Summary
Background
Arthroscopic sub-acromial decompression (decompressing the sub-acromial space by removing bone spurs and soft tissue arthroscopically) is a common surgery for subacromial shoulder pain, but its effectiveness is uncertain. We did a study to assess its effectiveness and to investigate the mechanism for surgical decompression.
Methods
We did a multicentre, randomised, pragmatic, parallel group, placebo-controlled, three-group trial at 32 hospitals in the UK with 51 surgeons. Participants were patients who had subacromial pain for at least 3 months with intact rotator cuff tendons, were eligible for arthroscopic surgery, and had previously completed a non-operative management programme that included exercise therapy and at least one steroid injection. Exclusion criteria included a full-thickness torn rotator cuff. We randomly assigned participants (1:1:1) to arthroscopic subacromial decompression, investigational arthroscopy only, or no treatment (attendance of one reassessment appointment with a specialist shoulder clinician 3 months after study entry, but no intervention). Arthroscopy only was a placebo as the essential surgical element (bone and soft tissue removal) was omitted. We did the randomisation with a computer-generated minimisation system. In the surgical intervention groups, patients were not told which type of surgery they were receiving (to ensure masking). Patients were followed up at 6 months and 1 year after randomisation; surgeons coordinated their waiting lists to schedule surgeries as close as possible to randomisation. The primary outcome was the Oxford Shoulder Score (0 [worst] to 48 [best]) at 6 months, analysed by intention to treat. The sample size calculation was based upon a target difference of 4·5 points (SD 9·0). This trial has been registered at ClinicalTrials.gov, number NCT01623011.
Findings
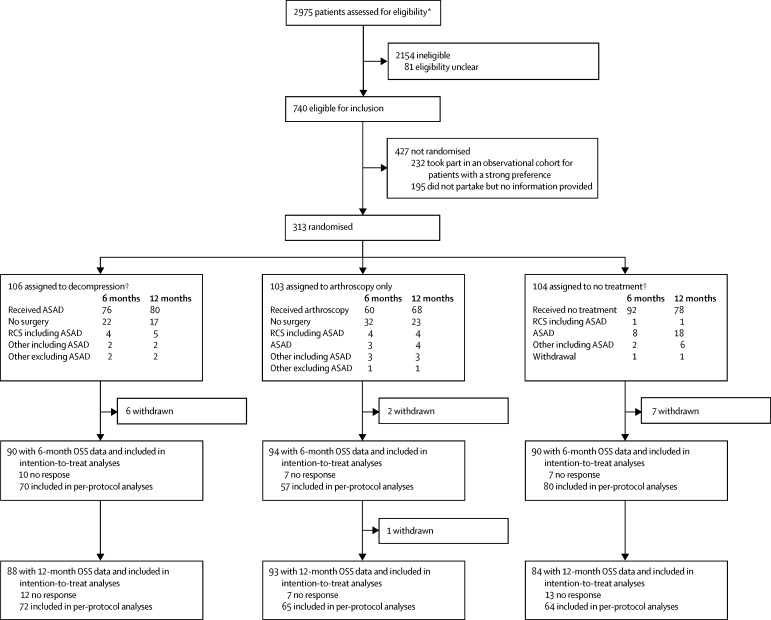
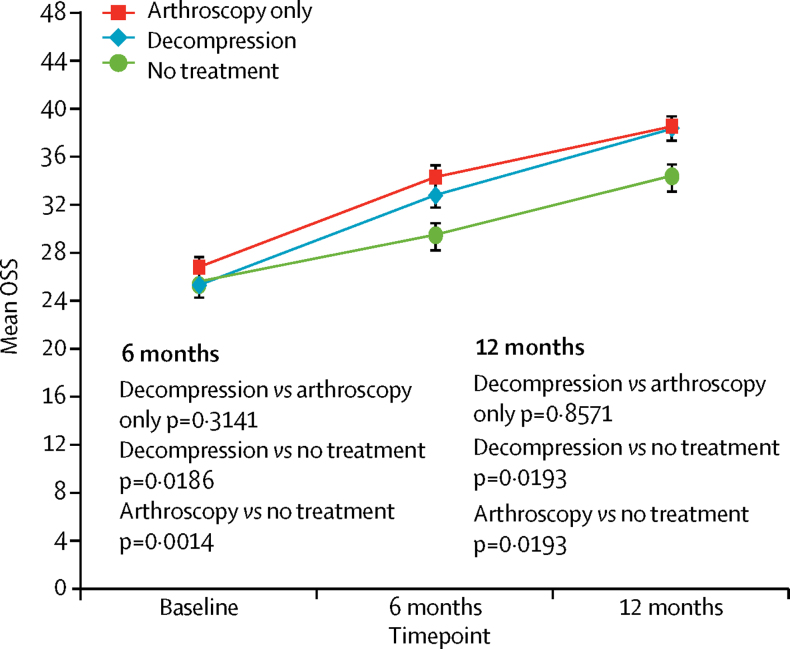
Between Sept 14, 2012, and June 16, 2015, we randomly assigned 313 patients to treatment groups (106 to decompression surgery, 103 to arthroscopy only, and 104 to no treatment). 24 [23%], 43 [42%], and 12 [12%] of the decompression, arthroscopy only, and no treatment groups, respectively, did not receive their assigned treatment by 6 months. At 6 months, data for the Oxford Shoulder Score were available for 90 patients assigned to decompression, 94 to arthroscopy, and 90 to no treatment. Mean Oxford Shoulder Score did not differ between the two surgical groups at 6 months (decompression mean 32·7 points [SD 11·6] vs arthroscopy mean 34·2 points [9·2]; mean difference −1·3 points (95% CI −3·9 to 1·3, p=0·3141). Both surgical groups showed a small benefit over no treatment (mean 29·4 points [SD 11·9], mean difference vs decompression 2·8 points [95% CI 0·5–5·2], p=0·0186; mean difference vs arthroscopy 4·2 [1·8–6·6], p=0·0014) but these differences were not clinically important. There were six study-related complications that were all frozen shoulders (in two patients in each group).
Interpretation
Surgical groups had better outcomes for shoulder pain and function compared with no treatment but this difference was not clinically important. Additionally, surgical decompression appeared to offer no extra benefit over arthroscopy only. The difference between the surgical groups and no treatment might be the result of, for instance, a placebo effect or postoperative physiotherapy. The findings question the value of this operation for these indications, and this should be communicated to patients during the shared treatment decision-making process.
Funding
Arthritis Research UK, the National Institute for Health Research Biomedical Research Centre, and the Royal College of Surgeons (England).
Introduction
Painful shoulders pose a substantial socioeconomic burden,1 accounting for 2·4% of all primary care consultations in the UK2 and 4·5 million visits to physicians annually in the USA.3 Subacromial pain accounts for up to 70% of all shoulder-pain problems4 and can impair the ability to work or do household tasks.5, 6 The mean annual total cost of treating patients with shoulder pain is estimated as €4139, with costs for sick leave and secondary care substantially adding to total costs.7
Research in context.
Evidence before this study
Shoulder pain is common and poses a substantial burden to society. Subacromial decompression was introduced in 1972 as a treatment for subacromial shoulder pain without high-level evidence and is now one of the most common surgical procedures in orthopaedics. The rationale is that the shoulder pain is caused by physical contact during arm movement between the rotator cuff tendons and a spur of bone or associated soft tissue projecting from the acromion of the scapula, and that surgical removal of the bone spur and soft tissue (decompression) eliminates this contact and, thereby cures or reduces symptoms. In 2009, we searched the scientific literature (assisted by Arthritis Research UK) before study submission with no language restrictions for reports published between 2000–09. We searched NLH, Embase, MEDLINE, PEDro; MetaRegister of Clinical Trials (active and archived registers), ClinicalTrials.gov, Centre for Reviews and Dissemination (DARE, NHS EED and HTA), Cochrane Database of Systematic Reviews, and the Cochrane Central Register of Controlled Studies databases, with the search terms “arthroscopy”, “arthroscopic”, “arthroscope”, “arthroscopies”, “acromioplasty”, “shoulder”, “rotator cuff”, “glenohumeral”, “acromioclavicular”, “supraspinatus”, “bicipital”, “subacromial”, “osteoarthritis”, “rheumatoid arthritis”, “calcific”, “tendonitis”, “impingement”, and “bursitis “. A systematic review of the effectiveness of surgery was limited by the quality of evidence but their findings showed no difference between patients treated with surgery and those treated with non-surgical options. The recommendation from this review is that more high-quality trials are undertaken. We did a randomised, pragmatic, parallel group, placebo-controlled trial to determine whether decompression compared with placebo (arthroscopy only) improved pain and function, whether decompression differed in outcome to no treatment, and whether placebo differed to no treatment.
Added value of this study
To our knowledge, this is the largest study to compare surgery with no treatment and is the first published trial of shoulder surgery to include a placebo comparison. Both types of surgery were better than no treatment, but the differences were not clinically significant, which questions the value of this type of surgery to patients. There were also no differences in outcomes between decompression surgery and placebo surgery (arthroscopy only) for these indications. The mechanism of the treatment effect in the patients who received surgery might be the result of placebo, postoperative physiotherapy, or other factors. Removal of bone spurs and soft tissue conferred no clear added benefit.
Implications of all the available evidence
During the past three decades, clinicians and patients with subacromial shoulder pain have accepted minimally invasive arthroscopic subacromial decompression surgery in the belief that it provides reliable relief of symptoms at low risk of adverse events and complications. However, the findings from our study suggest that surgery might not provide clinically significant benefit over no treatment, and that there is no benefit of decompression over arthroscopy only. These results should be shared with patients considering surgery.
An anatomical cause for subacromial pain has been proposed, whereby mechanical contact occurs between the rotator cuff tendons and the overlying acromion or bone spur that often forms at the anteroinferior margin of the acromion, narrowing the subacromial space. The narrowing makes physical contact more likely, particularly in certain positions of the arm (known as a painful arc).8 The condition is sometimes referred to as impingement. Although many patients with subacromial pain are treated with, and will respond to, non-operative treatment alone,9 surgical intervention is often used as an early treatment choice or in recalcitrant cases. This intervention involves decompressing the subacromial space by removing the bone spur and any involved soft tissue arthroscopically, a procedure known as arthroscopic subacromial decompression. The indications for surgery are persistent and severe subacromial shoulder pain combined with functional restrictions that are resistant to conservative measures. However, the effectiveness of this procedure is uncertain. Some reports suggest that surgery can be no more effective than exercise therapy,10 whereas others report good outcomes from surgery.11 The number of patients undergoing subacromial decompression in England (UK) rose by seven times from 2523 in 2000 to 21 355 in 2010.12
Some studies have tried to assess the effectiveness of subacromial decompression against a control. One randomised controlled trial13 compared decompression plus subacromial bursectomy with bursectomy alone and reported no significant difference in clinical outcome between groups. Such studies support the theory that undergoing a surgical intervention for subacromial pain results in a significant placebo effect and that removal of the subacromial bone spur and soft tissue might not be necessary. No randomised trials have been reported with patients with subacromial pain to assess whether decompression is more effective than a diagnostic investigative arthroscopy, or doing nothing (no treatment).
The objectives of our Can Shoulder Arthroscopy Work? (CSAW) study were twofold: to compare shoulder surgery with and without the essential surgical element (surgical decompression vs arthroscopy only) to investigate the mechanism for surgical decompression, and to compare surgery (decompression and arthroscopy only for subacromial shoulder pain against no treatment to assess the effectiveness of surgical intervention.
Methods
Study design and participants
The detailed study design and protocol has been published previously.14 We did a multicentre, randomised, pragmatic, parallel group, placebo-controlled, three-group trial. 32 hospital sites in the UK (with 51 surgeons recruiting or delivering both surgical interventions) were open for recruitment. Two sites (with two surgeons) were subsequently closed without recruiting any participants. 11 surgeons were involved in the recruitment but did not operate. Therefore, patients were recruited at 30 sites and operated on by 38 surgeons.
Methodologically, the most desirable control comparisons for assessing treatment benefit are placebo or sham intervention and no treatment. An entirely sham procedure (ie, a completely simulated surgery) can pose recruitment issues, therefore we used a placebo surgical intervention in which the accepted critical surgical element (bone and tissue removal in this instance) was omitted.15 For this study, the placebo intervention was investigational arthroscopy only.
The study tested three main superiority hypotheses. First, that there is no difference in outcomes between decompression and arthroscopy only. This comparison accounts for the placebo effect of surgery and informs whether the proposed critical surgical element, removal of bone and soft tissue, is necessary. Secondly, that no difference in outcomes exists between decompression and no treatment. The third hypothesis is that there is no difference in outcomes between arthroscopy only and no treatment to evaluate the benefit of arthroscopy without removing bone, bursa and soft tissue.
The study was designed under the ethical supervision of an academic ethicist (JS) with placebo trial experience.15 UK National Health Service (NHS) ethical approval was obtained on Feb 2, 2012, from the National Research Ethics Service South Central Oxford B (Research Ethics Committee Reference 12/SC/0028). Local NHS research and development approvals were obtained for each recruiting centre. The study was accepted onto the UK Clinical Research Network portfolio (12104).
Operating surgeons had a large amount of experience (29 of 38 were consultants for more than 5 years and 26 of 38 had done more than 20 decompressions in the previous year), and most had done between 40–60 procedures. Patients had to have subacromial pain of at least 3 months' duration with intact rotator cuff tendons, be eligible for arthroscopic surgery, and to have previously completed a non-operative management programme that included both exercise therapy and at least one steroid injection. Diagnosis was confirmed by a consultant shoulder surgeon. Detailed exclusion criteria are in the protocol and included patients with a full-thickness torn rotator cuff (identified with MRI, ultrasound, or x-ray). Patients with partial-thickness tears were not excluded but the location, nature, and extent of the partial-thickness tear was recorded. Informed consent of patients was obtained by the participating surgeon or suitably qualified local centre study personnel after a reflection period of 48 h, subsequent to study introduction and taken at the baseline measurement appointment immediately before baseline measurement. Consent was requested for all study interventions and assessments, including voice recording of appointments for qualitative research investigative purposes.16
Randomisation and masking
We randomly assigned participants (1:1:1) to arthroscopic subacromial decompression, investigational arthroscopy only, or no treatment (attendance of one reassessment appointment with a specialist shoulder clinician 3 months after study entry, but no intervention). Arthroscopy only was a placebo as the essential surgical element (bone and soft tissue removal) was omitted. We did the randomisation with a computer-generated minimisation system.
Patients randomly assigned to surgery were not told which procedure they had undergone, decompression or arthroscopy only, to ensure they were masked to assignment; however, patients assigned to no treatment were not masked to assignment. Masked assessment was done in all groups. The randomisation was done by a site research officer using an automated computer generated minimisation system, minimising for age (<40, 40–55, or ≥56 years), sex, baseline Oxford Shoulder Score (<19, 19–26, 27–33, or ≥34 years), and recruiting site.
Procedures
Patients underwent standardised postoperative care and exercise therapy in both surgical groups. Patients received the appropriate surgical treatment for any unexpected pathology, such as repair for full thickness rotator cuff tear, under the same anaesthetic. We deemed these patients non-compliant with allocated treatment.
Decompression was done according to routine practice under general anaesthetic. Further details of the procedure are in the protocol,14 and involved removal of bursa and soft tissue within the subacromial space, release of the coracoacromial ligament, and removal of the subacromial bone spur through posterior and lateral portals. Arthroscopy only was also done under general anaesthetic through a posterior portal. Patients underwent routine investigational arthroscopy of the joint, rotator cuff tendons, and subacromial bursa, with the operation done in exactly the same manner as decompression. A lateral skin incision was made to simulate a lateral portal but no instruments were introduced through this incision. The intervention did not involve surgical removal of any bone, bursal tissue, other soft tissue or release of the coracoacromial ligament. The procedure involved inspection and irrigation of the glenohumeral joint (arthroscopy) and the subacromial bursa (bursoscopy). For all surgical patients, surgical pathology details were recorded at operation. Postoperative physiotherapy involved advice and between one and four routine treatment sessions.
No treatment (monitoring) involved patients attending one reassessment appointment with a specialist shoulder clinician 3 months after entering the study but with no planned intervention. The patients in the no-treatment group had no prescribed physiotherapy or steroid injections.
Outcomes
The primary outcome measure for the study was the Oxford Shoulder Score assessed at 6 months after randomisation. This is a patient-reported questionnaire and an effective measure of change in pain and function in shoulders over time (scale 0 [worst] to 48 [best]).17
Secondary outcome measures were the Oxford Shoulder Score at 12 months after randomisation, a modified Constant-Murley Shoulder Score (for shoulder function and range of motion),18 PainDETECT—a questionnaire for neuropathic pain components,19 Quantitative Sensory Testing (for pain and pain thresholds),20 adverse events (both expected and unexpected were recorded by trial co-ordinators at local sites and reported to the CSAW office), EQ-5D-3L (EuroQol 5 dimensions 3 level index; quality of life), EuroQol visual analogue scale (EQ VAS), treatment expectations, patient perception or satisfaction,21 and anxiety and depression (measured by the Hospital Anxiety and Depression [HADS] Score).22
Baseline assessments of the pain and quality of life measures were also were undertaken. Participating surgeons coordinated their surgery waiting lists to ensure surgical patients were scheduled for surgery as close to randomisation as possible. Patients were followed up at 6 and 12 months after randomisation, with patients in the no-treatment group having an additional reassessment at 3 months after randomisation. Each follow-up involved questionnaires and a clinical assessment in clinic. Operation details were also collected at the time of surgery. Patients with delays in receiving surgery of longer than 4 months completed additional questionnaires at the time of surgery and also 1 year after surgery. This further data collection was implemented early in the recruitment period once the length of the surgical waiting times in some sites became clear.
Statistical analysis
A full description of the sample size calculation and analysis plan is in the published protocol14 together with the statistical analysis plan. The sample size was based on the primary outcome measure, Oxford Shoulder Score at 6 months after randomisation and was calculated using a minimum clinically important difference approximation based on half of the SD of the change in the score from before to after treatment, which was estimated to be nine points (ie, a target difference of 4·5 points). Using a two-sided t test, 90% power to detect a difference in the Oxford Shoulder Score of 4·5 (SD 9·0), with a two-sided 5% level of significance (α) required a sample size of 85 participants in each group. Accounting for loss to follow-up of up to 15%, 100 participants were required in each group. A target of 300 participants (100 per treatment group) was set. No adjustment was made for multiple comparisons.
The principal analysis of the primary and all secondary outcomes was done according to the randomised group (intention-to-treat), irrespective of compliance without imputation for missing data. Per-protocol analyses (ie, inclusion of randomised patient if they received the allocated intervention at the specified timepoint) were also done for the primary outcome and Oxford Shoulder Score at 1 year. Additional sensitivity analyses for the two outcomes addressed delays in receiving surgery by using post-surgery follow-up data (decompression vs arthroscopy only, and also excluding participant data where surgery was just before [within 2 months] of the follow-up timepoint). Other sensitivity analyses assessed the sensitivity to model assumptions and other factors (bootstrapping for possible non-normality, unadjusted analysis to assess the effect of adjustment for minimisation factors and mixed-model analyses to maximise use of available outcome data), and missing data (using multiple imputation model for missing at random and the rctmiss command in Stata for missing not-at-random assumptions to quantify the potential effect of missing data).
Three separate two-way comparisons (ie, decompression vs arthroscopy only, decompression vs no treatment, and arthroscopy only vs no treatment) were done for all outcome analyses. For each comparison, linear regression analysis was used to compare the Oxford Shoulder Score at 6 months after randomisation, adjusting for the minimisation factors of sex and age (as a continuous factor), baseline Oxford Shoulder Score (as a continuous factor) and site (using cluster robust standard errors, implemented via the cluster option in Stata). Information from the participant's age and baseline Oxford Shoulder Score were recorded in two places, namely the randomisation system and the baseline form (later entered onto the trial database). Where there were discrepancies between the records, the information from the trial database was used in this analysis. The Oxford Shoulder Score at 12 months, and modified Constant-Murley, PainDETECT, Quantitative Sensory Testing, HADS, EQ VAS, and EQ-5D-3L Index score [UK tariff] and visual analogue scale) measures were analysed in a similar manner (adjusting for the appropriate baseline). Complications, treatment expectations, and patient satisfaction were analysed using a Fisher's exact test or χ2 test for trend. The statistical analysis plan was reviewed by an independent Data Monitoring Committee, and finalised and approved by a Trial Steering Committee before unmasking the data to study investigators. All statistical testing was done at the two-sided 5% significance level, and 95% CIs with Stata 14.2. This trial has been registered at ClinicalTrials.gov, number NCT01623011.
Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
Between Sept 14, 2012, and June 16, 2015, we randomly assigned 313 patients (of 740 eligible patients [appendix]) to treatment: 106 to decompression surgery, 103 to arthroscopy only, and 104 to no treatment, figure 1. The study groups were well balanced on all baseline characteristics (table 1). The median number of operations per surgeon was two (IQR 1–6). Further details on reasons for non-compliance, missing data, and the per-protocol population are in the appendix. 24 (23%), 43 (42%), and 12 (12%) of the decompression, arthroscopy only, and no-treatment groups, respectively, did not receive their assigned treatment by 6 months. At 1 year, 19 (18%), 35 (34%), and 26 (25%) of the decompression, arthroscopy only, and no-treatment groups, respectively, had not received their assigned treatment. Median time to surgery (any type) was 90 days (IQR 58–123), 82 days (56–134), and 217 days (111–262) for decompression, arthroscopy only, and no treatment groups, respectively.
Figure 1.
Trial profile
OSS=Oxford Shoulder Score. ASAD=arthroscopic subacromial decompression. AO=arthroscopy only. RCS=rotator cuff surgery. *Based on data received from 23 sites and data imputed for nine sites. †Median time to any type of surgery was 58, 56, and 217 days in the decompression, arthroscopy only, and no treatment groups, respectively. ‡One rotator cuff surgery did not involve decompression.
Table 1.
Baseline characteristics of the intention-to-treat population
| Decompression (n=106) | Arthroscopy only (n=103) | No treatment (n=104) | |
|---|---|---|---|
| Female | 54 (51%) | 52 (50%) | 52 (50%) |
| Age | 52·9 (10·3) | 53·7 (10·5) | 53·2 (10·2) |
| Previously received injections in study shoulder | 2 (1–3) for n=105 | 2 (1–3) | 2 (1–3) |
| Oxford Shoulder Score | 25·2 (8·5) | 26·7 (8·8) | 25·5 (8·3) |
| Modified Constant-Murley Score | 39·4 (13·9), n=102 | 43·1 (15·5), n=101 | 38·3 (14·2), n=100 |
| PainDETECT Score | 11·7 (6·6), n=105 | 11·0 (5·9) | 11·9 (6·6), n=100 |
| HADS Depression Score | 5·0 (3·8), n=105 | 5·0 (3·7), n=102 | 5·7 (4·2) |
| HADS Anxiety Score | 6·3 (4·3) | 6·3 (4·2) | 6·9 (4·5) |
| EQ VAS | 65·8 (19·4) | 69·7 (19·2) | 64·4 (23·2) |
| EQ-5D-3L Index | 0·52 (0·30), n=105 | 0·55 (0·29), n=102 | 0·50 (0·33) |
Data are n (%), mean (SD), or median (IQR). Oxford Shoulder Score range 0 to 48. Modified Constant-Murley Score range 0 to 100. PainDETECT score −1 to 38. Hospital Anxiety and Depression Scale (HADS) Depression and Anxiety Score range 0 to 21. EuroQol 5 dimensions 3 level index (EQ-5D-3L) range −0·59 to 1·0. EuroQol visual analogue scale (EQ VAS) range 0–100. A higher score indicates a better state for all measures except for HADS and PainDETECT.
At 6 months, data for the Oxford Shoulder Score were available for 90 patients assigned to decompression, 94 to arthroscopy, and 90 to no treatment. Mean Oxford Shoulder Score did not differ between the two surgical groups at 6 months (decompression 32·7 points [SD 11·6] vs arthroscopy 34·2 points [9·2]; mean difference −1·3 points (95% CI −3·9 to 1·3, p=0·3141). Both surgical groups showed a small benefit over no treatment (mean 29·4 points [SD 11·9], decompression was higher by 2·8 points [95% CI 0·5–5·2], p=0· 0186; mean difference vs arthroscopy by 4·2 points [1·8–6·6], p=0·0014) but these differences were not clinically important (table 2, figure 2).
Table 2.
Oxford shoulder score outcome analyses
|
Mean (SD); n |
Mean difference (95% CI); p value |
|||||
|---|---|---|---|---|---|---|
| Decompression | Arthroscopy only | No treatment | Decompression vs arthroscopy only | Decompression vs no treatment | Arthroscopy only vs no treatment | |
| Intention to treat | ||||||
| OSS at 6 months | 32·7 (11·6); n=90 | 34·2 (9·2); n=94 | 29·4 (11·9); n=90 | −1·3 (−3·9 to 1·3); 0·3141 | 2·8 (0·5 to 5·2); 0·0186 | 4·2 (1·8 to 6·6); 0·0014 |
| OSS at 1 year | 38·2 (10·3); n=88 | 38·4 (9·3); n=93 | 34·3 (11·8); n=84 | 0·3 (−2·9 to 3·5); 0·8571 | 3·9 (0·7 to 7·1); 0·0193 | 3·6 (0·6 to 6·6); 0·0193 |
| Per protocol | ||||||
| OSS at 6 months | 34·7 (10·9); n=70 | 35·5 (8·8); n=57 | 29·4 (11·8); n=80 | −0·7 (−4·2 to 2·8); 0·6971 | 4·6 (1·7 to 7·5); 0·0030 | 5·3 (2·7 to 7·8); 0·0002 |
| OSS at 1 year | 39·4 (9·4); n=72 | 38·1 (9·6); n=65 | 33·8 (12·3); n=64 | 1·6 (−1·9 to 5·1); 0·3526 | 5·7 (2·9 to 8·4); 0·0003 | 3·9 (0·7 to 7·0); p=0·0175 |
| Multiple imputation* | ||||||
| OSS at 6 months | 32·5 (11·3); n=106 | 34·5 (8·8); n=103 | 29·6 (11·3); n=104 | −1·3 (−3·9 to 1·3); 0·3237 | 3·1 (0·7 to 5·5); 0·0139 | 4·1 (1·8 to 6·4); 0·0013 |
| OSS at 1 year | 38·3 (9·7); n=106 | 38·4 (8·9); n=103 | 34·4 (10·8); n=104 | 0·5 (−2·6 to 3·7); 0·7338 | 4·1 (0·7 to 7·5); 0·0202 | 3·3 (0·3 to 6·4); 0·0338 |
The primary outcome was the Oxford Shoulder Score (OSS) at 6 months.
Means and SDs including imputed values, averaged over the 20 imputation runs using predictive mean matching.
Figure 2.
Oxford Shoulder Score in the intention-to-treat analyses
Data are mean (95% CI) shown at follow-up timepoints. OSS= Oxford Shoulder Score.
At both 6 months and 1 year follow-up, all groups had on average better mean Oxford Shoulder Score scores (table 2, figure 2). The per-protocol analyses showed similar results (appendix) and the results were not sensitive to missing data. Similarly, sensitivity analyses had consistent results. Using post-surgery follow-up did not change the findings nor did exclusion of data for assessments done less than 2 months after surgery (appendix). Mixed model and bootstrapped analyses also produced very similar results (appendix). A 12-point difference missing not-at-random assumption did not change the decompression versus arthroscopy only results (appendix).
Nearly all secondary outcomes (table 3, appendix) reflect the same pattern as the primary outcome. The modified Constant-Murley score also showed no difference between decompression and arthroscopy only at 6 months and 1 year, but both surgical groups were better than no treatment at both timepoints. PainDETECT, EQ-5D-3L Index, EQ VAS, and HADS results also showed similar trends for no difference between the two surgical groups at 6 months, but differences were noted between each surgical group and no treatment (if not reaching statistical significance in all analyses). More patients in both surgical groups (decompression and arthroscopy only) considered themselves to have improved after treatment than in the no-treatment group at 6 months (appendix), although not in all comparisons at 1 year. Similarly, more patients in the surgical groups were pleased with their outcome than in the no-treatment group at 6 months, although at 1 year only some of the corresponding results were significant. Other patient-reported outcomes (appendix) were mostly not significant between any of the groups for either timepoint.
Table 3.
Secondary outcomes for pain and quality of life
|
Mean (SD); n |
Mean difference (95% CI); p value |
|||||
|---|---|---|---|---|---|---|
| Decompression | Arthroscopy only | No treatment | Decompression vs arthroscopy only | Decompression vs no treatment | Arthroscopy only vs no treatment | |
| At 6 months | ||||||
| Modified Constant-Murley | 56·5 (21·8); n=82 | 57·6 (17·7); n=84 | 45·4 (21·3); n=83 | 0·3 (−4·1 to 4·7); 0·8972 | 9·3 (4·1 to 14·6); 0·0012 | 9·1 (3·1 to 15·2); 0·0045 |
| PainDETECT | 8·4 (7·1); n=81 | 7·9 (5·7); n=82 | 10·2 (6·3); n=80 | 0·1 (−1·8 to 2·0); 0·9036 | −1·7 (−3·5 to 0·0); 0·0559 | −1·9 (−3·7 to 0·0); 0·0502 |
| HADS Depression | 3·6 (4·0); n=88 | 3·6 (3·9); n=91 | 5·5 (4·4); n=89 | 0·2 (−0·8 to 1·2); 0·6738 | −1·1 (−1·8 to −0·4); 0·0040 | −1·3 (−2·2 to −0·3); 0·0100 |
| HADS Anxiety | 5·1 (4·0); n=87 | 5·6 (4·6); n=92 | 6·7 (4·7); n=88 | −0·1 (−1·0 to 0·8); 0·7368 | −0·8 (−1·5 to −0·2); 0·0168 | −0·6 (−1·4 to 0·1); 0·1096 |
| EQ VAS | 74·2 (20·3); n=89 | 72·8 (20·2); n=93 | 67·8 (22·1); n=89 | 3·1 (−3·5 to 9·7); 0·3393 | 6·4 (2·2 to 10·7); 0·0043 | 3·4 (−1·4 to 8·2); 0·1601 |
| EQ5D-3L Index | 0·65 (0·29); n=89 | 0·67 (0·26); n=93 | 0·52 (0·36); n=89 | 0·00 (−0·09 to 0·08); 0·9308 | 0·12 (0·04 to 0·21); 0·0076 | 0·12 (0·02 to 0·21); 0·0154 |
| At 1 year | ||||||
| Modified Constant-Murley | 66·2 (19·9); n=76 | 64·9 (17·2); n=81 | 56·7 (22·1); n=70 | 2·7 (−2·7 to 8·2); 0·3087 | 8·3 (2·5 to 14·1); 0·0067 | 4·9 (0·9 to 8·9); 0·0173 |
| PainDETECT Score | 8·5 (7·1); n=67 | 7·3 (5·7); n=72 | 9·8 (7·6); n=69 | 0·4 (−1·4 to 2·2); 0·6541 | −1·5 (−3·7 to 0·7); 0·1721 | −1·8 (−4·3 to 0·7); 0·1536 |
| HADS Depression | 3·2 (3·5); n=84 | 3·5 (3·7); n=88 | 4·4 (4·0); n=78 | −0·1 (−0·7 to 0·5); 0·6906 | −0·7 (−1·5 to 0·2); 0·1208 | −0·5 (−1·3 to 0·2); 0·1452 |
| HADS Anxiety | 5·2 (4·1); n=83 | 5·7 (4·5); n=87 | 5·9 (4·2); n=81 | −0·1 (−0·9 to 0·6); 0·7474 | −0·1 (−1·0 to 0·8); 0·8220 | 0·0 (−1·0 to 1·1); 0·9215 |
| EQ VAS | 73·7 (21·0); n=85 | 75·9 (20·0); n=91 | 73·4 (22·4); n=82 | −0·4 (−4·4 to 3·7); 0·8530 | 0·0 (−4·3 to 4·2); 0·9947 | 0·3 (−5·1 to 5·7); 0·9050 |
| EQ-5D-3L Index | 0·74 (0·28); n=86 | 0·73 (0·27); n=92 | 0·66 (0·33); n=80 | 0·04 (−0·03 to 0·10); 0·2750 | 0·08 (0·00 to 0·16); 0·0517 | 0·05 (−0·04 to 0·13); 0·2644 |
HADS=Hospital Anxiety and Depression Scale. EQ-5D-3L=EuroQol 5 dimensions 3 levels. EQ VAS=EuroQol visual analogue scale.
There were six study-related complications, all frozen shoulders (two in each group). The comparisons of any complications were not significant (decompression vs arthroscopy only p>0·9999; decompression vs no treatment, p>0·9999; arthroscopy only vs no treatment, p≥0·9999). Two further participants had trauma-related injuries during the study (not considered study-related complications) that affected their study shoulder (one car accident [in the no-treatment group], and one fall due to slipping [in the decompression group]). Two participants (both in the arthroscopy only group) required further surgery for pain. One of these patients underwent decompression, the other received superior labrum anterior posterior debridement. Details on non-surgical treatments received are in the appendix.
Discussion
This study showed that shoulder pain improved substantially from baseline with subacromial decompression, arthroscopy only, and no treatment, and that the magnitude of difference between surgery (both surgical groups) and no treatment was statistically but of uncertain clinical significance. The difference between surgery and no treatment might be attributable to other factors including a surgical placebo effect, or to other unidentified effects of arthroscopic assessment of the joint and bursa, or to rest and postoperative physiotherapy associated with surgery.
There were also no differences in outcome between the two surgical groups at any timepoint. This finding suggests that the treatment effect is not due to the principal clinical justification for the surgery, which is the removal of bone, bursa, and soft tissue to relieve impingement on the underlying tendons during movement of the arm.
Consistent findings were noted for all the patient-reported and clinical outcomes used. The only other randomised trial reported in this field13 compared arthroscopic subacromial decompression with arthroscopic resection of the bursa and release of the coracoacromial ligament attached to the anterior acromion. Findings from this study showed no difference in outcome between the two groups, but both included substantial surgical resection of tissue that might be the cause of mechanical symptoms and impingement.
One conclusion from our study is that both arthroscopic subacromial decompression and arthroscopy only are effective, regardless of mechanism, and could be used as a treatment strategy for patients with subacromial impingement. Another more plausible conclusion is that surgery, although statistically better than no treatment, does not provide patients with a clinically important benefit. The focus of this study was an assessment of the role of surgery, and comparison groups were chosen to enable this. In the light of the results, other management strategies apart from surgery clearly should be assessed.
We are not aware of any published randomised trials investigating the effectiveness of arthroscopic subacromial decompression surgery compared with a placebo control, despite this being the most common arthroscopic shoulder operation.23 Placebo-controlled trials of surgery are not common but have been shown to be feasible, ethical, and of value.24 There have been trials involving placebo surgery in the knee and these have resulted in changes in practice.25, 26 Although harms associated with arthroscopy are rare, they do occur and include infection and venous thromboembolism.27, 28, 29
The strengths of this study were the use of a randomised placebo-controlled design with three groups (including both placebo and no-treatment arms), multiple follow-up assessments, and the use of valid patient-reported outcome measures. Additionally, the pragmatic nature of the study, and the wide range of sites and surgeons increased the generalisability of the results. Masking of the assessors and patients to the specific surgical intervention was also a strength with regard to the comparison of the decompression and arthroscopy only groups; however, masking of participants in the no-treatment arm was not possible, which could have affected reported outcomes in this group.
The major study limitation was the level of non-compliance to treatment allocation. Some patients assigned to surgery improved while waiting and did not proceed with surgery, whereas others assigned to no treatment chose to undergo decompression surgery. However, the findings of the trial were consistent when analysed both as randomised and per protocol. Similarly, missing data did not account for the absence of benefit of decompression over arthroscopy only. Patients in both surgical groups received a package of care consisting of surgery and subsequent postoperative physiotherapy advice on mobilisation and exercises. This postoperative physiotherapy could have affected outcome, and therefore we remain unsure of the mechanism for the benefit gained in the surgical groups. Our study also did not address long-term recurrence of any pain and problems beyond 1 year; however, the likelihood of one of the surgical groups achieving greater benefit beyond 1 year, when no difference existed previously, seems improbable. We did not adjust for multiple comparisons as per the protocol and the statistical analysis plan given the nature of three groups, although published views regarding this differ.30, 31
A further limitation was that patients could not be masked to treatment in the no treatment group, and they therefore might have perceived their treatment to be inferior to surgery. This perception could have adversely affected the outcome (nocebo effect). Another limitation is the waiting-list effect in the surgical groups, which might have had the potential to affect interpretation. We undertook additional follow-up assessments for the few patients who had significant delays waiting for surgery to enable appropriate comparisons.
In conclusion, we showed that, in patients with persistent subacromial shoulder pain due to impingement, improvement in Oxford Shoulder Scores with arthroscopic subacromial decompression did not differ to that achieved with arthroscopy only (placebo surgery). Although both types of surgery provide greater symptom improvement than no treatment, this difference was of uncertain clinical significance. The findings (which should be communicated to patients during the shared treatment decision-making process) question the value of this type of surgery for these indications, and might discourage some surgeons from offering decompression surgery and dissuade some patients from undergoing the surgery.
Acknowledgments
Acknowledgments
This study was funded by Arthritis Research UK (Clinical Studies Grant 19707), the National Institute for Health Research (NIHR) Oxford Biomedical Research Centre (BRC; previously the Biomedical Research Unit), and the Royal College of Surgeons (England). The NIHR Oxford BRC is based at the Oxford University Hospitals NHS Foundation Trust and run in partnership with the University of Oxford, funded by the NIHR. The University of Oxford sponsored the study. JC held a Medical Research Council Methodology Fellowship (G1002292) for part of the duration of the study. The Nuffield Department of Orthopaedics, Rheumatology and Musculoskeletal Sciences coordinated the study via the Surgical Intervention Trials Unit from the Royal College of Surgeons (England) Surgical Trials Initiative. We would like to thank the British Elbow and Shoulder Society for supporting and promoting this trial in the UK, all participants for their involvement in the study, and acknowledge The Oxford Clinical Trials Research Unit, the National Institute of Health Research Clinical Research Network, and The Nuffield Department of Orthopaedics, Rheumatology and Musculoskeletal Sciences (NDORMS) for their support. We particularly thank Marion Campbell and Alison McDonald and ChaRT (University of Aberdeen, UK) for their early guidance, and also thank Caroline Wilson (Quintet Research Group, School of Social and Community Medicine, University of Bristol, UK); Ben Dean (Investigator for the CSAW Tissue Sample Study, NDORMS, University of Oxford, UK); Patrick Julier (Oncology Clinical Trials Office, University of Oxford); Jeremy Lewis (St George's University of London, UK); Richard Gray (University of Oxford); Helen Higham (Oxford University Hospitals NHS Trust, UK); and the principal investigators and their teams at each of the CSAW sites. The views expressed in this report are those of the authors and do not necessarily reflect the views of the funders.
Contributors
DJB and AJC were co-chief investigators. JLR, CC, JAC, SG, and AJ were co-applicants on the grant application to Arthritis Research UK. IT, IR, AG, JM, and JS were collaborators and were involved in the design of the study and its implementation. JM and JS provided expertise on physiotherapy outcomes and ethics, respectively. NM was the study co-ordinator. KW provided neurophysiology input. DJB, AJC, and JLR were responsible for writing the manuscript. BAS, IR, and JAC did the statistical analysis. JLD and MJ were qualitative researchers involved in the qualitative research investigation aspect of the study. All authors read and approved the final manuscript.
Trial Steering Committee
Anthony Jones, University of Manchester, Chair. Amar Rangan, James Cook University Hospital, Independent Clinical Member. James D Hutchinson, University of Aberdeen, Independent Clinical Member. Dair Farrar-Hockley, Patient Representative. Veronica Conboy, Torbay Hospital, Principal Investigator and non-independent member. Data Monitoring Committee Matthew Costa, University of Warwick, Chair. Louise Stanton, University of Southampton, Independent Senior Statistician. Stephen Brealey, University of York, Independent Clinical Member. Megan Bowers, University of Southampton, Independent Senior Statistician.
CSAW Study Group
Philip Ahrens, Royal Free London NHS Foundation Trust (Hampstead). Cheryl Baldwick, North Devon Healthcare NHS Trust. Mark Brinsden, Plymouth Hospitals NHS Trust. Harry Brownlow, Royal Berkshire NHS Foundation Trust. David Burton, County Durham and Darlington NHS Foundation Trust. Muhammad Sohail Butt, The Dudley Group NHS Foundation Trust. Andrew Carr, Oxford University Hospitals NHS Foundation Trust. Charalambos P Charalambous, Blackpool Teaching Hospitals NHS Foundation Trust. Veronica Conboy, Torbay and South Devon NHS Foundation Trust. Lucy Dennell, The Queen Elizabeth Hospital King's Lynn NHS Foundation Trust. Oliver Donaldson, Yeovil District Hospital NHS Foundation Trust. Steven Drew, University Hospitals Coventry and Warwickshire NHS Trust Amitabh Dwyer, Hinchingbrooke Health Care NHS Trust. David Gidden, Northampton General Hospital NHS Trust. Peter Hallam, Norfolk and Norwich University Hospitals NHS Foundation Trust. Socrates Kalogrianitis, University Hospitals Birmingham NHS Foundation Trust. Cormac Kelly, The Robert Jones and Agnes Hunt Orthopaedic Hospital NHS Foundation Trust. Rohit Kulkarni, Aneurin Bevan University Health Board. Tim Matthews, Cardiff and Vale University Health Board. Julie McBirnie, NHS Lothian. Vipul Patel, Epsom and St Helier Hospitals NHS Trust. Chris Peach, University Hospital of South Manchester NHS Foundation Trust. Chris Roberts, The Ipswich Hospital NHS Trust. David Robinson, Worcestershire Acute Hospitals NHS Trust. Philip Rosell, Frimley Health NHS Foundation Trust. Dan Rossouw, Royal Free London NHS Foundation Trust (Barnet). Colin Senior, Dorset County Hospital NHS Foundation Trust. Bijayendra Singh, Medway NHS Foundation Trust. Soren Sjolin, West Suffolk NHS Foundation Trust. Geoffrey Taylor, Buckinghamshire Healthcare NHS Trust. Balachandran Venkateswaran, The Mid Yorkshire Hospitals NHS Trust. David Woods, Great Western Hospitals NHS Foundation Trust.
Authors' information
DJB: Professor of Musculoskeletal Sciences, Co-Director, Royal College of Surgeons (Eng) Surgical Intervention Trials Unit (SITU), Nuffield Department of Orthopaedics, Rheumatology and Musculoskeletal Sciences, University of Oxford. AJC: Nuffield Professor of Orthopaedic Surgery, Head of Dept The Nuffield Dept of Orthopaedics, Rheumatology and Musculoskeletal Sciences, University of Oxford. JLR: Professor of Orthopaedic Surgery and Musculoskeletal Science, and Academic Consultant Shoulder & Elbow Surgeon, Nuffield Dept of Orthopaedics, Rheumatology and Musculoskeletal Sciences, Botnar Research Centre, University of Oxford. IR: Statistician, SITU, Statistician for the CSAW Study, Nuffield Dept of Orthopaedics, Rheumatology and Musculoskeletal Sciences, University of Oxford. JAC: Associate Professor, Methodologist, Centre for Statistics in Medicine, Deputy Director SITU, University of Oxford. CC: Portfolio Manager, SITU, CSAW Study Trial Manager, Nuffield Dept of Orthopaedics, Rheumatology and Musculoskeletal Sciences, University of Oxford. NM: Clinical Trials Study Coordinator, CSAW Study Coordinator, Nuffield Dept of Orthopaedics, Rheumatology and Musculoskeletal Sciences, University of Oxford. BAS: Medical Statistician, OCTRU, Nuffield Dept of Orthopaedics, Rheumatology and Musculoskeletal Sciences, University of Oxford. JLD: Professor of Social Medicine, Quintet Recruitment Intervention lead for School of Social and Community Medicine, University of Bristol and NIHR Collaboration for Leadership in Applied Health Research and Care (CLAHRC) West at University Hospitals Bristol NHS Trust. SG: Consultant Trauma & Orthopaedic Surgeon, Clinical Lecturer in Orthopaedics, Trauma Unit, Oxford University Hospitals NHS Trust. JS: Uehiro Professor in Practical Ethics, Director of the Institute for Science and Ethics, Co-Director, Oxford Geoengineering Programme, Director of The Oxford Centre for Neuroethics and Director of the Oxford Uehiro Centre for Practical Ethics, Oxford Centre for Neuroethics, University of Oxford. JM: Clinical Physiotherapy Specialist, Lead Clinical Physiotherapy Specialist, Nuffield Dept of Orthopaedics, Rheumatology and Musculoskeletal Sciences, University of Oxford. AG: Professor of Health Economics, Director of the Health Economics Research Centre, Health Economics Research Centre, Nuffield Dept of Population Health, University of Oxford. MJ: Senior Research Associate/Lecturer in Qualitative Health Science, The Quintet Research Group, School of Social and Community Medicine, University of Bristol. IT: Nuffield Professor of Anaesthetic Science, Head of the Nuffield Department of Clinical Neurosciences, University of Oxford. AJ: Associate Professor, Nuffield Dept of Orthopaedics, Rheumatology and Musculoskeletal Sciences, University of Oxford, Senior Statistician, NIHR Musculoskeletal Biomedical Research Unit, University of Oxford. KW: Research Fellow, Investigator for the CSAW Neuroimaging Observational Study, Nuffield Dept of Orthopaedics, Rheumatology and Musculoskeletal Sciences, University of Oxford.
Declaration of interests
We declare no competing interests.
Contributor Information
David J Beard, Email: david.beard@ndorms.ox.ac.uk.
Andrew J Carr, Email: andrew.carr@ndorms.ox.ac.uk.
CSAW Study Group:
Philip Ahrens, Cheryl Baldwick, Mark Brinsden, Harry Brownlow, David Burton, Muhammad Sohail Butt, Andrew Carr, Charalambos P Charalambous, Veronica Conboy, Lucy Dennell, Oliver Donaldson, Steven Drew, Amitabh Dwyer, David Gidden, Peter Hallam, Socrates Kalogrianitis, Cormac Kelly, Rohit Kulkarni, Tim Matthews, Julie McBirnie, Vipul Patel, Chris Peach, Chris Roberts, David Robinson, Philip Rosell, Dan Rossouw, Colin Senior, Bijayendra Singh, Soren Sjolin, Geoffrey Taylor, Balachandran Venkateswaran, and David Woods
Supplementary Material
References
- 1.Urwin M, Symmons D, Allison T. Estimating the burden of musculoskeletal disorders in the community: the comparative prevalence of symptoms at different anatomical sites, and the relation to social deprivation. Ann Rheum Dis. 1998;57:649–655. doi: 10.1136/ard.57.11.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Linsell L, Dawson J, Zondervan K. Prevalence and incidence of adults consulting for shoulder conditions in UK primary care; patterns of diagnosis and referral. Rheumatology. 2006;45:215–221. doi: 10.1093/rheumatology/kei139. [DOI] [PubMed] [Google Scholar]
- 3.Oh LS, Wolf BR, Hall MP, Levy BA, Marx RG. Indications for rotator cuff repair: a systematic review. Clin Orthop Relat Res. 2007;455:52–63. doi: 10.1097/BLO.0b013e31802fc175. [DOI] [PubMed] [Google Scholar]
- 4.Mitchell C, Adebajo A, Hay E, Carr A. Shoulder pain: diagnosis and management in primary care. BMJ. 2005;331:1124–1128. doi: 10.1136/bmj.331.7525.1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harkness EF, Macfarlane GJ, Nahit ES, Silman AJ, McBeth J. Mechanical and psychosocial factors predict new onset shoulder pain: a prospective cohort study of newly employed workers. Occup Environ Med. 2003;60:850–857. doi: 10.1136/oem.60.11.850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van der Windt DAWM, Thomas E, Pope DP. Occupational risk factors for shoulder pain: a systematic review. Occup Environ Med. 2000;57:433–442. doi: 10.1136/oem.57.7.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Virta L, Joranger P, Brox JI, Eriksson R. Costs of shoulder pain and resource use in primary health care: a cost-of-illness study in Sweden. BMC Musculoskelet Disord. 2012;13:17. doi: 10.1186/1471-2474-13-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Neer CS. Anterior acromioplasty for the chronic impingement syndrome in the shoulder. J Bone Joint Surg Am. 2005;87:1399. doi: 10.2106/JBJS.8706.cl. [DOI] [PubMed] [Google Scholar]
- 9.Cummins CA, Sasso LM, Nicholson D. Impingement syndrome: Temporal outcomes of nonoperative treatment. J Shoulder Elbow Surg. 2009;18:172–177. doi: 10.1016/j.jse.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 10.Coghlan JA, Buchbinder R, Green S, Johnston RV, Bell SN. Surgery for rotator cuff disease. Cochrane Database Syst Rev. 2008;1 doi: 10.1002/14651858.CD005619.pub2. CD005619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dorrestijn O, Stevens M, Winters JC, van der Meer K, Diercks RL. Conservative or surgical treatment for subacromial impingement syndrome? A systematic review. J Shoulder Elbow Surg. 2009;18:652–660. doi: 10.1016/j.jse.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 12.Judge A, Murphy RJ, Maxwell R, Arden NK, Carr AJ. Temporal trends and geographical variation in the use of subacromial decompression and rotator cuff repair of the shoulder in England. Bone Joint J. 2014;96-B:70–74. doi: 10.1302/0301-620X.96B1.32556. [DOI] [PubMed] [Google Scholar]
- 13.Henkus HE, de Witte PB, Nelissen RGHH, Brand R, van Arkel ERA. Bursectomy compared with acromioplasty in the management of subacromial impingement syndrome: a prospective randomised study. J Bone Joint Surg Br. 2009;91:504–510. doi: 10.1302/0301-620X.91B4.21442. [DOI] [PubMed] [Google Scholar]
- 14.Beard D, Rees J, Rombach I. The CSAW Study (Can Shoulder Arthroscopy Work?)—a placebo-controlled surgical intervention trial assessing the clinical and cost effectiveness of arthroscopic subacromial decompression for shoulder pain: study protocol for a randomised controlled trial. Trials. 2015;16:210. doi: 10.1186/s13063-015-0725-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Savulescu J, Wartolowska K, Carr A. Randomised placebo-controlled trials of surgery: ethical analysis and guidelines. J Med Ethics. 2016;42:776–783. doi: 10.1136/medethics-2015-103333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Donovan JL, Rooshenas L, Jepson M. Optimising recruitment and informed consent in randomised controlled trials: the development and implementation of the Quintet Recruitment Intervention (QRI) Trials. 2016;17:283. doi: 10.1186/s13063-016-1391-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dawson J, Rogers K, Fitzpatrick R, Carr A. The Oxford shoulder score revisited. Arch Orthop Trauma Surg. 2009;129:119–123. doi: 10.1007/s00402-007-0549-7. [DOI] [PubMed] [Google Scholar]
- 18.Constant CR, Murley AH. A clinical method of functional assessment of the shoulder. Clin Orthop Relat Res. 1987;214:160–164. [PubMed] [Google Scholar]
- 19.Freynhagen R, Baron R, Gockel U, Tolle TR. PainDETECT: a new screening questionnaire to identify neuropathic components in patients with back pain. Curr Med Res Opin. 2006;22:1911–1920. doi: 10.1185/030079906X132488. [DOI] [PubMed] [Google Scholar]
- 20.Gwilym SE, Keltner JR, Warnaby CE. Psychophysical and functional imaging evidence supporting the presence of central sensitization in a cohort of osteoarthritis patients. Arthritis Rheum. 2009;61:1226–1234. doi: 10.1002/art.24837. [DOI] [PubMed] [Google Scholar]
- 21.Rees JL, Dawson J, Hand GCR. The use of patient-reported outcome measures and patient satisfaction ratings to assess outcome in hemiarthroplasty of the shoulder. J Bone Joint Surg Br. 2010;92:1107–1111. doi: 10.1302/0301-620X.92B8.22860. [DOI] [PubMed] [Google Scholar]
- 22.Bjelland I, Dahl AA, Haug TT, Neckelmann D. The validity of the Hospital Anxiety and Depression Scale. An updated literature review. J Psychosom Res. 2002;52:69–77. doi: 10.1016/s0022-3999(01)00296-3. [DOI] [PubMed] [Google Scholar]
- 23.Chahal J, Mall N, MacDonald PB. The role of subacromial decompression in patients undergoing arthroscopic repair of full-thickness tears of the rotator cuff: a systematic review and meta-analysis. Arthroscopy. 2012;28:720–727. doi: 10.1016/j.arthro.2011.11.022. [DOI] [PubMed] [Google Scholar]
- 24.Wartolowska K, Judge A, Hopewell S. Use of placebo controls in the evaluation of surgery: systematic review. BMJ. 2014;348:g3253. doi: 10.1136/bmj.g3253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moseley JB, O'Malley K, Petersen NJ. A controlled trial of arthroscopic surgery for osteoarthritis of the knee. N Engl J Med. 2002;347:81–88. doi: 10.1056/NEJMoa013259. [DOI] [PubMed] [Google Scholar]
- 26.Sihvonen R, Paavola M, Malmivaara A. Arthroscopic partial meniscectomy versus sham surgery for a degenerative meniscal tear. N Engl J Med. 2013;369:2515–2524. doi: 10.1056/NEJMoa1305189. [DOI] [PubMed] [Google Scholar]
- 27.Thorlund JB, Juhl CB, Roos EM, Lohmander LS. Arthroscopic surgery for degenerative knee: systematic review and meta-analysis of benefits and harms. BMJ. 2015;350:h2747. doi: 10.1136/bmj.h2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carr A. Arthroscopic surgery for degenerative knee. BMJ. 2015;350:h2983. doi: 10.1136/bmj.h2983. [DOI] [PubMed] [Google Scholar]
- 29.Kise NJ, Risberg MA, Stensrud S, Ranstam J, Engebretsen L, Roos EM. Exercise therapy versus arthroscopic partial meniscectomy for degenerative meniscal tear in middle aged patients: randomised controlled trial with two year follow-up. BMJ. 2016;354:i3740. doi: 10.1136/bmj.i3740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wason JM, Stecher L, Mander AP. Correcting for multiple-testing in multi-arm trials: is it necessary and is it done? Trials. 2014;15:364. doi: 10.1186/1745-6215-15-364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schulz KF, Grimes DA. Multiplicity in randomised trials I: endpoints and treatments. Lancet. 2005;365:1591–1595. doi: 10.1016/S0140-6736(05)66461-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.