Abstract
Sleep related breathing disorders cause obstruction of the upper airway which can be alleviated by continuous positive airway pressure (CPAP) therapy, oral devices or surgical intervention. Non-surgical treatment modalities are not always accepted by patients and in order to attain successful surgical outcomes, evaluation of the upper airway is necessary to carefully select the patients who would benefit from surgery. There are numerous techniques available to assess the upper airway obstruction and these include imaging, acoustic analysis, pressure transducer recording and endoscopic evaluation. It is essential to note that the nocturnal obstructive upper airway has limited muscle control compared to the tone of the upper airway lumen during wakefulness. Thus, if one were to attempt to identify the anatomical segments contributing to upper airway obstruction in sleep related breathing disorders; it must be borne in mind that evaluation of the airway must be performed if possible when the patient is awake and asleep albeit during drug induced sleep. This fact as such limits the use of imaging techniques for the purpose. Drug induced sleep endoscopy (DISE) was pioneered at Royal National Throat, Nose and Ear Hospital, London in 1990 and initially introduced as sleep nasendoscopy. The nomenclature and the technique has been modified by various Institutions but the core value of this evaluation technique remains similar and extremely useful for identifying the anatomical segment responsible for obstructing the upper airway during sleep in patients with sleep related breathing disorders. There have been numerous controversies that have surrounded this technique but over the last two decades most of these have been addressed and it now remains in the forefront of methods of evaluating the upper airway obstruction. A variety of sedative agents and different grading systems have been described and efforts to unify various aspects of the technique have been made. This article will look at its usefulness and advantages and will discuss some important contributions made to the field of evaluation of the upper airway using DISE.
Keywords: Drug induced sleep endoscopy (DISE), upper airway obstruction, sleep related breathing disorders
Introduction
Georgalas et al., addressed an important issue for evaluation of the upper airway for obstructive sleep apnea (OSA) surgery using an evidence based approach and concluded that sedation endoscopy was a useful technique in helping with appropriate patient selection (1).
Drug induced sleep endoscopy (DISE) was pioneered at Royal National Throat, Nose and Ear Hospital in London in 1991 but was initially introduced with a different name of Sleep Nasendoscopy (2). Prior to drug induced sedation, endoscopic evaluation had been reported in natural sleep by Borowiecki in 1978 (3). However, this technique was thought to be time consuming as the whole night of sleep recording was subsequently evaluated for the anatomical events. Thus, the technique of DISE which offered a reasonable snap-shot of the obstructive upper airway anatomy in a much shorter timescale was introduced.
Since the inception of DISE, various sedative agents have been utilized to achieve the pharmacological sleep and these will be discussed in section A in a little more detail and an overview will be presented.
Furthermore, in section B, some of the controversies surrounding DISE such as the subjectiveness of the assessment, depth of sedation at which the obstruction should be assessed and the fact that drug induced sleep is not identical to natural physiological sleep will also be covered.
Finally, in section C, the impact of DISE on selecting patients for oral appliances (OA), its usefulness in evaluating the dynamic upper airway anatomy of continuous positive airway pressure (CPAP) treatment failures and its role in decision making for surgical procedures offered to patients with SRBD will also be discussed.
Sedative agents overview
Sedative agents play a central role for DISE procedure. The ideal sedative agent should provide a level of sedation which simulates the natural sleep without affecting the sleeping neurophysiology and upper airways collapsing performance. Up to now, this agent does not exist, but the current sedative agents applied during DISE procedure should be as alike as possible to the ideal one. Studies analyzing DISE patterns and simultaneous polysomnography demonstrated no significant changing in AHI and SaO2 in relation with basic polysomnography in the same patient, but a modification in sleep macrostructure: REM stage suppression, increasing of NREM 1 and NREM 3 sleep stages, but no difference in NREM 2 sleep stage (4,5). Propofol, midazolam, and, recently, dexmedetomidine represent the most common sedative agents routinely administrated during DISE, in single or combined modality. The ideal sedation depth is essential, consisting of a stable pattern of light-sedation, defining as the transition from consciousness to unconsciousness (loss of response to verbal stimulation: modified Ramsay score of 5) (6).
Propofol
Propofol’s (2-6-diisopropylphenol) exact mechanism of action for sedation is not completely known. It is considered a global central nervous system depressant that activates GABA-A receptors directly, at hypothalamic regions. One of the main advantages of propofol consists of fairly rapid sedation induction and a quick metabolization (7). EEG analysis during propofol sedation showed the induction of slow-waves’ sleep is similar to those found in natural NREM sleep but derived from different cortical areas, which are active during natural sleep (8). Therefore, propofol sedation acts through different mechanisms than natural sleep. Literature data clearly reported a direct relation between propofol concentrations increased and upper airways collapsibility and decreasing genioglossus muscle tone. These effects are dose-dependent, highlighting the importance of administrating as lower dosage of propofol as possible, and the importance of performing DISE by means of target controlled infusion (TCI) systems (9-11), which represent the preferred option for infusion (starting dose: 1.5–3.0 µg/mL; increasing rate 0.2–0.5, x times until the sedation level at right observation window have been obtained). If the TCI system is not available, then either a standard pump system or manual bolus technique could be utilized (Table 1) (12). Pre-operative upper airways assessment during DISE, performed using propofol, has been associated with good surgical outcomes: partial or complete anterior-posterior collapse pattern at the velum level and/or at base of the tongue has been identified as the most frequent upper airways pattern of collapse in OSA surgical patients, responders to single or multilevel surgery, whereas the complete concentric collapse pattern at the velum level and/or at the base of the tongue has been observed in OSA surgical patients, non-responders to surgical treatment (14,15).
Table 1. Sedative agents dosages for drug induced sleep endoscopy (DISE) (12,13).
| Sedative agents | Bolus technique | Pump infusion | Target controlled infusion |
|---|---|---|---|
| Propofol | Starting dose: 30–50 mg with increasing rate of 10 mg every 2 min or 1 mg/kg with increasing rate of 20 mg every 2 min (12) | Delivering dose: 50–100 mL/60 min (12) | (Single modality) starting dose: 1.5–3.0 ìg/mL; increasing rate 0.2–0.5, x times until the sedation level at the right observation window have been obtained; (Combined modality): starting dose of 1.5–3.0 ìg/mL; increasing rate 0.2–0.5, x times until the sedation level at the right observation window have been obtained (12) |
| Midazolam | (Single Modality) starting dose: 0.03 mg/kg, observe for 2–5 min, increasing rate of 0.03 mg/kg after 2–5 min, and of 0.015 mg/kg after 5 min, until the sedation level at the right observation window have been obtained (12); (Combined modality): single bolus, starting dose of 0.05 mg/kg (12) | – | – |
| Dexmedetomidine | Starting dose: 1.5 μg/kg over 10 minutes; maintenance infusion rate: 1.5 μg/kg/h | – | Starting dose: 1 μg/kg for 10 minutes, followed by an infusion at a rate of 1 μg/kg/hour (13) |
Midazolam
Midazolam is a benzodiazepine, agonist for gamma-aminobutyric acid A (GABA-A) receptor, which can act as an anxiolytic, an anticonvulsant, and also demonstrates muscle relaxant effects (16). Midazolam can also induce a depressant effect on the central respiratory drive, causing a decreasing in the ventilator response to a rise in CO2 levels. Midazolam was the sedative agent initially used in DISE and represents still an appropriate anesthetic agent for sleep endoscopy, providing a stable NREM sleep stages 1 and 2, which is the most percentage of time spent during midazolam sedation. Moreover, the critical closing pressure (Pcrit) was not significantly different during natural sleep and midazolam sedation (17-19). Bolus technique is the unique modality of Midazolam single administration, starting with a dosage of 0.03 mg/kg, and increasing of 0.03 mg/kg after 2–5 minutes, and of 0.015 mg/kg, after five minutes, if the patient is not completely asleep. Combined techniques consist in a single bolus of Midazolam (starting dose of 0.05 mg/kg), followed by Propofol TCI infusion (Table 1) (20).
Dexmedetomidine
The exact mechanism of Dexmedetomidine sedative effect is not completely known. It is a selective alpha-2 adrenergic receptor agonist, which seems to act on the locus coeruleus (LC) or to the preoptic hypothalamus to decrease wakefulness, with almost no effect on respiratory depression (21). Comparing with propofol and midazolam, dexmedetomidine provides a state of sedation closer to natural sleep and lesser upper airways muscular relaxing effect, even at the increased anesthetic dosage (13,21,22). Otherwise, Dexmedetomidine is characterized by a slightly longer onset of action (5–10 minutes), and patients take longer timing to recover (5). The sedative action of Dexmedetomidine can be reached by means of bolus technique (starting dose: 1.5 µg/kg over 10 minutes; maintenance infusion rate: 1.5 µg/kg/h) or TCI (starting dose: 1 µg/kg for 10 minutes, followed by an infusion at a rate of 1 µg/kg/hour) (Table 1).
Controversies surrounding DISE
Critics amongst us would raise various issues against DISE and argue that drug induced sleep is not identical to natural physiological sleep. When during the process of performing DISE, should the findings of the upper airway obstruction be considered as relevant is another question that may be posed as the depth of sedation would vary with the dose of sedative agent administered? This in turn could alter the degree or severity of the obstructive upper airway. The observation during the process may differ slightly when compared amongst various assessors and would therefore raise issues about uniformity of the findings recorded. There are various grading systems available for documenting the findings so these poses yet another issue.
Natural versus drug induced sleep
Early work on this topic was reported in 1996 by Sadoka et al., in a study looking at sleep parameters in natural sleep and comparing it during sleep induced by diazepam and concluded that the findings were similar for non-REM sleep (23). More recent novel studies have addressed this problem by comparing respiratory events during DISE using propofol with natural sleep (4,24). They looked at apnea and hypopnea events and also detected some events in keeping with central apneas. They also commented on oxygen desaturations as well as on position of patients. In both these studies the patients were attached with the sleep study equipment during the DISE procedure but the former (4) actually looked at sleep stages in more detail and concluded that during propofol induced sleep REM was not attained but very similar features were note with regard non-REM sleep. Of course, the limitation of these studies is the constraint in the duration of DISE not being identical to that of natural physiological sleep. However, when looking at respiratory events lot of similarities were identified.
Depth of sedation
How deep the sedation is during DISE plays an important role in accurately ascertaining the anatomical segment obstructing the upper airway. In general, 2 to 3 repeat cycles of snoring, hypoxia, obstruction with apnea and breakthrough with snoring again are observed during DISE to ensure thorough assessment. If sedation is too deep, then more tongue base and hypopharyngeal obstruction may be noted. More objectively, the use of bispectral (BIS) analysis during DISE has been useful in monitoring the correct depth of sedation. Studies utilizing BIS with midazolam alone and with combination of midazolam with propofol have demonstrated similar findings with regards depth of sedation during DISE (18,25).
Observer variations
With all practical procedures there is a learning curve and the same applies to DISE as numerous facts need to be taken into account before finalizing the findings of the upper airway evaluation (26). With modern technology, DISE can be recorded and the recording can be played back with the supervision of a senior clinician to go through the analysis in a more thorough manner. There are multiple studies that have addressed the issue of inter-observer agreement using such techniques and demonstrated satisfactory correlation and agreement in general (27,28). Further validation of this technique with multiple observer and duplication of procedure conducted on different days with similar findings has also been reported (29).
Grading systems and classification
Various institutions have reported their own grading or classification systems but to date there has not been an agreement as to which one is perfect or ideal as per the report by the European position paper on DISE (12). In essence the differing grading system has a substantial similarity in that the anatomical involvement looks at the commonly affected location of palatal, tongue base and epiglottis and takes in to consideration of lateral wall collapse as well as multi-level problems. An excellent systematic review and meta-analysis specifically on grading or classification system utilizing DISE technique has been recently reported (30). Essentially, most of the grading systems emphasize on identification of the anatomical segment of the pharynx that predominantly contributes to the upper airway obstruction and as most of these patients have a multi-level obstruction, the documentation of this allows an individually tailored management plan for these patients. Naturally, it would be useful to attain a single grading system that can be used universally and this is being addressed by the group that published the European position paper on the topic.
Impact of DISE in patient selection for non-surgical and surgical treatments
DISE has been extremely useful to the ENT surgeons in understanding, identifying and possibly alleviating some of the upper airway obstruction where possible and the role of the ENT surgeons in this multi-disciplinary field of sleep related breathing disorders has been highlighted (31). Patients can be offered multiple treatment modalities and would include CPAP therapy, OA such as mandibular advancement devices (MAD), positional therapy or surgery or indeed a combination of any of these. DISE has been found to be useful for some of these treatment options in patient selection and has also aided in identifying why some patients have failed CPAP therapy. Similarly, when surgery fails repeat assessment of upper airway obstruction by performing DISE may through some light on the residual problem.
DISE and non-surgical treatment modalities
Non-surgical treatment options such as CPAP and MAD are commonly recommended as first line conservative treatment measures but the compliance and adherence rates in general are not very good. DISE has been useful for both these groups of treatment in terms of predicting success in potential MAD users and in helping CPAP failures in understanding the reason for failure. However, it does not help in patient selection for positional therapy where the sleep study would determine the predominance of supine position during natural sleep.
CPAP is the recognized first line treatment for moderate or severe OSA. However, the long-term compliance rate is thought to be in the range of 40–85% (32-34). When a patient is experiencing difficulty with using CPAP, it could be due to the fact that there is an underlying anatomical problem in the upper airway at a single level or multiple levels affecting the nose, oropharynx and/or hypopharynx. In these cases, it would be worthwhile for the patients to be assessed by ENT surgeons who would most likely perform DISE to ascertain where exactly the problem is (31,35). DISE may demonstrate an obvious problem such as occlusion of the laryngeal inlet caused by posterior retraction of the epiglottis (Figure 1). This could subsequently be rectified by giving the patient an oral appliance such as MAD which may open up the laryngeal inlet (Figure 2) or by surgical laser wedge resection achieving similar results (Figure 3). In some patients with CPAP failure, DISE may demonstrate significant oropharyngeal collapse caused by tonsillar hypertrophy and palatal vibrations (Figure 4) thus tremendously increasing the pressure requirement for CPAP therapy and in these carefully selected patients, oropharyngeal surgery would substantially decrease the CPAP pressure and facilitate CPAP utilization or indeed in some may totally alleviate the need for this treatment modality (36).
Figure 1.
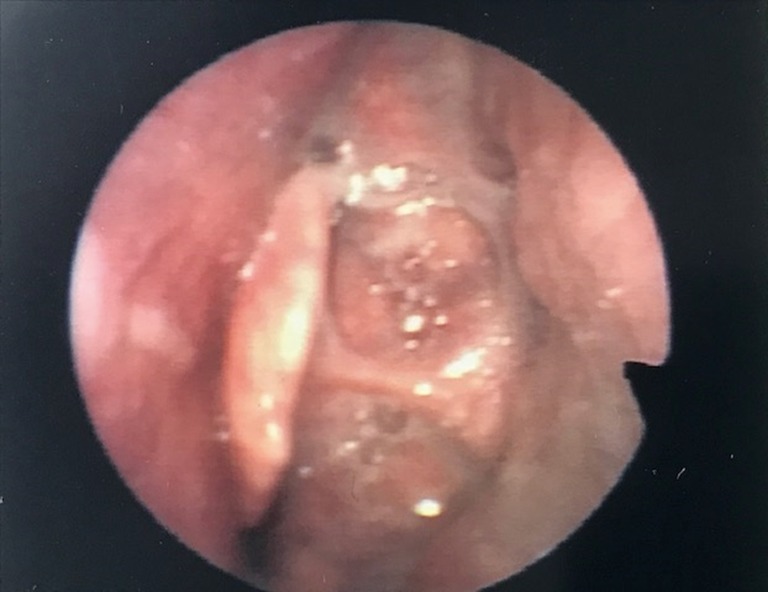
Occlusion of laryngeal inlet by retraction of epiglottis that may interfere with CPAP use. CPAP, continuous positive airway pressure.
Figure 2.
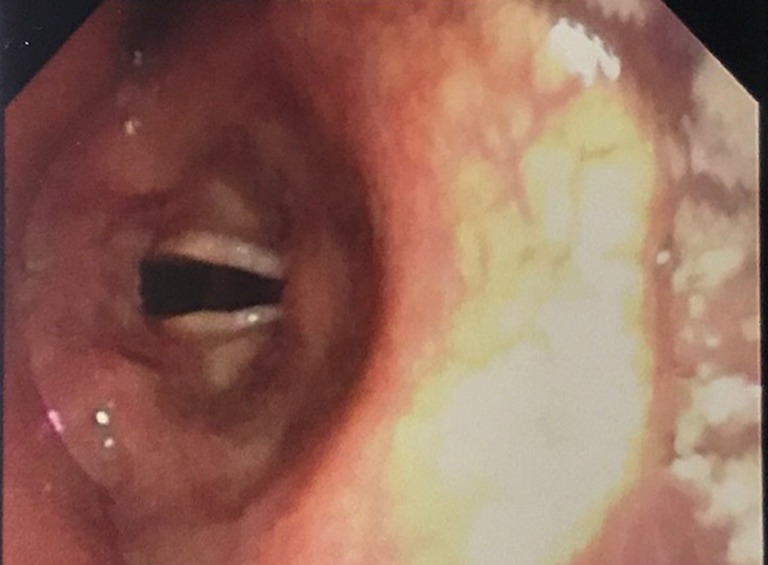
Laryngeal inlet visible with mandibular advancement device in situ.
Figure 3.
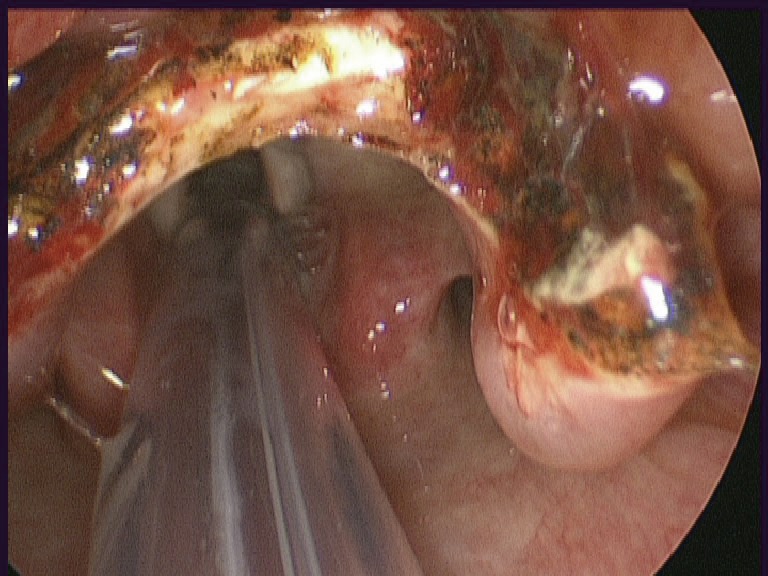
Larynx visible following partial resection of epiglottis after trans-oral robotic surgery.
Figure 4.
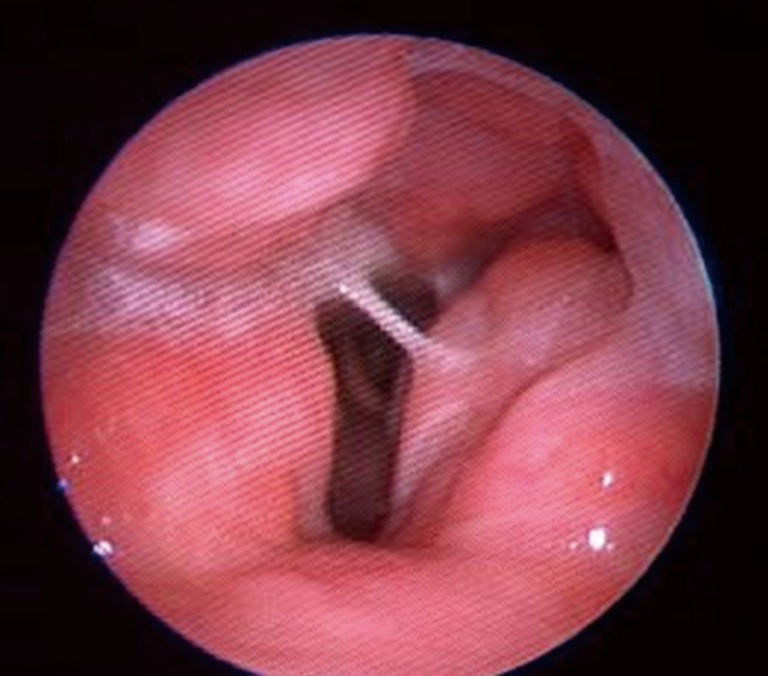
Endoscopic view during DISE demonstrating lateral wall about to collapse as a result of tonsillar hypertrophy and palatal vibrations. DISE, drug Induced sleep endoscopy.
With regards the use of MAD in patients with SRBD, number of studies have advocated the use of DISE in order to predict successful outcomes with this treatment modality (37,38). During DISE gentle protrusion of the jaw by 3–5 mm would mimic what a mandibular advancement device would do for that particular individual whilst asleep in terms of improving the upper airway dimensions and thus the obstruction and snoring. The improvement was demonstrated by performing the DISE with and without the device in situ.
DISE and surgical treatment options
Koutsourelakis et al. (15) tested the hypothesis that DISE variables can predict the outcome of upper airway surgery and concluded that there was indeed a positive response in patients with OSA. With single-level palatal surgery, patient selection with DISE has attained better long-term outcomes (39). However, in more than 50% of patients the upper airway obstruction is anatomically multi-segmental (40) and would require multi-level surgical intervention. Numerous studies have advocated multi-level surgery involving the soft palate and the base of tongue as being safe and successful (41,42).
More aggressive surgery using the trans-oral approach and addressing the hypopharynx in particular can be useful in treating the CPAP failures who whilst having DISE performed demonstrate that the problem is at the level of the base of tongue and/or the epiglottis (43,44).
In patients who are unable to tolerate CPAP and standard surgical techniques to correct the upper airway anatomy has failed, it is necessary to consider these patients for the recently introduced neuro-stimulation techniques to overcome the failure of the dilator muscle tone. The aim here is to initiate hypoglossal nerve stimulation during obstructive episodes and activate the main tongue protrusion muscle namely the genioglossus. A number of different systems have been proposed and these include Inspire, ImThera and Nyoxah, however, sufficient data at present only exists with the first system (45). Long-term data looking at outcome measures for this system are indeed encouraging (46). The key feature in patient selection for this treatment includes detailed screening of the upper airway obstruction using DISE (47).
Surgical failures can occur and in order to understand the mechanisms better, it may be useful to visualize the dynamic upper airway obstruction in slow motion during DISE and can be facilitated by using a stroboscopic light source (48).
Conclusions
The management of sleep-related breathing disorders should entail a multi-disciplinary approach. The treatment modality may include both non-surgical and surgical approaches and indeed in some cases combined and adjunctive treatments may need to be considered. Thus surgery could be combined with the use of oral appliance to attain optimum benefit or surgery could be performed to facilitate better use of CPAP and improve its compliance and adherence.
In cases where treatment response is positive, concerns are not raised but if treatment fails then further evaluation becomes critical. Careful selection of patients for appropriate treatment prior to implementing it may avoid failures and result in better outcomes.
In SRBD, it is important to note that the muscle tone during upper airway obstruction would vary in different stages of sleep and therefore the contrast in the muscle tone or lack of between wakefulness and sleep would be substantial. This would therefore suggest that the evaluation of the upper airway should be conducted during sleep in conjunction with the clinical examination performed when the patient is awake (49).
Other developments in the field of endoscopic evaluation are constantly being made and amongst this is comparison of three awake procedures to findings of DISE (50).
DISE continues to become more and more popular as a selection tool amongst otolaryngologists considering upper airway surgery in patients with SRBD and is preferred to imaging, sound analysis or pressure transducer recordings as it is the only evaluation technique to date that can offer a three-dimensional visualization of the upper airway anatomy during sleep. Some of the controversies regarding DISE have already been addressed and further work in order to enhance the validity of this technique is encouraged (51).
Acknowledgements
None.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Georgalas C, Garas G, Hadjihannas E, et al. Assessment of obstruction level and selection of patients for obstructive sleep apnoea surgery: an evidence-based approach. J Laryngol Otol 2010;124:1-9 10.1017/S002221510999079X [DOI] [PubMed] [Google Scholar]
- 2.Croft CB, Pringle M. Sleep nasendoscopy: a technique of assessment in snoring and obstructive sleep apnoea. Clin Otolaryngol Allied Sci 1991;16:504-9. 10.1111/j.1365-2273.1991.tb01050.x [DOI] [PubMed] [Google Scholar]
- 3.Borowiecki B, Pollak CP, Weitzman ED, et al. Fibro-optic study of pharyngeal airway during sleep in patients with hypersomnia obstructive sleep-apnea syndrome. Laryngoscope 1978;88:1310-3 10.1288/00005537-197808000-00010 [DOI] [PubMed] [Google Scholar]
- 4.Rabelo FA, Küpper DS, Sander HH, et al. Polysomnographic evaluation of propofol-induced sleep in patients with respiratory sleep disorders and controls. Laryngoscope 2013;123:2300-5. 10.1002/lary.23664 [DOI] [PubMed] [Google Scholar]
- 5.Carrasco Llatas M, Agostini Porras G, Cuesta González MT, et al. Drug-induced sleep endoscopy: a two drug comparison and simultaneous polysomnography. Eur Arch Otorhinolaryngol 2014;271:181-7. 10.1007/s00405-013-2548-3 [DOI] [PubMed] [Google Scholar]
- 6.Charakorn N, Kezirian EJ. Drug-Induced Sleep Endoscopy. Otolaryngol Clin North Am 2016;49:1359-72. 10.1016/j.otc.2016.06.002 [DOI] [PubMed] [Google Scholar]
- 7.Kotani Y, Shimazawa M, Yoshimura S, et al. The experimental and clinical pharmacology of propofol, an anesthetic agent with neuroprotective properties. CNS Neurosci Ther 2008;14:95-106. 10.1111/j.1527-3458.2008.00043.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Murphy M, Bruno MA, Riedner BA, et al. Propofol anesthesia and sleep: a high-density EEG study. Sleep 2011;34:283-91A. 10.1093/sleep/34.3.283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eastwood PR, Platt PR, Shepherd K, et al. Collapsibility of the upper airway at different concentrations of propofol anesthesia. Anesthesiology 2005;103:470-7. 10.1097/00000542-200509000-00007 [DOI] [PubMed] [Google Scholar]
- 10.Hillman DR, Walsh JH, Maddison KJ, et al. Evolution of changes in upper airway collapsibility during slow induction of anesthesia with propofol. Anesthesiology 2009;111:63-71. 10.1097/ALN.0b013e3181a7ec68 [DOI] [PubMed] [Google Scholar]
- 11.De Vito A, Agnoletti V, Zani G, et al. The importance of drug-induced sedation endoscopy (D.I.S.E.) techniques in surgical decision making: conventional versus target controlled infusion techniques-a prospective randomized controlled study and a retrospective surgical outcomes analysis. Eur Arch Otorhinolaryngol 2017;274:2307-17. 10.1007/s00405-016-4447-x [DOI] [PubMed] [Google Scholar]
- 12.De Vito A, Carrasco Llatas M, Vanni A, et al. European position paper on drug-induced sedation endoscopy (DISE). Sleep Breath 2014;18:453-65. 10.1007/s11325-014-0989-6 [DOI] [PubMed] [Google Scholar]
- 13.Arain SR, Ebert TJ. The efficacy, side effects, and recovery characteristics of dexmedetomidine versus propofol when used for intraoperative sedation. Anesth Analg 2002;95:461-6. [DOI] [PubMed] [Google Scholar]
- 14.Soares D, Sinawe H, Folbe AJ, et al. Lateral oropharyngeal wall and supraglottic airway collapse associated with failure in sleep surgery. Laryngoscope 2012;122:473-9. 10.1002/lary.22474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koutsourelakis I, Safiruddin F, Ravesloot M, et al. Surgery for obstructive sleep apnea: sleep endoscopy determinants of outcome. Laryngoscope 2012;122:2587-91 10.1002/lary.23462 [DOI] [PubMed] [Google Scholar]
- 16.Griffin CE, Kaye AM, Bueno FR. Benzodiazepine pharmacology and central nervous system-mediated effects. Ochsner J 2013;13:214-23. [PMC free article] [PubMed] [Google Scholar]
- 17.Genta PR, Eckert DJ, Gregorio MG, et al. Critical closing pressure during midazolam-induced sleep. J Appl Physiol (1985) 2011;111:1315-22. 10.1152/japplphysiol.00508.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abdullah VJ, Lee DL, Ha SC, et al. Sleep endoscopy with midazolam: sedation level evaluation with bispectral analysis. Otolaryngol Head Neck Surg 2013;148:331-7. 10.1177/0194599812464865 [DOI] [PubMed] [Google Scholar]
- 19.Cho JS, Soh S, Kim EJ, et al. Comparison of three sedation regimens for drug-induced sleep endoscopy. Sleep Breath 2015;19:711-7. 10.1007/s11325-015-1127-9 [DOI] [PubMed] [Google Scholar]
- 20.Hewitt RJ, Dasgupta A, Singh A, et al. Is sleep nasendoscopy a valuable adjunct to clinical examination in the evaluation of upper airway obstruction? Eur Arch Otorhinolaryngol 2009;266:691-7. 10.1007/s00405-008-0831-5 [DOI] [PubMed] [Google Scholar]
- 21.Alexopoulou C, Kondili E, Diamantaki E, et al. Effects of dexmedetomidine on sleep quality in critically ill patients: a pilot study. Anesthesiology 2014;121:801-7. 10.1097/ALN.0000000000000361 [DOI] [PubMed] [Google Scholar]
- 22.Yoon BW, Hong JM, Hong SL, et al. A comparison of dexmedetomidine versus propofol during drug-induced sleep endoscopy in sleep apnea patients. Laryngoscope 2016;126:763-7. 10.1002/lary.25801 [DOI] [PubMed] [Google Scholar]
- 23.Sadaoka T, Kakitsuba N, Fujiwara Y, et al. The value of sleep nasendoscopy in evaluation of patients with suspected sleep-related breathing disorders. Clin Otolaryngol 1996;21:485-9. 10.1111/j.1365-2273.1996.tb01095.x [DOI] [PubMed] [Google Scholar]
- 24.Gobbi R, Baiardi S, Mondini S, et al. Technique and preliminary analysis of drug-induced sleep endoscopy with online polygraphic cardiorespiratory monitoring in patients with obstructive sleep apnea syndrome. JAMA Otolaryngol Head Neck Surg 2017;143:459-65. 10.1001/jamaoto.2016.3964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Babar-Craig H, Rajani NK, Bailey P, et al. Validation of sleep nasendoscopy for assessment of snoring with bispectral index monitoring. Eur Arch Otorhinolaryngol 2012;269:1277-9. 10.1007/s00405-011-1798-1 [DOI] [PubMed] [Google Scholar]
- 26.Vroegop AV, Vanderveken OM, Wouters K, et al. Observer variation in drug-induced sleep endoscopy: experienced versus non-experienced ear, nose and throat surgeons. Sleep 2013;36:947-53. 10.5665/sleep.2732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kezirian EJ, White DP, Malhotra A, et al. Interrater reliability of drug-induced sleep endoscopy. Arch Otolaryngol Head Neck Surg 2010;136:393-7. 10.1001/archoto.2010.26 [DOI] [PubMed] [Google Scholar]
- 28.Carrasco-Llatas M, Zerpa-Zerpa V, Dalmau-Galofre J, Reliability of drug-induced sedation endoscopy: inerobserver agreement. Sleep Breath 2017;21:173-9. 10.1007/s11325-016-1426-9 [DOI] [PubMed] [Google Scholar]
- 29.Rodriguez-Bruno K, Goldberg AN, McCulloch CE, et al. Test-retest reliability of drug-induced sleep endoscopy. Otolaryngol Head Neck Surg 2009;140:646-51. 10.1016/j.otohns.2009.01.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dijemeni E, D'Amone G, Gbati I. Drug-induced sedation endoscopy (DISE) classification systems: a systematic review and meta-analysis. Sleep Breath 2017. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Virk JS, Kotecha B. Otorhinolaryngological aspects of sleep-related breathing disorders. J Thorac Dis 2016;8:213-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Turnbull CD, Bratton DJ, Craig SE, et al. In patients with minimally symptomatic OSA can baseline characteristics and early patterns of CPAP usage predict those who are likely to be longer-term users of CPAP. J Thorac Dis 2016;8:276-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kakkar RK, Berry RB. Positive airway pressure treatment for obstructive sleep apnea. Chest 2007;132:1057-72. 10.1378/chest.06-2432 [DOI] [PubMed] [Google Scholar]
- 34.Donovan LM, Boeder S, Malhotra A, et al. New developments in the use of positive airway pressure for obstructive sleep apnea. J Thorac Dis 2015;7:1323-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Virk JS, Kotecha B. When continuous positive airway pressure (CPAP) fails. J Thorac Dis 2016;8:E1112-21. 10.21037/jtd.2016.09.67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chisholm E, Kotecha B. Oropharyngeal surgery for obstructive sleep apnoea in CPAP failures. Eur Arch Otorhinolaryngol 2007;264:51-5. 10.1007/s00405-006-0139-2 [DOI] [PubMed] [Google Scholar]
- 37.Johal A, Hector MP, Battagel JM, et al. Impact of sleep nasendoscopy on the outcome of mandibular advancement splint therapy in subjects with sleep-related breathing disorders. J Laryngol Otol 2007;121:668-75. 10.1017/S0022215106003203 [DOI] [PubMed] [Google Scholar]
- 38.Battagel JM, Johal A, Kotecha BT. Sleep nasendoscopy as a predictor of treatment success in snorers using mandibular advancement splints. J Laryngol Otol 2005;119:106-12. 10.1258/0022215053419916 [DOI] [PubMed] [Google Scholar]
- 39.Iyngkaran T, Kanaglingam J, Rajeswaran R, et al. Long-term outcomes of laser-assisted uvulopalatoplasty in 168 patients with snoring. J Laryngol Otol 2006;120:932-8. 10.1017/S002221510600209X [DOI] [PubMed] [Google Scholar]
- 40.Kotecha BT, Hannan SA, Khalil HM, et al. Sleep nasendoscopy: a 10-year retrospective audit study. Eur Arch Otorhinolaryngol 2007;264:1361-7. 10.1007/s00405-007-0366-1 [DOI] [PubMed] [Google Scholar]
- 41.MacKay SG, Carney AS, Woods C, et al. Modified uvulopalatopharyngoplasty and coblation channeling of the tongue for obstructive sleep apnea: a multi-centre australian trial. J Clin Sleep Med 2013;9:117-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pang KP, Siow JK, Tseng P. Safety of multilevel surgery in obstructive sleep apnea: a review of 487 cases. Arch Otolaryngol Head Neck Surg 2012;138:353-7. 10.1001/archoto.2012.130 [DOI] [PubMed] [Google Scholar]
- 43.Vicini C, Montevecchi F, Campanini A, et al. Clinical outcomes and complications associated with TORS for OSAHS: a benchmark for evaluating an emerging surgical technology in a targeted application for benign disease. ORL J Otorhinolaryngol Relat Spec 2014;76:63-9. 10.1159/000360768 [DOI] [PubMed] [Google Scholar]
- 44.Arora A, Chaidas K, Garas G, et al. Outcome of TORS to tongue base and epiglottis in patients with OSA intolerant of conventional treatment. Sleep Breath 2016;20:739-47. 10.1007/s11325-015-1293-9 [DOI] [PubMed] [Google Scholar]
- 45.Sommer JU, Hörmann K. Innovative surgery for obstructive sleep apnea: Nerve stimulator. Adv Otorhinolaryngol 2017;80:116-24. 10.1159/000470880 [DOI] [PubMed] [Google Scholar]
- 46.Strollo PJ, Jr, Soose RJ, Maurer JT, et al. Upper-airway stimulation for obstructive sleep apnea. N Engl J Med 2014;370:139-49. 10.1056/NEJMoa1308659 [DOI] [PubMed] [Google Scholar]
- 47.Vanderveken OM, Maurer JT, Hohenhurst W, et al. Evaluation of drug-induced sleep endoscopy as a patient selection tool for implanted upper airway stimulation for obstructive sleep apnea. J Clin Sleep Med 2013;9:433-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kotecha B, Kumar G, Sands R, et al. Evaluation of upper airway obstruction in snoring patients using digital video stroboscopy. Eur Arch Otorhinolaryngol 2013;270:2141-7. 10.1007/s00405-013-2370-y [DOI] [PubMed] [Google Scholar]
- 49.Hewitt RJ, Dasgupta A, Singh A, et al. Is sleep nasendoscopy a valuable adjunct to clinical examination in the evaluation of upper airway obstruction? Eur Arch Otorhinolaryngol 2009;266:691-7. 10.1007/s00405-008-0831-5 [DOI] [PubMed] [Google Scholar]
- 50.Lovato A, Kotecha B, Vianello A, et al. Nasal and oral snoring endoscopy: novel and promising diagnostic tools in OSAS patients. Eur Arch Otorhinolaryngol 2015;272:1793-9. 10.1007/s00405-014-3473-9 [DOI] [PubMed] [Google Scholar]
- 51.Vanderveken OM. The global and evident need to increase the validity and uniformity when performing drug-induced sleep endoscopy. Sleep Breath 2017. [Epub ahead of print]. 10.1007/s11325-017-1543-0 [DOI] [PubMed] [Google Scholar]


