Abstract
Background
The physicians often confuse the early symptoms of Frontotemporal dementia (FTD) with Alzheimer dementia (AD), leading to misdiagnosis. There are some cognitive tests to discriminate between AD and behavioral variant FTD (bvFTD), and the INECO Frontal Screening (IFS) is a promising test for this purpose.
Objective
To assess the performance of the IFS to differentiate patients with AD from patients with bvFTD, compared with the Frontal Assessment Battery (FAB).
Methods
A prospective study with 117 patients of our cognitive unit (35 case-patients with AD, 34 case-patients with bvFTD, and 48 control subjects). They were submitted to the following successive phases of evaluation: 1) screening; 2) dementia diagnosis; and 3) dementia sub-type diagnosis. The IFS and FAB were blind and independently applied by one neurologist to all the participants to end of phase 1 (screening), before to the definitive diagnosis establishment.
Results
bvFTD showed a lower performance than AD patients on the IFS total score (F(1, 66) = 70.10, p < 0.01) and FAB total score (F(1, 66) = 17.91, p < 0.01). The IFS and FAB showed a sensitivity of 94.12% (95%CI = 80.3–99.2) and 82.3% (95%CI = 65.4–93.2), and a specificity of 94.2% (95%CI = 80.8–99.3) and 48.5% (95%CI = 31.3, 66.1), respectively. The IFS showed significantly superior discriminatory accuracy than the FAB (AuCIFS = 0.98; AuCFAB = 0.73, p < 0.00001).
Conclusion
The IFS is useful for discriminating between AD and bvFTD patients. The performance of the IFS to differentiate patients with AD from patients with bvFTD is greater than FAB.
Keywords: Frontotemporal dementia, Executive dysfunction, Neuropsychology, Predictive value of tests, Prospective studies
Highlights
-
•
Early symptoms of FTD are confused with AD.
-
•
In low-income countries is necessary to dispose of validated brief cognitive tests for discriminating between dementia of healthy individuals.
-
•
IFS and FAB are useful cognitive tests for discriminating dementia, but IFS has a good performance to discriminate between AD and FTD.
1. Introduction
Frontotemporal dementia (FTD) constitutes approximately 2% of all cases of dementia among people older than 65 years old in Latin America [1], and it is suspected that would be up to 15% people of 65 years or less [2]. Currently, the physicians are recognizing the late symptoms of FTD, but its early symptoms may be confused with Alzheimer dementia (AD) leading to misdiagnosis. Wisconsin Card Sorting Test (WCST), verbal fluency test (VFT) and Trail Making Test (TMT) are useful tools to detect the executive dysfunctions early showed by patients with FTD [3].
The current diagnostic criteria [4] consider executive functions (EF) impairments and social cognition problems as key elements for behavioral variant FTD (bvFTD) diagnosis. A neuropsychological assessment is the gold standard for assessing dementia, but unfortunately it is complex and need highly trained personnel, incurring high costs for health systems. Thus, in low-income countries it is necessary to assess the validity of brief cognitive tests developed for discriminating between dementia and healthy individuals and to properly differentiate between AD and bvFTD.
Although several cognitive screening tools have desirable diagnostic and psychometric properties [5], few have been designed to specifically evaluate executive functions. The INECO Frontal Screening (IFS) [6] is an easy-to-administer and brief (approximately 10 min) test, which does not need complex equipment and could be performed at primary care level. This test was designed to provide health professionals with an easy to use screening tool to detect frontal impairment in everyday clinical settings or even at bedside. The IFS was designed to include several subtests in order to measure, in an efficient way, as many EF as possible. Previous studies have shown promising diagnostic performance [6], [7], [8], [9], [10]. Thus, it arises as an encouraging alternative for early detection of dementia cases.
Previously, we evaluated the performance of Addenbrooke's Cognitive Examination (ACE) in patients with dementia from Peru [11], but our results showed that it poorly differentiate between AD and bvFTD. Thereby, the goal of the present study was to assess the clinical usefulness of the IFS to differentiate (a) patients with dementia from healthy controls, and (b) patients with bvFTD from patients with AD and to compare its performance with the Frontal Assessment Battery (FAB) [10], another screening test designed to assess executive functions.
2. Materials and methods
2.1. Participants
We designed a prospective study with 117 patients of the Cognitive Impairment Diagnosis and Dementia Prevention Unit of the International Clinic from Lima, Peru. Using convenience sampling we performed the recruitment between July 2011 and July 2013. Three groups were studied: 1) 48 control subjects; 2) 35 case-patients with diagnosis of probable AD mild-moderate; and 3) 34 case-patients with diagnosis of probable bvFTD.
For case-patients, we included subjects older than 60 years with dementia diagnosis by DSM-IV criteria [12]. The diagnosis of probable AD and bvFTD were performed according to NINCDS-ADRDA [13] and Neary Consensus criteria [14], respectively. Clinical Dementia Rating Scale (CDR) established the AD stage. This scale was also employed to the bvFTD patients' assessment. The control group consisted of patients' relatives or healthy volunteers.
In order to match relevant demographic characteristic among patients and control groups, control subjects were divided into two groups. A first control group consisted of 23 healthy participants matched by age, gender and years of education with the AD patients. The second control group consisted of 25 healthy participants matched by age, gender and years of education with the bvFTD patients.
We excluded those subjects who were not Spanish native speakers, participants with low education level (< 4 years of education), or with sensory or physical limitations (visual, auditory, or other physical deficits) that could affect the performance in cognitive tests. Furthermore, we excluded participants with history of diseases associated with secondary cognitive impairment (e.g. depression, hypothyroidism, central nervous system infections such as VIH or syphilis, severe encephalic traumatism, sub-dural haematoma, deficit of B12 vitamin, chronic hepatopathy or nephropathy, and addiction or abuse of substances), as well as subjects with cerebrovascular deficit suspected (score higher than 4 in the Hachinski index).
All participants provided written informed consent. The ethics committee of the Universidad San Martin de Porres approved the study.
2.2. Procedures
The subjects underwent a standard examination battery including the following successive evaluations: 1) screening; 2) dementia diagnosis; and 3) dementia sub-type diagnosis. In the screening phase, the evaluators applied the Mini Mental State Examination (MMSE) [15], the clock drawing test – Mano's version (CDT-Mv) [16] and the Pfeffer Functional Activities Questionnaire (PFAQ) [17]. Those patients with at least one positive test for dementia were submitted to the second evaluation (applied by a second evaluator) with MMSE and CDT-Mv. For dementia detection with the MMSE, the cut offs were as following: 1) 27 points in subjects higher than 7 years of education; 2) 23 points in subjects with 4–7 years of education; 3) 22 points in subjects with 1–3 years of education; and 4) 18 points for illiterate. CDT-Mv and PFAQ detected cognitive impairment with a score of 0–10 and 0–5, respectively. All cases of cognitive impairment confirmed in second evaluation were submitted to the next phase.
For dementia diagnosis our protocol included: 1) Beck depression index (for detection of depressive symptoms) and Addenbrooke's Cognitive Examination; 2) laboratory tests (hemoglobin, glucose, urea, creatinine, aspartate aminotransferase, alanine aminotransferase, albumin, globulin, vitamin B12, folic acid, VDRL, Elisa VIH, T3, T4 and TSH, and serum electrolytes such as sodium, potassium and chlorine); and 3) brain CT scans/MRN. All these tests were applied to investigate the presence of secondary causes of dementia.
In the last phase, we identified those patients with probable dementia. The dementia sub-type diagnosis was based on a consensus between neurologists and neuropsychologist, according to the results of all diagnostic tests. The neuropsychological battery included the following tests: Rey Auditory Verbal Learning Test [18], Logical Memory Subtest of Weschler Memory Scale - Revised [19], Trail Making Test A and B [20], Rey-Osterrieth Complex Figure Test [18], Boston Naming Test [21], Wisconsin Card Sorting Test [22], Letter-Number (subtest of Weschler Adult Intelligent Scale III) [19], and Digit Span. Neuropsychiatric symptoms were assessed by means of the Neuropsychiatric Inventory [23].
All evaluations were done blind and independently by one neurologist who applied the IFS and FAB to all the participants to end of phase 1 (screening), before to the definitive diagnosis establishment.
2.3. INECO Frontal Screening (IFS)
The IFS is an screening test to assess executive functions [24], which includes the following eight subtests: motor programming (3 points); conflicting instructions (3 points); motor inhibitory control (3 points); backward digit span (6 points); verbal working memory (2 points); spatial working memory (4 points); abstraction capacity (3 points); and verbal inhibitory control (6 points). The IFS has a maximum possible total score of 30 points and takes < 10 min to be administered and scored.
2.4. Frontal Assessment Battery (FAB)
The FAB [25] consists of six subtests, which assess conceptualization, conflicting instructions, motor programming, sensitivity to interference, motor inhibitory control, and prehension behavior. Each subtest is scored on a maximum of 3 points, rendering a total maximum score of 18.
2.5. Data analysis
All statistical analyses compared the AD patients and bvFTD patients groups with their respective control groups. Moreover, we compared the performance of AD and bvFTD patients. The demographic, neuropsychological and experimental data were compared between groups using ANOVA and Tukey's HSD post-hoc tests (when appropriate). When analyzing categorical variables (gender) chi square test were applied. Taking the age differences between AD and bvFTD groups into account, we considered it as covariable in all comparisons between these two groups. We reported only effects that were still significant after covariation. The p value for all statistical tests was set at 0.05.
In order to compare the usefulness of the IFS and FAB, we determined the sensitivity and specificity of each test to discriminate between (a) healthy controls and patients with dementia, and (b) AD and bvFTD patients. This was done by means of a receiver-operating characteristic (ROC) curve, detecting the optimal cut-off scores. The area under the ROC curve was used as a measure of discriminatory accuracy.
3. Results
Descriptive data for demographic and cognitive profiles are shown in Table 1.
Table 1.
Demographic and cognitive profiles of patients and controls.
| AD (n = 35) mean (SD) | AD controls (n = 23) mean (SD) | AD vs. CTR | FTD (n = 34) mean (SD) | FTD vs. CTR | FTD controls (n = 25) mean (SD) | AD vs. FTD | ||
|---|---|---|---|---|---|---|---|---|
| Demographics | Age (years) | 73.57 (3.8) | 73.78 (3.3) | 0.22 | 67.08 (4.04) | 0.53 | 66.52 (2.4) | < 0.01 |
| Gender (F:M) | 19:16 | 9:14 | 0.26 | 18:16 | 0.14 | 18:7 | 0.91 | |
| Education (years) | 11.8 (2.8) | 10.69 (2.5) | 0.13 | 11.73 (2.66) | 0.13 | 12.8 (2.3) | 0.92 | |
| General cognitive state | CDR | 0.88 (0.21) | 0.00 (0.00) | < 0.01 | 0.83 (0.23) | < 0.01 | 0.00 (0.00) | 0.38 |
| MMSE | 21.51 (3.04) | 28.78 (0.8) | < 0.01 | 26.11 (1.64) | < 0.01 | 29.04 (0.3) | < 0.01 | |
| ACE | 67.00 (5.26) | 92.34 (5.2) | < 0.01 | 76.61 (4.90) | < 0.01 | 93.40 (2.6) | < 0.01 | |
| Executive functions | IFS total score | 20.02 (2.10) | 27.00 (1.3) | < 0.01 | 14.55 (2.16) | < 0.01 | 27.04 (0.8) | < 0.01 |
| Motor series | 2.74 (0.44) | 2.73 (0.44) | 0.97 | 2.02 (0.38) | < 0.01 | 2.84 (0.37) | < 0.01 | |
| Conflictive instructions | 1.97 (0.51) | 2.86 (0.34) | < 0.01 | 2.61 (0.55) | 0.16 | 2.80 (0.40) | < 0.01 | |
| Motor inhibitory control | 1.77 (0.42) | 2.13 (0.34) | < 0.01 | 0.73 (0.66) | < 0.01 | 2.48 (0.50) | < 0.01 | |
| Digit span | 3.6 (0.84) | 5.60 (0.58) | < 0.01 | 3.32 (0.58) | < 0.01 | 5.44 (0.50) | 0.12 | |
| Verbal working memory | 1.80 (0.40) | 2.00 (0.00) | < 0.01 | 0.73 (0.61) | < 0.01 | 2.00 (0.00) | < 0.01 | |
| Spatial working memory | 2.57 (0.55) | 3.60 (0.49) | < 0.01 | 2.70 (0.46) | < 0.01 | 3.72 (0.45) | 0.28 | |
| Abstraction capacity | 2.00 (0.42) | 2.82 (0.38) | < 0.01 | 1.14 (0.55) | < 0.01 | 2.60 (0.50) | < 0.01 | |
| Verbal inhibitory control | 3.57 (0.50) | 5.21 (0.59) | < 0.01 | 1.26 (0.66) | < 0.01 | 5.16 (0.74) | < 0.01 | |
| FAB total score | 13.54 (1.78) | 16.39 (0.8) | < 0.01 | 11.97 (1.52) | < 0.01 | 16.52 (0.9) | < 0.01 | |
| Verbal fluency | 2.11 (0.58) | 2.82 (0.38) | < 0.01 | 2.32 (0.72) | 0.10 | 2.60 (0.50) | 0.19 | |
| Conceptualization | 2.28 (0.66) | 2.95 (0.20) | < 0.01 | 1.88 (0.47) | < 0.01 | 2.88 (0.33) | < 0.01 | |
| Prehension behavior | 2.65 (0.48) | 2.91 (0.28) | < 0.01 | 2.26 (0.44) | < 0.01 | 2.88 (0.33) | < 0.01 | |
| Phonological fluency | 7.94 (2.07) | 19.27 (1.5) | < 0.01 | 7.79 (2.54) | < 0.01 | 20.88 (2.5) | 0.79 | |
| TMT-B | 181 (28.8) | 55.17 (9.0) | < 0.01 | 205.73 (38.7) | < 0.01 | 58.8 (10.4) | < 0.01 | |
| WCST categories | 2.97 (0.56) | 5.17 (0.38) | < 0.01 | 2.44 (0.89) | < 0.01 | 5.24 (0.43) | 0.06 | |
| WCST errors | 6.68 (1.62) | 0.86 (0.69) | < 0.01 | 12.32 (2.96) | < 0.01 | 0.88 (0.60) | < 0.01 |
Significant differences between groups are indicated in bold. CDR = Clinical Dementia Rating, MMSE = Mini Mental State Examination, ACE = Addenbrook's Cognitive Examination, IFS = INECO Frontal Screening, FAB = Frontal Assessment Battery, TMT-B = Trail Making Test B, WCST = Wisconsin Card Sorting Test.
3.1. Demographic data
There were no significant differences in age between AD patients and controls (F(1,56) = 1.48, p = 0.22) or bvFTD patients and controls (F(1,57) = 0.349 p = 0.53). As expected AD were significantly older than bvFTD patients (F(1,67) = 45.93, p < 0.01). No differences in years of formal education were observed between AD patients and controls or bvFTD patients (F(1,56) = 2.30, p = 0.13) and controls (F(1,57) = 2.24 p = 0.13). No differences were observed neither in gender between AD patients and controls (X2(1) = 1.25, p = 0.26) or bvFTD patients and controls (X2(1) = 2.16, p = 0.14).
3.2. General cognitive state assessment
AD (F(1,56) = 395.20, p < 0.01) and bvFTD patients (F(1,57) = 310.16, p < 0.01) showed lower CDR scores than controls. However, no differences in CDR scores were observed between AD and bvFTD patients (F(1,67) = 0.76, p = 0.38). Both AD (F(1,56) = 124.18, p < 0.01) and bvFTD patients (F(1,57) = 68.42, p < 0.01) exhibited lower MMSE total scores than controls. Furthermore, AD showed lower performance than bvFTD patients in the MMSE (F(1,67) = 60.56, p < 0.01). Moreover, AD (F(1,56) = 461.02, p < 0.01) and bvFTD patients (F(1,57) = 241.42, p < 0.01) showed a lower performance than controls on the ACE total score. Furthermore, AD showed lower performance than bvFTD patients in the ACE (F(1,67) = 61.58, p < 0.01).
3.3. Executive functions assessment
3.3.1. INECO Frontal Screening (IFS)
3.3.1.1. AD patients vs. controls
AD patients showed a lower performance than controls on the IFS total score (F(1, 56) = 199.90, p < 0.01). A detailed comparison of the performance on each of the eight IFS subtests indicated that AD patients also exhibited a lower performance on subtests of conflictive instructions (F(1, 56) = 54.14, p < 0.01), motor inhibitory control (F(1, 56) = 11.40, p < 0.01), digit backward span (F(1, 56) = 98.37, p < 0.01), verbal working memory (F(1, 56) = 5.55, p < 0.05), spatial working memory (F(1, 56) = 52.10, p < 0.01), abstraction capacity (F(1, 56) = 57.00, p < 0.01) and verbal inhibitory control (F(1, 56) = 127.74, p < 0.01).
3.3.1.2. bvFTD patients vs. controls
bvFTD patients also showed a lower performance than controls on the IFS total score (F(1, 57) = 746.0, p < 0.01). A detailed comparison of the performance on each of the eight IFS subtests indicated that bvFTD patients exhibited a lower performance on subtests of motor programming (F(1, 57) = 64.76, p < 0.01), motor inhibitory control (F(1, 57) = 119.85, p < 0.01), digit backward span (F(1, 57) = 208.99, p < 0.01), verbal working memory (F(1, 57) = 104.10, p < 0.01), spatial working memory (F(1, 57) = 69.80, p < 0.01), abstraction capacity (F(1, 57) = 106.58, p < 0.01) and verbal inhibitory control (F(1, 57) = 445.36, p < 0.01).
3.3.1.3. AD vs. bvFTD patients
bvFTD showed a lower performance than AD patients on the IFS total score (F(1, 66) = 70.10, p < 0.01). A detailed comparison of the performance on each of the eight IFS subtests indicated that bvFTD patients exhibited a lower performance on the motor programming subtest (F(1, 66) = 47.58, p < 0.01), though a significant effect of age was observed (p = 0.04). AD performed worse than bvFTD patients on the conflictive instructions subtest (F(1, 66) = 18.79, p < 0.01). However, bvFTD showed lower scores than AD patients on motor inhibitory control (F(1,66) = 61.28, p < 0.01), verbal working memory (F(1,66) = 43.60, p < 0.01), abstraction capacity (F(1,66) = 36.17, p < 0.01) and verbal inhibitory control (F(1, 66) = 151.94, p < 0.01).
3.3.2. Frontal Assessment Battery (FAB)
3.3.2.1. AD patients vs. controls
AD patients showed a lower performance than controls on the FAB total score (F(1, 56) = 49.98, p < 0.01). A comparison of the performance on the subtests unique to the FAB indicated that AD patients exhibited a lower performance on conceptualization (F(1, 56) = 21.72, p < 0.01), verbal fluency (F(1, 56) = 26.52, p < 0.01), and prehension behavior (F(1, 56) = 5.24, p < 0.05).
3.3.2.2. bvFTD patients vs. controls
bvFTD patients also showed a lower performance than controls on the FAB total score (F(1, 57) = 171.31, p < 0.01). A detailed comparison of the performance on the subtests unique to the FAB indicated that bvFTD patients exhibited a lower performance on conceptualization (F(1, 57) = 80.37, p < 0.01) and prehension behavior (F(1, 57) = 33.58, p < 0.01).
3.3.2.3. AD vs. bvFTD patients
bvFTD showed a lower performance than AD patients on the FAB total score (F(1, 66) = 17.91, p < 0.01). A detailed comparison of the performance on the subtests unique to the FAB showed that bvFTD patients exhibited a lower performance than AD patients on the conceptualization (F(1,66) = 6.70, p < 0.05) and prehension behavior (F(1, 66) = 6.29, p < 0.05) subtests.
3.3.3. Other executive functions measures
3.3.3.1. AD patients vs. controls
AD patients showed a lower performance than controls on phonological fluency (F(1, 56) = 523.34, p < 0.01), the TMT-B test (F(1, 56) = 409.10, p < 0.01), the number of categories achieved (F(1, 56) = 264.11, p < 0.01) and the number of errors made on the WCST (F(1, 56) = 262.52, p < 0.01).
3.3.3.2. bvFTD patients vs. controls
bvFTD patients also showed a lower performance than controls on phonological fluency (F(1, 57) = 384.00, p < 0.01), the TMT-B test (F(1,57) = 340.69, p < 0.01), the number of categories achieved (F(1, 57) = 207.89, p < 0.01) and the number of errors made on the WCST (F(1, 57) = 360.77, p < 0.01).
3.3.3.3. AD vs. bvFTD patients
Compared to AD, bvFTD patients took more time to complete the TMT-B (F(1,66) = 16.29, p < 0.01) and made more preservative errors on the WCST (F(1, 66) = 68.05, p < 0.01).
3.4. Receiver operating characteristic (ROC) curve analyses
A cutoff score of 23.5 points (out of 30) on the IFS was associated with a sensitivity of 97.1%, 95% confidence interval (95%CI) = [89.9, 99.9], and a specificity of 97.9%, 95%CI = [88.9, 99.9], for the detection of dementia (AD and bvFTD). Regarding the FAB, a cut-off score of 14.5 (out of 18) showed a specificity of 81.1%, 95%CI = [69.9, 89.5] and a sensitivity of 97.9%, 95%CI = [88.3, 99.9]. As shown by Fig. 1, the discriminatory accuracy (patients with dementia vs. healthy controls) of the IFS (AuC = 0.99, SE = 0.001) was superior to that of the FAB (AuC = 0.95, SE = 0.01), and this difference was statistically significant (z = 1.84, p < 0.05).
Fig. 1.
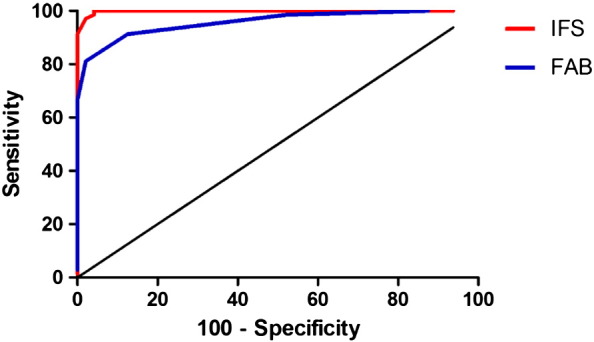
ROC curve for the discrimination between dementia patients and healthy control subjects by IFS and FAB tests.
In comparing the capacity to discriminate between types of dementia (AD vs. bvFTD), the IFS showed a sensitivity of 94.12%, 95%CI = [80.3, 99.2] and specificity of 94.2%, 95%CI = [80.8, 99.3], using a cut-off score of 17.5 points. On the contrary, a 13-point cut-off score on the FAB showed a sensitivity of 82.3%, 95%CI = [65.4, 93.2], but a low specificity of 48.5%, 95%CI = [31.3, 66.1]. Again, the IFS showed superior discriminatory accuracy (AuC = 0.98, SE = 0.009) than the FAB (AuC = 0.73, SE = 0.05), and this difference was statistically significant (z = 5.57, p < 0.00001), as revealed by Fig. 2.
Fig. 2.
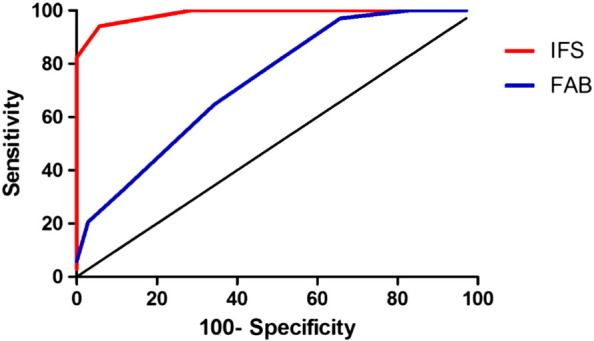
ROC curve for the discrimination between AD patients and FD patients by IFS and FAB tests.
4. Discussion
The goal of this study was to assess the clinical usefulness of the IFS to differentiate (a) patients with dementia from healthy controls, and (b) patients with bvFTD from patients with AD. Moreover, we compared the performance of the IFS with that of the FAB. Our results showed that the IFS have a good sensitivity and specificity to discriminate between patients with dementia and healthy controls, and between AD and bvFTD patients. Furthermore, we found that the discrimination accuracy of the IFS is higher than that of the FAB. These findings are consistent with those previously reported by other researchers [6], [10], [26], [27]. Specifically, our results support those of a previous study carried out in Argentina [6] showing that the IFS accurately discriminate between demented patients and healthy controls as well as between AD and bvFTD patients. Moreover, the present results agree with those of a previous study [10] reporting that, relative to the FAB, the IFS showed better capability to differentiate between AD and bvFTD. Finally, our findings suggest that although the FAB may be useful for discriminating between patients with dementia and healthy individuals, this screening test has a low specificity for discriminating between AD and bvFTD patients. Even though some studies [28], [29], [30] have shown that the FAB may be useful to discriminate between AD and bvFTD, our results did not support this observation. Instead of this, our findings are consistent with previous evidence [26], [27] suggesting that the FAB does not accurately differentiate AD from bvFTD patients.
Additionally, based on the analysis of the performance on IFS subtests, our findings suggest that the conflictive instructions subtest would be the most accurate for AD detection and subtests of verbal working memory, abstraction capacity and verbal inhibitory control would be the most accurate for bvFTD detection. These findings are consistent with those a previous study using the same screening tools [10].
In comparison with other brief tests assessing the general cognitive state such as MMSE and ACE, executive dysfunction was better detected with both IFS and FAB tests [6], [25], [32], [33], [34]. Thus, patients with bvFTD have lower scores than AD patients in these tests, while patients with AD have lower scores than patients with bvFTD in the general cognitive screening tests such as MMSE and ACE. Based on these findings, we believe that both MMSE and ACE may not be the best option in patients with bvFTD because those tests are not efficient for assessing the dysexecutive changes frequently observed in frontal pathologies. Thus, MMSE and ACE could not detect early cases of bvFTD.
Curiously, in applying the MMSE and ACE testing we have shown that patients with bvFTD showed performances in the domains of memory and reasoning similar to those of patients with AD, despite the younger age of patients with bvFTD. This finding has been previously reported [35]. Finally, our study showed that other executive functions tests, such as TMT-B test and the number of errors made on the WCST, could be interesting tools to differentiate between patients with bvFTD, AD, and healthy individuals.
This study has some limitations. First, our sample was relatively small. Thus, the future research should include larger sample sizes with patients with multiple dementia sub-types to approximate a more realistic scenario. Second, the comparison groups were homogeneous in years of education. Future studies should evaluate whether the results are consistent across groups with different educational levels.
However, the importance of our study lies in three points. First, in low-income countries is necessary to dispose of validated brief cognitive tests for discriminating between dementia of healthy individuals and, simultaneously, it to discriminate between dementia subtypes. For example, in Peru the only validated cognitive tests for screening dementia are MMSE, PDR-M, T@M and ACE. Second, the dysexecutive manifestations of behavioral variant bvFTD [36] can only be detected by the use of complex tests requiring trained personnel for its enforcement, such as phonological fluency, TMT-B test, and WCST [37]. Tests with these properties are Mini SEA [38], FAB and IFS [10].
In conclusion, the IFS is a test with a good performance for screening executive functions in patients with dementia. Moreover, the IFS is useful in discriminating between AD and bvFTD patients. We recommend the inclusion of the IFS screening test in screening protocols for dementia for the early detection of bvFTD.
Conflicts of interest
The authors declare that there are no conflicts of interest.
References
- 1.Custodio N., Perez E.H., Lira D. Prevalence of Frontotemporal dementia in community-based studies in Latin America. A systematic review. Dement. Neuropsychol. 2013 doi: 10.1590/S1980-57642013DN70100005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Knopman D.S., Roberts R.O. Estimating the number of persons with frontotemporal lobar degeneration in the US population. J. Mol. Neurosci. 2011;MN 45:330–335. doi: 10.1007/s12031-011-9538-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gregory C.A., Serra-Mestres J., Hodges J.R. Early diagnosis of the frontal variant of frontotemporal dementia: how sensitive are standard neuroimaging and neuropsychologic tests? Neuropsychiatry Neuropsychol Behav. Neurol. 1999;12:128–135. [PubMed] [Google Scholar]
- 4.Rascovsky K., Hodges J.R., Knopman D. Sensitivity of revised diagnostic criteria for the behavioural variant of Frontotemporal dementia. Brain J. Neurol. 2011;134:2456–2477. doi: 10.1093/brain/awr179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cullen B., O'Neill B., Evans J.J. A review of screening tests for cognitive impairment. J. Neurol. Neurosurg. Psychiatry. 2007;78:790–799. doi: 10.1136/jnnp.2006.095414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Torralva T., Roca M., Gleichgerrcht E. INECO Frontal Screening (IFS): a brief, sensitive, and specific tool to assess executive functions in dementia. J. Int. Neuropsychol. Soc. 2009;JINS 15:777–786. doi: 10.1017/S1355617709990415. [DOI] [PubMed] [Google Scholar]
- 7.Bruno D., Torralva T., Marenco V. Utility of the INECO Frontal Screening (IFS) in the detection of executive dysfunction in patients with relapsing-remitting multiple sclerosis (RRMS) Neurol. Sci. Off. J. Ital. Neurol. Soc. Ital. Soc. Clin. Neurophysiol. 2015 doi: 10.1007/s10072-015-2299-6. [DOI] [PubMed] [Google Scholar]
- 8.Silva T., Monteiro L., Lopes E. INECO Frontal Screening: an instrument to assess executive dysfunction in schizophrenia. Span. J. Psychol. 2014;17 doi: 10.1017/sjp.2014.22. [DOI] [PubMed] [Google Scholar]
- 9.Moreira H.S., Lima C.F., Vicente S.G. Examining Executive Dysfunction with the Institute of Cognitive Neurology (INECO) Frontal Screening (IFS): normative values from a healthy sample and clinical utility in Alzheimer's disease. J. Alzheimers Dis. 2014;JAD 42:261–273. doi: 10.3233/JAD-132348. [DOI] [PubMed] [Google Scholar]
- 10.Gleichgerrcht E., Roca M., Manes F. Comparing the clinical usefulness of the Institute of Cognitive Neurology (INECO) Frontal Screening (IFS) and the Frontal Assessment Battery (FAB) in frontotemporal dementia. J. Clin. Exp. Neuropsychol. 2011;33:997–1004. doi: 10.1080/13803395.2011.589375. [DOI] [PubMed] [Google Scholar]
- 11.Custodio N., Lira D., Montesinos R. Usefulness of the Addenbrooke’s Cognitive Examination (Spanish version) in Peruvian patients with Alzheimer’s disease and Frontotemporal dementia. Vertex B. Aires Argent. 2012;23:165–172. [PubMed] [Google Scholar]
- 12.American Psychiatric Association. APA . American Psychiatric Association; Washington, DC: 1994. Diagnostic and Statistical Manual of Mental Disorders DSM-IV. [Google Scholar]
- 13.McKhann G., Drachman D., Folstein M. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 14.Neary D., Snowden J.S., Gustafson L. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology. 1998;51:1546–1554. doi: 10.1212/wnl.51.6.1546. [DOI] [PubMed] [Google Scholar]
- 15.Folstein M.F., Folstein S.E., McHugh P.R. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 16.Custodio N., García A., Montesinos R. Validation of the clock drawing test - Manos' version - as a screening test for detection of dementia in older persons of Lima, Peru. Rev. Peru. Med. Exp. Salud Pública. 2011;28:29–34. doi: 10.1590/s1726-46342011000100005. [DOI] [PubMed] [Google Scholar]
- 17.Quiroga P., Albala C., Klaasen G. Validation of a screening test for age associated cognitive impairment, in Chile. Rev. Médica Chile. 2004;132:467–478. doi: 10.4067/s0034-98872004000400009. [DOI] [PubMed] [Google Scholar]
- 18.Rey A. L'examen physiologique dans le cas d'encephalopathie traumatique [Psychological examination in a case of traumatic encephalopathy] Arch. Psychol. 1941;286–340 [Google Scholar]
- 19.Wechsler D. Psychological Corporation; 1997. Wechsler Memory Scale. [Google Scholar]
- 20.Partington J.E., Leiter R. Partington's pathway test. Psychopharmacol. Serv. Cent. Bull. 1949;9–20 [Google Scholar]
- 21.Kaplan E., Goodglass H., Weintraub S. Lea & Febiger; Philadelphia: 1983. Boston naming test. [Google Scholar]
- 22.Nelson H.E. A modified card sorting test sensitive to frontal lobe defects. Cortex. 1976;12:313–324. doi: 10.1016/s0010-9452(76)80035-4. [DOI] [PubMed] [Google Scholar]
- 23.Cummings J.L., Mega M., Gray K. The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology. 1994;44:2308–2314. doi: 10.1212/wnl.44.12.2308. [DOI] [PubMed] [Google Scholar]
- 24.Torralva T., Roca M., Gleichgerrcht E. A neuropsychological battery to detect specific executive and social cognitive impairments in early frontotemporal dementia. Brain J. Neurol. 2009;132:1299–1309. doi: 10.1093/brain/awp041. [DOI] [PubMed] [Google Scholar]
- 25.Dubois B., Slachevsky A., Litvan I. The FAB: a Frontal Assessment Battery at bedside. Neurology. 2000;55:1621–1626. doi: 10.1212/wnl.55.11.1621. [DOI] [PubMed] [Google Scholar]
- 26.Castiglioni S., Pelati O., Zuffi M. The frontal assessment battery does not differentiate frontotemporal dementia from Alzheimer's disease. Dement. Geriatr. Cogn. Disord. 2006;22:125–131. doi: 10.1159/000093665. [DOI] [PubMed] [Google Scholar]
- 27.Lipton A.M., Ohman K.A., Womack K.B. Subscores of the FAB differentiate frontotemporal lobar degeneration from AD. Neurology. 2005;65:726–731. doi: 10.1212/01.wnl.0000174437.73416.7b. [DOI] [PubMed] [Google Scholar]
- 28.Appollonio I., Leone M., Isella V. The Frontal Assessment Battery (FAB): normative values in an Italian population sample. Neurol. Sci. Off. J. Ital. Neurol. Soc. Ital. Soc. Clin. Neurophysiol. 2005;26:108–116. doi: 10.1007/s10072-005-0443-4. [DOI] [PubMed] [Google Scholar]
- 29.Iavarone A., Ronga B., Pellegrino L. The Frontal Assessment Battery (FAB): normative data from an Italian sample and performances of patients with Alzheimer's disease and frontotemporal dementia. Funct. Neurol. 2004;19:191–195. [PubMed] [Google Scholar]
- 30.Slachevsky A., Villalpando J.M., Sarazin M. Frontal assessment battery and differential diagnosis of frontotemporal dementia and Alzheimer disease. Arch. Neurol. 2004;61:1104–1107. doi: 10.1001/archneur.61.7.1104. [DOI] [PubMed] [Google Scholar]
- 32.Ihnen J., Antivilo A., Muņoz-Neira C. Chilean version of the INECO Frontal Screening (IFS-Ch) Dement. Neuropsychol. 2013 doi: 10.1590/S1980-57642013DN70100007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mitchell A.J. A meta-analysis of the accuracy of the mini-mental state examination in the detection of dementia and mild cognitive impairment. J. Psychiatr. Res. 2009;43:411–431. doi: 10.1016/j.jpsychires.2008.04.014. [DOI] [PubMed] [Google Scholar]
- 34.Rodriguez del Alamo A., Catalán Alonso M.J., Carrasco Marín L. FAB: a preliminar Spanish application of the frontal assessment battery to 11 groups of patients. Rev. Neurol. 2003;36:605–608. [PubMed] [Google Scholar]
- 35.Malloy P., Tremont G., Grace J. The Frontal Systems Behavior Scale discriminates frontotemporal dementia from Alzheimer's disease. Alzheimers Dement. J. Alzheimers Assoc. 2007;3:200–203. doi: 10.1016/j.jalz.2007.04.374. [DOI] [PubMed] [Google Scholar]
- 36.Broe M., Hodges J.R., Schofield E. Staging disease severity in pathologically confirmed cases of frontotemporal dementia. Neurology. 2003;60:1005–1011. doi: 10.1212/01.wnl.0000052685.09194.39. [DOI] [PubMed] [Google Scholar]
- 37.Rami L., Serradell M., Bosch B. Standard values of frontal cognitive functioning tests for those over 60 years of age. Rev. Neurol. 2007;45:268–271. [PubMed] [Google Scholar]
- 38.Bertoux M., Delavest M., de Souza L.C. Social Cognition and Emotional Assessment differentiates frontotemporal dementia from depression. J. Neurol. Neurosurg. Psychiatry. 2012;83:411–416. doi: 10.1136/jnnp-2011-301849. [DOI] [PubMed] [Google Scholar]


