Abstract
Background and study aim
Due to the scarcity of specific data on endoscopic ultrasound (EUS)-guided fine-needle biopsies (SharkCore) FNB in the evaluation of pancreatic lesions, we performed a prospective study of the diagnostic performance of EUS SharkCore FNB in patients with pancreatic lesions. The aim of this study was to evaluate the diagnostic accuracy.
Patients and methods
Single-center prospective study of 41 consecutive patients referred for EUS-FNB from October 2015 to April 2016 at our center. EUS-FNB was obtained in a predefined setting regarding the procedure and pathological evaluation. Data regarding demographics, lesion, technical parameters, and diagnostic accuracy were obtained.
Results
The study included 41 consecutive patients (22 males (54 %); median age 68 years). The average size of the lesions was 28 mm (median: 30 mm). A diagnostic specimen was identified in 40 (98 %) cases during microscopy with an average of 2.4 passes. The route was trans-duodenal in 20 cases (49 %). The histological diagnosis of the specimens was malignant in 29 cases (71 %), benign in 8 (20 %), suspicious in 2 (5 %), atypical in 1 (2 %) and in 1 (2 %) no material for microscopic evaluation was obtained. This led to a diagnostic accuracy of 93 %, sensitivity of 91 % and a specificity of 100 %. 2 cases (5 %) of self-limiting bleeding were observed. The diagnosis at follow up was malignant in 32 (78 %) of the patients.
Conclusions
EUS-FNB of pancreatic mass lesions with the SharkCore needle produced specimens with a diagnostic accuracy of 93 %. The procedure was safe and easy to perform, and these data support the use of EUS-FNB in a routine setting.
Introduction
Endoscopic ultrasound -guided fine-needle aspiration (EUS-FNA) has for many years been recommended when evaluating mass lesions of the pancreas where microscopic analysis was deemed necessary for a clear-cut diagnosis 1 . FNA allows evaluation of cytological features suggestive or diagnostic of malignancy with high sensitivity and specificity, and the diagnostic accuracy is even higher when an on-site pathologist is present to evaluate the quality of the obtained material 2 3 4 5 . However, due to lack of resources, this scenario is not possible in many centers 3 4 . FNAs are also faced with other problems. Chronic pancreatitis can cause atypical cellular changes making the distinction from well-differentiated neoplasia based solely on cytological criteria difficult. In such difficult cases, the possibility to cut additional sections from a formalin-fixed and paraffin embedded tissue block with application of immunohistochemical markers may increase the diagnostic accuracy. Moreover, some entities such as mesenchymal tumors, autoimmune pancreatitis (AIP), lymphoma 6 and a few rare pancreatic primary tumors are difficult to assess by FNA. Such lesions are therefore better analyzed based on EUS-guided fine needle biopsies (FNB) or tissue fragments obtained by EUS-FNA also making it possible to perform molecular and genetic analyses for personalized treatment 7 8 9 .
Since 2002 it has been possible to perform core biopsy guided by EUS using the so-called Quick-Core needle (Cook Endoscopy Inc, Limerick, Ireland), and more recently the ProCore needle from the same manufacturer has been evaluated 10 11 . Currently, there is no standard regarding needle type, size, or biopsy technique when performing pancreatic EUS-FNB. A recently published meta-analysis comparing ProCore FNB and standard FNA did not demonstrate a significant difference regarding sample adequacy, accuracy or acquisition of histological material for evaluation. The ProCore needle, however, established the diagnosis with significant fewer passes 12 . Experience with a novel FNB needle (SharkCore FNB needle, Medtronic, Dublin, Ireland) demonstrated excellent pathologic diagnostic performance also with a minimum of passes 13 14 .
Due to the lack of specific data on EUS SharkCore FNB in the evaluation of pancreatic lesions, we performed a prospective study of the diagnostic performance of EUS SharkCore FNB in patients with pancreatic mass-forming lesions. The aim of this study was to evaluate the diagnostic accuracy of the needle. EUS-FNB was only performed if it would have impact on the treatment strategy 15 .
Patients and methods
Patients referred for EUS-FNB from October 1, 2015 to April 30, 2016 were included in this consecutive and prospective single-center trial, until 40 patients had histological or sufficient cytological material for microscopic evaluation. The center is a high-volume center performing around 1000 EUS procedures including 350 tissues samples a year (combination of FNA and FNB). All the participating endoscopists were experienced and had performed more than 150 procedures including tissue sampling prior to start of this study. Inclusion criteria were presence of a solid pancreatic mass on computed tomography (CT) and/or magnetic resonance imaging and/or EUS, clinical need for an exact diagnosis, age > 18 years, and ability to provide informed consent. After EUS-FNB the final diagnosis (benign/malignant) was established using 1 of the following methods: (1) definite proof of malignancy on a surgical specimen or biopsy of a metastatic lesion; (2) malignant diagnosis on EUS-FNB and clinical/imaging follow-up compatible with malignant disease; or (3) no proof of malignancy on neither EUS-FNB nor during clinical/imaging follow-up for at least 6 months. Patients with a resectable mass on EUS and/or CT went directly for surgery. This study concerns patients with a suspected but doubtful or non-resectable mass. Non-resectable masses were biopsied for final diagnosis before oncological treatment. Complications were identified by retrospective review of the electronic health records after at least 6 months both in house and in the referring hospitals.
The study was approved by The Regional Committees on Health Research Ethics for Southern Denmark and registered in the ClinicalTrial.gov database (NCT03016637). Written and informed consent was obtained from all enrolled patients.
EUS-FNB
EUS-FNB was only performed if the result would have impact on the treatment strategy, i. e. if there was a clinical need for an exact diagnosis of the histological type of pancreatic mass lesion. EUS-FNB was performed using the SharkCore needle, guided by a convex array echo-endoscope (Pentax EG-3870UTK; Pentax Europe, GmbH, Hamburg, Germany). The endoscopist decided whether to use the 25-G or the 22-G needle, and the pancreatic mass lesion was punctured using the trans-duodenal or trans-gastric approach. Prior to puncturing the target lesion, the stylet was retracted a few millimeters, and the tip of the needle was advanced into the target tissue under endosonographic guidance. The stylet was then retracted, while multiple movements of the needle within the lesion were performed (slow pull technique) 16 . If the second pass did not result in macroscopically visible core fragments as judged by the endoscopist, suction was applied. Suction was released before removal of the needle from the target. 1 to 3 needle passes were performed. Tissue was recovered in formalin by pushing air and eventually the stylet through the needle. All samples were processed at the Department of Pathology, Odense University Hospital (OUH). No pathologist was present in the endoscopy room.
Specimen preparation and evaluation
The SharkCore biopsies were fixed in formalin (6 – 24 hours) end embedded in paraffin. Thirteen serial sections were cut from the paraffin embedded tissue blocks. Section no. 1 and 13 were stained with hematoxylin & eosin (H&E), while section no. 2 was stained with Alcian blue periodic acid-Schiff (AbPAS pH 2.7). Sections 3 – 12 were initially left unstained. If necessary for the final diagnosis, some of these sections were immunohistochemically stained for markers such as CD56, monoclonal CEA, chromogranin A, CK7, CK8, CK20, IMP3, Ki67, maspin, S100P, SMAD4, synaptophysin or VHL. Additional markers were used when necessary. All cases were evaluated and signed out by 1 of 4 pathologists specialized in gastrointestinal pathology.
Statistics
Continuous data are presented with mean and standard deviation. Results of diagnostic tests are reported with 95 % confidence intervals. Stata v. 12 (StataCorp, USA) was used.
Results
The study included 41 consecutive patients, 22 males (54 %) and 19 females (46 %), with an average age of 68 years (range 36 – 88). 91 % of the patients were in WHO performance status 0 – 1. 40 patients (98 %) had a CT performed prior to EUS evaluation and EUS-FNB ( Table1 ). The final diagnosis was malignant in 32 (78 %) patients.
Table 1. Clinical data on 41 EUS-FNB patients.
| Gender | |
| Female | 19 (46 %) |
| Male | 22 (54 %) |
| Age | |
| Average | 68 ± 11 years |
| Range | 36 – 88 years |
| WHO Performance Status | |
| 0 | 13 (32 %) |
| 1 | 24 (59 %) |
| 2 | 3 (7 %) |
| 3 | 1 (2 %) |
| Diagnostic workup before EUS-FNB | |
| CT | 40 (98 %) |
| PET-CT | 2 (5 %) |
| ERCP | 5 (12 %) |
| Final diagnosis | |
| Malignant | 32 (78 %) |
| Benign | 9 (22 %) |
| Final diagnosis based on | |
| Histology – FNB | 30 (73 %) |
| Histology – Surgery | 7 (17 %) |
| Follow-up | 4 (10 %) |
EUS-FNB procedure
7 endosonographers performed the 41 EUS-FNBs included in this study. The 22-G needle was used in 38 (93 %) cases and the 25 G needle in 3 (7 %). An average of 2.4 passes was used (range 1 – 3), and the biopsies were obtained by the slow pull-technique in 39 (95 %) cases. The average size of the pancreatic lesions was 28 ± 11 mm (median: 30 mm). The access route was trans-duodenal in 20 cases (49 %) and trans-gastric in 21 (51 %) cases. 2 cases (5 %) of self-limited bleeding were observed during the biopsy procedure, but none of the patients required additional treatment or follow-up. There were not registered any additional complications requiring contact to our institution or the referring hospital. The endosonographers identified and documented tissue cores or fragments in all patients.
Microscopic evaluation of the EUS-FNBs
A diagnostic specimen was identified in 40 (98 %) cases during microscopy, and the quality of the obtained biopsy was considered “good” in 39 (95 %) cases. Details of the microscopic examination are shown in Table 2 . Immunohistochemistry was used in 38 (93 %) of the cases as a supplement to H&E and AbPAS. Histological diagnosis of EUS-FNB specimens was malignant in 29 cases (71 %) and benign in 8 cases (20 %). 2 (5 %) specimens were considered suspicious of malignancy, whereas 1 only consisted of a cytological smear of atypical cells (2 %) and another (2 %) contained several blood cylinders but no material relevant for microscopic evaluation. Among the malignant tumors, 90 % were classified as ductal adenocarcinomas of the pancreas ( Fig.1a and Fig.1b ) and the remaining 10 % as neuroendocrine tumors (NETs) ( Fig. 1c and Fig. 1d ). 3 specimens were indicative of an intraductal-papillary mucinous neoplasm (IPMN) ( Fig. 1e and Fig. 1f ). 1 of the IPMN cases was also suspicious of malignancy ( Table 2 ).
Table 2. Histological evaluation of EUS-FNB.
| Quality of specimen as assessed by the pathologist (n = 41) | |
| Good | 39 (95 %) |
| Poor | 1 (2 %) |
| No diagnostically useful specimen obtained | 1 (2 %) |
| FNB histological diagnosis | |
| Malignant | 29 (71 %) |
|
26 (90 %) |
|
3 (10 %) |
| Suspicion of malignancy | 2 (5 %) |
|
1 (50 %) |
|
1 (50 %) |
| Atypical cells | 1 (2 %) |
|
1 (100 %) |
| Benign | 8 (20 %) |
|
2 (25 %) |
|
2 (25 %) |
|
2 (25 %) |
|
1 (12.5 %) |
|
1 (12.5 %) |
| No diagnostically specimen obtained (blood cylinders) | 1 (2 %) |
| Gold standard / final histologic diagnosis | |
| Adenocarcinoma | 29 (71 %) |
| NET | 3 (7 %) |
| Benign lesion | 9 (22 %) |
FNB, fine-need biopsy; IPMN, intraductal papillary mucinous neoplasm; NET, neuroendocrine tumor
Fig. 1.
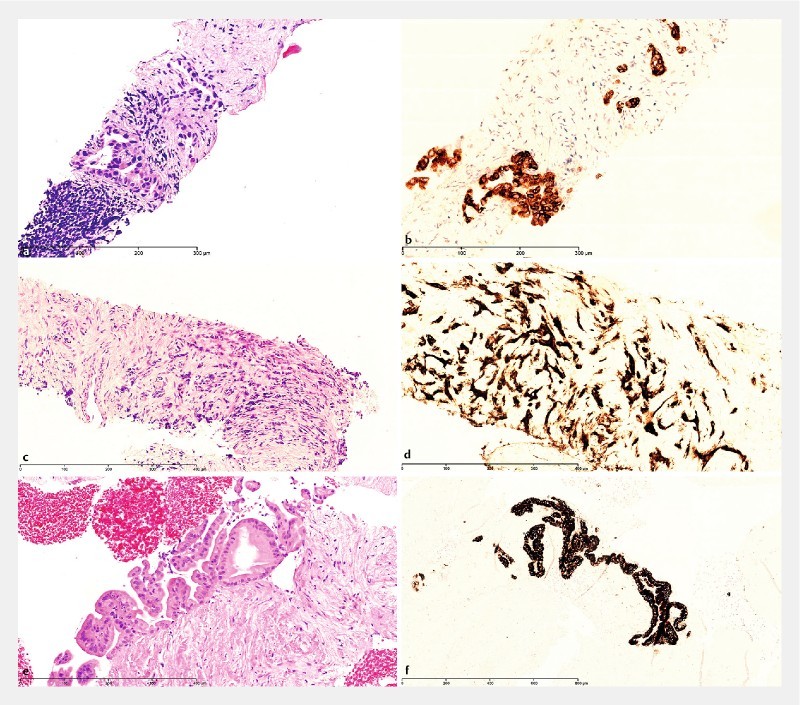
Microscopic examples of EUS-guided SharkCore biopsies from the pancreas. a H&E and b IMP3 immunostaining of ductal adenocarcinoma of the pancreas. c H&E and d insulin immunostaining of insulin-producing neuroendocrine tumor of the pancreas. e H&E and f MUC5AC immunostaining of intraductal-papillary mucinous neoplasm (IPMN) with gastric-type epithelium, low-grade dysplasia.
Follow-up
None of the cases with a benign histological diagnosis turned out to be malignant after at least 6 months of follow-up with clinical evaluation and imaging. The patient with atypical cells had a histologically confirmed malignant tumor, detected at follow-up by laparoscopic ultrasound (LUS) guided core needle biopsy. Of the 2 patients in whom malignancy was suspected, 1 had a malignant tumor diagnosed by a second SharkCore biopsy. In the calculation of the sensitivity, only conclusive malignant histological diagnoses were considered malignant, whereas cases specified as “atypical cells” and “suspicious of malignancy” were considered benign. This led to a sensitivity of 91 % and a specificity of 100 %, and an overall accuracy of 93 % ( Table 3 ). There were no false-positive and 3 (7 %) false-negative specimens observed in this study. The overall correlation between EUS-FNB and gold standard is shown in Fig. 2 . Follow up for non-malignant cases is shown in Table 4 .
Table 3. Diagnostic accuracy of EUS-FNB using the Sharkcore needle.
| Intention to treat – N = 41 | |
| Sensitivity | 0.91 (0.75 – 0.98) |
| Specificity | 1.00 (0.66 – 1) |
| Positive predictive value | 1.00 ( 0.88 – 1) |
| Negative predictive value | 0.75 (0.43 – 0.95) |
| Overall accuracy | 93 % (80 – 98 %) |
(95 % confidence intervals); EUS-FNB, endoscopic ultrasound-guided fine-need biopsy
Fig. 2.
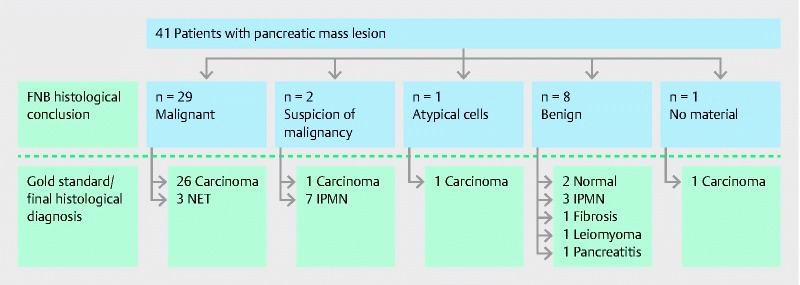
Flowchart of patients undergoing EUS-FNB for pancreatic mass lesions. Blue: FNB Histological conclusion. White: Gold standard (final histological diagnosis/follow up)
Table 4. Follow up of non-malignant cases plus number of procedures during the follow-up period of 6 months.
| Patient number | FNB | EUS + Biopsy | LUS + Biopsy | CT |
Final
diagnosis |
| 4 | Benign (suspicion of leiomyomatous tumor) | – | + | + | Leiomyoma |
| 5 | Benign (IPMN with LGD) | + + | – | + + | IPMN – LGD |
| 6 | Benign (pancreatitis) | – | – | + | Pancreatitis |
| 13 | Benign (pancreatitis) | + | + | + | Pancreatitis |
| 15 | Atypical (fibrosis) | – | + | – | Adenocarcinoma |
| 16 | Non-tissue (blood clots) | + | – | – | Adenocarcinoma |
| 19 1 | Benign (fibrosis) | + | – | + + | PANIN-2 |
| 25 | Suspicion of malignancy (HGD) | + | – | – | Adenocarcinoma |
| 28 | Benign (fibrosis) | – | – | + | Pancreatitis |
| 34 | Suspicion of malignancy (IPMN) | + + | – | + + | PANIN |
| 35 | Benign (normal tissue) | + (– biopsy) | – | + + | Normal pancreas |
| 40 | Benign (IPMN with LGD) | + + | – | + + | IPMN – LGD |
CT, computed tomography; EUS, endoscopic ultrasound; FNB, fine-needle biopsy; HGD, high-grade dysplasia; IPMN, intraductal papillary mucinous neoplasm; LGD, low-grade dysplasia; LUS, laparoscopic ultrasound; PAN IN, pancreatic intraepithelial neoplasia
The surveillance was stopped after 3 months due to severer psychiatric disease.
Discussion
EUS plays a central role in evaluation of pancreatic masses, and if cytological proof of malignancy is clinically relevant for optimal decision-making, cytological specimens can be obtained using EUS-FNA. Test performance of standard EUS-FNA needles has been evaluated in several prospective studies on patients with solid pancreatic mass lesions. A meta-analysis demonstrated a pooled sensitivity for EUS-FNA of 86.8 % (95 % confidence interval [CI], 85.5 – 87.9) and a pooled specificity of 95.8 % (95 % CI, 94.6 – 96.7) 17 . Despite these data the diagnostic yield may vary significantly, and the EUS-FNA technique and the reported data suffer from several limitations. Endosonographer experience 18 , institution volume, presence or absence of on-site pathologist 3 4 5 19 , number of passes and needles used 20 , cytological rating of indeterminate diagnoses 21 , presence of concomitant chronic pancreatitis, and success rates outside clinical trials are just a few of the relevant issues.
To circumvent some of these problems, several attempts have been undertaken to obtain EUS-FNB for histological analyses. Initial efforts were directed toward using large-caliber (18 G) needles 22 , and the Quick-Core and the ProCore biopsy needles demonstrated diagnostic accuracy ranging from 42 % to 90 % 10 12 23 24 . However, large-caliber needles may reduce maneuverability in the duodenum and increase the rate of complications, and focus has turned towards EUS-FNB with 25G and 22G needles. Preliminary retrospective data using the 25G and 22G SharkCore FNB needles in pancreatic lesions provided a pathological diagnostic yield of 86 % to 95 % with a mean number of passes of only 2 13 14 . In addition, the SharkCore needle seemed able to provide significantly larger histological specimens than the standard EUS-FNA needle 25 . The reported lower number of passes with EUS-FNB was in concordance with a meta-analysis comparing standard FNA and ProCore FNB 12 . However, the diagnostic yield was identical between the standard FNA needle and the FNB needle, and a subset analysis of pancreatic biopsies from a recent randomized study were unable to detect any differences in the cellularity yield, mean number of passes and reported adverse events 26 .
Both EUS-FNA and FNB may sometimes fail to provide sufficient material to establish the final diagnosis. Studies suggest that EUS re-biopsy in patients with previous non-diagnostic EUS-FNA from pancreatic lesions will lead to higher accuracy rates 27 , but this also means additional EUS investigations and use of needles, increasing the costs. Therefore, any new EUS-FNB needle should be monitored (and preferably tested against standard EUS-FNA needles in a randomized trial) regarding manoeuvrability, tumor access, diagnostic accuracy, number of passes, safety profile and overall costs until a final diagnosis is reached.
Using the SharkCore needle in a prospective setting, we obtained tissue suitable for microscopic analysis in the majority of cases (98 %), and the overall diagnostic accuracy for evaluating pancreatic tumors was 93 %. A similar high diagnostic yield of 86 % to 95 % and 87 % for pancreatic lesions has been published using the SharkCore needle and the ProCore needle, respectively in retrospective studies 12 13 14 . An interesting finding in the present study was the high proportion (49 %) of FNB procedures being performed through the duodenum with the endoscope in a J-flexed position. We experienced no needle handling problems in this position. The 25-G needle was used in only 3 early cases (1 through the stomach), and after these initial experiences the 22 G was chosen as standard for all EUS-FNB procedures. The needle flexibility and the trans-duodenal access have been reported as limitations especially with the Quick-Core needle 11 23 but also with the ProCore needle, where resistance to removing the stylet was hard in 18 % and removal impossible in 2 % 10 . We experienced no problems related to the use or removal of the stylet from the SharkCore needle in any position, but difficulty in puncturing the target was reported in 1 case. However, sufficient histological material was obtained from this lesion, leading to suspicion of a leiomyoma. In all samples a visible core was identified by the endosonographer. However, the inadequate sample consisted of several blood cylinders at microscopy. To optimize macroscopic evaluation of the specimens, we have tried photo documentation and macroscopic measuring of the tissue cores. None of the measures mentioned had a convincing effect.
A randomized study has shown the superiority of the fanning technique compared to the standard technique during EUS-FNA of solid pancreatic mass lesions 28 . It is easy to apply the same technique with the SharkCore needle, but there are no such data regarding this needle and it was not specifically investigated in the present study. A mean of 2.4 passes were performed until a macroscopic specimen was obtained as judged by the endoscopist, and this correlated with a diagnostically conclusive biopsy specimen for microscopic evaluation in 95 % of the cases. 1 specimen (2.5 %) contained only cytological material and 1 (2.5 %) only blood without any relevant tissue for analysis. Studies evaluating the diagnostic accuracy of the SharkCore needle have reported a higher diagnostic accuracy when comparing histological to cytological material, and in both studies a histological biopsy was obtained with a median number of 2 needle passes 13 14 . However, the potential clinical relevance of a low number of needle passes (i. e. regarding the rate of complications) is unknown.
We experienced no complications apart from 2 small self-limited bleeds during the procedure, which were asymptomatic. In the only other study reporting on EUS SharkCore FNB-related complications, a mild pancreatitis was reported in 2.9 %. This is slightly higher than expected since a systematic review found procedure-related pancreatitis in 0.4 % at pancreatic EUS-FNA 29 . However, larger prospective studies are needed to establish the true risk profile of the SharkCore needle.
Thus, EUS-guided biopsy plays a significant role in up to one-third of patients suspected of having pancreatic cancer, and the actual clinical impact of EUS-guided biopsy will probably increase, as more (rare) pancreatic diseases may be detected when EUS-FNA and EUS-FNB are used more frequently 15 . Based on these first prospective data, EUS-guided SharkCore biopsy may provide more material for pathological evaluation and thus make a substantial contribution to the area of minimal invasive diagnostics.
There are several limitations to this single-center non-comparative trial, and the results may not reflect practices or technologies used at other institutions. The relatively high number of malignant tumors included may have biased the results. Another critical point in the present study is the use and definition of the criterion-standard reference method. Ideally, when the EUS-FNB was negative, histological confirmation based on surgical specimens would be the criterion standard, but this cannot be obtained for ethical reasons in this type of patients. Therefore, clinical follow-up for at least 6 months with repeated imaging procedures was used in these patients. Although not ideal, this method is a well-accepted reference standard. 7 different endosonographers performed the biopsies, but we do not consider this a true limitation, but rather a reflection of every day routine in a large and busy endoscopic unit. In addition, the lack of complications and technical problems may indicate that the FNB needle is easy to use and without a learning curve.
Conclusion
In conclusion, EUS-FNB of pancreatic mass lesions with the SharkCore needle produced specimens with a diagnostic accuracy of 93 %. The procedure was safe and easy to perform, and these preliminary data support the use of EUS-FNB in a routine setting where establishing the histological type of pancreatic tumors is clinically relevant. Future prospective and comparative studies should focus on safety, diagnostic accuracy and costs.
Footnotes
Competing interests None
References
- 1.Storm A C, Lee L S. Endoscopic ultrasound-guided techniques for diagnosing pancreatic mass lesions: Can we do better? World J Gastroenterol. 2016;22:8658–8669. doi: 10.3748/wjg.v22.i39.8658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nelsen E M, Buhler D, Soni A V et al. Endoscopic ultrasound in the evaluation of pancreatic neoplasms-solid and cystic: A review. World J Gastrointest Endosc. 2015;7:318–327. doi: 10.4253/wjge.v7.i4.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iglesias-Garcia J, Larino-Noia J, Abdulkader I et al. Rapid on-site evaluation of endoscopic-ultrasound-guided fine-needle aspiration diagnosis of pancreatic masses. World J Gastroenterol. 2014;20:9451–9457. doi: 10.3748/wjg.v20.i28.9451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ecka R S, Sharma M. Rapid on-site evaluation of EUS-FNA by cytopathologist: an experience of a tertiary hospital. Diagn Cytopathol. 2013;41:1075–1080. doi: 10.1002/dc.23047. [DOI] [PubMed] [Google Scholar]
- 5.Khan M A, Grimm I S, Ali R et al. A meta-analysis of endoscopic ultrasound-fine-needle aspiration compared to endoscopic ultrasound-fine-needle biopsy: diagnostic yield and the value of onsite cytopathological assessment. Endosc Int Open. 2017;5:E363–E375. doi: 10.1055/s-0043-101693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhutani M, Koduru P, Lanke G et al. The emerging role of endoscopic ultrasound-guided core biopsy for the evaluation of solid pancreatic masses. Minerva Gastroenterol Dietol. 2015;61:51–59. [PubMed] [Google Scholar]
- 7.Brais R J, Davies S E, O'Donovan M et al. Direct histological processing of EUS biopsies enables rapid molecular biomarker analysis for interventional pancreatic cancer trials. Pancreatology. 2012;12:8–15. doi: 10.1016/j.pan.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 8.Christensen L, Mortensen M B, Detlefsen S. Breast Carcinoma With Unrecognized Neuroendocrine Differentiation Metastasizing to the Pancreas: A Potential Diagnostic Pitfall. Int J Surg Pathol. 2016;24:463–467. doi: 10.1177/1066896916632909. [DOI] [PubMed] [Google Scholar]
- 9.Cheng B, Zhang Y, Chen Q et al. Analysis of Fine-Needle Biopsy Versus Fine-Needle Aspiration in Diagnosis of Pancreatic and Abdominal Masses: A Prospective, Multicenter, Randomized Controlled Trial. Clin Gastroenterol Hepatol. 2017 doi: 10.1016/j.cgh.2017.07.010. [DOI] [PubMed] [Google Scholar]
- 10.Iglesias-Garcia J, Poley J, Larghi A et al. Feasibility and yield of a new EUS histology needle: results from a multicenter, pooled, cohort study. Gastrointest Endosc. 2011;73:1189–1196. doi: 10.1016/j.gie.2011.01.053. [DOI] [PubMed] [Google Scholar]
- 11.Levy M J, Jondal M L, Clain J et al. Preliminary experience with an EUS-guided trucut biopsy needle compared with EUS-guided FNA. Gastrointest Endosc. 2003;57:101–106. doi: 10.1067/mge.2003.49. [DOI] [PubMed] [Google Scholar]
- 12.Bang J Y, Hawes R, Varadarajulu S. A meta-analysis comparing ProCore and standard fine-needle aspiration needles for endoscopic ultrasound-guided tissue acquisition. Endoscopy. 2016;48:339–349. doi: 10.1055/s-0034-1393354. [DOI] [PubMed] [Google Scholar]
- 13.DiMaio C J, Kolb J M, Benias P C et al. Initial experience with a novel EUS-guided core biopsy needle (SharkCore): results of a large North American multicenter study. Endosc Int Open. 2016;4:E974–E979. doi: 10.1055/s-0042-112581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kandel P, Tranesh G, Nassar A et al. EUS-guided fine needle biopsy sampling using a novel fork-tip needle: a case-control study. Gastrointest Endosc. 2016;84:1034–1039. doi: 10.1016/j.gie.2016.03.1405. [DOI] [PubMed] [Google Scholar]
- 15.Mortensen M B, Pless T, Durup J et al. Clinical impact of endoscopic ultrasound-guided fine needle aspiration biopsy in patients with upper gastrointestinal tract malignancies. A prospective study. Endoscopy. 2001;33:478–483. doi: 10.1055/s-2001-14966. [DOI] [PubMed] [Google Scholar]
- 16.Nakai Y, Isayama H, Chang K J et al. Slow pull versus suction in endoscopic ultrasound-guided fine-needle aspiration of pancreatic solid masses. Dig Dis Sci. 2014;59:1578–1585. doi: 10.1007/s10620-013-3019-9. [DOI] [PubMed] [Google Scholar]
- 17.Puli S R, Bechtold M L, Buxbaum J L et al. How good is endoscopic ultrasound-guided fine-needle aspiration in diagnosing the correct etiology for a solid pancreatic mass?: A meta-analysis and systematic review. Pancreas. 2013;42:20–26. doi: 10.1097/MPA.0b013e3182546e79. [DOI] [PubMed] [Google Scholar]
- 18.Shahidi N, Ou G, Lam E et al. When trainees reach competency in performing endoscopic ultrasound: a systematic review. Endosc Int Open. 2017;5:E239–E243. doi: 10.1055/s-0043-100507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jhala N C, Grimm I S, Ali B et al. Providing on-site diagnosis of malignancy on endoscopic-ultrasound-guided fine-needle aspirates: should it be done? Ann Diagn Pathol. 2007;11:176–181. doi: 10.1016/j.anndiagpath.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 20.Erickson R A, Sayage-Rabie L, Beissner R S. Factors predicting the number of EUS-guided fine-needle passes for diagnosis of pancreatic malignancies. Gastrointest Endosc. 2000;51:184–190. doi: 10.1016/s0016-5107(00)70416-0. [DOI] [PubMed] [Google Scholar]
- 21.Virk R K, Gamez R, Mehrotra S et al. Variation of cytopathologistsʼ use of the indeterminate diagnostic categories “atypical” and “suspicious for malignancy” in the cytologic diagnosis of solid pancreatic lesions on endoscopic ultrasound-guided fine-needle aspirates. Diagn Cytopathol. 2017;45:3–13. doi: 10.1002/dc.23565. [DOI] [PubMed] [Google Scholar]
- 22.Binmoeller K F, Thul R, Rathod V et al. Endoscopic ultrasound-guided, 18-gauge, fine needle aspiration biopsy of the pancreas using a 2.8 mm channel convex array echoendoscope. Gastrointest Endosc. 1998;47:121–127. doi: 10.1016/s0016-5107(98)70343-8. [DOI] [PubMed] [Google Scholar]
- 23.Sakamoto H, Kitano M, Komaki T et al. Prospective comparative study of the EUS guided 25-gauge FNA needle with the 19-gauge Trucut needle and 22-gauge FNA needle in patients with solid pancreatic masses. J Gastroenterol Hepatol. 2009;24:384–390. doi: 10.1111/j.1440-1746.2008.05636.x. [DOI] [PubMed] [Google Scholar]
- 24.Gerke H, Rizk M K, Vanderheyden A D et al. Randomized study comparing endoscopic ultrasound-guided Trucut biopsy and fine needle aspiration with high suction. Cytopathology. 2010;21:44–51. doi: 10.1111/j.1365-2303.2009.00656.x. [DOI] [PubMed] [Google Scholar]
- 25.Jovani M, Abidi W M, Lee L S. Novel fork-tip needles versus standard needles for EUS-guided tissue acquisition from solid masses of the upper GI tract: a matched cohort study. Scand J Gastroenterol. 2017;52:784–787. doi: 10.1080/00365521.2017.1306879. [DOI] [PubMed] [Google Scholar]
- 26.Othman M O, Abdelfatah M M, Padilla O et al. The cellularity yield of 3 different 22-gauge endoscopic ultrasound fine needle aspiration needles. Diagn Cytopathol. 2017;45:426–432. doi: 10.1002/dc.23689. [DOI] [PubMed] [Google Scholar]
- 27.Ainsworth A P, Hansen T, Fristrup C W et al. Indications for and clinical impact of repeat endoscopic ultrasound. Scand J Gastroenterol. 2010;45:477–482. doi: 10.3109/00365520903453174. [DOI] [PubMed] [Google Scholar]
- 28.Bang J Y, Magee S H, Ramesh J et al. Randomized trial comparing fanning with standard technique for endoscopic ultrasound-guided fine-needle aspiration of solid pancreatic mass lesions. Endoscopy. 2013;45:445–450. doi: 10.1055/s-0032-1326268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang K X, Ben Q W, Jin Z D et al. Assessment of morbidity and mortality associated with EUS-guided FNA: a systematic review. Gastrointest Endosc. 2011;73:283–290. doi: 10.1016/j.gie.2010.10.045. [DOI] [PubMed] [Google Scholar]


