Abstract
Groundbreaking results concerning ischemic stroke (IS) hyperacute treatment worldwide were published in 2014 and 2015. We aimed to compare functional status after 3 months in patients treated with intra-arterial thrombectomy (IAT) and those treated with intravenous thrombolysis (IVT) alone in Joinville, Brazil.
From the Joinville Stroke Registry, we extracted and compared all consecutive IVT patients treated with r-tPA within 4.5 h in the period 2009–2011 versus all consecutive IAT treated within 6 h with the Solitaire FR device plus IVT in the period 2012–2014.
We registered 82 patients in the IVT group and 31 patients in the IAT group. At hospital admission, patients in the IAT group were significantly younger (p < 0.001), had a higher educational level (p = 0.001), had a slightly higher prevalence of atrial fibrillation (p = 0.057) and had more severe strokes measured by the NIH stroke scale (p = 0.011). After 90 days, 45% of patients in the IAT group and 27% in the IVT group were independent (0–1 points) according to the modified Rankin scale (adjusted odds ratio: 4.53; 95% CI: 1.22 to 16.75). Symptomatic hemorrhage was diagnosed in 10% of patients in both groups (p = 1.0). The 90-day case-fatality was 39% (32/82) in the IVT group and 26% (8/31) in the IAT group (p = 0.27). In this small cohort, a greater rate of functional independence was achieved in patients treated with IAT plus IVT, compared with patients treated with IVT lysis alone. Our “real-world” findings are consistent with results of controlled, randomized clinical trials.
Keywords: Ischemic stroke, Stroke thrombolysis, Mechanical thrombectomy, Cohort
Highlights
-
•
Incidence of ischemic stroke have been increasing in low and middle income countries ( LMIC) over last 3 decades.
-
•
Combined intravenous – endovascular approach opened a new era in treatment of ischemic stroke with absolute risk reduction of functional dependency.
-
•
How far these data might be translated to LMIC settings?
1. Introduction
In December 2014 and during 2015, a landmark was set in the contemporary treatment of ischemic stroke (IS) when the results of MR CLEAN (Multicenter randomized clinical trial of endovascular treatment for acute ischemic stroke in the Netherlands), ESCAPE (Randomized Assessment of Rapid Endovascular Treatment of Ischemic Stroke), EXTEND IA (A multicenter, randomized, controlled study to investigate EXtending the time for Thrombolysis in Emergency Neurological Deficits with Intra-Arterial therapy), SWIFTPRIME (Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke) and REVASCAT (Thrombectomy within 8 h after symptom onset in ischemic stroke) trials framed a new landscape for neurologists worldwide [1], [2], [3], [4], [5].
However, similarly to the new paradigm of care introduced by the relevance of stroke units in 1993, the evidence of effectiveness of mechanical endovascular treatment translates into huge challenges for IS care, particularly in low- and middle-income (LMIC) countries [6]. For example, in 2013, only 82 stroke centers, including 45 public hospitals had recombinant tissue-type plasminogen activator (r-tPA) in their emergency units [7], which means that less than 2% of all Brazilian hospitals (6700) according to National Registration of Health Establishments. How many stroke centers are prepared to implement these new approaches? How much infrastructure do we need in our programs to teach new skills required for endovascular treatment, for stroke and neuroradiology residents? Moreover, its well known that the findings of randomized clinical trials are quite distinct from the “real world” even in well structured settings [8].
Joinville is an industrial city located in Southern Brazil [9]. In the last Brazilian census (2010), the Joinville population included 515,288 inhabitants in an area of 1130 km2 [9]. The city has two stroke centers, three general hospitals (with computed tomography — CT — available 24 h/d, 7 d/wk), and 1 public rehabilitation care facility, totaling 1078 beds [9]. Since 2005, stroke patients have been transported by the national emergency medical service [Serviço de Atendimento Móvel de Urgência (SAMU)] [10], which uses a standard checklist based on the Cincinnati Stroke Scale [11].
The intravenous thrombolysis and first stroke unit in the country started to function in Joinville in 1997 [12], [13]. From 2005 until 2011, the adjusted incidence of thrombolysis for IS increased significantly from 1.4 (95% CI 0.6–2.9) in 2005 to 9.8 (7.3–12.9) per 100,000 in 2011 [12]. Results of the five main clinical trials of endovascular treatment for acute IS, published in 2014 and 2015, indicated that further improvements in outcome could be achieved by intra-arterial thrombectomy (IAT) as an add-on treatment to patients undergoing intravenous thrombolysis (IVT) or to patients not eligible for this intervention. In these studies, rates of functional independence (mRS score: 0–2) at 3 months ranged from 33% to 71% in patients treated with IAT ± IVT, compared with 19% to 40% of patients in the groups treated with usual care, which included IVT up to 4.5 h after IS [1], [2], [3], [4], [5]. Whether similar results can be obtained in a developing country is still unknown.
After 2011, our team started rescue IAT when the patient with IS presented NIHSS above 10 points, in 6 h time window, with cervical and cranial angiotomography defining a vascular occlusion site. Therefore, we aimed to compare functional status after 3 months between patients with IS who underwent IAT plus intravenous r-tPA versus intravenous thrombolysis (IVT) alone in Joinville, Brazil, using a historical cohort design. We hypothesized that the outcomes of patients treated with IAT + IVT would be better than those of patients treated with IVT alone.
2. Material and methods
2.1. The Joinville population registry
Cohort data were retrospectively extracted from the Joinville Stroke Registry. The Joinvile Stroke Registry is an ongoing population-based stroke data bank started in 2005 and supported by law since 2013 [14]. The registry uses the ideal methodology proposed by Sudlow and Warlow [15] as well as the Stroke-Steps modular program proposed by the WHO (first step for all hospital cases, second step for checking of death certificates and third step to ascertain mild events) [16]. The detailed methods of cohort recruitment have been described elsewhere [17].
After having obtained written informed consent from all patients or their relatives, the Joinville Stroke Register research nurses recorded the biochemical, electrocardiographic and radiological results. A neurologist was responsible for the NIH and ASPECTS scores [18], [19], OCSP and TOAST classifications [20], [21]. Baseline demographic data, risk factors, and length of stay were also registered. Local ethics committees approved the use of patients' retrospective data.
2.2. Groups
We specifically assessed performance of IVT, IAT and usual care in patients included in the Joinville Stroke Registry between 2009–2011 and 2012–2014. From 2009 to 2011, IVT was performed with intravenous r-tPA within 4.5 h time-window criteria (ECASS III) [22]. From 2012 until 2014, patients eligible for IVT were treated with both IVT and endovascular catheterization with a Solitaire FR device, up to 6 h after symptom onset [23]. We compared outcomes from two groups of patients within two time periods: patients treated with IVT alone from 2009 to 2011 (GroupIVT); patients treated with IVT + IAT from 2012 to 2014 (GroupIVT + IAT). In addition, in order to evaluate whether outcomes within the two study periods would be influenced by possible differences in usual care, we compared outcomes from patients treated with usual care (no thrombolysis), not included in the IVT group from 2009 to 2011 or IAT plus IVT from 2012 to 2014, using the same inclusion criteria.
2.3. Evaluation of outcomes
Functional independence was evaluated using the mRS, which ranges from 0 (no symptoms) to 6 (death) [24]. mRS scores were assessed at one month (face to face) and at 90 days (by telephone), by interviewers who were blinded to patient groups [24].
2.4. IVT protocol
The routine of stroke thrombolysis and stroke investigation followed the guidelines proposed by the Brazilian Society of Cerebrovascular Diseases [25]. The same protocol, based on the National Institute of Neurological Disorders and Stroke (NINDS) trial [26], was used in all of the centers that admitted IS patients in the acute phase. Clinical severity was measured by the NIH stroke scale (NIHSS) [20]. Following a concise clinical and neurological examination by a neurologist, a head CT was performed and eligibility for thrombolytic treatment was determined. The time of onset of symptoms, the interval between hospital arrival (door time) and the interval between hospital arrival and onset of tPA bolus infusion (needle time) were registered. To evaluate the extension of ischemic lesions in CT, we used the ASPECTS (Alberta Stroke Program Early CT score) [21]. A second CT was performed within 48 h after admission to ascertain intracerebral hemorrhage (ICH) in all patients who received r-tPA. CTs were also performed at any time, if clinical deterioration occurred. Symptomatic ICH was diagnosed if there was any neurological clinical worsening or NIH decline ≥ 4, and a parenchymal hematoma type 2 in the control brain CT as proposed in ECASS II [27], [28]. A neuroradiologist (PSCM) reviewed all imaging results.
2.5. IVT + IAT protocol
The following criteria were used for administration of IAT in addition to IVT: age ≥ 18 years, NIHSS > 10, non-lacunar syndrome or partial anterior cerebral infarction according to OCSP classification or small artery occlusion (SAO) according to TOAST classification. The OCSP classification assigns an ischaemic stroke subtype according to clinical signs and symptoms, modified where appropriate by the site and size of the underlying infarction on brain imaging. Whilst this method does not distinguish CE from LAA stroke, it does allow separate categorization of lacunar strokes (mainly presumed to be due to SAO). All endovascular procedures were performed by the same neuroradiology team (PSCM, HA and PW), who have followed a guideline for endovascular ischemic stroke intervention [29]. Time of groin puncture and time of vascular reperfusion were registered. Patients who awoke with stroke symptoms were excluded from the symptom-to-door time calculation.
IV r-tPA was started inside the CT room (0.9 mg/kg) and the patient was transferred to the angiography suite. IAT was performed via a femoral artery approach. For the anterior circulation, an 8F Corail ballon-guided catheter (Balt) was placed in the internal carotid artery. For posterior circulation an 6F Chaperon catheter (Microvention) was navigated to vertebral artery. Using the combination of a 0.014 microguide-wire and a 0.021-inch Rebar microcatheter (Medtronic), the occlusion site was accessed and the stent retriever Solitaire (Medtronic) was deployed. After 5 min, the ballon of the guiding catheter was inflated, the stent retriever and the micro catheter were pulled back together through the ballon-guided catheter, during continuous manual aspiration to reverse the flow inside the catheter. A control angiogram was performed to determine the immediate reperfusion status. Arterial patency in preprocedure, post thrombectomy, and post procedure angiograms were classified by the modified Thrombolysis in Cerebral Infarction (mTICI) scores [30]. This system classifies revascularization in 5 grades: grade 0, no perfusion; grade 1, perfusion past the initial obstruction, but limited distal branch filling with little or slow distal perfusion; grade 2a, partial perfusion with less than half of the vascular distribution of the occluded artery (e.g., filling and perfusion in one M2 division distal to M1 occlusion); grade 2b, partial perfusion of the half or greater of the vascular distribution of the occluded vessel (e.g., filling and perfusion in ≥ 2 M2 segments distal to M1 occlusion); and grade 3, full perfusion with filling all distal branches. Primary outcome success was defined as complete revascularization resulting in a mTICI grade 2b or 3 [30].
2.6. Statistical analysis
We compared baseline results between patients in GroupIVT and GroupIVT + IAT. Differences among patient subgroups were evaluated using the χ2 test, the t-test, or the Mann–Whitney U test, as appropriate. All tests were two-tailed. Characteristics that showed an association with this outcome (p < 0.05) in univariate analysis were included in the multivariate logistic model. The model was adjusted for age (grouped as 54 years or younger, 55–74 years and 75 years or older), NIH stroke scale (assumed as 4 categories: 4–8 or mild, 9–13 or moderate, 14–22 or severe and > 22 or coma), atrial fibrillation, years of education, OCSP classification and group (GroupIVT, GroupIVT + IAT). The main outcomes were measured by the modified Rankin scale score at 30 and 90 days after intervention, where 0 to 1 points indicated very favorable outcome, 0 to 2 points indicated functional independence, 3 to 5 points functional dependence and 6 points indicated death [24]. The primary outcome was mRS 0–2 at 90 days and secondary outcome was mRS 0–1 at 90 days. Due to small sample size groups, propensity score matching was not applicable. Statistical analysis was carried out using the Statistical Package for Social Sciences, version 17.0 (SPSS Inc., Chicago, Ill., USA). The study was approved by the Ethics in Research Committees of all involved hospitals and universities. The reporting of this study conforms to the STROBE statement [31].
3. Results
We registered 82 consecutive patients who underwent IVT between January 2009 and December 2011, and 31 consecutive patients who underwent mechanical thrombectomy with Solitaire FR device between January 2012 and November 2014. In the IVT group, we excluded 1 patient who was less than 18 years old, 9 patients with lacunar IS (LACS or SAO) and 29 patients with NIHSS ≤ 10 points. In the IAT group, we excluded two patients with lacunar IS (LACS or SAO) and five patients with proven occlusion of a large vessel, but NIH below 11 points. The baseline demographic, socioeconomic, clinical, and biochemical data are shown in Table 1. The IAT group was younger [62 versus 71 years old (p < 0.001)], had a higher educational level (p = 0.001) and a slightly higher prevalence of atrial fibrillation (23% versus 9%; p = 0.057). At hospital admission, the patients in IAT group also had clinically more severe strokes in OCSP classification (TACS: 77% versus 56%; p < 0.003) and higher median in NIHSS [(19 versus 16; p = 0.011); Table 2]. The infarct size, measured by ASPECTS score, and procedure times were similar between the groups.
Table 1.
Demographic, socioeconomic, clinical and biochemical characteristics between tPA intravenous lysis (IVT) versus intra-arterial thrombectomy (IAT).
| Intravenous r-tPA (n = 82) | IA Thrombectomy (n = 31) |
P value | |
|---|---|---|---|
| Demographic | |||
| Age (SD) | 71.1 (12.3) | 61.7 (11.6) | < 0.001 |
| Sex, men (%) | 44 (53.7) | 19 (61.3) | 0.528 |
| Education level, n (%) | 0.038 | ||
| < 11 years | 61 (89.7) | 15 (71.4) | |
| > 11 years | 7 (10.3)* | 6 (28.6)β | |
| Social class, * n (%) | 0.328 | ||
| A | 2 (2.4) | 0 | |
| B1 | 1 (1.2) | 1 (3.2) | |
| B2 | 11 (13.4) | 5 (16.1) | |
| C1 | 30 (36.6) | 12 (38.7) | |
| C2 | 15 (18.3) | 6 (19.4) | |
| D | 20 (24.4) | 3 (9.7) | |
| E | 0 | 0 | |
| Unknown | 3 (3.7) | 4 (12.9) | |
| Cardiovascular risk factors† | |||
| Hypertension | 63 (76.8) | 23 (74.2) | 0.807 |
| Diabetes | 19 (23.2) | 7 (22.6) | 1.0 |
| Dyslipidemia | 23 (28.0) | 5 (16.1) | 0.229 |
| Smoking | 8 (9.8) | 6 (19.4) | 0.203 |
| Atrial fibrillation | 7 (8.5) | 7 (22.6) | 0.057 |
| Previous stroke/TIA | 7 (8.5) | 1 (3.2) | 0.442 |
| Myocardial infarction | 9 (11) | 2 (6.5) | 0.724 |
| CHF | 5 (6.1) | 3 (9.7) | 0.682 |
| Vascular claudication | 1 (1.2) | 1 (3.2) | 0.475 |
| Glucose admission | |||
| Mean (SD); mmol/L | 7.3 (2.8) | 7.5 (4.5) | 0.432 |
| Proportion ≥ 10 mmol | 7 (9.3) | 3 (10.3) | 1.0 |
| Cholesterol admission | |||
| Mean (SD); mmol/L | 4.4 (1.5) | 4.3 (1.2) | 1.0 |
| Proportion ≥ 6 mmol | 54 (72) | 17 (58.6) | 0241 |
Social class according to Brazilian Criteria of Economic Classification based on 2013 National Household Sample Survey. Amounts per year in US dollars. Class: A = 64,020; B1 = 27,468; B2 = 19,980; C1 = 8256; C2 = 4572; D–E: 2016. The Brazilian gross domestic product per capita at purchasing power parity according to the World Bank in 2013 was US 14,997 per year in 2013. †TIA: transient ischemic attack CHF: cardiovascular failure; Missing data in ⁎ 14 cases; β 10 cases.
Table 2.
Clinical stroke severity, thrombolysis times, and thrombectomy data.
| Intravenous r-tPA (n = 82) |
IA Thrombectomy (n = 31) |
P value | |
|---|---|---|---|
| NIHSS score* (median-IQR) | |||
| Admission | 16 (13.5–21) | 19 (17–24) | 0.011 |
| OCSP classification§, n (%) | 0.003 | ||
| TACS | 46 (56.1) | 24 (77.4) | |
| PACS | 31 (37.8) | 2 (6.5) | |
| POCS | 5 (6.1) | 5 (16.1) | |
| TOAST classification# | |||
| CE | 39 (47.6) | 14 (45.2) | 0.141 |
| LAA | 21 (25.6) | 13 (41.9) | |
| UND | 22 (26.8) | 4 (12.9) | |
| ASPECTS† (median-IQR) | 10 (2) | 10 (1) | 0.171 |
| Procedure times (minutes/median-IQR) | |||
| Symptom-door | 77 (78) | 75 (84) | 0.779 |
| Symptom-needle | 170 (80) | 178 (101.5) | 0.550 |
| Symptom to groin puncture | . | 217 (135–255) | . |
| Groin revascularization | . | 38 (26–50) | . |
| Vessel occlusions | |||
| Cervical ICA | . | 11 (35) | . |
| Terminus ICA | . | 2 (6) | . |
| M1 | . | 13 (39) | . |
| M2 | . | 0 (0) | . |
| Basilar | . | 5 (16) | . |
| Reperfusion proportion | . | 27 (87) | |
| Final mTICI grade§ | |||
| 0 | . | 4 | . |
| 1 | . | 1 | . |
| 2 A | . | 4 | . |
| 2B | . | 8 | . |
| 3 | . | 14 | . |
| Symptomatic intracranial hemorrhage | 10 (12.2) | 3 (9.7) | 1.000 |
Thrombolysis times median (IQR: interquartile range), or n (%); ⁎ NIHSS: National Institutes of Health Stroke Scale; §OCSP: Oxfordshire Community Stroke Project classification. TACI: total anterior circulation syndrome; PACI: partial anterior circulation syndrome; POCI: posterior circulation infarction; # TOAST classification: SAO: small artery occlusion; LAA: large artery atherosclerosis; CE: cardioembolic. †ASPECTS: Alberta Stroke Program Early CT Score; ICA: internal carotid artery; M1/2: middle cerebral artery; mTICI grade: modified Thrombolysis in Cerebral Infarction score.
In the IAT group, 81% (25/32) received IV r-tPA. The median time from symptom to groin puncture was 217 min and 38 min from groin puncture to revascularization. After intra-arterial thrombectomy, 71% (22/31) had a good or complete revascularization (mTICI 2B or 3). Symptomatic intracranial hemorrhage (PH2) was found in 12% (10/92) of the IVT group and in 10% (3/31) of the IAT group (p = 1.000). One patient had arterial rupture during the intra-arterial procedure.
Table 3 shows functional status measured by modified Rankin scale at one and 3 months after the procedures. One month after the procedures, the proportion of favorable outcome (mRS: 0–2) was 48% in the IAT group versus 31% in the IVT group [OR 2.14 (CI 95%, 0.92 to 4.99)]. As expected, these proportions improved after 90 days, when favorable status (mRS: 0–2) was 55% in the IAT group versus 37% in IVT (OR 2.11; 95% CI, 0.91 to 4.87). Table 4 shows that, after adjusting for age, education level, atrial fibrillation prevalence, stroke severity (NIHSS at admission) and clinical stroke presentation (OCSP classification), the patients who underwent the IAT procedure had an odds of favorable outcome only at very favorable outcome strata (mRS: 0–1; OR 4.53; 95% CI, 1.22 to 16.75). For the 0–1 mRS outcome, the absolute risk reduction was 18.3%. (95% CI: − 1.64% to 38.3%. The NNT (number needed to treat) was 6.
Table 3.
Outcomes at 30 days and 90 days.
| Outcome | Intravenous r-tPA (n = 82) |
IA Thrombectomy (n = 31) |
Odds ratio (95% CI)* |
|---|---|---|---|
| mRankin score at 30 days, n (%) | |||
| 0–1 | 19 (23.2)† | 10 (32.3) | 1.58 (0.64 to 3.93) |
| 0–2 | 25 (30.5) | 15 (48.4) | 2.14 (0.92 to 4.99) |
| 3–5 | 29 (35.4) | 9 (29.0) | 0.75 (0.31 to 1.84) |
| 6 | 28 (34) | 7 (23) | 0.56 (0.22 to 1.47) |
| mRankin score at 90 days, n (%) | |||
| 0–1 | 22 (27.2) | 14 (45.2) | 2.25 (0.95 to 5.31) |
| 0–2 | 30 (37.0) | 17 (54.8) | 2.11 (0.91 to 4.87) |
| 3–5 | 20 (24.4) | 6 (19.4) | 0.74 (0.27 to 2.07) |
| 6 | 32 (39) | 8 (26) | 0.54 (0.22 to 1.36) |
| mRankin score at 90 days P value† | |||
| Median (IQR) | 4 (1 to 6) | 2 (1 to 6) | 0.065 |
| Case-fatality at 90 days, n (%) | 32 (39) | 8 (26) | 0.270 |
*Values were adjusted by logistic regression for age (categories), educational level, atrial fibrillation, NIHSS at admission (categories) and OCSP classification; †Mann–Whitney test.
Table 4.
Logistic regression analysis of favorable outcome defined as mRS 0 to 1 at 90 days.
| Variables | Adjusted odds ratio (95% CI) | P value |
|---|---|---|
| Age | 0.94 (0.89 to 0.98) | 0.01 |
| Education level | ||
| < 11 years | 0.81 (0.19 to 3.47) | 0.77 |
| ≥ 11 years (reference) | ||
| Atrial fibrillation | 0.49 (0.08 to 2.91) | 0.43 |
| NIHSS at admission | 0.98 (0.87 to 1.12) | 0.84 |
| OCSP classification | ||
| TACS (reference) | 0.009 | |
| PACS | 3.58 (0.90 to 14.21) | 0.935 |
| POCS | 1.14 (0.17 to 7.93) | 0.001 |
| Mechanical thrombectomy | 4.53 (1.22 to 16.75) | 0.02 |
When functional independence (mRS 0–2) was chosen as an outcome, the results were no longer significant. After 3 months, the overall mRS median was 2 (IQR: 1–6) in the IAT group against 4 (1–6) in IVT (p = 0.065).
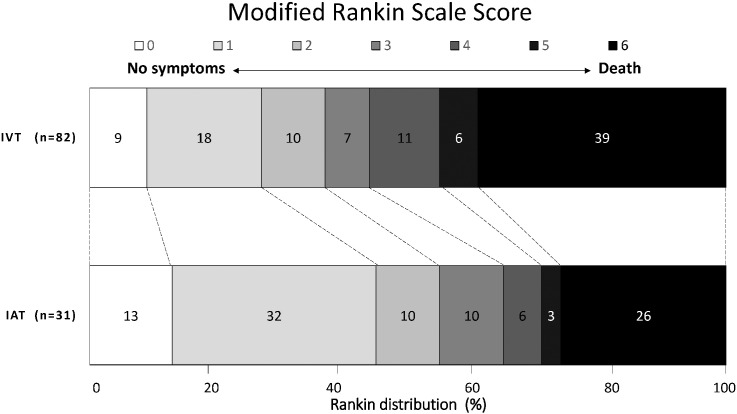
After 3 months, the shift toward better outcomes in favor of the IAT was consistent for all categories of mRS, including death (Fig. 1). The percentages of patients are shown in each cell, according to score on the mRS.
Fig. 1.
Modified Rankin scale scores at 90 days between IVT (r-tPA) alone versus intra-arterial thrombectomy (IAT) and IVT for ischemic stroke.
To know whether the standard of care was well balanced over time, we compared 546 consecutive patients with IS (270 in 2009–2011 and 276 in 2012–2014) who did not receive intravenous thrombolysis and had the same inclusion criteria used in the primary analysis (i.e., no small artery strokes or lacunar syndrome, NIHHSS > 10 and above 18 years old). The baseline data including age, socio-economic status, cardiovascular risk factors, stroke severity, length of stay, hospital distribution and outcomes were similar (Tables A.1–A.3; Appendix). As expected, all outcomes in the 30 and 90 days between no lysis in the period 2009–2011 versus no lysis in the period 2012–2013 were similar (Table A.3).
4. Discussion
In this small cohort sample, we found a benefit for patients with IS who underwent IVT within 6 h plus intravenous r-tPA compared with those who underwent IVT alone. This benefit was observed in 3 months for patients with favorable outcome (mRS: 0–1) without an increase in mortality. At the same time, 55% of patients in the IAT group were independent (mRS: 0–2) compared with 37% in IVT, whereas 24% of patients in the IAT group were dependent (mRS: 3–5) versus 19% in the IVT group.
All mechanical thrombectomies were performed with a Solitaire® stent retriever by the same team in two of four city hospitals (one public and one private). Table 5 compares our findings with those of five RCT on intra-arterial thrombolysis for IS [32]. The clinical stroke severity, the proportion of intravenous rt-PA use in mechanical thrombectomy, the successful reperfusion rate, the proportion of independent patients and device complications were similar with our cohort. However, the proportion of symptomatic hemorrhage and mortality were worse in our series, mainly in the IVT group. This could be explained by lower educational e economic levels of our patients. In IVT group, 85% (70/82) had ≤ 4 years of education and 48% (15/31) IAT group; 79% (65/82) of IVT group and 68% (21/31) of IAT group earn no more than 8300 USD per year. Indeed, low socioeconomic groups also have lower survival and greater stroke severity than high socioeconomic groups. [33]. Moreover, it is well known that patients in observational studies have more severe presentations severe and have more comorbidities and complications than patients in experimental studies [8].
Table 5.
Summary comparing data of five clinical trials and a cohort in Joinville/Brazil (JOINVASC IA).
| Study N IAT/IVT |
NIHSS range |
IV rt-PA | TICI 2B/3 |
LSN to groin (mdn) |
mRS 0–2 at 90 days |
sICH |
Device complications | Mortality |
||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IVT | IAT | IVT | IAT | IVT | IAT | IVT | IAT | |||||
| MR CLEAN 500 233/267 |
18 (14–21) |
17 (14–22) |
90% | 59% | 200 | 29% | 53% | 2.7% | 3.6% | Embol. 13 | 22% | 21% |
| EXTENDED IA 70 35/35 |
17 (12–20) |
16 (13–20) |
76% | 72% | 200 | 29% | 53% | 2.7% | 3.6% | Perf.1 | 19% | 10% |
| ESCAPE 315 165/150 |
13 (9–19) |
17 (13–20) |
100% | 86% | 210 | 40% | 71% | 6% | 0% | Perf.1 Embol.2 |
20% | 9% |
| SWIFT PRIME 196 98/98 |
17 (13–19) |
17 (13–20) |
98% | 88% | 224 | 36% | 60% | 3% | 0% | SAH 4 | 12% | 9% |
| REVASCAT 206 103/103 |
17 (12–19) |
17 (14–20) |
73% | 66% | 269 | 28% | 44% | 1.9% | 1.9% | Perf.5 Embol.5 |
16% | 18% |
| JOINVASC IA 113 31/82 |
16 (14–21) |
19 (17–24) |
81% | 71% | 217 | 37% | 55% | 12% | 10% | Perf.1 | 39% | 26% |
NIHSS indicates baseline National Institute of Neurological Disorders and Stroke Scale; rt-PA: recombinant tissue-type plasminogen activator; TICI 2b/3: patients in IAT group achieving thrombolysis in cerebral infarction grade 2b or 3 reperfusion according to Thrombolysis in Cerebral Infarction Score. LSN: last time seen normal to groin puncture; mRS: functional independence at modified Rankin scale; sICH: symptomatic intracerebral hemorrhage after IVT or IAT; Embol. Catheter embolization; Perf. Vessel perforation.
Our study had several limitations. First, functional assessment was obtained by telephone: Some would argue that the reliability of this method is low and cannot be recommended [34] However, a trained nurse was in charge of all phone interactions with both groups and was not aware of each patient's treatment group. Therefore, if information bias had occurred, it would have occurred in both groups of patients. Second, there was no imaging-based patient selection bias [35], [36], as the neuroimaging resources were the same over the two periods, essentially, CT and angio-CT in the public hospital and CT, angio-CT plus MRI in the private hospital. Third, the identification of proximal artery occlusion by image was a pragmatic and simple approach used in recent clinical trials. However, we did not have these data for patients in the IVT group. In fact, it is possible that the IVT group had patients with no large occlusion who were included in the analysis, i.e., who would be excluded if we had angio-CT data over the period 2009–2011 time. Therefore, we decided to exclude three patients with proven large vessel occlusion with NIHSS bellow 11 points from the IAT group. Fourth, as a single-center data ascertainment, our sample size is relatively small, i.e., has low statistical power. Fifth, the stroke team was the same all the time and we understand that the standard of care was well balanced because the outcomes of patients with no intervention beyond the usual treatment did not change from 2009 to 2014. However, we cannot prove that rehabilitation care was administered by the same team over time in our setting. Our strengths are the effectiveness of IAT in hyperacute IS in a “real world” setting from a middle-income country where stroke is the leading cause of death and whose population has an increasing life expectancy [7]. It is clear that the new findings from RCT create a historical turning point for Brazilian neurologists and neuroradiologists as well as in medical infrastructure, including urgent expansion in the number of stroke care units [6], [7], [12].
5. Conclusion
This is a small cohort study, so the results should be interpreted with caution. However, after the consistent results of effective treatment of moderate to severe neurological deficits due to proximal artery occlusion in IS with stent retrievers [6], [32], we believe that our findings represent a small step forward in the body of evidence for widespead use of endovascular reperfusion therapy. Further investigations in prospective cohorts from low- and middle-income countries are needed to compare with our findings.
Contributors
NC: study concept and design, coordination of the registry, statistical analysis, draft of manuscript, critical review of manuscript for intellectual content. PSCM: study concept, design, data collection and critical review. AC: manuscript draft and critical review. ALL, CHCM, HA, PW, VN, VV and ACG: data collection. SM and ARRG: study concept and statiscal analysis.
Funding
This study was entirely funded by Medtronic/Covidien (e-CPA 189918) Neurovascular. The sponsor had no role in the study design, data collection, data analysis, data interpretation or writing of the report. The corresponding author had full access to all of the data in the study and had the final responsibility for the decision to submit for publication.
Competing interests
NLC, PSCM, ALL and CHCM are consultants in educational projects for Medtronic/Convidien Neurovascular.
Ethical approval
The Joinville Stroke Registry and this study were approved by the local ethics committee.
Provence and peer review
Not commissioned; externally peer reviewed.
Acknowledgements
We thank the Joinville Health Secretary, which supports the Joinville Stroke Registry.
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.ensci.2016.04.002.
Appendix A. Supplementary data
Supplementary tables.
References
- 1.Berkhemer O.A., Fransen P.S., Beumer D. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med. 2015;372(1):11–20. doi: 10.1056/NEJMoa1411587. [DOI] [PubMed] [Google Scholar]
- 2.Goyal M., Demchuk A.M., Menon B.K., ESCAPE Trial Investigators Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med. 2015;372(11):1019–1030. doi: 10.1056/NEJMoa1414905. [DOI] [PubMed] [Google Scholar]
- 3.Campbell B.C., Mitchell P.J., Kleinig T.J. Endovascular therapy for ischemic stroke with perfusion-imaging selection EXTEND-IA Investigators. N Engl J Med. 2015;372(11):1009–1018. doi: 10.1056/NEJMoa1414792. [DOI] [PubMed] [Google Scholar]
- 4.Saver J.L., Goyal M., Bonafe A. Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. SWIFT PRIME Investigators. N Engl J Med. 2015;372(11):2285–2295. doi: 10.1056/NEJMoa1415061. [DOI] [PubMed] [Google Scholar]
- 5.Jovin T.G., Chamorro A., Cobo E. Thrombectomy within 8 hours after symptom onset in ischemic stroke. REVASCAT Trial Investigators. N Engl J Med. 2015;372(11):2296–2306. doi: 10.1056/NEJMoa1503780. [DOI] [PubMed] [Google Scholar]
- 6.Donnan G.A. Endovascular therapy: the dawning of a new era. Int J Stroke. 2015;10(4):463-463. doi: 10.1111/ijs.12514. [DOI] [PubMed] [Google Scholar]
- 7.Martins S.C.O., Pontes‐Neto O.M., Alves C.V. Past, present, and future of stroke in middle‐income countries: the Brazilian experience. Int J Stroke. 2013;8(A100):106–111. doi: 10.1111/ijs.12062. [DOI] [PubMed] [Google Scholar]
- 8.Dreyer N.A., Tunis S.R., Berger M. Why observational studies should be among the tools used in comparative effectiveness research. Health Aff. 2010;29(10):1818–1825. doi: 10.1377/hlthaff.2010.0666. [DOI] [PubMed] [Google Scholar]
- 9.Instituto Brasileiro de Geografia e Estatística — IBGE. Brazil 2010 census. http://censo2010.ibge.gov.br/resultados. (Accessed 02 April 2015)
- 10.Ministério da Saúde, Portal da Saúde. http://portal.saude.gov.br/portal/saude/visualizar_texto.cfm?idtxt=30273&janela=1 (accessed May 2013).
- 11.Kothari R.U., Pancioli A., Liu T. Cincinnati Prehospital Stroke Scale: reproducibility and validity. Ann Emerg Med. 1999;33(4):373–378. doi: 10.1016/s0196-0644(99)70299-4. [DOI] [PubMed] [Google Scholar]
- 12.Moro C.H., Gonçalves A.R., Longo A.L. Trends of the incidence of ischemic stroke thrombolysis over seven years and one-year outcome: a population-based study in Joinville, Brazil. Cerebrovasc Dis Extra. 2013;3(1):156–166. doi: 10.1159/000356984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cabral N.L., Moro C., Silva G.R. Study comparing the stroke unit outcome and conventional ward treatment. Arq Neuropsiquiatr. 2003;61(2 A):188–193. doi: 10.1590/s0004-282x2003000200006. [DOI] [PubMed] [Google Scholar]
- 14.Prefeitura Municipal de Joinville. Available at: https://www.joinville.sc.gov.br/noticia/4720-Lei+oficializa+Banco+de+Dados+de+Registros+de+AVC+em+Joinville.html
- 15.Sudlow C.L., Warlow C.P. Comparing stroke incidence worldwide: what makes studies comparable? Stroke. 1996;27(3):550–558. doi: 10.1161/01.str.27.3.550. [DOI] [PubMed] [Google Scholar]
- 16.The WHO STEPwise Approach to Stroke Surveillance. Overview and Manual (Version 2.0). Noncommunicable Diseases and Mental Health. World Health Organization. (http://www.who.int/entity/ncd_surveillance/steps/en (accessed December 7, 2013)).
- 17.Cabral N.L., Gonçalves A., Longo A. Trends in stroke incidence, mortality and case-fatality rates in Joinville, Brazil: 1995–2006. J Neurol Neurosurg Psychiatry. 2009;80(7):749–754. doi: 10.1136/jnnp.2008.164475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brott T., Adams H.P., Olinger C.P. Measurements of acute cerebral infarction: a clinical examination scale. Stroke. 1989;20(7):864–870. doi: 10.1161/01.str.20.7.864. [DOI] [PubMed] [Google Scholar]
- 19.Barber P.A., Demchuk A.M., Zhang J. ASPECTS study group: validity and reliability of a quantitative computed tomography score in predicting outcome in hyperacute stroke before thrombolytic therapy. Lancet. 2000;355(9216):1670–1674. doi: 10.1016/s0140-6736(00)02237-6. [DOI] [PubMed] [Google Scholar]
- 20.Bamford P., Sandercock M., Dennis J. Classification and natural history of clinically identifiable subtypes of cerebral infarction. Lancet. 1991;337(8756):1521–1526. doi: 10.1016/0140-6736(91)93206-o. [DOI] [PubMed] [Google Scholar]
- 21.Adams H.P., Bendixen B.H., Kappelle L.J. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in acute stroke treatment. Stroke. 1993;24(1):35–41. doi: 10.1161/01.str.24.1.35. [DOI] [PubMed] [Google Scholar]
- 22.Hacke W., Kaste M., Bluhmki E. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008;359(13):1317–1329. doi: 10.1056/NEJMoa0804656. [DOI] [PubMed] [Google Scholar]
- 23.Saver J., Jahan R., Levy Solitaire flow restoration device versus the Merci Retriever in patients with acute ischaemic stroke (SWIFT): a randomised, parallel-group, non-inferiority trial. Lancet. 2012;380(9849):1241–1249. doi: 10.1016/S0140-6736(12)61384-1. [DOI] [PubMed] [Google Scholar]
- 24.Sulter G., Steen C., De Keyser J. Use of the Barthel index and modified Rankin scale in acute stroke trials. Stroke. 1999;30(8):1538–1541. doi: 10.1161/01.str.30.8.1538. [DOI] [PubMed] [Google Scholar]
- 25.Oliveira-Filho J., Martins S.C.O., Pontes-Neto O.M. Guidelines for acute ischemic stroke treatment: part I. Arq Neuropsiquiatr. 2012;70(8):621–629. doi: 10.1590/s0004-282x2012000800012. [DOI] [PubMed] [Google Scholar]
- 26.Marier J.R. The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. 1995;333(24):1581–1587. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- 27.Schneider D., Diez-Tejedor E., Trouillas P. Randomised double-blind placebo-controlled trial of thrombolytictherapy with intravenous alteplase in acute ischaemic stroke (ECASS II). Second European-Australasian Acute Stroke Study Investigators. Lancet. 1998;352(9136):1245–1251. doi: 10.1016/s0140-6736(98)08020-9. [DOI] [PubMed] [Google Scholar]
- 28.Berger C., Fiorelli M., Steiner T. Hemorrhagic transformation of ischemic brain tissue. Asymptomatic or symptomatic? Stroke. 2001;32(6):1330–1335. doi: 10.1161/01.str.32.6.1330. [DOI] [PubMed] [Google Scholar]
- 29.Lavine S.D., Cockroft K., Hoh B. Training Guidelines for Endovascular Ischemic Stroke Intervention: an International multi-society consensus document. AJNR Am J Neuroradiol. 2016 doi: 10.3174/ajnr.A4766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zaidat O.O., Yoo A.J., Khatri P. Recommendations on angiographic revascularization grading standards for acute ischemic stroke: a consensus statement. Stroke. 2013;44(9):2650–2663. doi: 10.1161/STROKEAHA.113.001972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Von Elm E., Altman D.G., Egger M. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Prev Med. 2007;45(4):247–251. doi: 10.1016/j.ypmed.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 32.Grotta J.C., Hacke W. Stroke neurologist's perspective on the new endovascular trials. Stroke. 2015;46(6):1447–1452. doi: 10.1161/STROKEAHA.115.008384. [DOI] [PubMed] [Google Scholar]
- 33.Cox A.M., McKevitt C., Rudd A.G. Socioeconomic status and stroke. Lancet Neurol. 2006;5(2):181–188. doi: 10.1016/S1474-4422(06)70351-9. [DOI] [PubMed] [Google Scholar]
- 34.Kasner S.E. Clinical interpretation and use of stroke scales. Lancet Neurol. 2006;5(7):603–612. doi: 10.1016/S1474-4422(06)70495-1. [DOI] [PubMed] [Google Scholar]
- 35.Albers G.W., Thijs V.N., Wechsler L. Magnetic resonance imaging profiles predict clinical response to early reperfusion: the diffusion and perfusion imaging evaluation for understanding stroke evolution (DEFUSE) study. Ann Neurol. 2006;60(5):508–517. doi: 10.1002/ana.20976. [DOI] [PubMed] [Google Scholar]
- 36.Lansberg M.G., Straka M., Kemp S. MRI profile and response to endovascular reperfusion after stroke (DEFUSE 2): a prospective cohort study. Lancet Neurol. 2012;11(10):860–867. doi: 10.1016/S1474-4422(12)70203-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary tables.