Abstract
This paper reported an unusual manifestation of a 19-year-old Chinese male patient presented with a complex phenotype of mitochondrial encephalomyopathy, lactic acidosis and stroke-like episodes (MELAS) syndrome and Kearns–Sayre syndrome (KSS). He was admitted to our hospital with the chief complaint of “acute fever, headache and slow reaction for 21 days”. He was initially misdiagnosed as “viral encephalitis”. This Chinese man with significant past medical history of intolerating fatigue presented paroxysmal neurobehavioral attacks that started about 10 years ago. During this span, 3 or 4 attack clusters were described during which several attacks occurred over a few days. The further examination found that the hallmark signs of this patient included progressive myoclonus epilepsy, cerebellar ataxia, hearing loss, myopathic weakness, ophthalmoparesis, pigmentary retinopathy and bifascicular heart block (Wolff–Parkinson–White syndrome). By young age the disease progression is characterized by the addition of migraine, vomiting, and stroke-like episodes, symptoms of MELAS expression, which indicated completion of the MELAS/KSS overlap syndrome. The m. A3243G mitochondrial DNA mutation and single large-scale mtDNA deletions were found in this patient. This mutation has been reported with MELAS, KSS, myopathy, deafness and mental disorder with cognitive impairment. This is the first description with a MELAS/KSS syndrome in Chinese.
Keywords: Mitochondrial DNA (mtDNA), MELAS, Kearns–Sayre syndrome, Point mutation, Myoclonus epilepsy
Highlights
-
•
A 19-year-old Chinese man presented MELAS/KSS overlap syndrome.
-
•
A3243G mtDNA mutation and large-scale mtDNA deletions were in him.
-
•
This is the first description in Chinese.
1. Introduction
Since the identification of the first pathogenic mutations in human mitochondrial DNA (mtDNA) in 1980, hundreds of large-scale mtDNA rearrangements and over 200 mtDNA point mutations have been described [1]. Different mtDNA mutations may demonstrate many presentations. Among the clinical presentations of mitochondrial disorders, there are several merges as distinctive entities: mitochondrial encephalomyopathy, lactic acidosis and stroke-like episodes (MELAS), myoclonus epilepsy with ragged-red fibers (MERRF), Kearns–Sayre syndrome (KSS), chronic progressive external ophthalmoplegia (CPEO) and Leigh disease.
Point mutations of mtDNA, mostly in transfer RNA (tRNA) genes, have been described in the patients with syndromes of MELAS and MERRF, usually causing a wide spectrum of syndromes [2]. MELAS syndrome is commonly associated with an A to G transition at nt 3243 of the mtDNA. In contrast, KSS is a mitochondrial disorder characterized by the early-aged onset of progressive external ophthalmoplegia or pigmentary retinopathy, together with at least one of a triad of cerebellar ataxia, heart block and elevated cerebrospinal fluid protein [3]. Typically, KSS is due to single large-scale mtDNA deletions and is almost always sporadic.
Although some mtDNA mutations tend to be associated with specific clinical syndromes, genotype–phenotype correlations are imprecise. Reportedly many individuals who harbor a pathogenic mtDNA mutation manifest overlapping features of typical mitochondrial syndromes [4]. Because there is presently no curative treatment to mitochondrial diseases, the early definitive diagnosis is very important.
Here, we report another case of an overlap syndrome with features of MELAS and KSS in which there are correspondingly double mutations of m. A3243G mitochondrial DNA mutation and single large-scale mtDNA deletions.
2. Patients and methods
On Jul. 11th 2013, a 19-year-old man was first admitted to our department with the chief complaint of “acute fever, headache and slow reaction for 3 weeks”. Some cold symptoms appeared on Jun. 20th, 2013, followed by obvious eye pain. Subsequently, he manifested fever, headache, malaise, nausea and vomiting. Meanwhile, his family found him unresponsive with cognitive disorders, memory and computing power loss. Past history: patient with significant past medical history of intoleratting fatigue presented paroxysmal neurobehavioral attacks that started about 10 years ago. Patient denies diabetes, drugs and food allergies, and infectious hepatitis and tuberculosis. No smoking, alcohol, nor illicit substance abuse. Family history: his mother had migraine headache for over 20 years and his grandmother was diagnosed with diabetes for 35 years. No other family members presented with similar features.
Neurological examination: T 38.6 °C, BP 110/70 mmHg, HR 80 bpm, weight 40 kg, height 139 cm. Awake and alert, fluent to the point, poor calculation. When written down, he is able to read it aloud. Speech is slightly hesitant with apparent word finding difficulties. He seems easily confused, perhaps contributed by hearing deficits. Round bilateral pupillary diameter of 3.0 mm, light responsive. The muscle bulk is small. His motor and sensation may normal.
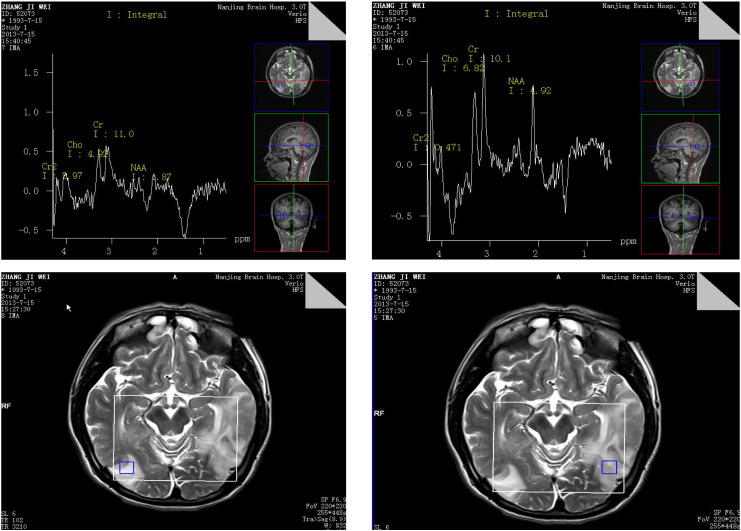
Brain magnetic resonance imaging showed large abnormal signal on the right temporal lobe and the left temporal lobe, with the impression of viral encephalitis or vascular lesions. Proton MRS showed an elevated lactate level in involved regions of the brain; the lactate peak disappeared in old areas of T2 prolongation (Fig.1). ECG showed WPW syndrome. CSF studies: cranial pressure 180 mm H2O, protein 0.71 g/L, glucose 3.5 mmol/L, chloro 118.2 mmol/L, IgG 43.6 mg/L. EEG showed atypical irregular 2.5–3.5 Hz spike and wave complexes. Based on the above history, symptoms and lab tests, he was initially misdiagnosed with “viral encephalitis”. After 2 days of anti-viral treatment, the patient presented significant psychiatric symptoms, such as the contents of hallucination, soliloquise and emotional irritability with no insight.
Fig. 1.
Large abnormal signal on the right temporal–parietal lobe and the left temporal lobe. Proton magnetic resonance spectroscopic imaging (MRS) showed that (1) the levels of N-acetylaspartate (NAA, a marker of neuronal density) were reduced in bilateral temporal and occipital lobes; (2) the levels of choline-containing compounds (Cho) were not obviously changed between the lesions and normal brain tissue (3) a creatine lactic acid peaks are showing in the lesions.
In order to further confirm the diagnosis, the min exercise testing of lactic acid was done. The venous blood lactate concentration was detected: 5.6 mmol/L at resting level (normal < 1.2), 12.1 mmol/L after 10 min movement (normal < 3.6), 11.6 mmol/L after 10 min rest (normal < 2.0). Laboratory tests revealed a mildly elevated creatine kinase of 398 U/L (normal < 200) with normal glucose, thyroid function tests, parathyroid hormone, and liver function panel.
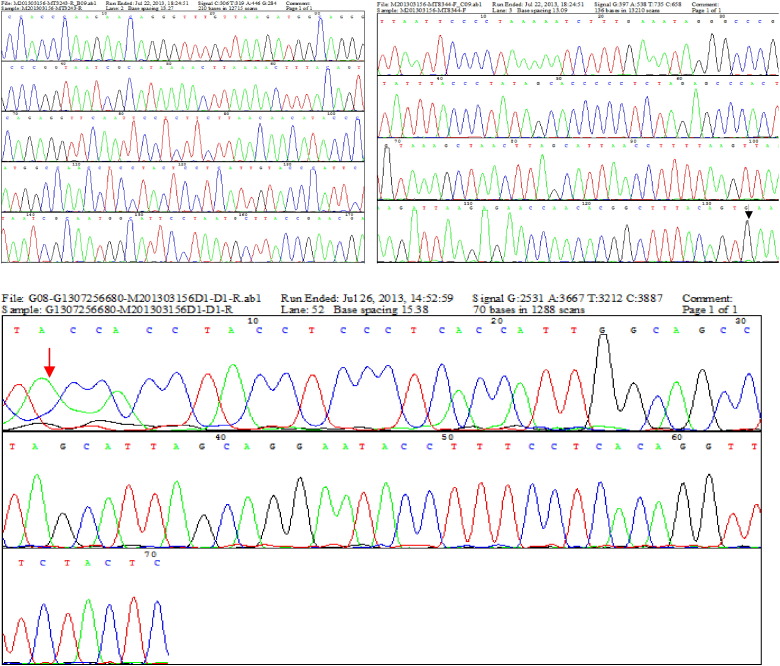
His mitochondrial gene was tested by bidirectional DNA sequencing analysis (Homo mtDNA No. NC-012920.1 as a control) with High-resolution agarose gel electrophoresis. Total genomic DNA was extracted from whole blood cells, isolated leukocytes and thrombocytes. We also compared the levels of the mtDNA mutation in blood with the level of urinary epithelial cells in this patient, as mutation levels are known to decrease in blood over time, while in some patients it may be absent. About 350 mL of the first morning urine was obtained after a 10 min-exercise, so as to increase the amounts of urinary epithelial cells. The heteroplasmic ratio of the mutant to normal mtDNA results of genetic tests showed the heteroplasmic ratio of the mutant to normal mtDNA: the m. A3243G mitochondrial DNA mutation (15% in leukocytes and 78% in urinary epithelial cells) and single large-scale mtDNA deletions (from 8470 to 13,446, totally 4977 bp, only found in urinary epithelial cells) in this patient (Fig.2).
Fig. 2.
Positive for the MELAS A3243G mitochondrial DNA mutation (marked by black arrow) and single large-scale mtDNA deletions (from 8470 to 13,446, totally 4977 bp, marked by red arrow) in this patient (urine). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Then the patient was diagnosed with mitochondrial encephalomyopathy and received nerve nutrition and symptomatic treatment. On Jul. 19th 2013, his family required discharged to outside hospital for further treatment.
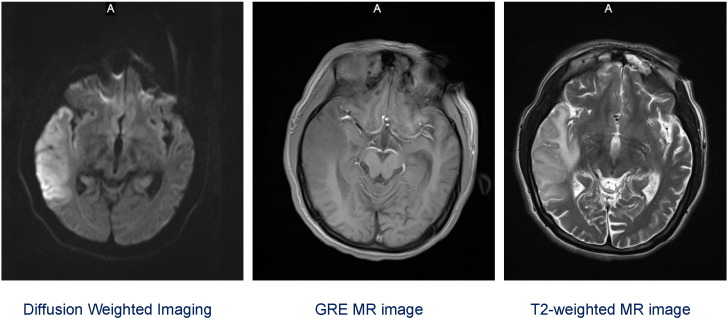
On Aug. 24th 2013, the patient was secondly admitted with the complaint of “acute right limb convulsions and weakness for 1 week”. He could not walk independently with progressive loss of balance and gait instability with leg weakness. He also suffered transient stroke-like episodes, consisting of initial headache and vomiting. In the ward, he developed myoclonus epilepsy. MRI again showed a large acute-to-subacute lesion over the right parietal–occipital–temporal region (Fig.3).
Fig. 3.
Repeated MRI on Aug. 24th 2013 showed a large acute-to-subacute lesion over the right parietal-occipital-temporal region. This lesion showed hypotensive signal on Gradient Echo Pulse Sequence (GRE) and hypertensive signal on T2 of MR image. Diffuse restriction diffusion in the right parietal–temporal convexity with mass effect. Appearance suggests an acute to subacute ischemic area with mild diffuse mass effect.
Neurological examination revealed mild clumsiness of rapid alternating movement and fine finger movements on the left, and a Romberg sign. Further examination showed atypical pigmentary retinopathy with normal optic discs, and abnormal eye movements with right eye amblyopia and exotropia, left exophoria, mild bilateral limitation of eye adduction, and saccadic pursuits.
So the patient's clinical manifestations suggest the presence of a MERRF/MELAS overlap syndrome.
3. Discussion
This patient's clinical manifestations of progressive cerebellar ataxia, frequent myoclonus seizures, recurrent stroke-like episodes, hearing loss, elevated resting lactate blood level, WPW, together with headache in the mother and diabetes in the grandmother, suggested a maternal inheritance mitochondrial disease. Partially female patients may even suffer from migraine-like headaches or early onset diabetes due to the point mutation at nt 3243 of the mtDNA [4]. Specifically, this patient appeared to have an overlap syndrome with features of both MELAS and KSS.
Cognitive impairment, migraine-like headaches, frequent myoclonus seizures, recurrent stroke-like episodes and elevated resting lactate blood level are features of the MELAS syndrome [2]. Brain MRI usually shows large lobe lesions, brain atrophy with or without basal ganglia calcification. In contrast, myoclonus epilepsy, heart block (WPW), pigmentary retinopathy, sensorineural hearing loss, premature graying pigmentary retinopathy, partial heart block, and diffuse white matter MRI alterations are features of the KSS syndrome [3]. Cerebellar ataxia is common in both conditions. According to the results of genetic tests, the patient was finally diagnosed with MELAS and Kearns–Sayre overlap syndrome. Although our existing data were sufficient to this diagnostics, it is a great mark of deficiency of our failing to undergo muscle biopsy to survey ragged-red fibers, due to the decline of the patient and his guardian.
Reportedly, adult-onset MELAS patients usually are mis-diagnosed with herpes encephalitis [5]. Patients suffering from encephalitis (infectious or autoimmune) typically also present with acute onset of cognitive/behavioral disturbances along with seizures. Imaging findings of encephalitis (especially herpes encephalitis) usually involves primarily the limbic system and temporal lobes. Repeated MRI brain showed a large acute-to-subacute lesion over the parietal–occipital–temporal region that was suspicious for focal encephalitis vs. inflammatory process vs. ischemia (though not following any vascular territory). Earlier in the patient's presentation, the recurring nature of the patients' symptoms with relative absence of symptoms between attack clusters would be somewhat unusual for an encephalitic presentation. Interestingly, in the previous report of the overlap syndrome with KSS, heart block is always manifested as WPW [6].
From a clinical point of view, our patient is strikingly different from the others, as he is the first example of MERRF/KSS overlap syndrome associated with the m. A3243G mitochondrial DNA mutation and single large-scale mtDNA deletions (from 8470 to 13,446, totally 4977 bp). The common single deletions of KSS syndrome is from 8468 to 13,446 in mtDNA [7].
The mtDNA point mutations may tend to be associated with different clinical syndromes, manifesting as heterogeneity of these mtDNA mutations. Several previous reports have described cases who had features of MERRF/KSS with m.3251A > G mutation [8] or m.3291T > C mutation [9]. Also, m.3291T > C mutation may be related to MERRF/MELAS overlap syndrome [10]. In addition, only A3243G mtDNA point mutation may tend to be associated with different clinical syndromes, such as KSS/MELAS [11], MERRF/MELAS [12] or MERRF [4]. As described, MERRF/MELAS overlap syndrome may also caused by a double pathogenic mutation in mitochondrial tRNA genes (m.8356T > C appeared homoplasmic and m.3243A > G) [6]. In contrast, compared to ours, those patients' genetic phenotype had notable differences.
In conclusion, there is a wide spectrum of syndromes presented with different phenotypes caused by various mutations of mtDNA. Our data reinforced the well-established concept of high clinical heterogeneity in mitochondrial disorders. This study is the first to describe the A3243G mtDNA mutation and single large-scale mtDNA deletions in association with the MELAS/KSS overlap syndrome. How this correlates with the clinical phenotype is still largely unanswered questions.
Conflict of interest
The authors declare that they have no conflict of interest.
Acknowledgments
This work has been supported by the two grants, one from the National Natural Science Foundation of China (Grant No. 81400981), and the other one from Nanjing Medical University Science and Technology Development Foundation of China (2013NJMU090). Thanks are due to Qing-ling Huang Prof. of radiological department in our hospital for the assistance with the analysis of magnetic resonance images.
References
- 1.Longo N. Mitochondrial encephalopathy. Neurol. Clin. 2003;21:817–831. doi: 10.1016/s0733-8619(03)00015-x. [DOI] [PubMed] [Google Scholar]
- 2.El-Hattab A.W., Adesina A.M., Jones J. MELAS syndrome: clinical manifestations, pathogenesis, and treatment options. Mol. Genet. Metab. 2015;116:4–12. doi: 10.1016/j.ymgme.2015.06.004. [DOI] [PubMed] [Google Scholar]
- 3.Phadke M., Lokeshwar M.R., Bhutada S. Kearns Sayre syndrome—case report with review of literature. Indian J. Pediatr. 2012;79:650–654. doi: 10.1007/s12098-011-0618-3. [DOI] [PubMed] [Google Scholar]
- 4.Pronicki M., Sykut-Cegielska J., Mierzewska H. Diversity of clinical symptoms in A3243G mitochondrial DNA mutation (MELAS syndrome mutation) Med. Sci. Technol. 2002;8:CR767–CR773. [PubMed] [Google Scholar]
- 5.Sharfstein S.R1., Gordon M.F., Libman R.B. Adult-onset MELAS presenting as herpes encephalitis. Arch. Neurol. 1999;56:241–243. doi: 10.1001/archneur.56.2.241. [DOI] [PubMed] [Google Scholar]
- 6.Nakamura M., Yabe I., Sudo A. MERRF/MELAS overlap syndrome: a double pathogenic mutation in mitochondrial tRNA genes. J. Med. Genet. 2010;47:659–664. doi: 10.1136/jmg.2009.072058. [DOI] [PubMed] [Google Scholar]
- 7.Mancuso M., Orsucci D., Angelini C. Redefining phenotypes associated with mitochondrial DNA single deletion. J. Neurol. 2015;262:1–9. doi: 10.1007/s00415-015-7710-y. [DOI] [PubMed] [Google Scholar]
- 8.Nishigaki Y., Tadesse S., Bonilla E. A novel mitochondrial tRNA(Leu(UUR)) mutation in a patient with features of MERRF and Kearns–Sayre syndrome. Neuromuscul. Disord. 2003;13:334–340. doi: 10.1016/s0960-8966(02)00283-3. [DOI] [PubMed] [Google Scholar]
- 9.Emmanuele V. MERRF and Kearns–Sayre overlap syndrome due to the mtDNA m.3291T > C mutation. Muscle Nerve. 2011;44:448–451. doi: 10.1002/mus.22149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu K., Zhao H., Ji K. MERRF/MELAS overlap syndrome due to the m.3291T > C mutation. Metab. Brain Dis. 2014;29:139–144. doi: 10.1007/s11011-013-9464-5. [DOI] [PubMed] [Google Scholar]
- 11.Wilichowski E1., Korenke G.C., Ruitenbeek W. Pyruvate dehydrogenase complex deficiency and altered respiratory chain function in a patient with Kearns–Sayre/MELAS overlap syndrome and A3243G mtDNA mutation. J. Neurol. Sci. 1998;157:206–213. doi: 10.1016/s0022-510x(98)00068-9. [DOI] [PubMed] [Google Scholar]
- 12.Mongini T., Doriguzzi C., Chiadò-Piat L. MERRF/MELAS overlap syndrome in a family with A3243G mtDNA mutation. Clin. Neuropathol. 2002;21:72–76. [PubMed] [Google Scholar]