Abstract
Background
In this study we investigated subjective measures of sleepiness and related our findings to dimensions of affect, fatigue, emotion, mood and quality of life based on a hypothetical multidimensional model of sleepiness.
Methods
Patients referred to a sleep clinic were assessed regarding their excessive daytime sleepiness (EDS), sleep complaints, routine and symptoms. Age, gender and body mass index (BMI), the Epworth Sleepiness Scale (ESS), the Stanford Sleepiness Scale (SSS), the Samn-Perelli fatigue Scale (SPS), the Global Vigor and Affect Scale (GVS and GAS, respectively), the Hospital Anxiety and Depression Scale (HADS-A and HADS-D, respectively), and the Positive and Negative Affect Schedule (PAS and NAS, respectively) scores were recorded.
Results
Fifty patients [25 male, 45.2 (18.7) years] completed the questionnaires. The ESS scores were positively correlated with SSS, SPS, HADS-A, HADS-D and NAS scores and negatively with GVS and GAS scores (P<0.05). The SPS (P<0.001) and HADS-A scores (P=0.002) were independently associated with the ESS scores (R2=0.532, adjusted R2 =0.4794, P<0.001).
Conclusions
A model of sleepiness that assesses dimensions of fatigue and anxiety could explain the symptom of subjective sleepiness better than the isolated use of the ESS.
Keywords: Affect, Epworth Sleepiness Scale (ESS), fatigue, mood, quality of life
Introduction
Background
Excessive daytime sleepiness (EDS) is a common complaint raised by patients referred to sleep laboratories. It can negatively impact individuals through lack of alertness and concentration, diminished memory, low mood and weakness (1). Untreated EDS can contribute to the breakdown of relationships with friends and family, and it is one of the cardinal symptoms reported by clinically anxious or depressed patients (2). Additionally, EDS has a significant effect on social health; it can cause reduced performance in the workplace and lead to unemployment1. Furthermore, approximately 20% of road traffic accidents in the UK are attributed to drivers affected by EDS (3), and it leads to a reduced quality of life (4).
Mood and sleepiness
In addition to any underlying sleep disorder, other medical conditions, such as depression, are frequently linked to EDS. Patients with depression are often referred to sleep laboratories with complaints of insomnia or, less frequently, with unusually long sleep of low quality (5). Some clinical rating scales for depression focus on fatigue and tiredness rather than EDS, and a potential association between depression, fatigue and sleepiness may be underestimated (6). However, there is an interaction between mood and sleepiness, as patients with underlying sleep disorders improve in mood when they are treated and, traditionally, depression used to be treated with sleep restriction (7). This association, however, is difficult to characterize as both sleep and mood are confounded by factors such as medication, comorbidities, alcohol consumption, drug use and anxiety (2).
Fatigue and sleepiness
Fatigue is a common complaint of patients referred to sleep laboratories as well. Although “sleepiness” and “fatigue” are distinct pathologies, these terms are sometimes used interchangeably and both act falsely as a synonym for “tiredness”. The ambiguity and overlap between EDS and fatigue can lead to inaccurate diagnosis and treatment of patients (8). EDS is defined as a high sleep propensity during the day, and it is the cardinal symptom of sleep disorders, whereas fatigue is defined as a feeling of exhaustion or strain linked to physical exhaustion, chronic diseases and psychiatric disorders (2). Despite these differences, there is a strong association between EDS and fatigue which is believed to be, in part, because these symptoms are operationalized in similar ways and often patients are cross-referred from Chronic Fatigue Services to sleep laboratories (8).
Quality of life
Sleepiness may dramatically affect a patient’s quality of life and is linked to changes in neurocognitive function, such as memory loss, impaired fine motor skills and abnormal executive function (9), as well as diminished emotional functioning that result in low mood and stress. Sleepiness can put strain on private and professional relationships. Sleepiness increases the risk of accidents, and patients are more likely to separate from partners and not progress in their careers (10). It is important to consider quality of life as an aspect of sleepiness when advising patients on treatment.
Quantification of sleepiness
The most commonly used measure to assess EDS in sleep centres is the Epworth Sleepiness Scale (ESS). However, the ESS measures situational sleep propensity without acknowledging other distinct, and potentially confounding aspects of the symptom such as affect, fatigue, emotion, mood and quality of life (2). The ESS was originally developed to measure sleep propensity in adults and has been particularly useful in sleep apnoea patients (11). In the sleep laboratory, EDS can be measured by performing the multiple sleep latency test (MSLT) as well and the mean sleep latency of several naps is an objective marker for EDS (12).
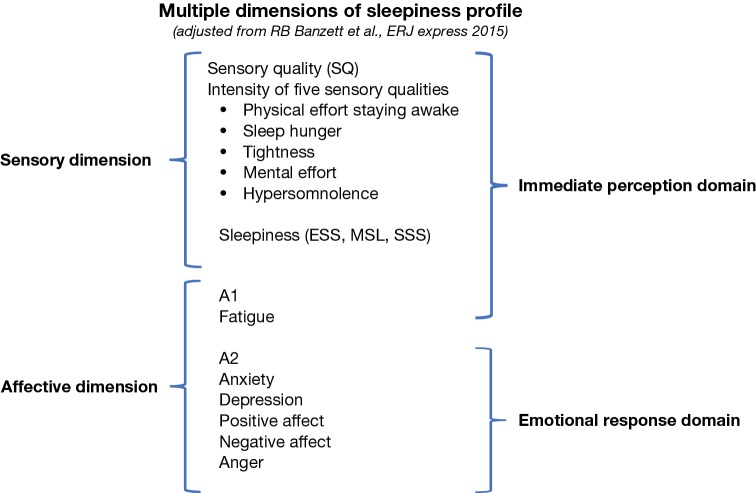
In the current study we hypothesised that subjective EDS as captured through ESS, can be more accurately described, by recording additional dimensions such as measures of affect, fatigue, emotion, mood and quality of life, providing important information for a successful diagnosis and onward management of the subjectively sleepy patient. We propose a multi-dimensional model of subjective sleepiness to better assess the symptom, based on profiles for other chronic symptoms such as pain and breathlessness (Figure 1), whereby sleepiness is a multi-factorial symptom associated with different sensory and affective dimensions that impact on the immediate perception and the emotional response of the patient.
Figure 1.
Schematic model of the components of sleepiness underlying the suggested multiple dimensions of sleepiness profile. The hypothetical model is divided into Sensory (SQ) and Affective dimensions, fatigue (A1) and emotional response (A2) (on the left) and into Immediate and Emotional Response Domains (on the right). Original picture modified from the “Multiple Dimensions of Dyspnoea Profile” according to Banzett et al. (13) (no permission required). ESS, Epworth Sleepiness Scale; MSL, Mean Sleep Latency; SSS, Stanford Sleepiness Scale.
Methods
This study assessed patients seeking specialty care who were referred to the outpatients clinic in the Lane Fox Unit and the Sleep Disorders Centre at Guy’s & St Thomas’ NHS Foundation Trust, London, UK during the period June to August 2016. The project was approved by the local review board (GSTT registration number: 2016-6172) and following informed consent, 50 patients completed the following questionnaires:
Sleepiness, as measured by the ESS and the Stanford Sleepiness Scale (SSS);
Fatigue, as assessed by the Samn-Perelli seven point fatigue scale (SPS);
Global vigor and affect, through the Global Vigor and Affect Scale (GVAS);
Anxiety and depression, as measured by the Hospital Anxiety and Depression Scale (HADS), and;
Positive and negative affect, through the Positive and Negative Affect Schedule (PANAS);
Questions regarding main sleep complaints and how EDS affects daily function, sleep routine and nighttime symptoms were included.
Age, gender, current medical conditions and regular medication were also recorded.
The ESS
The ESS is a single 8-item questionnaire that measures subjective EDS. Patients rate how likely they are to fall asleep in different situations based on their recent habits. The items are scored either as 0 (“I would never fall asleep”), 1 (“Slight chance of falling asleep”), 2 (“Moderate chance of falling asleep”) or 3 (“High chance of falling asleep”), giving a total score ranging between 0–24 points. Patients with scores higher than 10 points are regarded as excessively sleepy and may have an underlying sleep disorder (11).
The SSS
The SSS is a 7-point scale that subjectively measures how alert a patient is at that moment in time. Possible scores range from 1 (“Feel active and vital; alert, wide awake) to 7 (“Almost in reverie; sleep onset soon; lost struggle to remain awake”). A score of 3 or more, at a time when the patient should be alert, indicates a patient is excessively sleepy (14).
The Samn-Perelli 7-point fatigue scale
The SPS is a 7-point scale that subjectively measures the patient’s level of fatigue at that moment in time. Possible scores range from 1 (“fully alert, wide awake”) to 7 (“completely exhausted, unable to function effectively”) (15). Scores of 5 or 6 are classified as ‘Fatigue Class II’ and scores of 7 are classified as ‘Fatigue Class I’ where the patient is considered to be severely fatigued. This scale was originally developed to assess levels of fatigue and alertness in pilots before take-off (16).
The GVAS
The GVAS subjectively measures global vigor and global affect through eight unipolar visual analogue scales. Four of these scales ask questions concerning global vigor (alertness, sleepiness, motivational loss and weariness) and the remaining four ask questions concerning global affect (happiness, sadness, calmness and tension). Each individual scale is scored between 0 and 100 and a total global vigor score (GVS) and a total global affect score (GAS) are calculated ranging between 0 and 100 points, where lower scores indicate lower levels of global vigor or global affect (for GVS and GAS formulas—see Supplementary material) (17,18).
The hospitalized anxiety and depression Scale
The HADS subjectively measures levels of anxiety (A) and depression (D) through a 14-item questionnaire. The HADS is divided into two 7-item subscales that are concerned with anxiety and depression independently. Each item is scored from 0 to 3 points and these individual scores are summed up to give a HADS-A and HADS-D score ranging from 0 to 21 points each. The higher the total score, the greater the severity of anxiety or depression the patient exhibits (19). Patients with scores of 8 or higher are considered severely anxious or depressed (20). The HADS was chosen rather than other validated anxiety and depression scales because it is designed to control for symptoms of anxiety and depression that are somatic and could thus be confounded by other physical illnesses (19).
The PANAS
The PANAS subjectively measures positive and negative affect independently through two 10-item mood scales. Positive affect is the subjective experience of positive moods such as joy, interest and alertness whereas negative affect refers to the subjective experience of negative moods such anxiety, hostility and disgust (21). Each item in the mood scale is an adjective, which the patients must score from 1 (“very slightly or not at all”) to 5 (“extremely”) depending on how well the adjective describes their present mood. These individual scores are summed up to give a Positive Affect Score (PAS) and a Negative Affect Score (NAS) ranging from 10–50 points each. The higher the total score the greater the positive or negative affect the patient exhibits. This scale is not meant to be definitive but it is an indicator of behaviours associated with either positive or negative affect (22).
Statistical analysis
EDS, as measured by ESS, using multiple dimensions (affect, fatigue, emotion, mood, and quality of life) was recorded in the sleep clinic. Data were collected using MS Excel 2007 (Microsoft Corporation, Seattle/WA, USA) and analysed with SPSS statistics Version 23 (IBM, New York/NY, USA). Following testing for normality using the Shapiro-Wilk test data were presented as mean (SD) or median (interquartile range, IQR), as indicated. Non-normally distributed data were analysed using the Spearman’s rho correlation coefficient, unless indicated differently. The correlations between ESS scores and the other questionnaires (SSS, SPS, GVAS, HADS, PANAS) were assessed. Independent t-tests were used to assess potential differences in questionnaire scores between genders. Stepwise multiple linear regression analysis was performed to determine which of the questionnaire scores, were the best determinants of the ESS score. The level of significance was defined as P<0.05.
Results
Characteristics
Fifty middle-aged patients (25 male) completed the questionnaires. On average, the patients’ ESS, SPS and HADS-D scores did not surpass clinical thresholds, suggesting the patients were not excessively sleepy, not considerably fatigued and not depressed. Furthermore, the patients had high GVS and GAS scores suggesting high vigor and affect. The patients had moderate PAS scores and low NAS scores, which suggests moderate positive and low negative affect. Based on the average SSS scores patients were sleepy and the HADS-A scores indicated that the patients were anxious (Table 1). Female patients rated themselves more fatigued than male patients on the SPS scale [mean 3.9 (SD 1.4) vs. 2.8 (1.5) points, P=0.01; see Tables S1 and S2]; there were no significant gender differences for the other questionnaire scales.
Table 1. Characteristics of the total cohort (n=50).
| Parameters | Mean/Median | Standard deviation/IQR |
|---|---|---|
| Age (years) | 45.2 | 18.7 |
| ESS score (points)* | 7.3 | 3.8–15.0 |
| SSS score (points)* | 3.0 | 1.5–4.0 |
| SPS score (points)* | 4.0 | 2.0–4.0 |
| GVS score (points)* | 66.3 | 49.4–83.8 |
| GAS score (points)* | 58.8 | 41.9–77.5 |
| HADS-A score (points)* | 8.0 | 3.0–12.0 |
| HADS-D score (points)* | 6.0 | 3.0–8.5 |
| PAS score (points)* | 25.0 | 16.0–33.3 |
| NAS score (points)* | 16.5 | 11.0–21.0 |
*, data presented as median and IQR. ESS, Epworth Sleepiness Scale (range, 0–24 points); SSS, Stanford Sleepiness Scale (range, 0–8 points); SPS, Samn-Perelli fatigue scale (range, 0–7 points); GVS, Global Vigor Score (range, 0–100 points); GAS, Global Affect Score (range, 0–100 points); HADS-A, Hospital Anxiety Scale (range, 0–21 points); HADS-D, Hospital Depression Scale (range, 0–21 points); PAS, Positive Affect Score (range, 10–50 points); NAS, Negative Affect Score (range, 10–50 points); IQR, interquartile range.
Correlations
There was a positive correlation between the ESS and other measures of sleepiness, anxiety and depression, a high correlation with fatigue as well as a low correlation with measures of negative affect, and a negative correlation between the ESS and measures of global vigor and affect (Table 2).
Table 2. Spearman’s rho correlation coefficients for the ESS vs. all other scores (n=50).
| Parameters | ESS | P value |
|---|---|---|
| SSS | r=0.499 | <0.001 |
| SPS | r=0.607 | <0.001 |
| GVS | r=-0.433 | 0.002 |
| GAS | r=−0.508 | <0.001 |
| HADS-A | r=0.460 | 0.001 |
| HADS-D | r=0.448 | 0.001 |
| PAS | r=−0.215 | 0.135 |
| NAS | r=0.303 | 0.033 |
ESS, Epworth Sleepiness Scale; SSS, Stanford Sleepiness Scale; SPS, Samn-Perelli fatigue Scale; GVS, Global Vigor Scale; GAS, Global Affect Scale; HADSA, Hospital Anxiety Score; HADS-D, Hospital Depression Scale; PAS, Positive Affect Scale; NAS, Negative Affect Score.
Multiple linear regression analysis
Stepwise multiple linear regression analysis was performed to determine which of the questionnaire scores (SPS, GVS, GAS, HADS-A, HADS-D, PAS and NAS) were the best determinants of the ESS score, after adjusting for demographics (age, gender and BMI). The regression model (F=10.000) showed that about 48% of the response variance (R2=0.532, adjusted R2=0.4794, P<0.001) was significantly associated with the following determinants: SPS (partial R2=0.158, P<0.001) and HADS-A (partial R2=0.114, P=0.002) (see Table S3).
Discussion
The most common method to assess sleepiness, the ESS, is associated with measures of fatigue, anxiety, depression and negative affect. There is an inverse correlation between the ESS and measures of global vigor and global affect. Our results suggest that symptoms of fatigue and anxiety explain about 48% of the variability in the subjectively reported EDS. These findings indicate that fatigue and anxiety are dimensions associated with sleepiness and that they influence the immediate perception as well as the emotional experience of the symptom.
The idea that fatigue could be an additional dimension of sleepiness is not new; studies have shown that up to 46% of patients with Chronic Fatigue Syndrome fulfill the minimal criteria for sleep disorders, which suggests that the ambiguity between sleepiness and fatigue is not just due to an inaccurate diagnosis but that these symptoms might co-exist (8). Although our findings show a strong correlation between measures of fatigue and subjective measures of sleepiness, there is no link with objective measures of sleepiness, suggesting sleepiness and fatigue are distinct (23,24). The observed correlation could be due to patients mislabeling fatigue as subjective sleepiness, highlighting that there is a need to develop better scales that can differentiate these two symptoms.
This study assessed subjective (ESS, SSS) sleepiness. Additional dimensions were measured by validated questionnaires, inviting speculation about the accuracy of the used methods. For example, patients with long-term sleepiness could potentially lack a frame of reference point of what it feels like to be less sleepy, which may hamper their ability to complete a more accurate self-assessment (11). This inaccuracy in self-assessment also may be, in part, why the ESS and SSS scales did not correlate. However, it could also be that the SSS is a scale for an ad-hoc response, whilst the ESS requires a long assessment period to allow for any changes. Also, the power of the used tests was calculated to detect large effects, weak associations may not have been discovered accurately. However, by choosing validated clinical tools to assess affect, fatigue, emotion, mood, and quality of life this study adopted a pragmatic clinical approach, which is comparable to outcomes obtained in the majority of studies in this field. Further studies will be required to replicate and give credence to our results.
A more detailed descriptive background of the patients would have been helpful to understand contributing domains of the patients’ social, cultural, economic and educational background. Although the patients’ medical condition and regular medication were recorded our findings need to be supported by further studies. These further studies could also focus more on the differences between genders when rating levels of fatigue. Also, our supportive evidence base for the hypothetical multi-dimensional model needs to be expanded upon with a systematic analysis of the used scales’ redundant and differentiating factors. Previously, validated item banks for assessing qualitative aspects of sleepiness had been developed (25), but the current study has extended earlier research by exploring unique domains (e.g., affect).
Conclusions
A rigorous analysis of the used scales’ redundant and differentiating factors is required to validate the usefulness a multidimensional model of sleepiness, and assessing dimensions of affect, fatigue, emotion, mood, and quality of life might assist to better understand subjective sleepiness in patients in whom a direct somatic cause cannot be found. This approach facilitates a more integrated approach, from a multi-disciplinary collaboration of services, towards patient care. Qualitative work on the sensory and affective dimensions, as well as the development of suitable measures to quantify the immediate perception and the emotional experience to the symptom, should be the focus of future research.
Acknowledgements
Dr Steier’s contributions was partially supported by the National Institute for Health Research (NIHR) Biomedical Research Centre based at Guy’s and St Thomas’ NHS Foundation Trust and King’s College London, UK. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health.
Supplementary
The Global Vigor and Affect Scale
To calculate the total global vigor score (GVS) and total global affect score (GAS) the scores from each visual analogue scale assessing “alertness”, “sleepiness”, “effort ”, “weariness”, “happiness”, “calmness”, “sadness” and “tenseness” were included into the following formulas:
| GVS = [(alert) + 300 – (sleepy) – (effort) – (weary)/4] |
| GAS = [(happy) + (calm) + 200 – (sad) – (tense)/4] |
Gender differences in questionnaire Scales
Since previous studies have highlighted that women have higher self-perceived fatigue than men (26), we specifically explored gender differences in patients Samn-Perelli seven point Scale (SPS) and the other questionnaire scale scores through independent t-tests. Indeed, female patients rated themselves more fatigued than male patients on the SPS scale (Table S1), where the difference in means was statistically significant (P=0.01, Table S2). There were no other significant differences in mean questionnaire scores between genders.
Multiple linear regression analysis (n=50)
Stepwise multiple linear regression analysis was performed to determine which of the questionnaire scores (SPS, GVS, GAS, HADS-A, HADS-D, PAS and NAS) were the best determinants of the ESS score, after adjusting for demographics (age, gender and BMI). The regression model showed that about 48% of the response variance was associated with the following determinants: SPS (partial R2=0.158, P<0.001) and HADS-A (partial R2=0.114, P=0.002) (Table S3).
Table S1. Group statistics of questionnaire scale scores for males (n=25) and females (n=25).
| Scales | Gender | Mean (points) | Standard deviation | Standard error mean |
|---|---|---|---|---|
| SPS | Female | 3.88 | 1.42 | 0.28 |
| Male | 2.76 | 1.54 | 0.31 | |
| SSS | Female | 3.12 | 1.36 | 0.27 |
| Male | 2.38 | 1.38 | 0.28 | |
| ESS | Female | 9.30 | 7.42 | 1.48 |
| Male | 8.24 | 6.33 | 1.27 | |
| GAS | Female | 58.10 | 18.93 | 3.79 |
| Male | 62.30 | 23.21 | 4.64 | |
| GVS | Female | 65.10 | 20.20 | 4.04 |
| Male | 69.30 | 22.22 | 4.44 | |
| HADS-A | Female | 8.60 | 5.26 | 1.05 |
| Male | 6.75 | 4.93 | 1.01 | |
| HADS-D | Female | 6.52 | 4.67 | 0.93 |
| Male | 6.17 | 4.54 | 0.93 | |
| PAS | Female | 24.92 | 8.27 | 1.65 |
| Male | 24.56 | 10.34 | 2.07 | |
| NAS | Female | 18.32 | 7.06 | 1.41 |
| Male | 15.44 | 7.97 | 1.60 |
SPS, Samn-Perelli Scale; SSS, Stanford Sleepiness Scale; ESS, Epworth Sleepiness Scale; GAS, Global Affect Scale; GVS, Global Vigor Scale; HADS-A, Hospital Anxiety Scale; HADS-D, Hospital Depression Scale; PAS, Positive Affect Scale; NAS, Negative Affect Scale.
Table S2. Independent samples t-test statistics for questionnaire scores between males and females with equal variances assumed.
| Scales | t-test for equality of means | ||||||
|---|---|---|---|---|---|---|---|
| t | df | Sig. (2-tailed) | Mean difference (points) | Std. error difference | 95% confidence interval of the difference | ||
| Lower | Upper | ||||||
| SPS | 2.675 | 48 | 0.010 | 1.1200 | 0.4187 | 0.2781 | 1.9619 |
| SSS | 1.902 | 47 | 0.063 | 0.7450 | 0.3916 | −0.0428 | 1.5328 |
| ESS | 0.543 | 48 | 0.589 | 1.060 | 1.951 | −2.863 | 4.983 |
| GAS | −0.701 | 48 | 0.487 | −4.200 | 5.990 | −16.244 | 7.844 |
| GVS | −0.699 | 48 | 0.488 | −4.200 | 6.005 | −16.274 | 7.874 |
| HADS-A | 1.269 | 47 | 0.211 | 1.850 | 1.457 | −1.082 | 4.782 |
| HADS-D | 0.269 | 47 | 0.789 | 0.353 | 1.315 | −2.293 | 2.999 |
| PAS | 0.136 | 48 | 0.892 | 0.360 | 2.649 | −4.966 | 5.686 |
| NAS | 1.353 | 48 | 0.182 | 2.880 | 2.129 | −1.401 | 7.161 |
SPS, Samn-Perelli Scale; SSS, Stanford Sleepiness Scale; ESS, Epworth Sleepiness Scale; GAS, Global Affect Scale; GVS, Global Vigor Scale; HADS-A, Hospital Anxiety Scale; HADS-D, Hospital Depression Scale; PAS, Positive Affect Scale; NAS, Negative Affect Scale.
Table S3. Stepwise multiple linear regression analysis of relationship of ESS with SPS, GVS, GAS, HADS-A, HADS-D, PAS and NAS.
| Steps | β (95%CI) | Standard β | P value |
|---|---|---|---|
| Step 1 | |||
| Constant | −0.15 (−10.56 to 10.26) | 0.97 | |
| Age | −0.06 (−0.18 to 0.06) | −0.15 | 0.35 |
| Gender | 0.73 (−3.13 to 4.59) | 0.05 | 0.70 |
| BMI | 0.37 (0.02 to 0.72) | 0.35 | 0.03 |
| Step 2 | |||
| Constant | −2.16 (−10.63 to 6.34) | 0.61 | |
| Age | 0.02 (−0.08 to 0.13) | 0.07 | 0.61 |
| Gender | −2.03 (−5.37 to 1.30) | −0.15 | 0.22 |
| BMI | 0.11 (−0.18 to 0.42) | 0.11 | 0.44 |
| SPS | 2.85 (1.69 to 4.01) | 0.65 | <0.001 |
| Step 3 | |||
| Constant | −6.08 (−14.16 to 2.00) | 0.13 | |
| Age | 0.01 (−0.08 to 0.11) | 0.04 | 0.71 |
| Gender | −2.22 (−5.25 to 0.80) | −0.16 | 0.14 |
| BMI | 0.22 (−0.06 to 0.50) | 0.21 | 0.11 |
| SPS | 2.16 (1.03 to 3.29) | 0.49 | <0.001 |
| HADS-A | 0.50 (0.19 to 0.81) | 0.37 | 0.002 |
Step 1 model adjusted R2=−0.039 (P=0.188), step 2 model adjusted R2=0.366, (P<0.001) and step 3 model adjusted R2=0.479 (P≤0.001). The threshold for stopping the stepwise regression was F=0.10. BMI, body mass index; SPS, Samn-Perelli Scale; ESS, Epworth Sleepiness Scale; GAS, Global Affect Scale; GVS, Global Vigor Scale; HADS-A, Hospital Anxiety Scale; HADS-D, Hospital Depression Scale; PAS, Positive Affect Scale; NAS, Negative Affect Scale.
Ethical Statement: The project was approved by the local review board (GSTT registration number: 2016-6172) and following informed consent.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Guilleminault C, Brooks SN. Excessive daytime sleepiness: a challenge for the practising neurologist. Brain 2001;124:1482-91. 10.1093/brain/124.8.1482 [DOI] [PubMed] [Google Scholar]
- 2.Slater G, Steier J. Excessive daytime sleepiness in sleep disorders. J Thorac Dis 2012;4:608-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Strohl KP, Brown DB, Collop N, et al. An official American Thoracic Society Clinical Practice Guideline: sleep apnea, sleepiness, and driving risk in noncommercial drivers. An update of a 1994 Statement. Am J Respir Crit Care Med 2013;187:1259-66. 10.1164/rccm.201304-0726ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Engleman HM, Douglas NJ. Sleep. 4: Sleepiness, cognitive function, and quality of life in obstructive sleep apnoea/hypopnoea syndrome. Thorax 2004;59:618-22. 10.1136/thx.2003.015867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Douglas N, Young A, Roebuck T, et al. Prevalence of depression in patients referred with snoring and obstructive sleep apnoea. Intern Med J 2013;43:630-4. 10.1111/imj.12108 [DOI] [PubMed] [Google Scholar]
- 6.Sharafkhaneh A, Giray N, Richardson P, et al. Association of psychiatric disorders and sleep apnea in a large cohort. Sleep 2005;28:1405-11. 10.1093/sleep/28.11.1405 [DOI] [PubMed] [Google Scholar]
- 7.Giedke H, Schwärzler F. Therapeutic use of sleep deprivation in depression. Sleep Med Rev 2002;6:361-77. 10.1053/smrv.2002.0235 [DOI] [PubMed] [Google Scholar]
- 8.Pigeon WR, Sateia MJ, Ferguson RJ. Distinguishing between excessive daytime sleepiness and fatigue: toward improved detection and treatment. J Psychosom Res 2003;54:61-9. 10.1016/S0022-3999(02)00542-1 [DOI] [PubMed] [Google Scholar]
- 9.Adams N, Strauss M, Schluchter M, et al. Relation of measures of sleep-disordered breathing to neuropsychological functioning. Am J Respir Crit Care Med 2001;163:1626-31. 10.1164/ajrccm.163.7.2004014 [DOI] [PubMed] [Google Scholar]
- 10.D'Ambrosio C, Bowman T, Mohsenin V. Quality of life in patients with obstructive sleep apnea: effect of nasal continuous positive airway pressure--a prospective study. Chest 1999;115:123-9. 10.1378/chest.115.1.123 [DOI] [PubMed] [Google Scholar]
- 11.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep 1991;14:540-5. 10.1093/sleep/14.6.540 [DOI] [PubMed] [Google Scholar]
- 12.American Academy of Sleep Medicine. International Classification of Sleep Disorders, 3rd ed. American Academy of Sleep Medicine, Darien, IL 2014. [Google Scholar]
- 13.Banzett RB, O'Donnell CR, Guilfoyle TE, et al. Multidimensional Dyspnea Profile: an instrument for clinical and laboratory research. Eur Respir J 2015;45:1681-91. 10.1183/09031936.00038914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoddes E, Zarcone V, Smythe H, et al. Quantification of sleepiness: a new approach. Psychophysiology 1973;10:431-6. 10.1111/j.1469-8986.1973.tb00801.x [DOI] [PubMed] [Google Scholar]
- 15.Samn S, Perelli L. Estimating aircrew fatigue: A technique with implications to airlift operations. Technical Report No. SAM-TR-82-21. Brooks AFB, TX: USAF School of Aerospace Medicine 1982:1-26.
- 16.Civil Aviation Safety Authority. Fatigue estimate outputs [Internet]. Biomathematical Fatigue Models Guidance Document. 2016 [cited 17 August 2016]. Available online: https://www.casa.gov.au/sites/g/files/net351/f/_assets/main/aoc/fatigue/fatigue_modelling.pdf
- 17.Monk TH. A Visual Analogue Scale technique to measure global vigor and affect. Psychiatry Res 1989;27:89-99. 10.1016/0165-1781(89)90013-9 [DOI] [PubMed] [Google Scholar]
- 18.Simons R, Wilschut ES, Valk PJ. Sleep and alertness in North Sea helicopter operations. Aviat Space Environ Med 2011;82:704-10. 10.3357/ASEM.2960.2011 [DOI] [PubMed] [Google Scholar]
- 19.Snaith RP, Zigmond AS. The hospital anxiety and depression scale. Br Med J (Clin Res Ed) 1986;292:344. 10.1136/bmj.292.6516.344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bjelland I, Dahl AA, Haug TT, et al. The validity of the Hospital Anxiety and Depression Scale. An updated literature review. J Psychosom Res 2002;52:69-77. 10.1016/S0022-3999(01)00296-3 [DOI] [PubMed] [Google Scholar]
- 21.Watson D, Clark LA, Carey G. Positive and negative affectivity and their relation to anxiety and depressive disorders. J Abnorm Psychol 1988;97:346-53. 10.1037/0021-843X.97.3.346 [DOI] [PubMed] [Google Scholar]
- 22.Crawford JR, Henry JD. The positive and negative affect schedule (PANAS): construct validity, measurement properties and normative data in a large non-clinical sample. Br J Clin Psychol 2004;43:245-65. 10.1348/0144665031752934 [DOI] [PubMed] [Google Scholar]
- 23.Pengo MF, Higgins S, Drakatos P, et al. Characterisation of sleep disturbances in postural orthostatic tachycardia syndrome: a polysomnography-based study. Sleep Med 2015;16:1457-61. 10.1016/j.sleep.2015.08.003 [DOI] [PubMed] [Google Scholar]
- 24.Neu D, Hoffmann G, Moutrier R, et al. Are patients with chronic fatigue syndrome just 'tired' or also 'sleepy'? J Sleep Res 2008;17:427-31. 10.1111/j.1365-2869.2008.00679.x [DOI] [PubMed] [Google Scholar]
- 25.Buysse DJ, Yu L, Moul DE, et al. Development and validation of patient-reported outcome measures for sleep disturbance and sleep-related impairments. Sleep 2010;33:781-92. 10.1093/sleep/33.6.781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen MK. The epidemiology of self-perceived fatigue among adults. Prev Med 1986;15:74-81. 10.1016/0091-7435(86)90037-X [DOI] [PubMed] [Google Scholar]