated centrally with the OTA. This is illustrated by a significant central × peripheral treatment interaction effect [F1,49=5.21, p<0.05] and a significant main effect of central treatment [F1,49, =14.28, p<0.01]. Subsequent 2-way ANOVA (central × peripheral treatment) for data from females showed a significant main effect of OTA treatment [F1,23 = 10.76; p<0.01] where OTA reduced the number of head dips compared to vehicle groups. There was no interaction effect, or R-DPN effect. In males, 2 way ANOVA revealed a significant peripheral × central treatment interaction [F1,26 = 5.02; p<0.05] with increases following R-DPN in the vehicle group and decreases following R-DPN in the OTA pretreated group. There was also a significant main effect of OTA [F 1,26= 7.53, p<0.05] with OTA treated groups being lower than vehicle treated. There was no effect of R-DPN.
Grooming
R-DPN treatment increased grooming time only in male rats and only following central OTA treatment. 3 way ANOVA showed a significant sex × central × peripheral treatment effect [F1,49=6.21, p<0.05] and no other interaction or main effects noted. Two way ANOVA of male data (central × peripheral treatment) revealed a significant peripheral × central treatment interaction [F 1,26= 6.79, p<0.05] with significant increases following R-DPN treatment in the OTA pretreated group only (Figure 4E).
Closed arm entries, and rearing showed no significant effects of sex, R-DPN or OTA treatment.
Corticosterone
3 way ANOVA revealed that R-DPN treatment caused a reduction in levels of corticosterone following the EPM test which was not apparent following treatment with the OT antagonist alone (i.e. peripheral VEH). This is illustrated by 3-way ANOVA (Gender, Central Trt and Periph Trt as factors) results showing a significant central × peripheral treatment interaction [F1,49=5.68, p<0.05]. As no significant interaction of Gender with Central and/or Peripheral treatment was found, 2-way ANOVA was performed using Central treatment and Peripheral treatment as factors. The two way ANOVA of levels of plasma CORT showed a peripheral × central treatment interaction [F1,21 =4.1; p<0.05], but no main effects of OTA or R-DPN treatment in females. Post hoc analysis shows that female rats treated peripherally with R-DPN, but centrally with VEH, exhibit significantly lower levels of CORT following the stressor compared to those treated peripherally and centrally with VEH(p<0.05, Figure 5A).In males, there as a significant main effect of centrally administered OTA [F1,26 =4.12; p<0.05] but no effect of peripheral R-DPN treatment and no significant interaction between the two factors.
Figure 5.
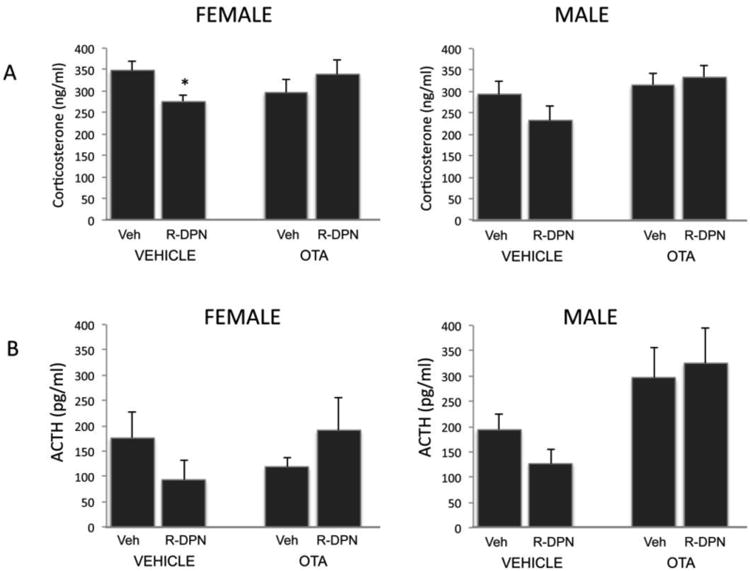
Effect of ICV treatment with an oxytocin receptor antagonist (OTA) and an ERβ agonist (R-DPN) given to gonadectomized female and male rats, on the corticosterone (CORT, Panel A) and ACTH (panel B) levels after behavior testing on the Elevated plus maze (EPM). Animals were tested on the EPM after 5 daily injections of R-DPN. OTA was given ICV 20′ prior to behavior testing and animals were killed 15 minutes after the end of the 5 min EPM session. Results of 3 way and 2-way ANOVA are found in the results section. Each bar represents the mean +/- SEM of 6-8 individuals. * indicates stressed groups that were significantly different (p<0.05) compared to non-stress controls after post-hoc testing
ACTH
Three way ANOVA (Sex × peripheral treatment × central treatment) revealed significant interactions between sex and central treatment [F1,49=3.96, p<0.05] and between central and peripheral treatment [F1,49=3.73, p<0.05] indicating that R-DPN treatment reduced stress-induced ACTH levels in male and female animals treated centrally with vehicle, but this effect was not present in animals pretreated with the OTA. Significant main effects of sex [F1,49 = 7.7, p<0.001] and central treatment [F1,49=6.74, p<0.05] were also noted. Subsequent 2-way ANOVA (central treatment × peripheral treatment) of plasma ACTH in either males or females showed a significant main effect of central OTA administration in males [F1,26 = 9.68; p<0.01, Figure 5B]. No significant main effect of R-DPN nor any interactions were noted.
Discussion
In this study we have demonstrated that the ERβ agonist, R-DPN, modulates anxiety and depressive-like behaviors as well as HPA axis responsivity to restraint-stress in a sex specific fashion. We found that the effects of ERβ agonism on behaviors in the elevated plus maze are similar between males and females, but that R-DPN effectively increased anxiolytic behaviors more effectively and consistently across parameters in females. Similarly, we also found a clear sex difference in the response to R-DPN in the forced swim test. Females responded to R-DPN with an anti-depressive like response by increasing time spent struggling, whereas males did not respond to treatment. Correspondingly, when examining the CORT and ACTH response to restraint stress, R-DPN treatment reduced the response in both sexes, but the effect appeared greater in females than males. Based on these data, we explored the effect of R-DPN treatment in behaviors in the EPM and we show that ICV treatment with an oxytocin receptor antagonist can block the effects of R-DPN on anxiolytic behaviors and restraint-induced hormone secretion. These data support the hypothesis that ERβ signaling pathways are linked with oxytocinergic pathways in the control of anxiety-like behaviors and HPA axis stress-responsivity.
For these studies we have utilized a biologically active isomer of the originally described ERβ agonist, diarylpropionitrile (DPN [42]. Diarylpropionitrile exists as a racemic mixture of two enantiomers, R-DPN and S-DPN. Previously, chiral separation of racemic DPN (rac-DPN) revealed that one enantiomer contained most of the ERβ selective activity, whereas the other was less potent and also contained residual ERα activity [31]. Based on the modeling studies of Sun et al [43], the biologically active enantiomer was presumed to be S-DPN and the non-active enantiomer, R-DPN [31]. Subsequently, using enantioselective synthesis, Carroll et al [44] determined that the isomer with the higher potency for ERβ in fact had the R-configuration. Therefore, to maintain consistency in nomenclature, we now use the designation R-DPN for the biologically active enantiomer (formerly labeled S-DPN) [31, 45].
Our studies demonstrate that activation of ERβ with R-DPN increases some anxiolytic behaviors in both male and female rats. Previously, rac-DPN was shown to have anxiolytic and anti-depressive-like properties when administered to either male or female rats [31, 46, 47, 48] and the biologically active R-enantiomer of DPN is anxiolytic when given to female mice [45]. These effects are opposite of those seen following peripheral administration of an ERα selective agonist. Moreover, the effects of rac-DPN could be blocked by co-administration of the nonselective ER antagonist tamoxifen [46], thereby verifying that the anxiolytic actions of DPN are ER mediated, and are likely due to activation of the beta subtype of ER. We have now directly compared the efficacy of R-DPN in gonadectomized male and female rats and demonstrated that R-DPN exerts similar anxiolytic properties in both sexes but is more effective in females. Examination of all anxiolytic behaviors, including open arm time and head dips showed a significant increase following R-DPN exposure in females. The effects of R-DPN on depressive-like behaviors in gonadectomized animals, as demonstrated by examining increases in time spent struggling in the FST, were also only seen in females. This sex difference in sensitivity to R-DPN is independent of circulating gonadal steroids since all animals were gonadectomized in these studies. It is possible that males may possess a higher threshold of sensitivity to R-DPN, but if present, this could differ by brain region. Of importance, a previous study using rac-DPN administration to intact males showed that it did not alter anxiolytic behaviors [49]. In that study, the lack of effect of rac-DPN in intact males was likely due to the presence of the gonad, and circulating testosterone levels. Moreover, testosterone can be metabolized to 5alpha dihydrotestosterone and subsequently to 5alpha androstane 3β 17β diol (3β diol), a compound that has been shown to bind ERβ and have anxiolytic properties of its own [50]. Hence, the presence of such an endogenous ERβ agonist in intact males, as a result of testosterone metabolism, could have masked any effects of further receptor activation with an ERβ agonist. However, this does not appear to be the case in the current studies since all animals were gonadectomized. Therefore, these data suggest the potential for an organizational effect of steroids, or a genetic sex difference, that may influence sex-specific sensitivity to ERβ agonist administration on depressive-like behaviors.
In these studies we also compared the effects of R-DPN treatment on behaviors in the forced swim test, as a measure of depressive-like behavior [31]. Our results demonstrate a sex difference in the response to ERβ agonist treatment where ovariectomized females responded to R-DPN with an increase in time spent struggling, indicative of an anti-depressent action, whereas males did not. Previous studies have indicated that there are sex differences in behavior in the forced swim test, with females showing increased time to immobility versus males [51, 52]. Our studies show that there is not a sex difference in behavior in the forced swim test in gonadectomized animals, but a sex difference arises when treated with an ERβ agonist. These results suggest that it is occupancy of the ERβ in the intact animals that may account for the previously reported sex differences and further, that females are more responsive than males to ERβ agonist treatment.
In rodents, estrogens have been shown to both increase and attenuate HPA reactivity to stressors [8, 10, 13]. It has been suggested that these contrasting reports of E2 action may in part reflect study designs using different doses or temporal windows of E2 treatment [13]. Moreover, the opposing actions of E2 on anxiolytic behaviors may be through differential actions at ERα and ERβ. Previous studies have shown that ERα activation can enhance HPA reactivity, resulting in elevations in stress-responsive CORT and ACTH secretion [15] that are likely occurring via changes in inhibitory input to the PVN since ERα is found in GABAergic neurons of the periPVN [31]. Alternatively, ERβ also is found within parvocellular and magnocellular neuropeptide neurons of the PVN [17, 18, 53], and ERβ activation has been shown to inhibit HPA reactivity [15, 31] through actions at the level of the PVN. Implants of ERβ agonists near the PVN are effective in reducing stress-responsive CORT and ACTH secretion in females [15].
We now show that R-DPN inhibits the CORT response to restraint stress in both male and female rats. Although similar effects of ERβ agonists to inhibit HPA activity have been reported separately for male [15] and female rats [31] or female mice [45], a direct sex comparison regarding sensitivity to ERβ agonist effects on HPA reactivity has not been reported. It is also apparent in these data sets that there is a sex difference in CORT levels in the basal state, and following stress, with higher levels seen in females. R-DPN treatment reduces only the post-stress rise in CORT and this effect appears to be greater in females, although the elevated levels of CORT in the non-stressed female group may account for much of this difference. These results are consistent with previous studies showing that the effect of ERβ agonist treatment is restricted to inhibiting post-stress levels of CORT and not basal levels [31, 46]. No effects of RDPN were seen in this study on ACTH levels and this may be due to the post-stress timing of blood collection in this study. Blood was collected at a time that was optimized to detect changes in plasma corticosterone levels and although we detected robust effects of stress, it is likely that the more rapid changes in plasma ACTH and the less robust changes as a result of RDPN treatment on ACTH levels could have been missed with the current study design using a single blood sampling paradigm.
The site of action for ERβ's regulation of anxiety like behaviors and HPA axis reactivity is currently not known. In considering the regulation of anxiety-like behaviors, our previous studies have shown that rac-DPN implants near the dorsal raphe nucleus were sufficient to increase TPH2 mRNA expression and reduce depressive like behaviors, but not alter anxiolytic behaviors [54]. These results seem to rule out the DRN as a primary site for ERβ action in controlling anxiety, although it is a likely site for regulating depressive-like behaviors. Other sites that might be involved include the medial amygdala and bed n. of the stria terminalis [45] based on studies examining c-Fos induction by EPM in wild type and ERβKO mice.
Estrogen receptor β is found at high levels in the PVN where it is co-expressed with oxytocin [17,18], and thus, these neurons are likely candidates for controlling not only HPA function but also stress related behaviors. In particular, oxytocin neurons in PVN have been shown to project to autonomic brain regions and constitute the bulk of oxytocinergic innervation of the forebrain [36]. Since oxytocin treatment has an anxiolytic effect in estrogen-treated female mice [55], it has been proposed that the role of oxytocin in the brain is to facilitate social encounters by reducing the related anxiety [55]. Oxytocin has also been shown to act in an anti-depressant fashion [28]. Thus, the possibility exists that ERβ may act through oxytocinergic pathways to forebrain areas, as well as within the PVN itself to control HPA reactivity to stress and stress-related behaviors.
Our studies now show that ICV administration of an OT receptor antagonist prior to behavior testing in the EPM prevents some of the actions of ERβ agonism on anxiolytic behaviors. Specifically, we found that R-DPN reduced closed arm time and correspondingly increased open arm time and open arm entries in animals treated centrally with vehicle and these effects were not seen in animals that were treated centrally with OTA. These results are consistent with the interpretation that the anxiolytic actions of R-DPN treatment are mediated through an oxytocinergic pathway. Similar effects were observed when assessing head dips from the open arm. There were also selected sex specific effects observed in these behavioral studies, suggesting that R-DPN is somewhat more effective in reducing anxiety-like behavior in females. The one exception to this was the increase in grooming seen following R-DPN treatment noted in male rats that were treated centrally with OTA. No such effects of R-DPN were seen in groups treated centrally with vehicle, or in females treated centrally with OTA. Since it is known that OT can enhance grooming behaviors in mice (56, 57), this raises the interesting possibility that ERβ can have activate anxiogenic circuits in the absence of oxytocin signaling through a pathway that is only present in males. The elucidation of such a mechanism requires further exploration.
It should also be noted that we did not observe an effect of the OT antagonist alone on CORT and ACTH secretion as has been observed previously (58). We can only conclude that the timing of our OT administration and sampling paradigm was unable to pick this up. Neumann et al, (58) examined ACTH and CORT secretion beginning 10 min after infusion, whereas we began studies at 20 min after infusion of OTA. Further, the effects they noted (increased ACTH and CORT) occurred within 5 min after the end of a 5 min behavior test, whereas we measured hormone levels at the end of a 20 min restraint. Thus, our data are actually consistent with those of Neumann et al (58) because the effects they observed were no longer significant after 15 min.
The possibility exists that the effects of OVT in modulating the effects of R-DPN could be through a non-specific action on activity. Our previous studies show that ERβ agonists do not alter total activity levels, as assessed using the open field arena [31, 46]. Although OVT has been previously reported to not influence locomotor activity when delivered to the medial preoptic area [59], it is still possible that there is an overall effect of OVT in these studies that could counter the effects of R-DPN.
Similar to the effects seen on anxiety, antagonism of oxytocin receptors can prevent some of the effects of ERβ agonist treatment on the CORT response to behavior testing in female rats. In male rats, a similar pattern of CORT and ACTH appears, but unlike the studies of expt. 1, examining restraint stress, a significant effect of R-DPN in males was not apparent in experiment 2 where treatment was also coupled with the potential stress of ICV cannulation. Similarly, some effects of R-DPN on behaviors (e.g. head dips) were not seen in experiment 2 and the stress of ICV cannulation may also play a role in these differences. A more detailed examination of the kinetics of CORT and ACTH secretion in ICV cannulated males before and after behavior testing on the EPM should be conducted in order to verify this apparent difference between the sexes. In both males and females however, there were no effects of R-DPN on CORT or ACTH secretion following OTA treatment, suggesting that ERβ utilizes, or influences, an oxytocinergic pathway to modulate HPA activity. Previous studies, examining the central effects of oxytocin on HPA reactivity have demonstrated that when administered to the PVN, oxytocin reduces HPA reactivity to stressors [35, 38]. This may be through dendritic release of oxytocin by PVN neurons, a mechanism that has been suggested as a potential way for oxytocin neurotransmission to affect the PVN in a paracrine, or autocrine action [35]. Alternatively, many oxytocin neurons in the PVN are pre-autonomic and thus it is possible that some of the effects that we see are through autonomic modulation of stress-responsive circuitry. In support of this, Hara et al [60] showed that, in the rat, exogenous estrogen attenuates the increased activation of oxytocinergic neurons observed in the parvocellular PVN of ovariectomized females in response to a psychological stressor.
Recent studies have also demonstrated that ERβ can regulate the oxytocin gene promoter [33, 61] through a composite response element, raising the possibility that ERβ works through genomic actions to increase oxytocin expression and thereby enhance oxytocinergic neurotransmission at the PVN, or in brain sites where OT PVN neurons project. The distribution of OT receptors in the brain is widespread [62] with particularly relevant populations found in the dorsal raphe [39], amygdala, bed nucleus of the stria terminalis [63] and PVN [64]. Moreover, oxytocin receptor expression has been shown to be regulated through ERα action [65, 66]. Thus, the anatomical and molecular overlap of the ERβ and oxytocin neural systems, and ERα with the OT receptor system suggests a potentially important two-tiered regulatory pathway that involves estrogen input through regulation of OT receptors by ERα and OT expression by ERβ [19, 66].
Summary/Conclusions
Our studies have demonstrated that the ERβ agonist, R-DPN, is anxiolytic and reduces the neuroendocrine response to restraint stress. The effects of ERβ agonists are similar between the sexes, whereas the ability of R-DPN to affect depressive-like behaviors appears to be female specific. The results of these studies also demonstrate that the effects of R-DPN are mediated by the downstream activation of oxytocin neurosecretory neurons and support the hypothesis that the ERβ mediated actions of T and E2 in controlling the level of stress-responsive HPA axis function involve an oxytocinergic pathway.
Highlights.
An ER beta agonist reduces anxiety more effectively in female versus male rats
An ER beta agonist reduces depressive like behaviors in female but not male rats
An ER beta agonist reduces CORT responses to restraint-stress
An Oxytocin antagonist prevents some of the actions of ER beta agonist.
Acknowledgments
The authors acknowledge the expert technical support of Jennifer Fromm. These studies were supported by USPHS grant NIH R01-NS039951 (RJH).
List of Abbreviations
- 3β-diol
15 alpha androstane 3β, 17β diol
- ACTH
adrenocorticotrophic hormone
- CORT
corticosterone
- CRH
corticotropin releasing hormone
- E2
estradiol
- HPA
hypothalamo-pituitary-adrenal
- PVN
paraventricular nucleus
- T
testosterone
- ERβ
estrogen receptor beta
- ERα
estrogen receptor alpha
- OT
oxytocin
- EPM
elevated plus maze
- VEH
vehicle
- AR
Androgen receptor
- ABA
gamma amino butyric acid
- ERβKO
Estrogen receptor beta knockout
- WT
wild type
- OTKO
oxytocin knockout
- FST
forced swim test
- R-DPN
R-diarylpropionitrile
- ICV
intracerebroventricular
- EDTA
ethylenediaminetetraacetic acid
- CSF
cerebrospinal fluid
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Critchlow V, Liebelt RA, Bar-Sela M, Mountcastle W, Lipscomb HS. Sex difference in resting pituitary-adrenal function in the rat. Am J Physiol. 1963;205:807–815. doi: 10.1152/ajplegacy.1963.205.5.807. [DOI] [PubMed] [Google Scholar]
- 2.Larkin JW, Binks SL, Li Y, Selvage D. The role of oestradiol in sexually dimorphic hypothalamic-pituitary-adrena axis responses to intracerebroventricular ethanol administration in the rat. J Neuroendocrinol. 2010;22:24–32. doi: 10.1111/j.1365-2826.2009.01934.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Seale JV, Wood SA, Atkinson HC, Harbuz MS, Lightman SL. Gonadal steroid replacement reverses gonadectomy-induced changes in the corticosterone pulse profile and stress-induced hypothalamic-pituitary-adrenal axis activity of male and female rats. J Neuroendocrinol. 2004;16:989–998. doi: 10.1111/j.1365-2826.2004.01258.x. [DOI] [PubMed] [Google Scholar]
- 4.Handa RJ, Nunley KM, Lorens SA, Louie JP, McGivern RF, Bollnow MR. Androgen regulation of adrenocorticotropin and corticosterone secretion in the male rat following novelty and foot shock stressors. Physiol Behav. 1994a;55:117–124. doi: 10.1016/0031-9384(94)90018-3. [DOI] [PubMed] [Google Scholar]
- 5.Iwasaki-Sekino A, Mano-Otagiri A, Ohata H, Yamauchi N, Shibasaki T. Gender differences in corticotropin and corticosterone secretion and corticotropin-releasing factor mRNA expression in the paraventricular nucleus of the hypothalamus and the central nucleus of the amygdala in response to footshock stress or psychological stress in rats. Psychoneuroendocrinology. 2009;34:226–237. doi: 10.1016/j.psyneuen.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 6.Rhodes ME, Kennell JS, Belz EE, Czambel RK, Rubin RT. Rat estrous cycle influences the sexual diergism of HPA axis stimulation by nicotine. Brain Res Bull. 2004;64:205–213. doi: 10.1016/j.brainresbull.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 7.Viau V, Meaney MJ. Variations in the hypothalamic-pituitary-adrenal response to stress during the estrous cycle in the rat. Endocrinology. 1991;129:2503–2511. doi: 10.1210/endo-129-5-2503. [DOI] [PubMed] [Google Scholar]
- 8.Burgess LH, Handa RJ. Chronic estrogen-induced alterations in adrenocorticotropin and corticosterone secretion, and glucocorticoid receptor-mediated functions in female rats. Endocrinology. 1992;131:1261–1269. doi: 10.1210/endo.131.3.1324155. [DOI] [PubMed] [Google Scholar]
- 9.Handa RJ, Nunley KM, Lorens SA, Louie JP, McGivern RF, Bollnow MR. Androgen regulation of adrenocorticotropin and corticosterone secretion in the male rat following novelty and foot shock stressors. Physiol Behav. 1994b;55:117–124. doi: 10.1016/0031-9384(94)90018-3. [DOI] [PubMed] [Google Scholar]
- 10.Serova LI, Harris HA, Maharjan S, Sabban EL. Modulation of responses to stress by estradiol benzoate and selective estrogen receptor agonists. J Endocrinol. 2010;205:253–262. doi: 10.1677/JOE-10-0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Viau V, Meaney MJ. Testosterone-dependent variations in plasma and intrapituitary corticosteroid binding globulin and stress hypothalamic-pituitary-adrenal activity in the male rat. J Endocrinol. 2004;181:223–231. doi: 10.1677/joe.0.1810223. [DOI] [PubMed] [Google Scholar]
- 12.Figueiredo HF, Bodie BL, Tauchi M, Dolgas CM, Herman JP. Stress integration after acute and chronic predator stress: differential activation of central stress circuitry and sensitization of the hypothalamo-pituitary-adrenocortical axis. Endocrinology. 2003;144:5249–5258. doi: 10.1210/en.2003-0713. [DOI] [PubMed] [Google Scholar]
- 13.Young EA, Altemus M, Parkison V, Shastry S. Effects of estrogen antagonists and agonists on the ACTH response to restraint stress in female rats. Neuropsychopharmacology. 2001;25:881–891. doi: 10.1016/S0893-133X(01)00301-3. [DOI] [PubMed] [Google Scholar]
- 14.Weiser MJ, Handa RJ. Estrogen impairs glucocorticoid dependent negative feedback on the hypothalamic-pituitary-adrenal axis via estrogen receptor alpha within the hypothalamus. Neuroscience. 2009;159:883–895. doi: 10.1016/j.neuroscience.2008.12.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lund TD, Hinds LR, Handa RJ. The androgen 5alpha-dihydrotestosterone and its metabolite 5alpha-androstan-3beta, 17beta-diol inhibit the hypothalamo-pituitary-adrenal response to stress by acting through estrogen receptor beta-expressing neurons in the hypothalamus. J Neurosci. 2006;26:1448–1456. doi: 10.1523/JNEUROSCI.3777-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Williamson M, Viau V. Androgen receptor expressing neurons that project to the paraventricular nucleus of the hypothalamus in the male rat. J Comp Neurol. 2007;503:717–740. doi: 10.1002/cne.21411. [DOI] [PubMed] [Google Scholar]
- 17.Hrabovszky E, Kallo I, Steinhauser A, Merchenthaler I, Coen CW, Petersen SL, Liposits Z. Estrogen receptor-beta in oxytocin and vasopressin neurons of the rat and human hypothalamus: Immunocytochemical and in situ hybridization studies. J Comp Neurol. 2004;473:315–333. doi: 10.1002/cne.20127. [DOI] [PubMed] [Google Scholar]
- 18.Suzuki S, Handa RJ. Estrogen receptor-beta, but not estrogen receptor-alpha, is expressed in prolactin neurons of the female rat paraventricular and supraoptic nuclei: comparison with other neuropeptides. J Comp Neurol. 2005;484:28–42. doi: 10.1002/cne.20457. [DOI] [PubMed] [Google Scholar]
- 19.Murakami G, Hunter RG, Fontaine C, Ribeiro A, Pfaff D. Relationships among estrogen receptor, oxyhtocin and vasopressin gene expression and social interaction in male mice. Eur J Neurosci. 2011;34:469–477. doi: 10.1111/j.1460-9568.2011.07761.x. [DOI] [PubMed] [Google Scholar]
- 20.Milner TA, Thompson LI, Wang G, Kievits JA, Martin E, Zhou P, McEwen BS, Pfaff DW, Waters EM. Distribution of estrogen receptor containing cells in the brains of bacterial artificial chromosome transgenic mice. Brain Res. 2010;1351:74–96. doi: 10.1016/j.brainres.2010.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nomura M, McKenna E, Korach KS, Pfaff DW, Ogawa S. Estrogen receptor-beta regulates transcript levels for oxytocin and arginine vasopressin in the hypothalamic paraventricular nucleus of male mice. Brain Res Mol Brain Res. 2002;109:84–94. doi: 10.1016/s0169-328x(02)00525-9. [DOI] [PubMed] [Google Scholar]
- 22.Patisaul HB, Scordalakes EM, Young LJ, Rissman EF. Oxytocin, but not oxytocin receptor is regulated by oestrogen receptor beta in the female mouse hypothalamus. J Neuroendocrinol. 2003;15:787–793. doi: 10.1046/j.1365-2826.2003.01061.x. [DOI] [PubMed] [Google Scholar]
- 23.Krezel W, Dupont S, Krust A, Chambon P, Chapman PF. Increased anxiety and synaptic plasticity in estrogen receptor beta -deficient mice. Proc Natl Acad Sci U S A. 2001;98:12278–12282. doi: 10.1073/pnas.221451898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Imwalle DB, Gustafsson JA, Rissman EF. Lack of functional estrogen receptor beta influences anxiety behavior and serotonin content in female mice. Physiol Behav. 2005;84:157–163. doi: 10.1016/j.physbeh.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 25.Amico JA, Mantella RC, Vollmer RR, Li X. Anxiety and stress responses in female oxytocin deficient mice. J Neuroendocrinol. 2004;16:319–324. doi: 10.1111/j.0953-8194.2004.01161.x. [DOI] [PubMed] [Google Scholar]
- 26.Bernatova I, Rigatto KV, Key MP, Morris M. Stress-induced pressor and corticosterone responses in oxytocin deficient mice. Exp Physiol. 2004;89:549–557. doi: 10.1113/expphysiol.2004.027714. [DOI] [PubMed] [Google Scholar]
- 27.Bodo C, Rissman EF. New roles for estrogen receptor beta in behavior and neuroendocrinology. 2006;27:217–232. doi: 10.1016/j.yfrne.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 28.Gimpl G, Fahrenholz F. The oxytocin receptor system: structure, function and regulation. Physiol Rev. 2001;81:629–683. doi: 10.1152/physrev.2001.81.2.629. [DOI] [PubMed] [Google Scholar]
- 29.Mantella RC, Vollmer RR, Li X, Amico JA. Female oxytocin-deficient mice display enhanced anxiety-related behavior. Endocrinology. 2003;144:2291–2296. doi: 10.1210/en.2002-0197. [DOI] [PubMed] [Google Scholar]
- 30.Mantella RC, Vollmer RR, Rinaman L, Lli X, Amico JA. Enhanced corticosterone conscentrantions and attenuated Fos expression in themedial amygdala of female oxytocin knockout mice exposed to psychogenic stress. Am J Phyusiol regul Integr Comp Physiol. 2004;287:R1494–1504. doi: 10.1152/ajpregu.00387.2004. [DOI] [PubMed] [Google Scholar]
- 31.Weiser MJ, Wu TJ, Handa RJ. Estrogen receptor-beta agonist diarylpropionitrile: biological activities of R- and S-enantiomers on behavior and hormonal response to stress. Endocrinology. 2009;150:1817–1825. doi: 10.1210/en.2008-1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hughes ZA, Liu F, Platt BJ, Dwyer JM, Pulicicchio CM, Zhang G, Schechter LE, Rosenzweig-Lipson S, Day M. WAY-200070, a selective agonist of estrogen receptor beta as a potential novel anxiolytic/antidepressant agent. Neuropharmacology. 2008;54:1136–1142. doi: 10.1016/j.neuropharm.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 33.Hiroi R, Lacagnina AF, Hinds LR, Carbone DG, Uht RM, Handa RJ. The Androgen Metabolite, 5alpha-Androstane-3beta,17beta-Diol (3beta-Diol), Activates the Oxytocin Promoter Through an Estrogen Receptor-beta Pathway. Endocrinology. 2013;154:1802–1812. doi: 10.1210/en.2012-2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schlosser SF, Almeida OF, Patchev VK, Yassouridis A, Elands J. Oxytocin-stimulated release of adrenocorticotropin from the rat pituitary is mediated by arginine vasopressin receptors of the V1b type. Endocrinology. 1994;135:2058–2063. doi: 10.1210/endo.135.5.7956927. [DOI] [PubMed] [Google Scholar]
- 35.Neumann ID. Stimuli and consequences of dendritic release of oxytocin within the brain. Biochem Soc Trans. 2007;35:1252–1257. doi: 10.1042/BST0351252. [DOI] [PubMed] [Google Scholar]
- 36.Knobloch HS, Charlet A, Hoffmann LC, Eliava M, Khrulev S, Cetin AH, Osten P, Schwarz MK, Seeburg PH, Stoop R, Grinevich V. Evoked axonal oxytocin release in the central amygdala attenuates fear response. Neuron. 2012;73:553–566. doi: 10.1016/j.neuron.2011.11.030. [DOI] [PubMed] [Google Scholar]
- 37.McCarthy MM, Altemus M. Central nervous system actions of oxytocin and modulation of behaviors in humans. Mol Med Today. 1997;3:269–275. doi: 10.1016/S1357-4310(97)01058-7. [DOI] [PubMed] [Google Scholar]
- 38.Neumann ID, Kromer SA, Toschi N, Ebner K. Brain oxytocin inhibits the (re)activity of the hypothalamo-pituitary-adrenal axis in male rats: involvement of hypothalamic and limbic brain regions. Regul Pept. 2000;96:31–38. doi: 10.1016/s0167-0115(00)00197-x. [DOI] [PubMed] [Google Scholar]
- 39.Yoshida M, Takayanagi Y, Inoue K, Kimura T, Young LJ, Onaka T, Nishimori K. Evidence that oxytocin exerts anxiolytic effects via oxytocin receptor expressed in serotonergic neurons in mice. J Neurosci. 2009;29:2259–2271. doi: 10.1523/JNEUROSCI.5593-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nomura M, Saito J, Ueta Y, Muglia LJ, Pfaff DW, Ogawa S. Enhanced uup- regulation of corticotropin-releasing hormone gene expression in response to restraint stress in the hypothalamic paraventriuclar nucleus of oxytocin gene-deficient male mice. J Neuroendocrinol. 2003;15:1054–1061. doi: 10.1046/j.1365-2826.2003.01095.x. [DOI] [PubMed] [Google Scholar]
- 41.Idris AI. Ovariectomy/orchidectomy in rodents. Methods Mol Biol. 2012;816:545–551. doi: 10.1007/978-1-61779-415-5_34. [DOI] [PubMed] [Google Scholar]
- 42.Carroll VM, Jeyakumar M, Carlson KE, Katzenellenbogen JA. Diarylpropionitrile (DPN) enatiomers: synthesis and evaluation of estrogen receptor β-selective ligands. J Med Chem. 2012;55:528–537. doi: 10.1021/jm201436k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Meyers MJ, Sun J, Carlson KE, Marriner GA, Katzenellenbogen BS, Katzenellenbogen JA. Estrogen receptor-beta potency-selective ligands: structure activity relationship studies of diarylpropionitriles and their acetylene and polar analogues. J Med Chem. 2001;44:4230–4251. doi: 10.1021/jm010254a. [DOI] [PubMed] [Google Scholar]
- 44.Sun J, Baudry J, Katzenellenbogen JA, Katzenellenbogen BS. Molecular basis for the subtype discrimination of the estrogen receptor –beta selective ligand, diarylpropionitrile. Mol Endocrinol. 2003;17:247–258. doi: 10.1210/me.2002-0341. [DOI] [PubMed] [Google Scholar]
- 45.Oyola MG, Portillo W, Reyna A, Foradori CD, Kudwa A, Hinds L, Handa RJ, Mani SK. Anxiolytic effects and neuroanatomical targets of estrogen receptor-beta (ERbeta) activation by a selective ERbeta agonist in female mice. Endocrinology. 2012;153:837–846. doi: 10.1210/en.2011-1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lund TD, Rovis T, Chung WC, Handa RJ. Novel actions of estrogen receptor-beta on anxiety-related behaviors. Endocrinology. 2005;146:797–807. doi: 10.1210/en.2004-1158. [DOI] [PubMed] [Google Scholar]
- 47.Walf AA, Frye CA. ERbeta-selective estrogen receptor modulators produce antianxiety behavior when administered systemically to ovariectomized rats. Neuropsychopharmacology. 2005;30:1598–1609. doi: 10.1038/sj.npp.1300713. [DOI] [PubMed] [Google Scholar]
- 48.Walf AA, Rhodes ME, Frye CA. Antidepressant effects of ERbeta-selective estrogen receptor modulators in the forced swim test. Pharmacol Biochem Behav. 2004;78:523–529. doi: 10.1016/j.pbb.2004.03.023. [DOI] [PubMed] [Google Scholar]
- 49.Patisaul HB, Burke KT, Hinkle RE, Adewale HB, Shea D. Systemic administration of diarylpropionitrile (DPN) or phytoestrogens does not affect anxiety-related behaviors in gonadally intact male rats. Horm Behav. 2009;55:319–328. doi: 10.1016/j.yhbeh.2008.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lund TD, Munson DJ, Haldy ME, Handa RJ. Dihydrotestosterone may inhibit hypothalamo-pituitary-adrenal activity by acting through estrogen receptor in the male mouse. Neurosci Lett. 2004;365:43–47. doi: 10.1016/j.neulet.2004.04.035. [DOI] [PubMed] [Google Scholar]
- 51.Martinez-Mota L, Ulloa RE, Herrera-Perez J, Chavira R, Fernandez-Guasti A. Sex and age differences in the impact of the forced swimming test on the levels of steroid hormones. Physiol Behav. 2011;104:900–9905. doi: 10.1016/j.physbeh.2011.05.027. [DOI] [PubMed] [Google Scholar]
- 52.Huynh TN, Krigbaum AM, Hanna JJ, Conrad CD. Sex differences and phase of light cycle modify chronic stress effects on anxiety and depressive-like behavior. Beh Br Res. 2011;222:212–222. doi: 10.1016/j.bbr.2011.03.038. [DOI] [PubMed] [Google Scholar]
- 53.Laflamme N, Nappi RE, Drolet G, Labrie C, Rivest S. Expression and neuropeptidergic characterization of estrogen receptors (ERalpha and ERbeta) throughout the rat brain: anatomical evidence of distinct roles of each subtype. J Neurobiol. 1998;36:357–78. doi: 10.1002/(sici)1097-4695(19980905)36:3<357::aid-neu5>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 54.Donner N, Handa RJ. Estrogen receptor beta regulates the expression of tryptophan-hydroxylase 2 mRNA within serotonergic neurons of the rat dorsal raphe nuclei. Neuroscience. 2009;163:705–718. doi: 10.1016/j.neuroscience.2009.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McCarthy MM. Estrogen modulation of oxytocin and its relation to behavior. Adv Exp Med Biol. 1995;395:235–245. [PubMed] [Google Scholar]
- 56.Meisenberg G, Simmons WH. Behavioral effects of intracerebroventricularly admininstered neurohypophyseal hormone analogs in mice. Pharmacol Biochem Behav. 1982;16:819–825. doi: 10.1016/0091-3057(82)90242-8. [DOI] [PubMed] [Google Scholar]
- 57.Amico JA, Vollmer RR, Karam JR, Lee PR, Li X, Konig JI, McCarthy MM. Centrally administered oxytocin elicits exaggerated grooming in oxytocin null mice. Pharmacol Biochem Behav. 2004;78:333–339. doi: 10.1016/j.pbb.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 58.Neumann ID, Torner L, Wigger A. Brain oxytocin: differential inhibition of neuroendocrine stress responses and anxiety-related behaviour in virgin, pregnant and lactating rats. Neuroscience. 2000;95:567–575. doi: 10.1016/s0306-4522(99)00433-9. [DOI] [PubMed] [Google Scholar]
- 59.Gil M, Bhatt R, Picotte KB, Hull EM. Oxytocin in the medial preoptic area facilitates male sexual behavior I the rat. Horm. Behav. 2011;59:435–443. doi: 10.1016/j.yhbeh.2010.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hara Y, Kohno T, Takamata A, Ueyama T, Morimoto K. Effects of estrogen on stress-induced activation of peptide nerons in PVN of ovariectomized rats. Ann N Y Acad Sci. 2008;1148:99–105. doi: 10.1196/annals.1410.042. [DOI] [PubMed] [Google Scholar]
- 61.Sharma D, Handa RJ, Uht RM. The ERβ ligand 5α-androstane, 3β, 17β-diol (3β- diol) regulates hypothalamic oxytocin (Oxt) gene expression. Endocrinology. 2012;153:2353–2361. doi: 10.1210/en.2011-1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Patchev VK, Schlosser SF, Hassan AH, Almeida OF. Oxytocin binding sites in rat limbic and hypothalamic structures: site specific modulation by adrenal and gonadal steroids. Neuroscience. 1993;57:537–543. doi: 10.1016/0306-4522(93)90003-x. [DOI] [PubMed] [Google Scholar]
- 63.Liberzon I, Young EA. Effects of stress and glucocorticoids on CNS oxytocin receptor binding. Psychneuroendocrinology. 1997;22:411–422. doi: 10.1016/s0306-4530(97)00045-0. [DOI] [PubMed] [Google Scholar]
- 64.Bealer SL, Lipschitz DL, Ramoz G, Crowley WR. Oxytocin receptor binding in the hypothalamus during gestation in rats. Am J Physiol Reg Integr Comp Physiol. 2006;291:R53–58. doi: 10.1152/ajpregu.00766.2005. [DOI] [PubMed] [Google Scholar]
- 65.Quinones-Jenab V, Jenab S, Ogawa S, Adan RA, Burbach JP, Pfaff DW. Effects of estrogen on oxytocin reeptor messenger ribonucleic acid expression in the uterus, pituitary and forebrain of the female rat. Neuroendocrinology. 1997;65:9–17. doi: 10.1159/000127160. [DOI] [PubMed] [Google Scholar]
- 66.Choleris E, Gustafsson JA, Korach KS, Muglia LJ, Pfaff DW, Ogawa S. An estrogen dependent four-gene micronet regulating social recognition: a study with oxytocin and estrogen receptor-alpha and beta knockout mice. Proc Natl acad Sic USA. 2003;100:6192–6197. doi: 10.1073/pnas.0631699100. [DOI] [PMC free article] [PubMed] [Google Scholar]


